Abstract
Recognition of nucleic acids by TLR9 expressed by human plasmacytoid dendritic cells (PDC) plays a key role in the defense against viral infections. Upon microbial pathogen stimulation, PDC secret large amounts of type I interferon and attend thereby both innate and adaptive immune mechanisms. Unmethylated CpG motifs, which are an integral part of bacterial or viral DNA are used in vitro and in vivo to activate the TLR9 pathway, while inhibitory oligodeoxynucleotide (iODN) are capable to depress TLR9 signaling. In this study we show that TTAGGG motif containing iODN efficiently block the TLR9 signaling in terms of herpes simplex virus (HSV) induced type I interferon production by PDC. However, iODN as well as control ODN still promote PDC maturation with upregulated expression of costimulatory molecules, major histocompatibility complex molecules and other signs for PDC maturation. Furthermore, iODN and control ODN incubated PDC show increased T cell stimulatory functions. Co-culture experiments with autologous T cells indicate that iODN treated PDC induce more CD4+CD25+Foxp3+ T regulatory cells from naïve CD4+ T cells and preincubation of HSV stimulated PDC with iODN upregulated T cells’ IFN-γ production. These data indicate that iODN while blocking type I interferon production by PDC, modify PDC activation and maturation as well as T cell priming and stimulation. The knowledge about the different functions of iODN on PDC elucidated here might be crucial for immunotherapeutic strategies in which iODN motifs are used to prevent the interaction of CpG-DNA with TLR9 to calm down specific immunological responses, since our data show that iODN might not only have inhibitory functions but moreover be effective activators of immune cells.
Keywords: Inhibitory oligodeoxynucleotide, dendritic cell, cell activation, cell surface molecules, cytokines
Introduction
Human plasmacytoid dendritic cells (PDC), also known as interferon-producing cells {1–3}, release high amounts of type I interferon (IFN) after pathogen challenge {3–5}. PDC selectively express Toll-like receptor (TLR)7 and TLR9 {1,2} and are capable to sense nucleic acids of microbial pathogens via pathogen recognition receptors, which are an integral part of the innate immune system. Upon stimulation with viral DNA, PDC secret high amounts of IFN-α/β {6–8}, which directly links PDC from the innate to the adaptive immune system and inhibits viral replication by activation of effector cells such as NK cells, macrophages and cytotoxic T cells {9}. Furthermore, type I IFN secreted by PDC modulates the maturation of bystander dendritic cells and promotes priming of CD4+ T cells toward Th1 immune responses {10}. Following cytokine secretion, PDC differentiate into DC with high expression of costimulatory- as well as surface molecules involved in antigen presentation {11–13}. Thus, PDC are regarded as potential therapeutic target cells in the treatment of autoimmune, neoplastic or infectious diseases.
Herpes simplex virus (HSV) triggers PDC type I IFN production with the help of TLR9 recognizing viral DNA in vivo and this can be mimicked in vitro using either ultraviolet (UV) inactivated HSV or purified genomic HSV DNA {6,8}. Mainly, two types of DNA sequence are able to modulate TLR9 signals in PDC. On one hand, immunostimulatory sequences (ISS), existing in most pathogen DNA, activate the TLR9 pathway, induce strong Th1 responses and are therefore used as adjuvants in different immunotherapeutic approaches {14}. On the other hand inhibitory or immunoregulatory DNA sequences (IRS) {15} which neutralize ISS activation via the TLR9 pathway. Inhibitory sequences exist in many species, such as viral DNA, mutated CpG sequences and repeat TTAGGG motifs in mammalian telomeres {16–19}. The most potent inhibitory sequence, 4 repeat (TTAGGG) sequence, is used in this study to modulate HSV induced PDC TLR9 signaling activation {19}. The exact mode of action of these IRS is unclear so far. Therefore, further investigations of IRS and their influence on immune cells are indispensable to understand TLR9 modulation in order to target these pathways for the treatment of diseases with inappropriate TLR9 signaling. However, studies on the functional impact of inhibitory oligodeoxynucleotide so far available are mainly limited to TLR9 signaling in murine models, while studies on inhibitory oligodeoxynucleotide induced changes of human PDC are rare. Therefore we tended to investigate phenotypic and functional changes induced by iODN on human PDC.
MATERIALS AND METHODS
Inhibitory oligodexoxynucleotide and UV inactivation of HSV
Inhibitory TTAGGG motif containing phosphorothioate ODN was obtained from Invitrogen, San Diego, USA. The sequence of the inhibitory oligodeoxynucleotide was: 5′-TTT AGG GTT AGG GTT AGG GTT AGG G -3′ (25 mer). The sequence of phosphorothioate control ODN to TTAGGG containing iODN was: 5′-TTC AAA TTC AAA TTC AAA TTC AAA-3′ (Sigma). To examine different iODN efforts, iODN 2088 (5′-TCC TGG CGG GGA AGT-3′, Invivogen, San Diego, USA) was also used in the study. The ODNs were dissolved in endotoxin free water supplied by the company. HSV type 1 strain 17+ was propagated on Vero cells in maintenance medium composed of Eagle’s Minimum Essential Medium (Biochrom, Berlin, Germany) supplemented with 10% fetal calf serum (FCS) (Biochrom) with Earle’s balanced salts (MEM-Earle). For virus stock preparation, Vero cells were infected with HSV at a multiplicity of infection (MOI) of 0.1. Cell culture supernatant was harvested 72 hours after inoculation and clarified by centrifugation at 1.931g for 10 min followed by 30 min-lasting centrifugation at 3.434g. Virus was inactivated in complete darkness by ultraviolet (UV) light at 254 nm. Samples were irradiated for 30 min in sterile petri dishes containing a magnetic stir bar stirring at low speed (thickness of virus suspension 1.5–2 mm, distance between virus suspension and mercury vapor lamp 15 cm). Determination of viral titer before and after UV radiation was performed by titration in microtiter plates using Vero cells starting with undiluted virus suspension. Additionally, supernatants of mock infected Vero cells were prepared as controls. Inactivated HSV and mock control was stored at −80°C before use. All materials in medium with working concentration to stimulate PDC were analyzed for endotoxin and have been shown to contain only very low endotoxin levels (<0.005 EU/ml) tested by Cambrex Bio Science Verviers, Belgium.
Isolation and stimulation of PDC
Peripheral blood mononuclear cells (PBMC) were isolated as described previously {20} from buffy coats of human healthy blood donors provided from the blood bank of the University of Bonn. PDC were isolated using human BDCA-4 MicroBead isolation Kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) as described before {20}. PDC were >95% BDCA2+CD123+ as measured by flow cytometry. PDC were diluted at 1 million cells/ml and seeded at 0.2 million cells/well into 96-well round-bottomed plates in Dulbecco’s Modified Eagle’s Medium medium supplemented with 10% heat-inactivated FCS, L-glutamine (2mmol/L), penicillin (100U/ml), streptomycin (100μg/ml) and recombinant IL-3 (10ng/ml). PDC were divided into four groups: PDC incubated with inactivated Vero cell supernatant (control group), PDC stimulated with UvHSV(MOI=1) (UvHSV group), PDC stimulated with 4μM TTAGGG containing iODN (iODN group) and PDC stimulated with UvHSV(MOI=1) combined with iODN(4μM) (iODN+UvHSV group). Before adding UvHSV, PDC were preincubated 40min with iODN to allow full uptake of iODN as described in the literature {21}. To examine different ODN’s stimulatory effects to PDC, TTAGGG motif containing iODN (iODN), iODN 2088 (iODN 2088) and Control ODN (Con ODN) were added to PDC at final concentration of 4μM, untreated PDC left as blank control. All PDC were cultured for 18 hours at 37°C, 5% CO2.
Cell surface and intracellular phenotyping of PDC by flow cytometry
After 18 hours of stimulation, supernatants of PDC were centrifuged and collected. Afterwards intracellular and extracellular staining has been performed as reported in detail elsewhere {20}. Monoclonal antibodies (mAb) used for flow cytometric stainings included: mAb against TLR9 (clone: 26C593.2) purchased from Imgenex Corp, San Diego, USA, mAb against CD83 (clone:HB15e), mAb against CD80 (clone:L307.4), mAb against CD86 (Clone:2331), mAb against HLA-A,B,C (clone:G46-2.6), mAb against HLA-DR (clone:G46-6), Phycoerythrin-Cy5 (PE-Cy5) labeled mAb against CD123 (clone:9F5) and respective isotype-matched control IgG were purchased from BD Biosciences, Heidelberg, Germany. The phycoerythrin (PE) labeled mAb against the blood-dendritic-cell antigen (BDCA)-2 (clone:AC144) was from Miltenyi Biotec GmbH. The anti-human FcεRIα chain mAb (clone: 22E7) was kindly provided by Dr. J. Kochan (Hoffmann-La Roche Inc., Nutley, NJ). After washing with PBS, the cells were measured with the help of a FACSCanto Flow Cytometer (BD Biosciences, San Jose, USA) and data were analyzed with BD FACSDiva software version 5.0.1. The antigen expression level was stated as Relative Fluorescence Index (RFI), RFI=(MFIantigen−MFIisotype control)/MFIisotype control.
Co-culture of PDCs with autologous naïve CD4+ T cell
After PDC isolation, naïve CD4+ T cells were isolated from the same PBMC. Briefly, naïve CD4+ T cells were enriched using a two-step approach with the help of magnetic microbead labeled antibodies (Miltenyi Biotec). Briefly, CD8+, CD14+, CD16+, CD19+, CD56+, CD36+, CD45RO+, CD123+, TCRγ/δ+, and CD235a+ cells were labeled with biotin-conjugated antibodies first and subsequently labeled with anti-biotin microbeads for magnetic depletion. Purity was assessed by staining with CD4 (Clone: RPA-T4) (BD Biosciences) and CD45RA (Clone: HI100) (BD Biosciences) antibodies and was about 99%. After 18 hours of stimulation, supernatants of PDC were collected and stored at −80°C, PDC were washed three times and co-cultured with autologous naïve CD4+ T cells at a ratio of 1:10 PDC/T cells in a 96-well round-bottomed plate. After 5–6 days of culture, cells were harvested, supernatants were collected and stored at −80 °C.
Forkhead box protein p3 (Foxp3) staining of T cells
At day 7 of co-culture, the cells were fixed and intracellularly stained for Forkhead box protein p3 (Foxp3) expression with FITC anti-human Foxp3 staining Set (eBiosciences, San Diego, USA) as described in the manufacture’s instruction. Briefly, T cells were stained with FITC labeled mAb against Foxp3 (clone: PCH101, eBiosciences), followed by extracellular staining with PE-Cy5 labeled mAb against CD4 and PE-labeled mAb against CD25 (clone:M-A25)(BD Biosciences). The percentage of Foxp3+CD4+CD25+ regulatory T cells was measured with the FACSCanto Flow Cytometer.
Mixed leukocyte reaction (MLR)
To assess the ability of differentially treated PDC to stimulate naïve autologous CD4+ T cells, mixed leukocyte reactions (MLR) have been performed. Autologous T cells (2×105) were cultured alone or with differentially treated PDC as described above at PDC/T cell ratios 1:10, 1:100, and 1:1000 for a total of 96 hours in 96-well round-bottomed plate. After 72 hours of co-culture 0.5 Ci (3H)thymidine (3HTdR, 5.0Ci/mM, Amersham Corp., UK) was added to the cells. Culture plates were frozen at −20°C for several days, harvested and counted in a Wallac Microbeta Jet 1450 Microplate Scintillation/Luminescence Counter (Long Island Scientific, NY, USA). Proliferation data were quantified as counts per minute (cpm).
ELISA assays of human IFN-α/β and IFN-γ
The amount of IFN-α and -β in the supernatant of PDC was measured with human IFN-α and IFN-β ELISA kit (PBL Biomedical Laboratories, Piscataway, USA). IFN-γ concentration in the cell culture supernatants was assessed with human IFN-γ ELISA kit (R&D Systems, Wiesbaden, Germany). ELISA assays were performed according to the manufacturer’s instruction.
Statistical analysis
Statistical analysis was performed using Wilcoxon-Test and SPSS 12.0 software (SPSS Inc, Chicago, USA). A p-value<0.05 was considered as statistically significant.
RESULTS
The inhibitory oligodeoxynucleotide blocks type I IFN production of PDC upon HSV stimulation
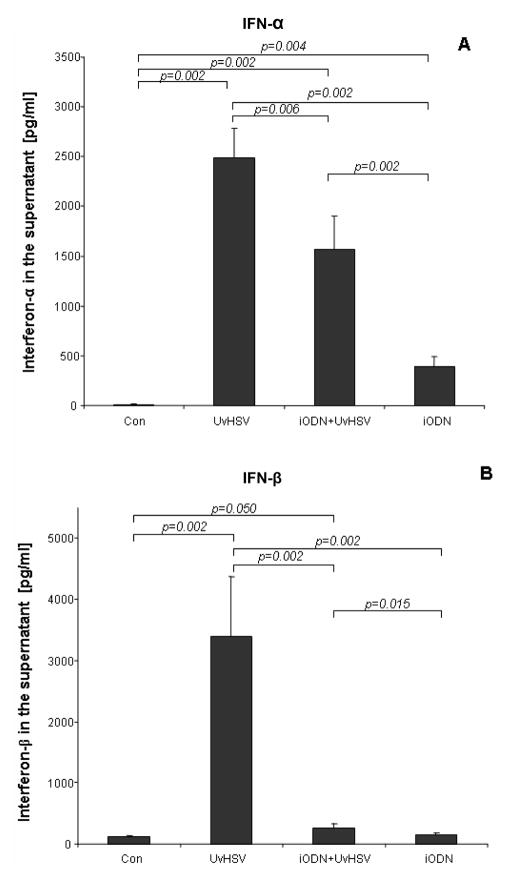
TLR9 signaling in PDC can be activated by viral challenge, including UvHSV {7} which leads to the production of type I IFN. Using a similar approach, we tended to evaluate PDC IFN secretion upon UvHSV (MOI=1) stimulation and the effect of iODN on UvHSV mediated TLR9 activation. After 18 hours of culture, PDC activated with UvHSV secreted high amounts of type I IFN (IFN-α/β) (Fig. 1A, B). In contrast, pre-incubation of PDC with iODN before UvHSV stimulation significantly suppressed both, UvHSV induced IFN-α and IFN-β secretion of PDC. Furthermore, addition of iODN alone induced slight IFN-α production by PDC (Fig. 1A). Together these data indicate that the iODN impedes type I interferon production by PDC upon viral TLR9 stimulation.
Figure 1. inhibitory oligodeoxynucleotide impaired type I IFN production of UvHSV treated PDC.
PDC were stimulated with Uv-activated Vero cell supernatant as mock control (Con), UvHSV alone (UvHSV), UvHSV in combination with iODN (iODN+UvHSV), or with iODN alone (iODN). 18 hours later, supernatants were harvested and the amount of type I IFN (IFN-α/β) in the cell culture supernatant was measured by ELISA. (A) IFN-α concentration in PDC supernatants is depicted on the y-axis (data from 12 different donors). (B) IFN-β concentration in PDC supernatants is depicted on the y-axis (data from 12 different donors). Error bars indicate the standard error of the mean (SEM). (*: p<0.05).
Incubation of PDC with inhibitory oligodeoxynucleotide promotes PDC activation and maturation
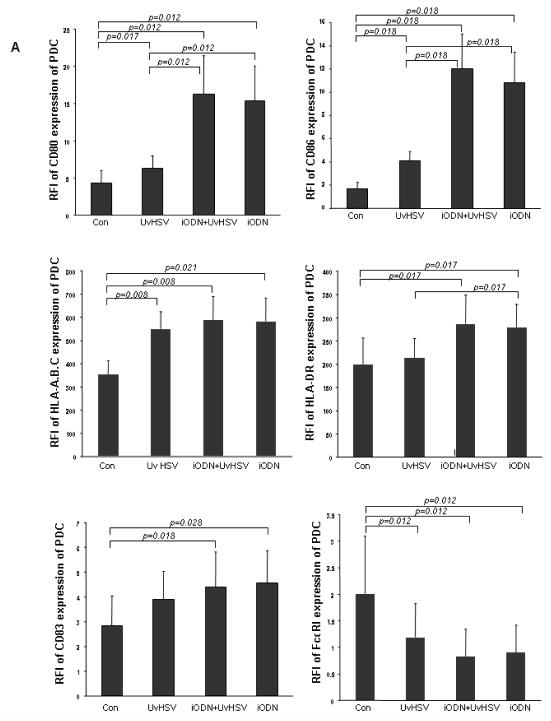
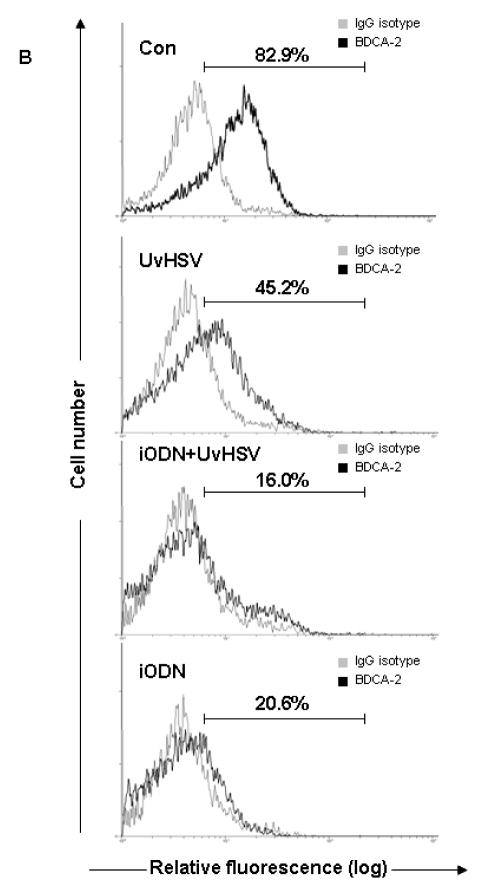
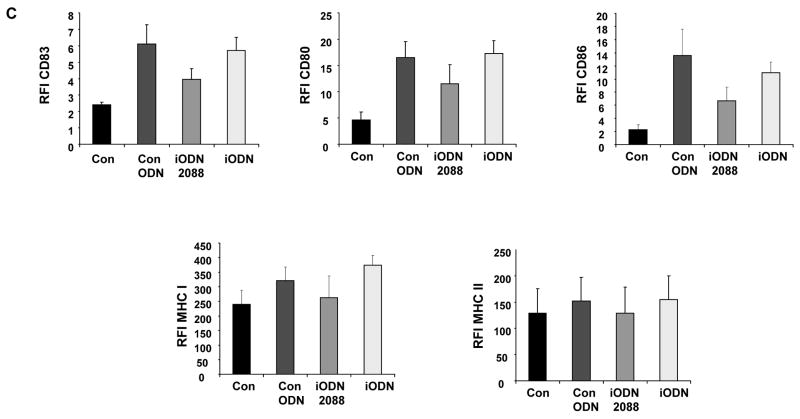
Viral challenge has been shown to induce PDC activation and maturation, mirrored by the up-regulation of major histocompatibility complex (MHC) molecules as well as costimulatory molecules on the surface of PDC. To examine both, the activation and maturation state of PDC under the different treatment conditions, phenotyping of PDC has been performed by flow cytometric staining. After UvHSV stimulation, PDC significantly upregulated costimulatory molecules, such as CD80 and CD86, and MHC molecules such as HLA-DR and HLA-A,B,C, as well as CD83, a typical DC maturation marker. Furthermore, the high affinity receptor for IgE, FcεRI was down-regulated after challenge with UvHSV, according to previous reports about a reciprocal counter-regulation of the TLR9 and FcεRI expression of PDC {22} (Fig. 2A). At the same time, BDCA-2, a PDC marker known to decrease its expression during PDC maturation, was significantly down-regulated after PDC activation with UvHSV {23} (Fig. 2B). Most interestingly, incubation of PDC with iODN with or without UvHSV promoted PDC activation and maturation of PDC with significantly higher expression of CD80, CD86 and CD83, HLA-DR and HLA-A,B,C, as well as significantly stronger down-regulation of BDCA-2 and FcεRI from the cell surface (Fig. 2A and 2B). In addition, we could show that another iODN (2088) with a GGGG motif as well as control ODN were able to induce upregulated CD80, CD86, CD83 and MHC I expression by PDC (Fig. 2C).
Figure 2. inhibitory oligodeoxynucleotide induces PDC activation and maturation.
After 18 hours of treatment, extracellular staining of mock (Con), UvHSV, iODN+UvHSV and iODN treated PDC has been performed. (A). Data were shown as mean value of the relative fluorescence index (RFI) depicted on the y-axis, data from 8 different donors, Error bars indicate SEM (B) BDCA-2 expression of differentially treated PDC is depicted as fluorescence intensity on the x-axis. This is one representative experiment of 9 independent experiments. Mean of the RFI of surface expression of CD80, CD86, CD83, MHC I and II of unstimulated PDC (Con), PDC incubated with iODN 2088 with GGGG-motif, iODN with TTAGGG motif and control ODN ± SEM of n=4 is shown (C).
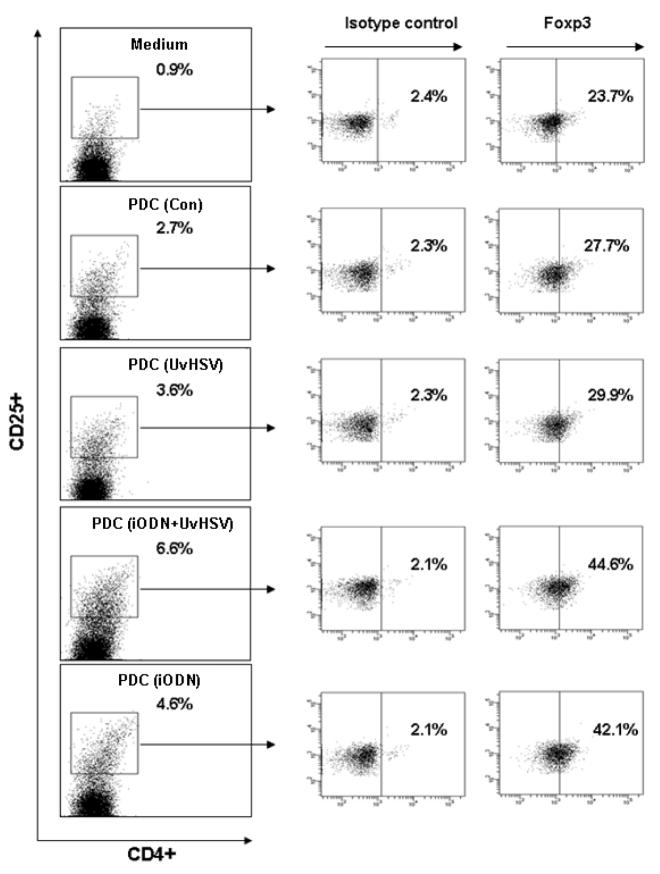
Enhanced ability of PDC to prime naïve CD4+CD45RA+ into CD4+CD25+Foxp3+ expressing T cells after inhibitory oligodeoxynucleotide incubation
Activated PDC have a distinct ability to derive naïve T cells into CD4+CD25+Foxp3+ regulatory T (Treg) cells {24}. To examine the capability of PDC to promote the outcome of CD4+CD25+ Foxp3+ Treg cells from naïve T cells, co-culture experiments of autologous PDC treated as previously described with naïve CD4+ T cell have been performed. As a result, iODN treated PDC induced more CD4+CD25+ expressing T cells compared to UvHSV activated PDC (Fig. 3 on the left side). Similarly, within CD4+CD25high T cells, iODN treated PDC induced more Foxp3 expressing CD4+CD25high T cells than PDC stimulated with UvHSV (MOI=1) (Fig. 3 on the right side). The mean percentage of CD4+CD25highFoxp3+ T cells within CD4+CD25high T cells co-cultured with differentially treated PDC was 22.80±2.57% for control PDC, 22.87±2.82% for PDC stimulated with UvHSV, 32.23±4.88% for PDC incubated with iODN combined with UvHSV and 32.1±5.04% for PDC incubated with iODN alone. Together these data indicate higher induction of Foxp3 expressing T cell subsets by incubation of PDC with iODN. Increased number of Foxp3+CD4+CD25high T cells was also observable after preincubation of PDC with iODN (2088) with a GGGG motif as well as control ODN (data not shown).
Figure 3. PDC stimulated with inhibitory oligodeoxynucleotide generate more CD4+CD25+Foxp3+ T regulatory cells from autologous naïve T cells.
After stimulation with iODN, UvHSV, iODN and UvHSV combination or mock infection (Con) for 18 hours, PDC subgroups were co-cultured at a ratio of 1:10 (PDC/T cells) with autologous naïve CD4+CD45RA+ T cells. 7 days later, the percentage of CD4+CD25highFoxp3+ T regulatory cells (right side of the figure) within the CD4+CD25high T cell fraction (left side of the figure) was measured by flow cytometry. One representative experiment of 5 independent experiments is shown.
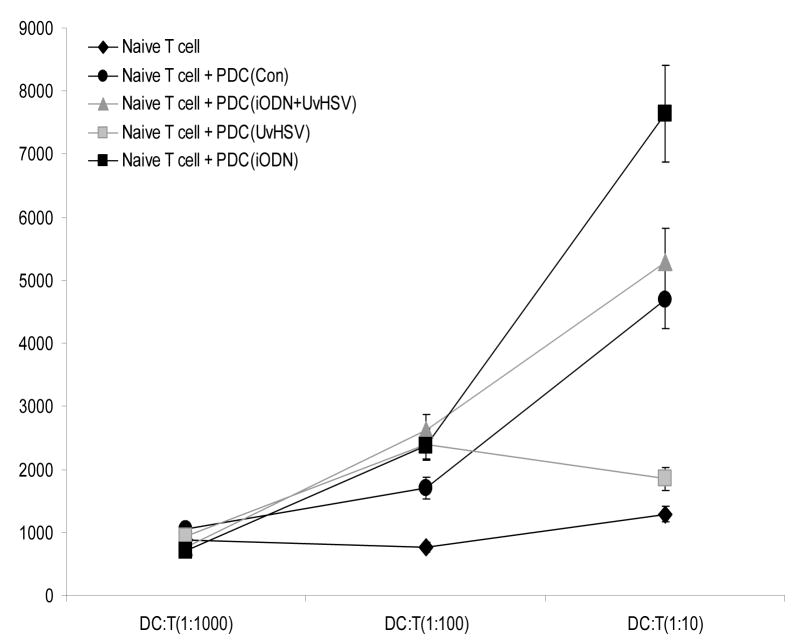
Enhanced stimulatory capacity of inhibitory oligodeoxynucleotide treated PDC towards autologous T cells
Stimulation of T cells is a characteristic and important feature of DC. Therefore in the next step, MLR assays have been performed to investigate the capacity of differentially treated PDC to stimulate autologous T cells. PDC did not exert any stimulatory functions towards naïve T cells at the ratio of 1:1000 since no T cell proliferation was observed among different groups. PDC stimulated with iODN, UvHSV, iODN and UvHSV exerted moderate stimulatory function toward naïve T cells at PDC/T cell ratio of 1:100. Interestingly, at the ratio of 1:10, PDC incubated with UvHSV depressed naive T cell proliferation while PDC stimulated with iODN maintained or increased their stimulatory ability (Fig. 4). Taken together, PDC cultured with iODN alone can promote CD4+ T cell proliferation. Further on, incubation of PDC with iODN (2088) with a GGGG motif as well as control ODN also profoundly increased the stimulatory capacity of PDC toward T cells (Fig. 4B).
Figure 4. inhibitory oligodeoxynucleotide incubated PDC induce high T cell proliferation.
After 3 days of co-culture with differentially treated PDC, T cell proliferation was measured by MLR. PDC were co-cultured with T cells at PDC/T cell ratios of 1:10, 1:100 and 1:1000. The incorporation of (3H)thymidine of proliferating T cells was indicated as counts per minute (cpm) on the y-axis. Average data from 6 independent experiments were shown. Error bars indicate SEM. (*p<0.05) (A). Mean of cpm values ± SEM of unstimulated PDC (Con), PDC incubated with iODN 2088 with GGGG-motif, iODN with TTAGGGG motif and control ODN (n=3, every experiment was triplicate) is shown, APC/T-cell ratio is depicted on the x-axis (B).
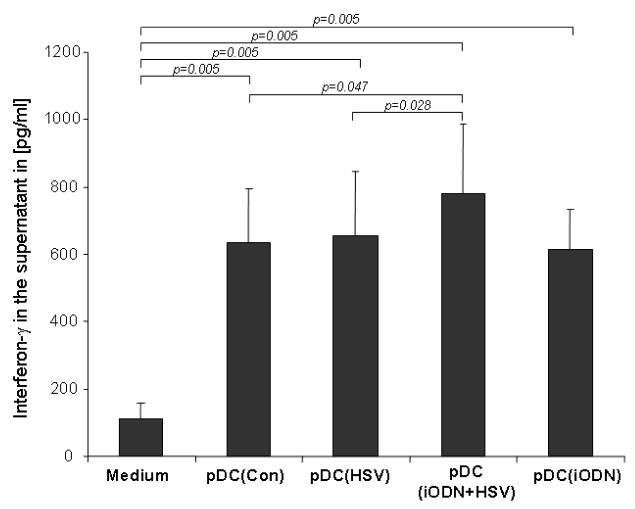
PDC incubated with inhibitory oligodeoxynucleotide and UvHSV prime naïve T cells into IFN-γ producing T cells
An important feature of PDC is their capacity to prime naïve T cells, virus stimulated PDC can prime naive CD4+ T cells to produce IFN-γ {11}. In order to assess in which way the different conditions modulate the T cell priming capacities of PDC, we performed co-culture experiments with autologous naïve T cells and measured IFN-γ secretion of co-cultured T cells in the cell culture supernatant. As a result incubation of PDC with iODN alone did not profoundly influence the priming of T cells into IFN-γ producing T cells, while the highest amount of IFN-γ releasing T cells could be achieved by PDC pre-incubated with iODN and subsequent activation via UvHSV (Fig. 5).
Figure 5. inhibitory oligodeoxynucleotide and HSV combination stimulated PDC prime naïve CD4+CD45RA+ T cells into IFN-γ producing T cells.
After 6 days of co-culture, the amount of IFN-γ in the supernatant of PDC co-cultured with T cells at a ratio of 1:10 was measured and indicated as pg/ml on the y-axis (data from 10 different donors). Error bars indicate SEM.
DISCUSSION
PDC are regarded as important facilitators between innate and adaptive immune responses due to their ability to secrete type I IFN upon microbial pathogen stimulation and their antigen presenting and T cell priming capacity {11–13}. TLR9 signaling induced by virus stimulation in PDC leads to the activation of signal transduction mechanisms which involve NF-κB and the adaptor protein MyD88 to orchestrate inflammatory cytokine production {25,26}. Accumulating data suggest that increased levels of IFN-α/β based on inappropriate TLR9 signaling in PDC play a significant role in the pathogenesis of autoimmune disorders such as Lupus Erythematosus (SLE) and Insulin-dependent diabetes mellitus (IDDM) {25}. Recent studies report further that PDC are critically involved in the initiation of psoriasis, a chronic inflammatory skin disorder through IFN-α production, while on the other hand blockage of IFN-α signaling with BDCA-2 antibody prevented the development of psoriasis in animal models {27}. Thus PDC represent promising cellular targets to modulate type I IFN induced immune disorders.
Here we demonstrated TTAGGG motif containing iODN could decrease type I IFN production but not decrease other cellular stimulatory functions of PDC in adaptive immunity in vitro. Furthermore inhibitory effect on TLR9 signaling did not result from degradation of TLR9 since TLR9 expression of PDC did not decrease and is rather slightly up-regulated (data not shown). The TTAGGG containing iODN dampens stronger IFN-β (about 90%) than IFN-α (about 40%) secretion, which might be related to the specific sequence of iODN used in this study. Interestingly, despite inhibition of TLR9 activation in UvHSV stimulated PDC preincubated with iODN, PDC still upregulated functional cell surface molecules and showed improved stimulatory properties toward T cells. These effects were reproducible by using another more potent iODN, which contains a GGGG motif indicating that the mechanism is not limited to a particular type of iODN. Further on, using phosphotioate control ODN, we could show that the stimulatory properties of iODN driven on PDC result most likely from the phosphotioate backbone and the DNA itself. Furthermore, PDC still can be stimulated to maturation by lose dose of iODNs (400nM) with upregulation of cell surface markor CD83, CD80, CD86, MHC I and II (data not shown). The higher induction of Foxp3+CD4+CD25+ T cell subset by iODN shown here might be of clinical relevance for diseases treated with iODN, such as autoimmune disorders, in which Foxp3-expressing T cells are critically involved in the underlying pathophysiologic mechanisms{28}. Therefore, potential changes in the induction of regulatory T cells by iODN or the activation stage of T cells, which might impact positively or negatively on the disease state should be evaluated further in murine models and additional futural studies. Together, our data strongly suggest that iODN might have bivalent effects on human PDC mirrored by suppressive capacities in terms of type I interferon production and via the induction of CD4+CD25+Foxp3+ T cell subtypes.
In recent years iODN have attracted much attention of researchers from different fields due to their remarkable attribute to neutralize CpG DNA stimulatory effects and the resulting potential of iODN to be therapeutically used for the treatment of autoimmune and inflammatory diseases {29}. The first iODN were identified as poly(GC)-rich sequences with ability to competitively inhibit the interaction of CpG with TLR9.16,30–32 In general, two types of inhibitory ODN exist {33}: GC-rich iCpG and poly(G) motifs such as the TTAGGG motifs containing iODN used in this study. The GC-rich iODN suppress TLR9 signaling in PDC and B cells and reduces CpG dependent activation of the signal transcription factors NF-κB and AP-1 {33, 34}. The latter iODN has broader immunosuppressive activities, leading to the suppression of proinflammatory signals and Th1 cytokines, including tumor-necrosis factor (TNF)-γ, IL-12, IL-6, and IL-18, with blockage of the phosphorylation of STAT1 and STAT4 {33, 34}. Several reports have demonstrated that TTAGGG motif containing iODN can effectively prevent or slow down the progression of autoimmune or inflammatory diseases in murine models {29}. Therefore much attention has been paid to TTAGGG motif containing iODN, which hold promise to represent useful therapeutic tools. However, in comparison to the rising knowledge about the signaling pathways affected by iODN, iODN related changes of the phenotype and functional characteristics of PDC and B cells have been less often investigated in the human system. In this study we showed that TTAGGG motif containing iODN were capable to block TLR9 signaling mediated via UvHSV. Most importantly, the possibility of concomitant activation of PDC and other immunocompetent cells by iODN requires further investigations to exclude or minimize the risk for unwanted side effects. The knowledge about the different specific and unspecific functions of iODN on PDC elucidated here might be crucial for immunotherapeutic strategies in which iODN motifs are used to prevent the disorder of TLR9 signaling to calm down specific immunological responses.
Acknowledgments
This study was supported in part by the NIH/NIAID contract N01 AI40029, grants from the DFG NO 454/1-3, NO454/2-3 and BONFOR. N.N. is supported by a Heisenberg-Fellowship of the DFG NO454/3-1.
ABBREVIATIONS
- PDC
Plasmacytoid dendritic cells
- ISS
Immunostimulatory oligonucleotide sequence
- IRS
Immunoregulatory oligonucleotide sequence
- MOI
Multiplicity of infection
- iODN
Inhibitory oligodeoxynucleotide
- RFI
Relative fluorescence index
- MFI
Mean fluorescence intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 3.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 4.Cederblad B, Lindahl M, Alm G. Stimulation of natural interferon-α/β-producing cells by Staphylococcus aureus. J Interferon Cytokine Res. 1996;16:7–16. doi: 10.1089/jir.1996.16.7. [DOI] [PubMed] [Google Scholar]
- 5.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 6.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–20. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–7. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 9.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;12:419–24. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 10.Krug A, Veeraswamy R, Pekosz A, Kanagawa O, Unanue ER, Colonna M, Cella M. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–26. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonteneau JF, Gilliet M, Larsson M, Dasilva I, Munz C, Liu YJ, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–6. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 13.Schlecht G, Garcia S, Escriou N, Freitas AA, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–15. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- 14.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–8. doi: 10.1038/nm1210. Review. [DOI] [PubMed] [Google Scholar]
- 15.Duramad O, Fearon KL, Chang B, Chan JH, Gregorio J, Coffman RL, Barrat FJ. Inhibitors of TLR-9 act on multiple cell subsets in mouse and man in vitro and prevent death in vivo from systemic inflammation. J Immunol. 2005;174:5193–200. doi: 10.4049/jimmunol.174.9.5193. [DOI] [PubMed] [Google Scholar]
- 16.Krieg AM, Wu T, Weeratna R, Efler SM, Love-Homan L, Yang L, Yi AK, Short D, Davis HL. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc Natl Acad Sci USA. 1998;95:12631–6. doi: 10.1073/pnas.95.21.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisetsky DS, Reich CF. Inhibition of murine macrophage IL-12 production by natural and synthetic DNA. Clin Immunol. 2000;96:198–204. doi: 10.1006/clim.2000.4897. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Lenert P, Weeratna R, McCluskie M, Wu T, Davis HL, Krieg AM. Identification of methylated CpG motifs as inhibitors of the immune stimulatory CpG motifs. Gene Ther. 2001;8:1024–32. doi: 10.1038/sj.gt.3301482. [DOI] [PubMed] [Google Scholar]
- 19.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 20.Novak N, Allam JP, Hagemann T, Jenneck C, Laffer S, Valenta R, Kochan J, Bieber T. Characterization of FcepsilonRI-bearing CD123 blood dendritic cell antigen-2 plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 2004;114:364–70. doi: 10.1016/j.jaci.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 21.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroeder JT, Bieneman AP, Xiao H, Chichester KL, Vasagar K, Saini S, Liu MC. TLR9- and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–31. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 23.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 24.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 25.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 26.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 27.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–43. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–16. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 29.Inohara, Chamaillard, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–83. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Klinman DM, Gursel I, Klaschik S, Dong L, Currie D, Shirota H. Therapeutic potential of oligonucleotides expressing immunosuppressive TTAGGG motifs. Ann N Y Acad Sci. 2005;1058:87–95. doi: 10.1196/annals.1359.015. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Lenert P, Weeratna R, McCluskie M, Wu T, Davis HL, Krieg AM. Identification of methylated CpG motifs as inhibitors of the immune stimulatory CpG motifs. Gene Ther. 2001;8:1024–32. doi: 10.1038/sj.gt.3301482. [DOI] [PubMed] [Google Scholar]
- 33.Lenert P, Stunz L, Yi AK, Krieg AM, Ashman RF. CpG stimulation of primary mouse B cells is blocked by inhibitory oligodeoxyribonucleotides at a site proximal to NF-κB activation. Antisense Nucleic Acid Drug Dev. 2001;11:247–56. doi: 10.1089/108729001317022241. [DOI] [PubMed] [Google Scholar]
- 34.Yamada H, Gursel I, Takeshita F, Conover J, Ishii KJ, Gursel M, Takeshita S, Klinman DM. Effect of suppressive DNA on CpG induced immune activation. J Immunol. 2002;169:5590–4. doi: 10.4049/jimmunol.169.10.5590. [DOI] [PubMed] [Google Scholar]
- 35.Shirota H, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides inhibit Th1 differentiation by blocking IFN-gamma- and IL-12-mediated signaling. J Immunol. 2004;173:5002–7. doi: 10.4049/jimmunol.173.8.5002. [DOI] [PubMed] [Google Scholar]
- 36.Shirota H, Gursel I, Gursel M, Klinman DM. Suppressive oligodeoxynucleotides protect mice from lethal endotoxic shock. J Immunol. 2005;174:4579–83. doi: 10.4049/jimmunol.174.8.4579. [DOI] [PubMed] [Google Scholar]