Introduction
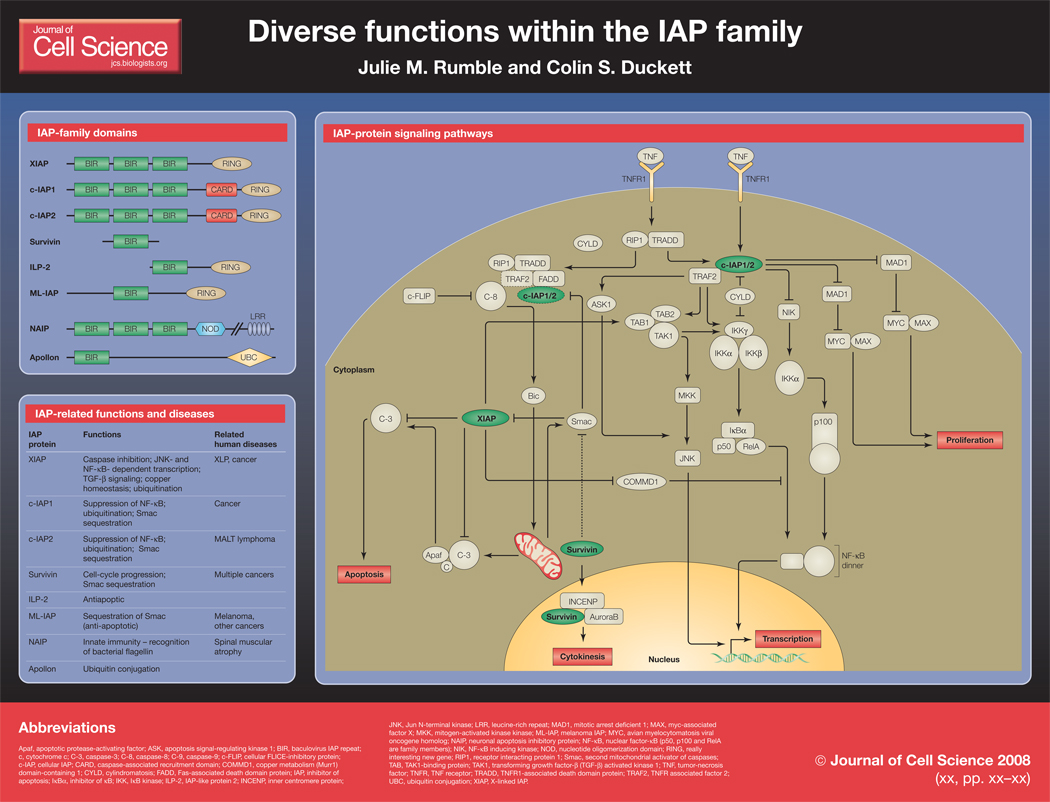
Inhibitors of apoptosis (IAPs) are a diverse group of signaling molecules that function in a wide range of cellular roles, from the inhibition of caspases to promotion of cell-cycle progression. These diverse functions may well be linked to the many different domains that are contained within different proteins in the family. IAP family members were first described as inhibitors of apoptosis in baculoviruses, and are therefore identified by the presence of baculovirus IAP repeat (BIR) domains. However, it is becoming increasingly evident that although the family-defining BIR domain is highly conserved, distinct BIRs, even within the same protein, have different functions (Eckelman et al., 2006; Srinivasula and Ashwell, 2008). Additionally, RING domains, which have been described in many proteins to have E3 ubiquitin ligase function, are present in several IAP family members. Two IAPs, c-IAP1 and c-IAP2, each contain a caspase activation and recruitment domain (CARD), which is thought to mediate protein-protein interactions. Furthermore, some unique protein domains are found within the family, including a nucleotide-binding oligomerization domain (NOD) and a leucine-rich repeat (LRR) in neuronal apoptosis inhibitory protein (NAIP) (Fritz et al., 2006), and a ubiquitin-conjugating domain (UBC) in Apollon (Bartke et al., 2004).
X-linked IAP (XIAP) is the best-characterized member of the IAP family and a potent inhibitor of caspases. Since it is overexpressed in numerous malignancies, it has been pursued as a potential therapeutic target for the treatment of cancers. In the past few years, several synthetic small-molecule IAP antagonists have been produced, and in addition to demonstrating therapeutic potential, studies performed using these drugs have been very informative to the field of IAP biology. Beyond their inhibitory effects on XIAP, these compounds also act on other members of the family, such as c-IAP1 and c-IAP2. These findings have greatly clarified the role of IAP proteins in apoptosis, but have also been instrumental in understanding how IAPs function in other pathways. Here we will place the recently revealed signaling pathways of these IAP proteins in the context of the delicately balanced network of cell death and activation.
Smac mimetics and IAP signaling
XIAP is thought to be the only member of the human IAP family that can directly bind and inhibit the caspases that carry out the cell death program (Eckelman et al., 2006; Srinivasula and Ashwell, 2008). XIAP binds to caspase-9 through the most carboxy-terminal BIR domain (BIR3), whereas caspases-3 and -7 bind through the middle of the three BIRs (BIR2). Recent data indicate that the C-terminal RING domain is required for XIAP’s antiapoptotic activity, however this may be directly related to reduced protein stability (Schile et al., 2008). Binding of XIAP to caspases is antagonized by several mitochondrial proteins, most notably second mitochondrial activator of caspases (Smac), which is released following loss of mitochondrial integrity during apoptosis (Vaux and Silke, 2003). The potential of this XIAP-Smac interaction to sensitize cells to apoptosis led to the pursuit of molecules that would mimic Smac and therefore inhibit XIAP as anti-cancer therapies (Holcik et al., 2001; Wright and Duckett, 2005). Several small molecules have been developed, which can induce death in tumor cells either alone or in combination with established chemotherapeutics. For example, certain tumor-necrosis factor (TNF)-related apopotosis-inducing ligand (TRAIL)-resistant tumor cells can be sensitized to TRAIL-induced death when used in combination with a Smac mimetic drug (Li et al., 2004).
Previous studies have shown that c-IAP1 binds via its BIR1 to a key intracellular signaling intermediate, TNF receptor-associated factor 2 (TRAF2), which is known to be essential for several signaling pathways. These two proteins transduce signals from TNF receptor family members, becoming part of the signaling complex of the TNF receptors. Additionally, c-IAP1 can bind caspases but is not thought to inhibit apoptotic death through this binding (Eckelman et al., 2006; Tenev et al., 2004). Interestingly, both proteins are degraded rapidly upon treatment with Smac mimetics, which confers a pro-apoptotic phenotype. This mechanism of death, induced by c-IAP1 degradation, appears to be dependent on TNF (Srinivasula and Ashwell, 2008; Wu et al., 2007; Varfolomeev and Vucic, 2008).
Smac mimetics have helped to elucidate functions for c-IAP1, showing that under normal conditions, c-IAP1 acts as an E3 ubiquitin ligase (probably through its RING domain) for nuclear factor-κB (NF-κB)-inducing kinase (NIK), maintaining low basal levels of NIK and preventing NF-κB signaling (Srinivasula and Ashwell, 2008; Wu et al., 2007; Varfolomeev and Vucic, 2008). The loss of c-IAP1 (through degradation by Smac or Smac mimetics, for example) results in phosphorylation and activation of downstream signaling molecules including NIK, ultimately leading to translocation of NF-κB dimers into the nucleus to activate κB-dependent transcription. NF-κB activation induces the expression of TNF, which can exert an autocrine effect on the cell. In the absence of c-IAP1, TNF receptor ligation results in the deubiquitination of the receptor-associated kinase RIP1 (receptor interacting protein 1) by the tumor-suppressor protein CYLD (cylindromatosis) and induction of a death-inducing complex, which includes RIP1, FADD (Fas-associated death-domain protein) and activated caspase-8 and culminates in cell death through caspase-3 activation (Wang et al., 2008). Smac mimetics have thus greatly enhanced our understanding of both the signaling functions of c-IAP1 and the consequences of TNF receptor signaling.
Other functions of IAPs
XIAP has also been implicated in other cellular processes besides the suppression of apoptosis, including the control of NF-κB-dependent transcription. TNF receptor ligation activates the canonical NF-κB pathway by phosphorylating the inhibitor of κB (IκB) kinase (IKK) complex, which results in phosphorylation and degradation of IκB and release of NF-κB dimers into the nucleus. The phosphorylation of the IKKs is performed by transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) (Hayden and Ghosh, 2008), a mitogen activated protein kinase kinase kinase (MAP3K) that is also involved in Jun N-terminal kinase (JNK) signaling. XIAP has been shown to bind TAK1 binding protein 1 (TAB1) through the most amino-terminal BIR (BIR1), which in turn activates TAK1. Activation of TAK1 results in both NF-κB and JNK activation and is thought to enhance the anti-apoptotic function of XIAP (Lu et al., 2007). XIAP BIR1, although homologous to BIR2 and BIR3, does not have caspase-inhibitory activity, and this role in the activation of TAK1 illustrates the diversity of functions within the IAP family.
XIAP has also been shown to interact with copper metabolism (Murr1) domain-containing 1 (COMMD1), a copper-metabolism protein, and through this interaction has effects on two different signaling pathways (Maine and Burstein, 2007; Mufti et al., 2007). The importance of COMMD1 in copper export from the cell is evidenced by loss of COMMD1, which results in a copper toxicosis syndrome that is common in Bedlington terriers. XIAP binds COMMD1 through its BIR3 domain and catalyzes its ubiquitination through the RING domain. This downregulation of COMMD1 by XIAP can thus increase intracellular copper levels. Homeostasis of copper metabolism is achieved through a negative-feedback loop, in which higher copper levels have a negative effect on XIAP, causing its inhibition and ultimate degradation. The second pathway of XIAP function through COMMD1 regulation is related to the role of COMMD1 as an inhibitor of NF-κB. COMMD1 has been shown to bind and catalyze the ubiquitination of DNA-bound RelA, a key NF-κB subunit, thus suppressing NF-κB-dependent transcription. As XIAP can in turn catalyze the ubiquitination of COMMD1, it can prevent this inhibition and allow NF-κB-dependent transcription to continue (Maine and Burstein, 2007; Mufti et al., 2007). The physiological consequences of the XIAP-COMMD1 interaction remain to be elucidated.
Two members of the IAP family whose functions are not yet fully understood are NAIP and BRUCE/Apollon, both of which contain unique domains to the IAP family. NAIP was the first mammalian member of the IAP family to be identified, and it was found in connection with spinal muscular atrophy, as it is contained within the locus responsible for the disease (Roy et al., 1995). In studies using mice deficient in one of the several paralogs of NAIP, NAIP5 has been shown to contribute to sensing of bacterial flagellin and subsequent activation of caspase-1 for pyroptotic cell death ( Carneiro et al., 2007; Delbridge and O'Riordan, 2007; Lightfield et al., 2008). This has been supported by work with the human protein (Vinzing et al., 2008), and represents perhaps the best-understood function of NAIP, as its anti-apoptotic properties remain unclear. BRUCE/Apollon, the largest member of the mammalian IAP family (Chen et al., 1999), is even more controversial. Interestingly, murine deficiency in BRUCE/Apollon leads to embryonic lethality due to excessive apoptosis of the placenta, which is a far more striking phenotype than that caused by loss of the better-characterized XIAP. It appears that the antiapoptotic function of BRUCE/Apollon resides in the C-terminal UBC domain, rather then the single BIR (Ren et al., 2005). BRUCE/Apollon has also recently been described to participate as a coordinator of multiple processes during cytokinesis (Pohl and Jentsch, 2008), which again illustrates the diversity of functions within the IAP family.
Survivin is the smallest member of the mammalian IAP family, containing only one BIR and no other functional domains (Sah et al., 2006; Altieri, 2003). Its simplicity belies its apparent importance for two separate processes that are crucial for cell homeostasis: cell-cycle regulation and inhibition of apoptosis. Interestingly, survivin has been reported to be upregulated in cancer cells, although it is possible that this upregulation is simply indicative of cancer cells cycling at a greater rate than the rest of the cell population, as survivin is regulated in a cell cycle-dependent manner. Evidence for the role of survivin in cell-cycle regulation is indicated by loss of the protein resulting in arrest or mitotic catastrophe in cycling cells. Survivin binds to the AuroraB kinase and the inner centromere protein (INCENP) during cytokinesis, and may have other functions in promoting cell division. Additionally, studies have suggested that survivin can inhibit cell death, possibly through binding of Smac to its single BIR, removing Smac’s ability to inhibit XIAP (Sah et al., 2006; Altieri, 2003).
Conclusion
Our understanding of the IAP family has clearly moved far beyond the originally described function as caspase inhibitors. It is increasingly apparent that the family-defining BIR domain evolved for signaling in concert with Smac, as the ability of IAPs to bind Smac is more widely retained than caspase inhibition. The interaction of BIRs with Smac has been shown to result in a variety of cellular outcomes, which have remarkable consequences in the context of cancer therapeutics. The other domains that are present in IAP family members, such as the RING domain, also clearly diversify the functions of these proteins to have effects in other cellular processes. Future studies will further clarify how each of these functions of IAPs participate in the maintenance of cellular homeostasis.
Abbreviations
- Apaf
apoptotic protease-activating factor
- ASK
apoptosis signal-regulating kinase 1
- BIR
baculovirus IAP repeat
- c
cytochrome c
- C-1
caspase-1
- C-3
caspase-3
- C-8
caspase-8
- C-9
caspase-9
- c-FLIP
cellular FLICE-inhibitory protein
- c-IAP
cellular IAP
- CARD
caspase-associated recruitment domain
- COMMD1
copper metabolism (Murr1) domain-containing 1
- CYLD
cylindromatosis
- FADD
Fas-associated death domain protein
- IAP
inhibitor of apoptosis
- IκBα
inhibitor of κB
- IKK
IκB kinase
- ILP-2
IAP-like protein 2
- INCENP
inner centromere protein
- JNK
Jun N-terminal kinase
- L
Legionella pneumophila
- LRR
leucine-rich repeat
- MAD1
mitotic arrest deficient 1
- MAX
myc-associated factor X
- MKK
mitogen-activated kinase kinase
- ML-IAP
melanoma IAP
- MYC
avian myelocytomatosis viral oncogene homolog
- NAIP
neuronal apoptosis inhibitory protein
- NF-κB
nuclear factor-κB (p50, p100 and RelA are family members)
- NIK
NF-κB inducing kinase
- NOD
nucleotide oligomerization domain
- RING
really interesting new gene
- RIP1
receptor interacting protein 1
- Smac
second mitochondrial activator of caspases
- TAB
TAK1-binding protein
- TAK1
transforming growth factor-β (TGF-β) activated kinase 1
- TNF
tumor-necrosis factor
- TNFR
TNF receptor
- TRADD
TNFR1-associated death domain protein
- TRAF2
TNFR associated factor 2
- UBC
ubiquitin conjugation
- XIAP
X-linked IAP
References
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- Bartke T, Pohl C, Pyrowolakis G, Jentsch S. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol Cell. 2004;14:801–811. doi: 10.1016/j.molcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Carneiro LA, Travassos LH, Girardin SE. Nod-like receptors in innate immunity and inflammatory diseases. Ann Med. 2007;39:581–593. doi: 10.1080/07853890701576172. [DOI] [PubMed] [Google Scholar]
- Chen Z, Naito M, Hori S, Mashima T, Yamori T, Tsuruo T. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochem Biophys Res Commun. 1999;264:847–854. doi: 10.1006/bbrc.1999.1585. [DOI] [PubMed] [Google Scholar]
- Delbridge LM, O'Riordan MX. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2007;19:10–16. doi: 10.1016/j.coi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, Henry T, Sun YH, Cado D, Dietrich WF, Monack DM, Tsolis RM, Vance RE. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008 doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Lin SC, Huang Y, Kang YJ, Rich R, Lo YC, Myszka D, Wu H. XIAP induces NF-κB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maine GN, Burstein E. COMMD proteins: COMMing to the scene. Cell Mol Life Sci. 2007;64:1997–2005. doi: 10.1007/s00018-007-7078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti AR, Burstein E, Duckett CS. XIAP: cell death regulation meets copper homeostasis. Arch Biochem Biophys. 2007;463:168–174. doi: 10.1016/j.abb.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl C, Jentsch S. Final stages of cytokinesis and midbody ring formation are controlled by BRUCE. Cell. 2008;132:832–845. doi: 10.1016/j.cell.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Ren J, Shi M, Liu R, Yang QH, Johnson T, Skarnes WC, Du C. The Birc6 (Bruce) gene regulates p53 and the mitochondrial pathway of apoptosis and is essential for mouse embryonic development. Proc Natl Acad Sci U S A. 2005;102:565–570. doi: 10.1073/pnas.0408744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164–171. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Schile AJ, Garcia-Fernandez M, Steller H. Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 2008;22:2256–2266. doi: 10.1101/gad.1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasula SM, Ashwell JD. IAPs: What’s in a Name? Molecular Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenev T, Zachariou A, Wilson R, Ditzel M, Meier P. IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat Cell Biol. 2004 doi: 10.1038/ncb1204. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Vucic D. (Un)expected roles of c-IAPs in apoptotic and NF-κB signaling pathways. Cell Cycle. 2008;7:1511–1521. doi: 10.4161/cc.7.11.5959. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Silke J. Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun. 2003;304:499–504. doi: 10.1016/s0006-291x(03)00622-3. [DOI] [PubMed] [Google Scholar]
- Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, Slevogt H, N'guessan PD, Gunther S, Schmeck B, Hippenstiel S, Flieger A, Suttorp N, Opitz B. NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol. 2008;180:6808–6815. doi: 10.4049/jimmunol.180.10.6808. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-α induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wright CW, Duckett CS. Reawakening the cellular death program in neoplasia through the therapeutic blockade of IAP function. J Clin Invest. 2005;115:2673–2678. doi: 10.1172/JCI26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Tschopp J, Lin SC. Smac mimetics and TNFα: a dangerous liaison? Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]