Abstract
IL-17, hallmark cytokine of the newly-described “Th17” population, signals through a novel subclass of receptors, yet surprisingly little is known about mechanisms of IL-17 receptor signaling. IL-17 activates target gene expression via NF-κB and two members of the C/EBP transcription factor family, C/EBPδ and C/EBPβ. It has been shown that TRAF6 and Act1 are upstream of NF-κB and C/EBPδ, but the signaling pathways regulating C/EBPβ remain entirely undefined. In this report, we demonstrate that IL-17 signaling leads to phosphorylation of two sites in the C/EBPβ “regulatory 2 domain” in a sequential, interdependent fashion. First rapid phosphorylation of Thr188 occurs within 15 minutes and is ERK-dependent. A second phosphorylation event targets Thr179, which requires prior Thr188 phosphorylation and GSK3β activity. We further show that the pathways leading to C/EBPβ phosphorylation are mediated by distinct subdomains within IL-17RA. Whereas phosphorylation of Thr188 is mediated by the previously-identified SEFIR/TILL domain, activation of Thr179 occurs through a motif located in the IL-17RA distal tail. Functionally, C/EBPβ phosphorylation mediates a negative signal, as blocking ERK and GSK3β upregulates IL-17-induced genes, and a C/EBPβ-Thr188 mutant enhances activation of a C/EBP-dependent reporter. Consistently, GSK3β overexpression inhibits IL-17 signaling to a C/EBP-dependent reporter, and GSK3β-deficient cells fail to induce phosphorylation at T179. Thus, IL-17 triggers dual phosphorylation of C/EBPβ, which down-modulates expression of inflammatory genes. This is the first detailed dissection of the IL-17-mediated C/EBP pathway and is also the first known example of a negative signal mediated by this unique receptor.
Introduction
A revolution in understanding T cell-mediated immunity occurred recently with the discovery of a new population of inflammatory T helper cells, distinct from the classic Th1 and Th2 subsets (1, 2). Termed “Th17,” these cells secrete a variety of pro-inflammatory cytokines that link adaptive and innate immunity by stimulating genes associated with inflammation (3). IL-17 (IL-17A), the signature cytokine of the Th17 subset, is the founding member of a novel subfamily of cytokines (4, 5). IL-17 plays a non-redundant role in host defense against extracellular microorganisms, and, conversely, is a key mediator of pathology in autoimmune disease. Indeed, antibodies to IL-17 or its receptors are presently in clinical trials for treating rheumatoid arthritis and other autoimmune diseases (6).
To date, our understanding of IL-17-mediated signal transduction remains surprisingly limited, in part because the IL-17 receptor family is unique in sequence and hence few inferences about signaling mechanisms can be made simply on the basis of homology to other receptor types. IL-17 binds to a transmembrane receptor complex composed of IL-17RA and IL-17RC, both members of a novel subfamily of cytokine receptors (7, 8). IL-17 signaling leads to activation of gene expression profiles similar to innate immune receptors such as Toll-like receptors and the IL-1R. Despite an unusual amino acid sequence, the IL-17RA intracellular tail contains elements that are functionally parallel to the TLR/IL-1R (TIR) superfamily, particularly a “SEFIR” domain that is related to to TIR domains (9, 10). The IL-17R and TIR-receptor signaling pathways converge on TRAF6, which activates the NF-κB and MAPK cascades (11). Whereas TLRs use proximal TIR-containing adaptors such as MyD88 and TRIF to engage TRAF6, IL-17RA uses the SEFIR-containing adaptor protein Act1/CIKS (12–14).
Analysis of IL-17 target genes has been informative in defining pathways activated by IL-17 by elucidating relevant downstream transcription factors. In particular, IL-17 target promoters are enriched for binding sites recognized by NF-κB and the CCAAT/Enhancer Binding protein (C/EBP) family (also known as NF-IL6) (15, 16). C/EBP sites are essential for IL-17-dependent induction of genes encoding IL-6, chemokines, acute phase proteins and other inflammation-related genes (7). However, regulation of the C/EBP family by IL-17 is poorly understood. We and others have shown that IL-17 induces rapid expression of C/EBPβ and C/EBPδ mRNA and protein (13, 16, 17) as well as nuclear complexes that bind C/EBP binding sites in vitro (15). Although C/EBPδ and C/EBPβ recognize similar DNA sequences and regulate many of the same target genes (18), they play non-redundant roles in IL-17 signaling pathways. Different subdomains within IL-17RA regulate expression of C/EBPβ and C/EBPδ, as a mutant form of IL-17RA activates C/EBPδ but not C/EBPβ (10). In other systems, C/EBPβ is actively regulated by various post-translational modifications, particularly phosphorylation, and can act either as a positive or negative transcriptional regulator (reviewed in (19)). Therefore, in this study we sought to evaluate C/EBPβ phosphorylation during IL-17-mediated signal transduction.
C/EBPβ contains a transcriptional activation domain in its N-terminus, a C-terminal DNA binding domain, and a pair of centrally-located “Regulatory Domains” (Fig. 3D) (19, 20). In particular, the Regulatory Domain 2 (RD2) is a Ser/Thr-rich region with multiple sites of potential phosphorylation. During IFNγ signaling, phosphorylation of Ser64 in the activation domain and Thr188 in the RD2 domain regulate C/EBPβ function (21, 22). During adipogenesis, inducible phosphorylation of two sites in the RD2 domain are required for C/EBPβ activity (23, 24), whereas dephosphorylation of C/EBPβ increases its ability to bind DNA and regulate transcription (25). Consequently, we hypothesized that IL-17 signaling might also lead to phosphorylation of C/EBPβ and hence modulate downstream target gene expression. Here, employing tandem mass spectrometry, we show that IL-17 signaling activates phosphorylation on two threonine residues within the C/EBPβ RD2 domain. This event is mediated by separable domains within IL-17RA, through ERK- and GSK3β-dependent mechanisms. Unexpectedly, phosphorylation of C/EBPβ exerts a down-modulatory effect on IL-17-induced gene expression, and thus reveals the first known negative signaling event mediated by the IL-17 receptor.
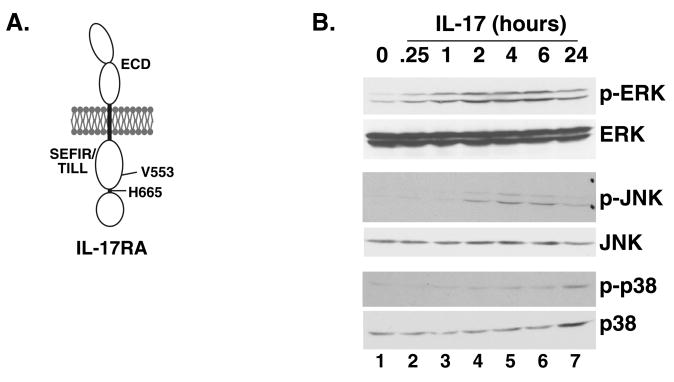
Figure 3. ERK and GSK3α/β activate phosphorylation of T188 and T179, mediated through disparate regions of the IL-17RA subunit.
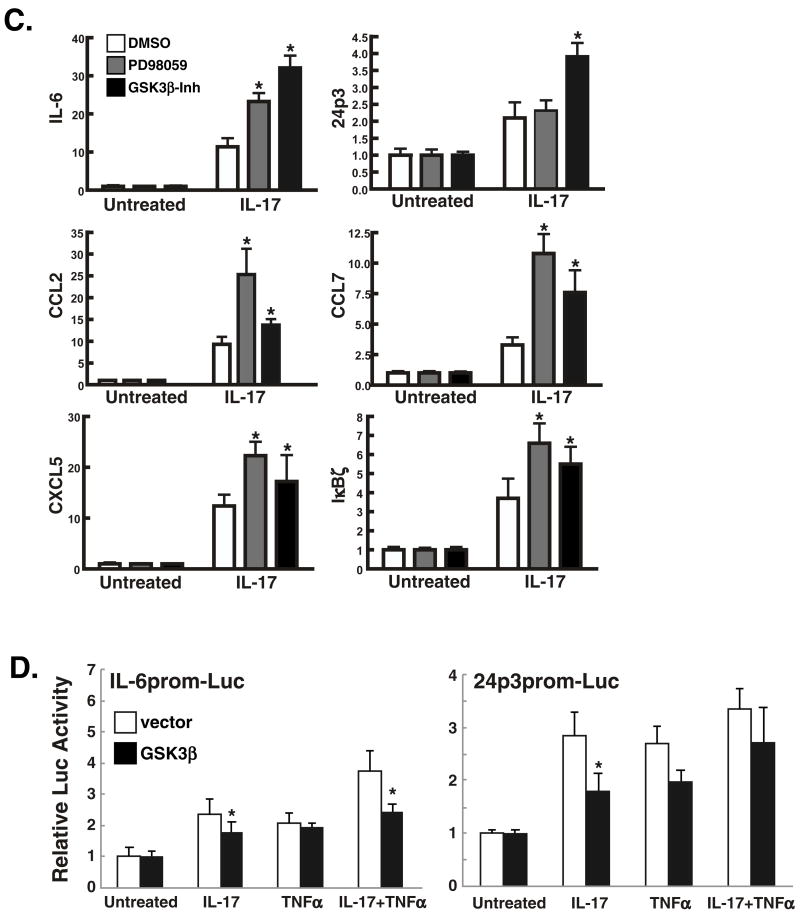
A. Domain structure of IL-17RA. The proximal functional motif within the murine IL-17RA cytoplasmic tail is the SEFIR/TILL domain, and a point mutation at V553 to histidine renders this domain incapable of activating ERK. We have also identified a region in the distal tail downstream of residue V665 that regulates expression of C/EBPβ (10). These mutants are used in Table 1. B. IL-17 induces rapid induction of ERK but delayed induction of other MAPK pathways. ST2 cells were stimulated with IL-17 (200 ng/ml) for the indicated times, and cell lystes were subjected to western blotting with anti-MAPK Abs, as indicated. C. Blockade of ERK or GSK3α/β upregulates expression of IL-17 target genes. ST2 cells were pre-treated with the ERK inhibitor PD98059 or GSK3α/β inhibitor I for 1 hour and total mRNA was prepared. Real time RT-PCR was performed in triplicate with primers to the indicated genes. *p<0.05. D. Over-expression of GSK3β inhibits IL-17-mediated induction of a C/EBP-dependent reporter. ST2 cells were co-transfected in triplicate with an IL-6-promoter-Luc (left) or a 24p3 promoter-Luc (right) together with a 10-fold excess of GSK3β plasmid, stimulated with IL-17 or TNFα, and luciferase activity assessed after 8 hours. *p<0.05.
Materials and Methods
Cell culture
ST2 and C/EBPβδ-deficient cells were cultured as described (16). GSK3β-deficient cells were described in (26). IL-17RAKO cell lines were prepared from IL-17RAKO mice generously provided by Amgen, and are described in (10). IL-17 and TNFα were from Peprotech (Rocky Hill, NJ). PD98095 and the GSK3β inhibitor I were from Calbiochem/EMD (Gibbstown, NJ).
Plasmids, luciferase assays and realtime PCR
IL-17RA constructs were described in (10). C/EBPβ mutations on the LAP background were created by PCR and expressed in pcDNA3 vectors. Real-time PCR, transfections and luciferase assays were performed as described (17).
EMSA, Western Blotting and Immunoprecipitation
EMSA, IP and western blotting and were performed as described (15). Abs used were anti-C/EBPβ (Santa Cruz Biotechnology, Santa Cruz CA) and anti-pT188 (Cell Signaling, Beverly MA). Abs to phospho-ERK, phospho-p38 MAPK, phospho-JNK were also from Cell Signaling.
In-Gel Digestion, TiO2-Tip Enrichment and Nano-ESI- Tandem MS
Immunoprecipitated C/EBPβ was resolved on 7.5% SDS-PAGE. Bands corresponding to ~38 kDa were excised and de-stained by 25 mM NH4HCO3/50% acetonitrile. Gel particles were incubated with 10 mM DTT at 56°C for 1 h and 55 mM iodoacetamide for 45 mins in the dark. Following in-gel tryptic digestion (sequence grade, Promega Pierce) at 37°C for18 h, vacuum-dried peptides were dissolved in 80%ACN/0.1%TFA and loaded on a Top tip-TiO2 micropipette tip (Glygen Corp. Columbia, MD). Phosphopeptides were eluted in NH4OH (pH10.5) and desalted using a Ziptip C18A (Millipore, Billerica, MA). Nano-ESI-MS experiments were performed on a Thermo Finnigan (LCQ) Advantage quadrupole ion trap mass spectrometer instrument (San Jose, CA) equipped with a custom Nano-ESI source and PANI emitters (27). Emitters were positioned ~2 cm from the mass spectrometer inlet and supplied with 4–4.5 KV spray voltage to form positive ions. Capillary temperature was 190°C. Mass spectra were acquired for 10 min. During the MS/MS experiments, manual selection of the parent ions was employed for the tandem-MS acquisitions. Data analysis was performed with the ProteinProspector system (http://prospector.ucsf.edu).
Results
Sequential phosphorylation of C/EBPβ following IL-17 signaling
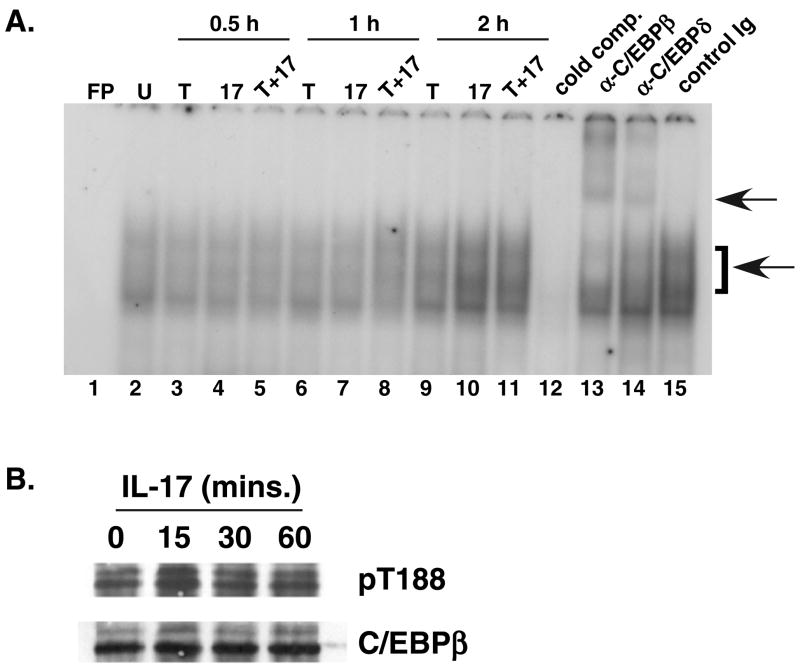
IL-17 is critical for mediating host defense to numerous infectious organisms, but the signaling mechanisms used by this cytokine are still poorly defined. We previously reported that induction of IL-17 target genes such as IL-6 and lipocalin-2/24p3 requires C/EBPβ and C/EBPδ (16, 17). IL-17 signal transduction leads to upregulation of C/EBPβ and C/EBPδ mRNA and protein, and increased binding of these factors to a C/EBP promoter element (15, 16). Both C/EBPβ and C/EBPδ can independently activate IL-17-inducible and C/EBP-dependent reporters, such as the 24p3 or IL-6 proximal promoters (17). To determine which form of C/EBP is important for downstream gene regulation, we performed EMSA supershift assays using the 24p3 C/EBP promoter element and antiC/EBP Abs (Fig. 1A). While both TFs were abundant in the shifted complex, a somewhat stronger supershift and a correspondingly reduced gel shift complex were seen with anti-C/EBPβ antibodies (Abs) compared to anti-C/EBPδ Abs (Fig. 1A). Therefore, we evaluated IL-17 regulation of C/EBPβ in more detail.
Figure 1. IL-17-mediated regulation of C/EBPβ. A. C/EBPβ is the dominant factor in IL-17-induced gel shift complexes.
ST2 stromal cells were stimulated with IL-17 (200 ng/ml, lanes 4, 7, 10), TNFα (2 ng/ml, lanes 3, 6, 9) or both cytokines (lanes 5, 8, 11–15) for 0.5 h (lanes 3–5), 1 h (lanes 6–8) or 2 h (lanes 9–15). Nuclear extracts were subjected to EMSA with a 32P-labeled C/EBP site from the 24p3 proximal promoter in the presence or absence of α-C/EBPβ or α-C/EBPδ Abs, as described in (15). Bottom arrow indicates the specific shifted complex; top arrow indicates the supershifted complex. FP, free probe. B. IL-17 induces phosphorylation on T188 in the RD2 domain of C/EBPβ. Nuclear extracts from IL-17-stimulated ST2 cells were subjected to western blotting with Abs to phospho-T188 (top) or total C/EBPβ (bottom).
In order to assess C/EBPβ regulation and function in the absence of potentially confounding influences from C/EBPδ, we established immortalized C/EBPβ/C/EBPδ doubly-deficient MEF cells (hereafter termed C/EBPβδKO) by stable transfection with the SV40 T antigen. We previously showed that these cells produce no IL-6 or 24p3 in response to IL-17 (16). Several studies have demonstrated that C/EBPβ is inducibly phosphorylated on the regulatory domain 2 (RD2), which is important for its transcriptional activity in certain settings such as interferon-γ stimulation and during adipogenesis (22, 23). To determine whether IL-17 triggers C/EBPβ phosphorylation, ST2 stromal cells were treated with IL-17, and C/EBPβ was immunoprecipitated (IP) and immunoblotted with Abs to C/EBPβ phospho-T188. Although commercially available antibodies recognizing C/EBPβ T188 show a high background level of nonspecific staining (Fig. 1B and data not shown), these data suggested that phosphorylation of T188 was increased after 15 min of IL-17 treatment, and reduced after 30 min (Fig. 1B).
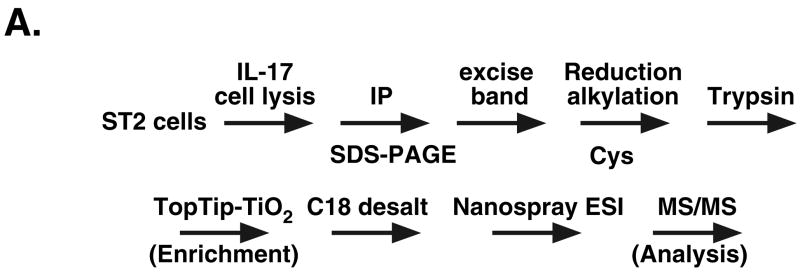
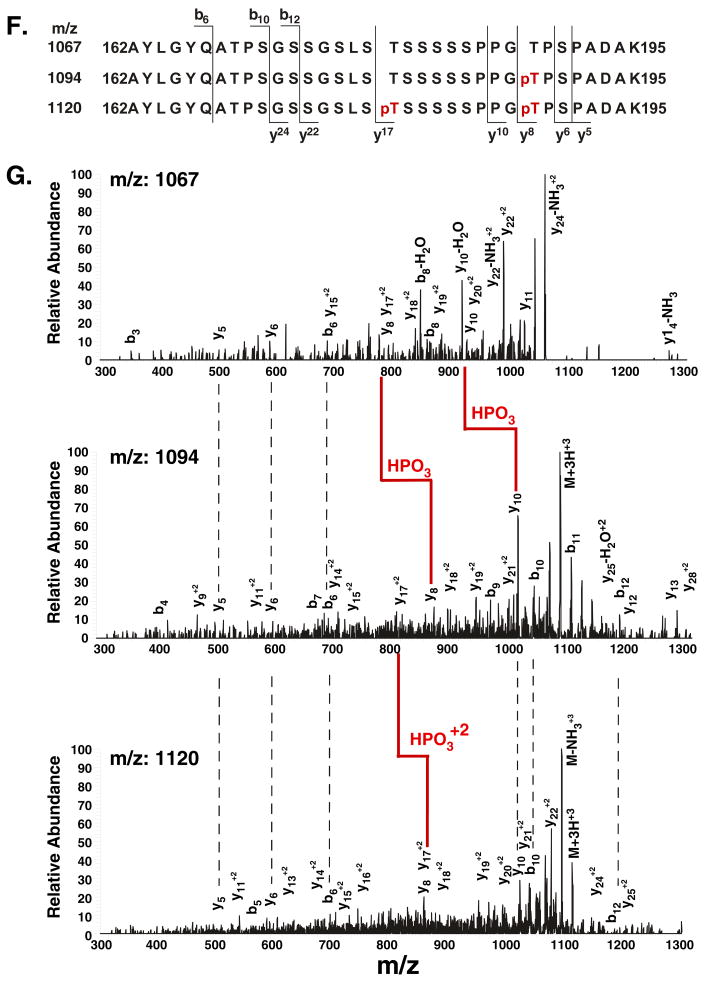
To confirm this finding in a more robust manner and to carry out a more detailed analysis of phosphorylation of the C/EBPβ RD2 domain than is possible with available antibodies, we performed tandem mass spectrometry (MS) on IP samples excised from SDS-PAGE in the molecular weight range corresponding to C/EBPβ. After in-gel tryptic digestion and TiO2-tip enrichment of phosphopeptides, nano-electrospray ionization (nESI) MS was used to evaluate phosphorylation in the RD2 domain (Fig 2A). A baseline MS profile was established in C/EBPβδKO cells transfected with a control plasmid (Supplementary Fig. 1A). Cells transfected with C/EBPβ exhibited a unique triply-charged ion at m/z ratio of 1067 (P1067), corresponding to a predicted tryptic fragment encoding the RD2 domain (Supplementary Fig. 1B). The identity of this peptide was then verified by the tandem MS spectrum, which indicated mainly y-ion series and some b-ion series fragments (see Fig. 2F). These structurally informative fragment ions provided sufficient sequence information to definitively assign this peptide as a non-phosphorylated form of the RD2 sequence (Fig. 2B, 2F, and Supplementary Table 1).
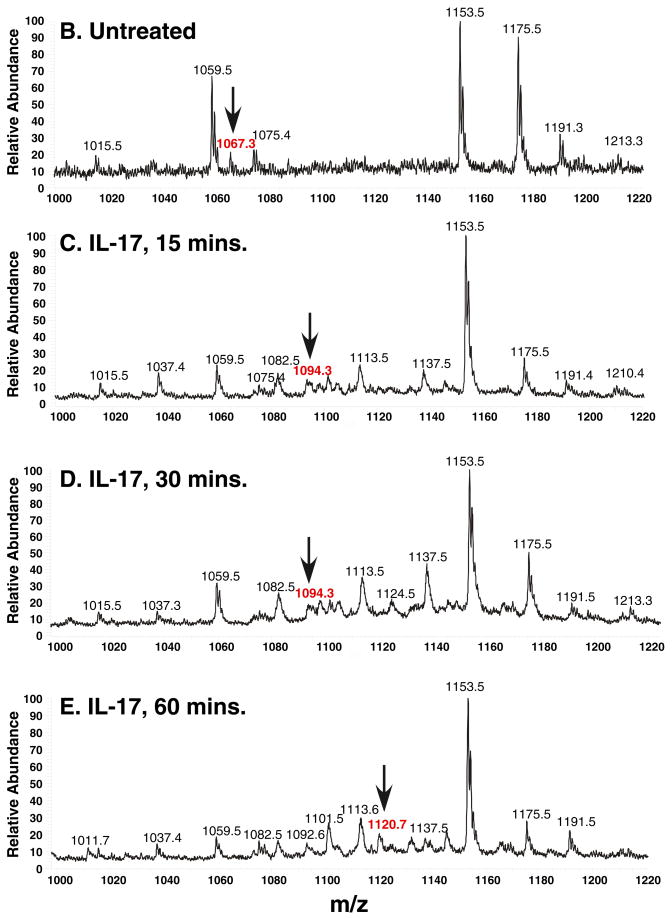
Figure 2. IL-17 induces dual, sequential phosphorylation of T188 and T179 by tandem mass spectrometry.
A. Schematic diagram of the tandem MS/MS procedure. B–E. MS spectra of C/EBPβ immunoprecipitates from ST-2 cells treated with nothing (B) or IL-17 for various time points (C–E) m/z= mass/charge. Arrows indicate the tryptic fragments containing the RD2 domain, which were further analyzed by collision-induced decay (see also Supplementary Table 1). F. Sequence of the tryptic fragment encoding RD2 and the b abnd y ions generated by collision-induced decay/MS. Specific phosphorylated residues are indicated in red. G. Tandem MS of the 1067, 1094 and 1120 peaks, indicating specific y and b ions and sites of phosphorylation.
When analyzing ST2 cells that had not been treated with cytokines, we observed the same P1067 peak (Fig. 2B), indicating that the C/EBPβ RD2 domain is not detectably phosphorylated in the absence of stimulation. After IL-17 treatment for 15–30 minutes, most major peaks matched the untreated sample, but P1067 was absent. However, a new triply charged peak at m/z 1094.3 (P1094) was detected, suggestive of a phosphorylation event (Fig. 2C–D). The P1094 was fragmented and found to represent a peptide composed of the same amino acid residues as P1067 plus a phosphate group (Fig. 2G, Supplementary Table 1). The tandem-MS spectrum verified that the P1094 y8-ion was mass-shifted by +80 Da and y9-y33 fragments were also shifted accordingly; however, the y2-y7 and b2-b17 fragments indicated no mass changes (Fig. 2F). These observations demonstrated conclusively that P1094 represents a C/EBPβ RD2 peptide with a single phosphorylation modification on T188.
After 60 minutes of IL-17 treatment, P1094 disappeared and a new peak at m/z 1120.8 (P1120) was detected (Fig. 2E). Comparing the MS/MS spectrum of P1120 to P1067, the masses of the y8-y16 ions shifted from their original value by +80 Da due to addition of a phosphate group at T188. Moreover, the y17-y23 fragment ion shifted from their original values by +160 Da due to the addition of a second phosphate group at T179. In addition, some of the singly- or doubly-charged b and y ions related to a phosphate group showed a characteristic neutral loss of a phosphoric acid (98kDa) due to a gas phase β-elimination reaction in the MS/MS spectrum, further proving their identity (Fig. 2G, Supplementary Table 1). Thus, P1120 represents a C/EBPβ RD2 peptide dually phosphorylated at both T188 and T179. Notably, the P1067, P1094 or P1120 peaks were present in samples from C/EBPβδKO cells transfected with C/EBPβ and treated with IL-17 at the same time course, indicating this is a general property of IL-17 signaling to C/EBPβ (Supplementary Fig. 1B–D). Collectively, these data show that IL-17 induces two sequential phosphorylation events within the C/EBPβ RD2 region.
IL-17 and TNFα exhibit considerable synergy in signaling, and these cytokines drive cooperative induction of C/EBPβ expression (16). Here, we observed that addition of low dose TNFα slightly accelerated the kinetics of T179 phosphorylation from 60 minutes to 30 minutes (Supplementary Table 2).
Regulation of C/EBPβ phosphorylation by distinct domains in IL-17RA
The IL-17RA cytoplasmic tail contains two distinct subdomains that engage downstream signaling pathways (Fig. 3A) (10). The best-characterized is termed “SEFIR,” which bears homology to the “TIR” domain motif located in Toll/IL-1 receptors (9, 13, 14). We previously identified an extension of the SEFIR domain that also has homology to TLR domains, termed a “TIR-like loop” (TILL). Mutation of a single residue within the TILL domain, V553H, eliminates IL-17-dependent activation of NF-κB, C/EBPδ and ERK, thus impairing downstream gene expression (10). To evaluate signaling events leading to C/EBPβ phosphorylation, IL-17RA-deficient fibroblasts stably expressing IL-17RA.V553H were stimulated with IL-17 for various times, and C/EBPβ phosphorylation was assessed. As shown, IL-17RA.V553H failed to induce detectable phosphorylation of C/EBPβ, because P1067 was detected in all samples while neither P1097 nor P1120 were present after IL-17 stimulation (Table 1).
Table 1. Distinct domains within IL-17RA regulate C/EBPβ phosphorylation.
IL-17RAKO cells stably expressing IL-17RA.V553H or IL-17RAΔ665 were treated with IL-17 for 0, 15, 30 or 30 minutes, and tandem MS/MS analysis was performed as described for Figure 2.
| IL-17RA mutant, duration IL-17 treatment | Unphosphorylated (m/z=1067) | pT188 (m/z=1095) | pT188, pT179 (m/z=1120) |
|---|---|---|---|
| V553H, untreated | + | − | − |
| V553H, 15 min | + | − | − |
| V553H, 30 min | + | − | − |
| V553H, 60 min | + | − | − |
| Δ665, untreated | + | − | − |
| Δ665, 15 min | − | + | − |
| Δ665, 30 min | − | + | − |
| Δ665, 60 min | − | + | − |
A second, more poorly defined functional domain in IL-17RA is located in the distal cytoplasmic tail downstream of residue 665. We have reported that this domain controls induction of C/EBPβ protein expression, but not NF-κB, ERK or C/EBPδ expression (10). In IL-17RAKO cells expressing IL-17RAΔ665, IL-17-induced T188 phosphorylation proceeded normally, since P1094 was present 15 min following IL-17 stimulation. However, in contrast to cells expressing a WT IL-17 receptor, P1094 remained in the sample even after 60 minutes of IL-17 treatment, whereas P1021 was not detected. Thus, while T188 phosphorylation can occur in the absence of this domain, T179 phosphorylation is blocked (Table 1). Therefore, the distal domain of IL-17RA regulates both T179 phosphorylation as well as C/EBPβ protein expression (10). Accordingly, we propose to term this region the “C/EBPβ-activating domain (C-BAD).” Surprisingly, homology searches of the C-BAD sequence against mammalian databases did not identify any other proteins with detectable similarity (unpublished observations), and thus this appears to be a new, unusual functional signaling motif.
C/EBPβ phosphorylation is mediated by ERK and glycogen synthase kinase-3
We next sought to determine the kinases responsible for IL-17-dependent C/EBPβ phosphorylation. The T188 site is located in a MAPK target sequence, and the MEKK/ERK pathway has been implicated in T188 phosphorylation in the context of adipogenesis (23). Moreover, recombinant C/EBPβ has been shown to be phosphorylated by purified MAPK in vitro (24, 28). The failure of the IL-17RA.V553H mutation to permit phosphorylation of CEBPβ is also consistent with a role for ERK, as we previously showed that IL-17RA.V553H cannot activate the ERK pathway (10) (Table 1). Moreover, the kinetics of T188 phosphorylation correlate closely with activation of ERK, but not other MAPK pathways such as JNK and p38-MAPK (Fig 3B). Specifically, IL-17 activates ERK p42/44 phosphorylation within 30 minutes. In contrast, activation of p38 MAPK and JNK is only evident after 4 hours, and is thus likely to be due to secondary effects rather than direct IL-17 stimulation. To determine whether blocking ERK reduced C/EBPβ phosphorylation, ST2 cells were pre-treated with a panel of kinase inhibitors prior to and during IL-17 stimulation, and C/EBPβ phosphorylation was evaluated by MS. Regardless of the duration of IL-17 stimulation, only the P1067 (unphosphorylated) peak was detected in samples treated with an ERK inhibitor (PD98059) (Table 2). However, since the IL-17RAΔ665 mutant fails to induce phosphorylation of T179 even though it can activate ERK (Table 1), we conclude that ERK activity alone is not sufficient to induce phosphorylation of T179. Prior in vitro studies have shown that phosphorylation of T179 requires a “priming” phosphorylation event at T188 (23). Accordingly, it is probable that ERK directly phosphorylates T188, which then renders C/EBPβ permissive for subsequent phosphorylation on T179 by a different kinase (see also Discussion).
Table 2. ERK and GSK3α/β inhibitors block phosphorylation of the C/EBPβ RD2 domain.
ST2 cells were treated with IL-17 for the indicated times in the presence of PD98095 (ERK inhibitor) or the GSK Inhibitor I and tandem MS/MS analysis was performed as described for Figure 2.
| Conditions | PD98095 | GSK- Inhibitor I | Unphosphorylated (m/z=1067) | pT188 (m/z=1095) | pT188, pT179 (m/z=1120) |
|---|---|---|---|---|---|
| Untreated | − | − | + | − | − |
| IL-17, 15 min | − | − | − | + | − |
| IL-17, 30 min | − | − | − | + | − |
| IL-17, 60 min | − | − | − | − | + |
| IL-17, 24 hrs | − | − | − | − | − |
| Untreated | + | − | + | − | − |
| IL-17, 15 min | + | − | + | − | − |
| IL-17, 30 min | + | − | + | − | − |
| IL-17, 60 min | + | − | + | − | − |
| Untreated | − | + | + | − | − |
| IL-17, 15 min | − | + | − | + | − |
| IL-17, 30 min | − | + | − | + | − |
| IL-17, 60 min | − | + | − | + | − |
Using additional kinase inhibitors, we found that phosphorylation at T179 but not T188 was blocked by a GSK3β inhibitor (Table 2). After IL-17 treatment for 15 min, the P1094 peak was detected in samples pre-treated with the GSK3β inhibitor, indicating that T188 phosphorylation proceeds normally. At 30 and 60 minutes, P1094 but not P1120 was detected (and verified by MS/MS, data not shown), suggesting that GSK3β specifically targets T179. To verify the role of GSK3β, we evalulated C/EBPβ phosphorylation in GSK3β-deficient MEF cells (26). While phosphorylation at T188 was clearly evident at 15 and 30 minutes, there was no evidence for the dually phosphorylated T188/T179 peak at 60 or 120 minutes. However, at these time points there was also no evidence for the unphosphorylated P1067 peak or the singly phosphorylated P1094 peak, indicating that additional post-translational modifications may occur (Table 3 and data not shown). Thus, it is clear that GSK3β is not necessary for T188 phosphorylation, but is an essential component of the pathway upstream of T179 phosphorylation. While we cannot determine whether GSK3β targets T179 directly, it has been demonstrated in vitro that purified GSK3β can phosphorylate this site during the process of adipogenesis (23).
Table 3. GSK3β-deficient cells fail to induce phosphorylation at T179.
ST2 cells were treated with IL-17 for the indicated times, and tandem MS/MS analysis was performed as described for Figure 2.
| Conditions | Unphosphorylated (m/z=1067) | pT188 (m/z=1095) | pT188, pT179 (m/z=1120) |
|---|---|---|---|
| IL-17, 15 min | − | + | − |
| IL-17, 30 min | − | + | − |
| IL-17, 60 min | − | − | − |
| IL-17, 120 mins | − | − | − |
C/EBPβ phosphorylation negatively regulates IL-17 target gene expression
To assess the functional relevance of C/EBPβ phosphorylation in IL-17R signaling, expression of several IL-17 target genes was assessed in ST-2 cells treated with ERK or GSK3β inhibitors. Realtime RT-PCR analyses showed that IL-17-induced IL-6 mRNA expression is enhanced after blocking either ERK or GSK3β (Fig. 3C). Similarly, expression of other IL-17 target genes are also enhanced by ERK or GSK3β inhibitors, including 24p3, CCL2, CCL7, CXCL5 and IκBζ (Fig 3C). These data suggested that, contrary to the activating role of C/EBPβ phosphorylation in adipogenesis or IFNγ signaling (22, 23), phosphorylation at T188 and T179 negatively regulates C/EBPβ transcriptional activity following IL-17 signaling. To further verify the inhibitory effects of C/EBPβ phosphorylation, we evaluated the effect of overexpression of GSK3β on IL-17-mediated induction of two C/EBP-dependent promoters (16). As shown in Fig. 3D, co-transfection of GSK3β with the IL-6 promoter-Luc or 24p3-promoter-Luc constructs led to reduced expression of the reporter following IL-17 signaling, either alone or in conjunction with TNFα. Collectively, these data show that ERK and GSK3β repress or at least help to limit IL-17 target gene expression.
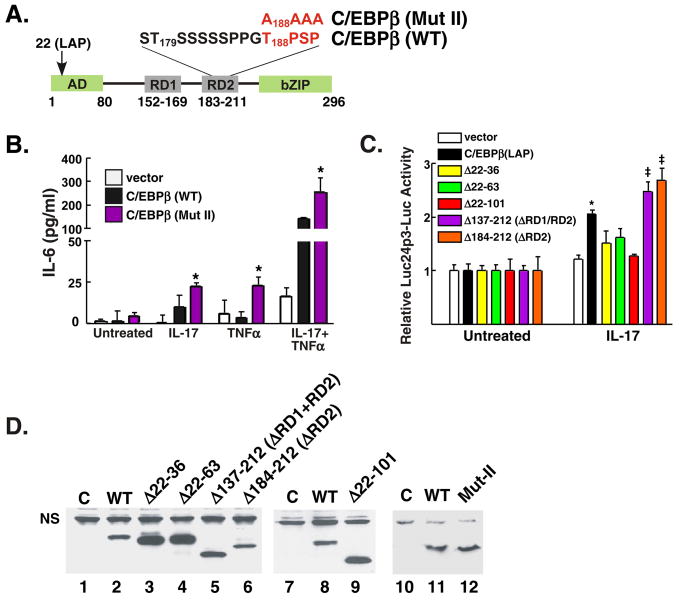
Since blocking kinases via pharmacologic inhibitors may have a broad impact on cell signaling, we tested the specific effect of a C/EBPβ construct mutated in the RD2 region (Fig. 4A). C/EBPβδKO cells were transiently transfected with WT or mutant C/EBPβ constructs, stimulated with IL-17, and IL-6 expression was assessed. Compared to wild type C/EBPβ, transfection of C/EBPβ-MutII (T188PSP -> AAAA) led to reproducibly enhanced IL-17-induced IL-6 production (Fig. 4B). Consistently, deletion of the RD2 region (residues 184–212) also led to increased expression of the C/EBP-dependent 24p3-luciferase reporter (Fig. 4C). In contrast, a deletion in all or part the N-terminal transcriptional activation domain (residues 22–101) failed to induce the reporter gene (Fig. 4C). We verified that all C/EBPβ mutants were expressed well in these experiments (Fig. 4D). Therefore, these data confirm that phosphorylation of the C/EBPβ RD2 domain serves to limit IL-17 signaling through the C/EBPβ pathway.
Figure 4. The RD2 domain of C/EBPβ limits IL-17-induced, C/EBPβ-dependent gene expression.
A. Diagram of C/EBPβ. Location of specific subdomains and mutation in RD2 domains is indicated. B. Mutation of T188 leads to enhanced IL-17-dependent signaling. C/EBPβδKO cells were transfected in triplicate with a vector control, C/EBPβ (LAP) or a mutant form of C/EBPβ in which residues 188–191 were mutated to Ala (“Mut II”). IL-6 in the supernatant of transfected cells was assessed in triplicate by ELISA. C. The N-terminal domain of C/EBPβ and not the RD2 domain is required for IL-17-induced activation of a C/EBP-dependent reporter. C/EBPβδKO cells were transfected in triplicate with the indicated C/EBPβ deletion mutants (on the LAP background) together with a 24p3-Luc promoter and normalized to a Renilla-Luc control. Results are presented as fold-increase over untreated for each construct. * p<0.05 compared to untreated sample; ‡ p<0.05 compared to WT-C/EBPβ. D. Expression of C/EPBβ mutants. Lysates from cells transfected in panel B were subjected to western blotting with anti-C/EBPβ Abs. C= control vector.
Discussion
IL-17 is the founding member of a new subfamily of cytokines (4). Several years ago, IL-17 was brought into prominence with the discovery of the new “Th17” T cell subset. Th17 cells mediate inflammatory events crucial for defense against extracellular bacteria, yeast and parasites (29). Although produced primarily by T cells, IL-17 functions as a classic innate cytokine, activating expression of neutrophil-activating factors (G-CSF, G-CSFR and CXC chemokines), innate cytokines (IL-6, IL-1, TNFα), anti-microbial factors (defensins, mucins) and acute phase proteins (lipocalins, CRP) (7).
The molecular mechanisms used by IL-17 to achieve inflammatory gene expression remain poorly defined. Using gene profiling as a tool to define endpoints of IL-17 signaling cascades, we have shown that many IL-17 target promoters require both NF-κB and C/EBP (15, 16). In contrast to TLR ligands or TNFα, IL-17 is a weak activator of NF-κB (16). However, IL-17 induces expression of C/EBPβ and C/EBPδ, which are required for IL-17 induction of multiple genes (13, 14, 16, 30, 31). To date, IL-17-mediated regulation of C/EBP proteins has not been explored. Here, we show for the first time that IL-17 triggers at least two sequential phosphorylation events in the C/EBPβ RD2 domain (Figs. 1–2). We further show that T188 phosphorylation is mediated by ERK and T179 phosphorylation is mediated by GSK-3β. Finally, C/EBPβ phosphorylation and ERK/GSK3 serve to limit IL-17-mediated signaling.
C/EBPβ can exist in 3 forms generated by alternative translation, LAP*, LAP and LIP (19). We previously found that IL-17 preferentially induces LAP* (15), which appears to have similar transactivating properties as LAP (FS, unpublished data). We never observe induction of LIP following IL-17 or TNFα treatment of various cell lines (data not shown).
Here, we show that IL-17-induced phosphorylation of C/EBPβ negatively regulates IL-17 target gene expression. Although unexpected given the positive role for C/EBP elements in IL-17 target genes, this finding is similar to studies of growth hormone regulation of c-fos (25). In that setting, phosphorylation of C/EBPβ by GSK3β decreases its capacity to bind DNA, at least in vitro, thereby reducing its ability to regulate transcription. However, those studies did not demonstrate whether C/EBPβ phosphorylation occurs in living cells, which we have now shown for IL-17 (Fig. 1B, 2). Our findings contrast work in adipogenesis, where acquisition of DNA binding capacity by C/EBPβ requires phosphorylation of T188 and T179 (23, 24). Mechanistically, phosphorylation of C/EBPβ may cause a conformational change that renders the DNA binding domain accessible (24). Those studies were also performed in purified biochemical systems, and the validity in intracellular signaling has not been substantiated. Clearly, regulation of C/EBPβ is complex, and the role of C/EBPβ phosphorylation seems to depend both on the upstream signals as well as the particular downstream promoter system being examined.
Although phosphorylation of C/EBPβ downregulates IL-17-induced gene expression, both C/EBPβ and C/EBPδ are nonetheless capable of activating IL-17 target genes. The IL-6 and 24p3 gene promoters contain essential C/EBP binding sites, deletion of which abrogates IL-17-mediated activation of expression (15, 16). C/EBPβδKO cells fail to respond to IL-17, suggesting that other C/EBP family members are not involved in the IL-17 pathway. Reconstitution of C/EBPβδKO cells with either C/EBPδ or C/EBPβ restores IL-17 signaling (16), and thus C/EBPβ can act as both a positive regulator IL-17 downstream signaling. The relative contribution of C/EBPδ and C/EBPβ has not been evaluated, and may depend on cellular environment. C/EBPβ is essential for driving cox-2 expression in macrophages but not fibroblasts (32). This is consistent with our findings in fibroblasts where C/EBPδ alone can activate IL-17-mediated IL-6 expression, and it is noteworthy that IL-17 signaling is most relevant in fibroblast-type cells such as stromal cells and osteoblasts (3). Moreover, the RD2 domain is inhibitory in L cells (of fibroblast origin) but not hepatocytes (20).
Alternatively, the inhibitory effect of phosphorylated C/EBPβ may be related to the kinetics of the response. We have used C/EBPβδKO cells to dissect the role of C/EBPβ in the absence of C/EBPδ, but the functional interplay between C/EBPδ and C/EBPβ is likely to be dynamic and biologically important. At baseline, C/EBPδ levels are almost undetectable; whereas, the LAP isoform of C/EBPβ predominates, exists in an unphosphorylated state and is transcriptionally active (15). Unphosphorylated LAP-C/EBPβ probably accounts for the rapid induction of C/EBP-dependent genes such as IL-6 and 24p3, which are detectable 30 minutes post-stimulation, before C/EBPδ is induced significantly (16). After 1 hour of IL-17, C/EBPδ is upregulated (15); at this time period, C/EBPβ converts to LAP*, becomes phosphorylated at T188 and T179 and becomes less transcriptionally active. Since C/EBP proteins form homodimers and heterodimers, their relative ratios will dictate the final effect on downstream gene expression. Ultimately, changes in C/EBPβ activity and expression may allow for a nuanced inflammatory response in vivo.
C/EBPβ is subject to other post-translational modifications and changes in subcellular location (reviewed in (19)). With respect to IL-17, additional phosphorylation of C/EBPβ may occur at later time points. Specifically, the P1120 MS peak, representing the dually phosphorylated pT188/pT179 RD2 fragment, disappears 24 after IL-17 treatment (Table 3). However, C/EBPβ is not restored to an unphosphorylated state, because P1067 is also absent. Therefore at least one other posttranslational modification within the RD2 fragment occurs. Further studies will be necessary to determine all the post-translational events that impact C/EBPβ.
The IL-17R family is unique in sequence, and thus it has been challenging to make predictions about signaling based on homology to other receptors (Fig. 5). IL-17RA contains a cytoplasmic motif termed a “SEFIR/TILL,” with homology to TIR motifs (9, 10). However, rather than engaging TIR adaptors such as MyD88, IL-17RA binds to Act1, a SEFIR-containing adaptor upstream of ERK and TRAF6 (10, 13, 14). Act1, p38-MAPK and ERK1/2 have been implicated in C/EBPβ and C/EBPδ transcriptional regulation (13, 30), and TRAF6 knockdown impaired C/EBPβ phosphorylation at T188 (13, 31). Consistently, mutagenesis or deletion of the IL-17RA SEFIR and TILL domains impairs Act1 recruitment and abrogates ERK signaling (10, 14), and here we show that the V553H mutant also impairs C/EBPβ phosphorylation (Table 1). Although IL-17-induction of MAPK is important for target gene expression (7), the underlying mechanism seems to involve increased mRNA stability via AU-rich regions in the 3′ untranslated region, (UTR) (33). The direct targets of the ERK pathway remain unknown. Here we demonstrate that ERK is directly connected to C/EBPβ via phosphorylation of T188 (Table 2, Fig. 5). Since the SEFIR/TILL domain is also upstream of CEBPδ and NF-κB, mutations within this region of IL-17RA are defective in mediating expression of all IL-17 target genes so far examined (10, 14). Therefore, we cannot use IL-17RA mutants to delineate the exclusive role of ERK. Moreover, Act1-independent signals may contribute to C/EBPβ phosphorylation, as Act1-deficient MEF cells were shown to activate delayed phosphorylation of ERK following IL-17 signaling (14).
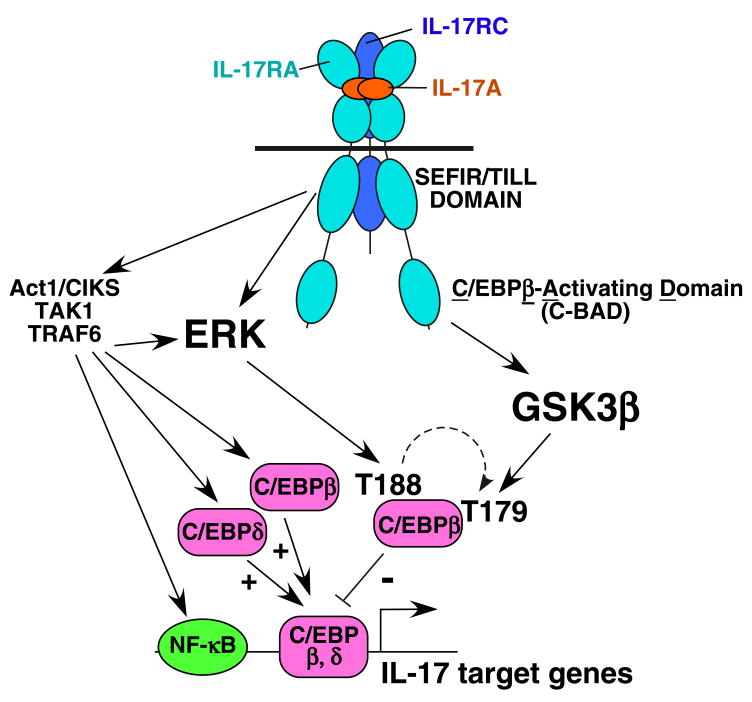
Figure 5. Model of IL-17 signal transduction.
IL-17 binds to its receptor, composed of at least two IL-17RA subunits (34) and IL-17RC (8). The SEFIR/TILL domain contains homology to TIR domains, and is an essential upstream modulator of NF-κB, TRAF6 and ERK signaling through engagement of Act1 and TRAF6 (10). Here, we show that ERK is upstream of T188 and T179 phosphorylation. However, since the IL-17RAΔ665 mutant can activate ERK but not T179, we conclude that ERK acts indirectly on T179, which has been shown previously in vitro (23). Our data with GSK3β inhibitors and GSK3β-deficient cells indicate that T179 but not T188 is a target of this kinase, and the distal “C-BAD” domain of IL-17RA is required for this event to proceed (Table 1).
We have identified a new functional domain in IL-17RA, located in the C-terminal region of IL-17RA. Specifically, a deletion at residue 665 has no effect on NF-κB, ERK or induction of C/EBPδ, but specifically repressed C/EBPβ-LAP* synthesis (10). We demonstrate that the IL-17RAΔ665 mutant permits phosphorylation of T188 but not T179 (Table 1, Fig. 5). Thus, phosphorylation at T179 correlates with LAP* induction kinetically, and both events are mediated by the IL-17RA distal tail. Interestingly, this domain bears no homology to other IL-17R family members, so it will be important to delineate the boundaries of this region and determine its other functions.
In summary, this study has expanded the picture of IL-17RA signal transduction by connecting ERK and GSK3β to phosphorylation of C/EBPβ and its and subsequent functional modulation.
Supplementary Material
Acknowledgments
IL-17RA-deficent fibroblast lines were generated from IL-17RAKO mice generously provided by Amgen. SLG and TDW were supported by the SUNY Buffalo Interdisciplinary Research fund. SLG was also supported by NIH-AR054389 and the Alliance for Lupus Research. DVK was supported by NIH-CA78282 and TDW by the UB Center of Excellence in Bioinformatics. BD and JRW were supported by the Canadian Institutes of Health Research. We thank L. Kane for critical reading and JP Malone for bioinformatics analysis of IL-17RA. Conflict of interest: SLG received honoraria and travel reimbursements from Amgen and Wyeth, and consults for Kinex Biopharma, Buffalo NY.
References
- 1.McGeachy MJ, Cua DJ. Immunity. 2008 Apr;28:445. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Dong C. Nat Rev Immunol. 2006 Apr;6:329. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Gaffen SL. Front Biosci. 2008;13:170. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal S, Gurney AL. J Leukoc Biol. 2002;71:1. [PubMed] [Google Scholar]
- 5.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Cytokine Growth Factor Rev. 2003;14:155. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 6.McInnes IB, Schett G. Nat Rev Immunol. 2007 Jun;7:429. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 7.Shen F, Gaffen SL. Cytokine. 2008;41:92. doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toy D, et al. J Immunol. 2006;177:36. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. Trends Biochem Sci. 2003 May;28:226. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 10.Maitra A, et al. Proc Natl Acad Sci, USA. 2007;104:7506. [Google Scholar]
- 11.Schwandner R, Yamaguchi K, Cao Z. J Exp Med. 2000;191:1233. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X. Cytokine. 2008 Feb;41:105. doi: 10.1016/j.cyto.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Chang SH, Park H, Dong C. J Biol Chem. 2006 Nov 24;281:35603. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 14.Qian Y, et al. Nat Immunol. 2007 Feb 4;8:247. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 15.Shen F, Hu Z, Goswami J, Gaffen SL. J Biol Chem. 2006;281:24138. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- 16.Ruddy MJ, et al. J Biol Chem. 2004;279:2559. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 17.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. J Leukoc Biol. 2005 Mar;77:388. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 18.Kalvakonalu D, Roy S. J Interferon Cytokine Res. 2005;25:757. doi: 10.1089/jir.2005.25.757. [DOI] [PubMed] [Google Scholar]
- 19.Ramji DP, Foka P. Biochem J. 2002;365:561. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams S, Baer M, Dillner A, Johnson P. EMBO J. 1995;14:3170. doi: 10.1002/j.1460-2075.1995.tb07319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy SK, et al. J Biol Chem. 2005 Jul 1;280:24462. doi: 10.1074/jbc.M413661200. [DOI] [PubMed] [Google Scholar]
- 22.Roy SK, et al. Proc Natl Acad Sci U S A. 2002 Jun 11;99:7945. doi: 10.1073/pnas.122075799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang QQ, et al. Proc Natl Acad Sci U S A. 2005 Jul 12;102:9766. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Tang QQ, Li X, Lane M. Proc Natl Acad Sci, USA. 2007;104:1800. doi: 10.1073/pnas.0611137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piwien-Pilipuk G, Van Mater D, Ross SE, MacDougald OA, Schwartz J. J Biol Chem. 2001 Jun 1;276:19664. doi: 10.1074/jbc.M010193200. [DOI] [PubMed] [Google Scholar]
- 26.Hoeflich KP, et al. Nature. 2000 Jul 6;406:86. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 27.Maziarz E, Lorenz S, White T, Wood T. J Am Soc Mass Spectrom. 2000;11:659. doi: 10.1016/s1044-0305(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 28.Gade P, Roy SK, Li H, Nallar SC, Kalvakolanu DV. Mol Cell Biol. 2008 Apr;28:2528. doi: 10.1128/MCB.00784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver CT, Hatton RD, Mangan PR, Harrington LE. Annu Rev Immunol. 2007;25:821. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 30.Cortez DM, et al. Am J Physiol Heart Circ Physiol. 2007 Dec;293:H3356. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 31.Patel DN, et al. J Biol Chem. 2007 Sep 14;282:27229. doi: 10.1074/jbc.M703250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorgoni B, Caivano M, Arizmendi C, Poli V. J Biol Chem. 2001 Nov 2;276:40769. doi: 10.1074/jbc.M106865200. [DOI] [PubMed] [Google Scholar]
- 33.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. J Immunol. 2007 Sep 15;179:4135. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 34.Kramer J, et al. J Immunol. 2006;176:711. doi: 10.4049/jimmunol.176.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.