Abstract
mKeima is an unusual monomeric red fluorescent protein (λemmax ~620 nm) that is maximally excited in the blue (λexmax ~440 nm). The large Stokes shift suggests that the chromophore is normally protonated. A 1.63 Å resolution structure of mKeima reveals the chromophore to be imbedded in a novel hydrogen bond network, different than in GFP, which could support proton transfer from the chromophore hydroxyl, via Ser142, to Asp157. At low temperatures the emission contains a green component (λemmax ~535 nm), enhanced by deuterium substitution, presumably resulting from reduced proton transfer efficiency. Ultrafast pump/probe studies reveal a rising component in the 610 nm emission with lifetime ~4 ps, characterizing the rate of proton transfer. Mutation of Asp157 to neutral Asn changes the chromophore resting charge state to anionic (λexmax ~565 nm, λemmax ~620 nm). Thus, excited state proton transfer (ESPT) explains the large Stokes shift. This work unambiguously characterizes green emission from the protonated acylimine chromophore of red fluorescent proteins.
The discovery of ESPT within a biological system was made with wild type Green Fluorescent Protein (GFP)1. Upon excitation of the protonated neutral chromophore (A band), the pKa drops by several units. Proton transfer from the hydroxyl group then takes place within ~12 picoseconds to generate an anionic intermediate I* that subsequently emits a green photon2-5. Excitation of the anionic chromophore (B band) gives rise to instantaneous green emission. Crystallographic studies2 revealed a “proton wire” connecting the chromophore hydroxyl group through a water molecule and Ser205 to acceptor Glu222. Glu222 was verified as a proton acceptor by ultrafast IR spectroscopy4, but proton migration to solvent is possible6. The rate of proton transfer is characterized by a 6-fold deuterium isotope effect1. ESPT has since been shown to form a basis for practical applications of GFP such as ratiometric GFP biosensors for pH7 and thiol-disulfide redox potential8, 9.
The tetrameric red fluorescent protein Keima, originally developed for use in dual-color fluorescence cross-correlation spectroscopy10, was derived by directed evolution from a nonfluorescent chromoprotein discovered in the stony coral Montipora sp. Dimeric and monomeric forms (mKeima, MW 25 kDa) were also produced. mKeima and GFP are both excited in the blue but their emission is well separated, which is advantageous for two-color labeling schemes in cell biology.
Fluorescent proteins that exhibit ESPT are useful for the study of the factors controlling the rates of proton transfer. The process can be initiated by light and observed on picosecond time scales. The details of the proton transfer pathways can be determined at atomic resolution and modified by mutagenesis, see for example references11-15. Here we describe our structural and spectroscopic studies of mKeima, which reveals a novel ESPT pathway from the chromophore hydroxyl through Ser142 to acceptor Asp157.
The steady state emission of mKeima has a strong dependence on temperature and deuterium substitution (Figures 1 and S1 in Supporting Information). At RT, the emission maximum is broad at ~620 nm, but is shifted 25 nm to the blue at 83 K. At low temperature, a blue shoulder with maximum at ~535 nm is apparent, the amplitude of which is enhanced and the curve slightly red shifted by deuterium substitution. We assign the blue shoulder to the S1→S0 transition of the protonated chromophore (Figure S1). Ultrafast pump-probe measurements of I* emission reveal a negative signal that achieves a maximum in about 20 ps (Figure S2). 40% of the signal amplitude occurs within the instrument response time of 500 fs and is interpreted as arising from stimulated emission from the red tail (Figure S1) of the A-band. The remainder, attributed to stimulated emission from the growing I* state, grows with a time constant of ~4 ps and is consistent with a proton transfer process of lifetime ~4 ps.
Figure 1.
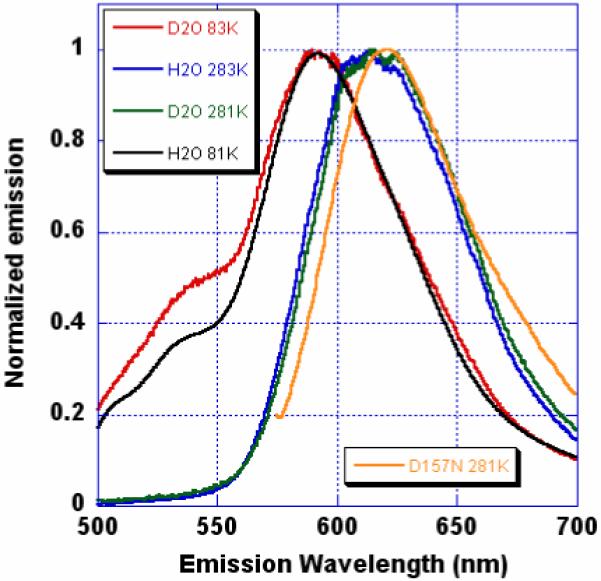
Emission spectra of mKeima (excitation 430 nm) as a function of temperature and solvent (H2O or D2O). Orange curve: emission of D157N mutant (excitation 565 nm) in H2O at 281K.
The 1.63 Å resolution atomic model (Table S1) is, as expected, very similar to that determined for the nonfluorescent chromoprotein Rtms516 (PDB ID 1MOU). Unlike in Rtms5, the acylimine chromophore is in the cis configuration, presumably due to the replacement of His146 in Rtms5 with Ser142 (Fig. S4B) in mKeima. In the intact, mature polypeptide, the chromophore is identical to that found in DsRed17. The imidazolidinone and phenyl rings are nearly coplanar, with torsion angle deviations about 5 and -13°; comparable to that seen in GFP and consistent with the fluorescence quantum yield of 0.24. However, 30-50% hydrolysis of the acylimine linkage is observed in all samples (Figs. S3 and S4), breaking the polypeptide backbone between Leu61 and Gln62 as found in the “kindling fluorescent protein”18,19. Compared to DsRed, Rtms5 and mKeima share an unusual placement of a positively charged group adjacent to the chromophore and close to the linkage between the two rings. This group is essential for formation of the red chromophore21. However it originates from the main chain at position 71 (Lys) in DsRed and 194 or 197 (Arg) in mKeima or Rtms5, respectively (Figs. S5A and S5B).
The most striking feature of mKeima is the putative proton relay, consisting of Ser142 and Asp157 (Figure 2). Mutation of charged Asp157 to neutral Asn changes the protonation state of the chromophore to anionic and red-shifts the emission slightly (Figure 1). The identical arrangement is found in two other green fluorescent proteins derived from reef organisms, KCy20 (PDB ID 2ZO6, CroOH-Ser141-Asp156) and asFP49921 (PDB ID 2C9I, CroOH-Ser143-Asp158). The excitation spectra of both proteins show clear evidence of a mixed population of the protonated and anionic chromophore states, both of which give rise to green fluorescence. From an evolutionary point of view, this equilibrium is presumably advantageous, possibly because the excitation spectrum is substantially broadened by the presence of two excitable species.
Figure 2.
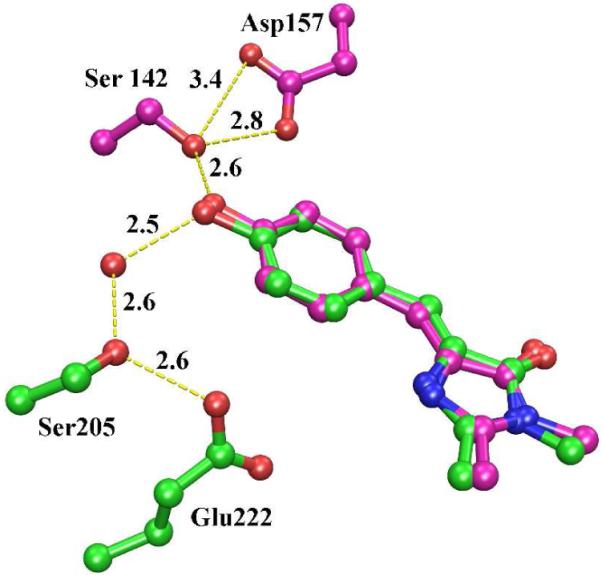
Ball and stick figure of the proton relay of mKeima (purple), showing the chromophore, Ser142 and Asp157. Hydrogen bonds are shown as dashed lines with lengths given in Å. The corresponding proton relay of GFP is superimposed in green. The isolated red sphere is a tightly bound water molecule within GFP.
The time resolved spectroscopic measurements are consistent with a proton transfer process of lifetime ~4 ps, whereas in GFP, lifetimes of ~3 and ~12 ps have been reported1, 22. Evidence is accumulating that the overall process of proton transfer is concerted23, 24. From data collected for GFP and its mutants14,23, as well as from computer simulations24, the rate limiting step for proton transfer is proposed to be from Ser205 to Glu222. In mKeima, the geometry of the proton transfer pathway appears to more optimal and has fewer degrees of freedom than within GFP, which may explain the difference in transfer rates.
Bonsma et al.25 reviewed spectroscopic evidence supporting the existence a photo-induced green (G’-) emitting form of DsRed (different from the immature green-emitting form) that absorbs and emits to the blue of the ‘mature’ red (R-) form. Our work provides evidence that the photoinduced G’- form is the neutral protonated chromophore, however, it remains to be determined whether the photo-induced form is the cis or trans isomer.
mKeima is the only red fluorescent protein shown to employ ESPT for emission, utilizing a novel proton relay different from that found in GFP and provides a new framework for study. It also provides a new system for the development of red fluorescent biosensors, which can take advantage of the equilibrium between the protonation states to provide ratiometric readout of cellular variables such as thiol-disulfide redox potential9, 26.
Note added in revision: Violot et al.27 recently reported a 2.6 Å resolution structure of mKeima at pH 8.0 in a different crystal form, revealing the chromophore isomer to be trans. Our 1.63 Å resolution map clearly indicates the cis isomer at >90% occupancy, but we cannot exclude up to 10% occupancy of trans. Conceivably, the isomeric state is determined by crystal contacts or differing buffer conditions.
Supplementary Material
Acknowledgement
This work was supported by grants from the National Science Foundation (MCB-0720420) and National Institutes of Health (GM042-18-16) to S.J.R. and the Binational US-Israeli Science Foundation to S.J.R. and D.H.
References
- (1).Chattoraj M, King BA, Bublitz GU, Boxer SG. Proc. Natl. Acad. Sci. USA. 1996;93:8362–8367. doi: 10.1073/pnas.93.16.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Brejc K, Sixma TK, Kitts PA, Kain SR, Tsien RY, Ormo M, Remington SJ. Proc. Natl. Acad. Sci. USA. 1997;94:2306–2311. doi: 10.1073/pnas.94.6.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Palm GJ, Zdanov A, Gaitanaris GA, Stauber R, Pavlakis GN, Wlodawer A. 3Nature Structural Biology. 1997;4:361–365. doi: 10.1038/nsb0597-361. [DOI] [PubMed] [Google Scholar]
- (4).Stoner-Ma D, Jaye AA, Matousek P, Towrie M, Meech SR, Tonge PJ. J. Am. Chem. Soc. 2005;127:2864–2865. doi: 10.1021/ja042466d. [DOI] [PubMed] [Google Scholar]
- (5).van Thor JJ, Ronayne KL, Towrie M, Sage JT. Biophys. J. 2008;95:1902–1912. doi: 10.1529/biophysj.108.129957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Agmon N. J. Phys. Chem. B. 2007;111:7870–8. doi: 10.1021/jp071403p. [DOI] [PubMed] [Google Scholar]
- (7).Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. Biophys. J. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hanson GT, McAnaney TB, Park ES, Rendell M, Yarbrough DK, Chu S, Xi L, Boxer SG, Montrose MH, Remington SJ. Biochemistry. 2002;41:15477–15488. doi: 10.1021/bi026609p. [DOI] [PubMed] [Google Scholar]
- (9).Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. J. Biol. Chem. 2004;279:22284–93. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- (10).Kogure T, Karasawa S, Arkai T, Saito K, Kinjo M, Miyawaki A. Nature Biotechnol. 2006;24:577–581. doi: 10.1038/nbt1207. [DOI] [PubMed] [Google Scholar]
- (11).Stoner-Ma D, Jaye AA, Ronayne KL, Nappa J, Meech SR, Tonge PJ. J. Am. Chem. Soc. 2008;130:1227–1235. doi: 10.1021/ja0754507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Shi X, Abbyad P, Shu X, Kallio K, Kanchanawong P, Childs W, Remington SJ, Boxer SG. Biochemistry. 2007;46:12014. doi: 10.1021/bi700904a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shu X, Kallio K, Kanchanawang P, Childs W, Boxer SG, Remington SJ. Biochemistry. 2007;46:12005. doi: 10.1021/bi7009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Shu X, Leiderman P, Gepshtein R, Smith NR, Kallio K, Huppert D, Remington SJ. Protein Science. 2007;16:2703–2710. doi: 10.1110/ps.073112007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Leiderman P, Genosar L, Huppert D, Shu X, Remington SJ, Solntsev K, Tolbert LM. Biochemistry. 2007;46:12026. doi: 10.1021/bi7009053. [DOI] [PubMed] [Google Scholar]
- (16).Prescott M, Ling M, Beddoe T, Oakley AJ, Hoegh-Guldberg O, Devenish RJ, Rossjohn J. Structure. 2003;11:275–284. doi: 10.1016/s0969-2126(03)00028-5. [DOI] [PubMed] [Google Scholar]
- (17).Yarbrough D, Wachter RM, Kallio K, Matz MV, Remington SJ. Proc. Natl. Acad. Sci. USA. 2001;98:462–467. doi: 10.1073/pnas.98.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Quillin ML, Anstrom DM, Shu X, O’Leary S, Kallio K, Lukyanov KA, Remington SJ. Biochemistry. 2005;44:5774–5787. doi: 10.1021/bi047644u. [DOI] [PubMed] [Google Scholar]
- (19).Wilmann PG, Petersen J, Dvenish RJ, Prescott M, Rossjohn J. J. Biol. Chem. 2005;280:2401–2404. doi: 10.1074/jbc.C400484200. [DOI] [PubMed] [Google Scholar]
- (20).Kikuchi A, Eiko Fukumura E, Karasawa S, Shiro Y, Miyawaki A. Biochemistry. 2009;48:5276–83. doi: 10.1021/bi801658p. [DOI] [PubMed] [Google Scholar]
- (21).Nienhaus K, Renzi F, Vallone B, Wiedenmann J, Nienhaus GU. Biophys. J. 2006;91:4210–20. doi: 10.1529/biophysj.106.087411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kennis JT, Larsen DS, van Stokkum IH, Vengris M, van Thor JJ, van Grondelle R. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17988–93. doi: 10.1073/pnas.0404262102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Henderson JN, Gepshtein R, Heenan JR, Kallio K, Huppert D, Remington SJ. J. Am. Chem. Soc. 2009;131:4176–4177. doi: 10.1021/ja808851n. [DOI] [PubMed] [Google Scholar]
- (24).Vendrell O, Gelabert R, Moreno M, Lluch JM. J. Phys. Chem. B. 2008;112:13443–52. doi: 10.1021/jp805049c. [DOI] [PubMed] [Google Scholar]
- (25).Bonsma S, Purchase R, Jezowski S, Gallus J, Konz F, Volker S. Chem Phys Chem. 2005;6:838–849. doi: 10.1002/cphc.200500005. [DOI] [PubMed] [Google Scholar]
- (26).Hanson GT, Aggeler R, Oglesbee D, Canon M, Capaldi RA, Tsien RY, Remington SJ. J. Biol. Chem. 2004;279:13044–53. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- (27).Violot S, Carpentier P, Blanchoin L, Bourgeois D. J. Am. Chem. Soc. 2009;XXXX:xxxx. doi: 10.1021/ja903695n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


