Abstract
Glutathione (GSH), the most abundant intracellular nonprotein thiol, is critical for many cellular functions. The rate-limiting step in GSH synthesis is catalyzed by glutamate cysteine ligase (GCL), a heterodimer composed of a catalytic (GCLC) and a modifier (GCLM) subunit. The tissue-specific regulation of GSH synthesis is poorly understood. We showed previously that gonadotropin hormones regulate ovarian GSH synthesis. In the present study, we sought to clarify the ovarian cell type-specific effects of follicle-stimulating hormone (FSH) and estradiol on GSH synthesis. Immature female rats were treated with estradiol to stimulate development of small antral follicles. Granulosa cells (GCs) from these follicles or whole follicles were cultured in serum-free media, with or without FSH and 17beta-estradiol. The GSH and GCLC protein and mRNA levels increased in GCs treated with FSH alone. The effects of FSH on GCLC and GCLM protein and mRNA levels, GCL enzymatic activity, and GSH concentrations in GCs were significantly enhanced by the addition of estradiol. Estradiol alone had no effects on GSH. Dibromo-cAMP mimicked and protein kinase A (PKA) inhibitors prevented FSH stimulation of GCL subunit protein levels. In cultured small antral follicles, FSH stimulated estradiol synthesis and robustly increased GCL subunit mRNA and protein levels and GSH concentrations. The GCL subunit mRNA expression increased in both the granulosa cells and theca cells of follicles with FSH stimulation. These data demonstrate that maximal stimulation of GSH synthesis by FSH in granulosa cells and follicles requires estradiol. Without estradiol, FSH causes lesser increases in GCL subunit expression via a PKA-dependent pathway.
Keywords: antioxidant, antral follicle, estradiol, follicle, follicle-stimulating hormone, glutamate cysteine ligase, glutathione, granulosa cells, protein kinase A, toxicology
Estradiol enhances follicle-stimulating hormone upregulation of glutamate cysteine ligase subunit expression increasing the synthesis of the antioxidant glutathione in small antral follicles and granulosa cells.
INTRODUCTION
Premature menopause, infertility, and decreased fecundity are among the serious consequences of human exposure to environmental toxicants. Infertility or impaired fecundity affects about 12% of American women [1]. Premature menopause afflicts about 1% of all women [2]. Women with early onset of natural or surgical menopause are at increased risk of cardiovascular disease, Alzheimer disease, and osteoporosis compared with age-matched controls [3–5]. In most cases, the causes of these conditions are unknown, but exposure to environmental toxicants likely is responsible for many more cases than is currently appreciated. Environmental chemicals that are known female reproductive toxicants include anticancer drugs, like cyclophosphamide [6–9]; occupational chemicals, like 4-vinylcyclohexene [10] and 2-bromopropane [11]; and environmental pollutants, like polycyclic aromatic hydrocarbons [12–14]. The tripeptide glutathione (GSH) is involved in the phase 2 detoxification of many of these female reproductive toxicants, as well as of reactive oxygen species that may be generated as a result of toxicant metabolism [15–21]. Our previous work has shown that depletion of GSH enhances and supplementation of GSH diminishes the toxicity of polycyclic aromatic hydrocarbons and cyclophosphamide to ovarian follicles and granulosa cells [22, 23].
Glutathione is present in every mammalian cell, and moderate levels are found in the ovary [21, 24–26]. Glutathione is synthesized in a two-step, ATP-dependent process. The first step, which forms γ-glutamylcysteine, is controlled by the rate-limiting enzyme glutamate cysteine ligase (GCL; EC 6.3.2.2). The second step requires the enzyme glutathione synthetase, which catalyzes the addition of glycine to the γ-glutamylcysteine to form GSH. Glutamate cysteine ligase is a heterodimer composed of a catalytic (GCLC) and a modulatory (GCLM) subunit. GCLC exhibits all catalytic activity, whereas GCLM decreases the Km of the enzyme for glutamate and raises the Ki for GSH [27]. Intracellular GSH concentrations reflect a balance between loss of GSH and synthesis of GSH. The GCL enzymatic activity is regulated by nonallosteric negative feedback inhibition by GSH [28]. Glutathione synthesis is also regulated via transcriptional and posttranscriptional regulation of Gclc and Gclm and by the availability of cysteine, which is the least abundant constituent amino acid of GSH [28, 29]. Despite this understanding of the general factors regulating GSH synthesis, much less is known about the regulation of tissue-specific rates of GSH synthesis.
We showed previously that ovarian GSH synthesis is regulated by the gonadotropin hormones. Ovarian GSH concentrations in rats varied with estrous cycle stage and were highest on estrus, the day of ovulation [24]. Equine chorionic gonadotropin (eCG) treatment of immature female rats to stimulate follicular development increased ovarian GSH concentrations and GCLM and GCLC protein levels without appreciable effects on whole-ovary GCL subunit mRNA [24, 25]. However, in vivo eCG treatment altered the intraovarian localization of Gclc and Gclm mRNA, increasing expression of both mRNAs in theca cells [25]. The mechanisms by which gonadotropins modulate GCL subunit expression in granulosa and theca cells are not known. Binding of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) to their cognate membrane-bound receptors results in the activation of intracellular signaling cascades, starting with the cyclic AMP/protein kinase A (cAMP/PKA) cascade and followed by several downstream cascades [30]. Activation of these signaling cascades stimulates synthesis of the ovarian steroids estradiol and progesterone and proliferation and differentiation of granulosa cells. We hypothesized that upregulation of GSH synthesis by FSH in follicles and granulosa cells also involves PKA activation and is mediated by stimulation of estradiol synthesis.
MATERIALS AND METHODS
Reagents
All chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO) or Fisher Scientific (Houston, TX) unless otherwise noted. Ovine FSH was purchased from the National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases (Dr. A.F. Parlow, Harbor UCLA Medical Center, Torrance, CA). Tissue culture media, trypsin, and RT-PCR primers were purchased from Invitrogen (Carlsbad, CA). RNeasy and SYBR Green RT-PCR kits were purchased from Qiagen (Valencia, CA). Antibodies for GCLC and GCLM were a gift from Dr. Terrance J. Kavanagh at the University of Washington, Seattle, Washington [31]. Progesterone radioimmunoassay kits were purchased from Diagnostic Systems Laboratories (Webster, TX), and estradiol radioimmunoassay kits were purchased from Siemens Medical Solutions Diagnostics (Los Angeles, CA). The PKA inhibitors 14–22 amide, myristoylated peptide inhibitor (PKI) and H89, as well as cycloheximide (CHX) were purchased from Calbiochem (Carlsbad, CA).
Animals
Female Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were housed under a 12L:12D schedule, with ad libitum access to standard laboratory rodent chow and deionized water. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine.
Granulosa Cell Culture
Primary granulosa cells were obtained from estradiol-primed rats and cultured according to the methods of Gonzalez-Robayna et al. [32]. Briefly, immature rats were given daily subcutaneous injections of 1.5 mg of 17β-estradiol in 0.2 ml of propylene glycol for 3 days beginning at 25 days of age. Animals were killed by carbon dioxide asphyxiation 24 h after the last injection, and ovaries were immediately excised and kept in Dulbecco modified Eagle medium (DMEM)/F12 media on ice. Granulosa cells were obtained from small antral follicles by needle puncture and were cultured at a density of 1 × 105 cells/cm2 in serum-free DMEM/F12 media with penicillin, streptomycin, and various treatments in tissue culture-treated six-well plates for up to 48 h. Treatments included 2, 20, and 200 ng/ml ovine FSH, 100 ng/ml 17β-estradiol, 1 mM dibutyryl cyclic AMP (dbcAMP), 10 μM PKA inhibitor H89, 50 μM PKI, or 10 μg/ml CHX. The concentrations of FSH were chosen to encompass non-preovulatory surge concentrations measured during the rat estrous cycle [33, 34]. The concentration of estradiol corresponded to concentrations measured in rat follicular fluid [35]. Estradiol was dissolved in 100% ethanol prior to adding to culture medium. Cycloheximide was dissolved in dimethylsulfoxide (DMSO) prior to addition to the culture medium. The final concentration of ethanol or DMSO in the culture medium was 0.1%. The same concentration of ethanol alone was added to the other groups in experiments that included estradiol-treated groups, and the same concentration of DMSO was added to the other groups in experiments that included CHX-treated groups.
Small Antral Follicle Culture
Twenty-five-day-old Sprague Dawley rats were primed with estradiol as described for granulosa cell culture. Ovaries were placed in Eagle minimum essential medium (MEM) media with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1% fatty acid-free bovine serum albumin, gassed with 95% O2 and 5% CO2, and kept on ice. Small antral follicles were dissected by hand from the ovaries with 27-gauge needles under a stereomicroscope using sterile technique and were kept at 4°C until time of culture as described previously [36, 37]. Cultures were gassed every 12 h with 95% O2 and 5% CO2 and were incubated at 37°C with gentle agitation. For each experiment, follicles from three rats were dissected, pooled, and then allocated to treatment groups. Between 10 and 20 follicles were routinely obtained from each rat. One experiment consisted of two to five observations per treatment (control or FSH), and each observation consisted of one or two culture vials containing four or six follicles. The number of follicles processed together for an observation depended on the end point being measured (e.g., eight follicles per observation for GSH assay, eight follicles for immunoblotting, six follicles for quantitative RT-PCR). Each experiment was repeated with follicles dissected from a second group of three rats, and the data were pooled for a final n = 4–9 observations per treatment.
GSH Assay
Granulosa cells were trypsinized and counted by trypan blue exclusion. Cells were pelleted by centrifugation and homogenized in TES-SB buffer (20 mM Tris, 1 mM ethylenediaminetetraacetic acid, 250 mM sucrose, 20 mM boric acid, and 2 mM l-serine) with protease inhibitors on ice using a Kontes handheld homogenizer and disposable pestle. Follicles were similarly homogenized in TES-SB buffer. A small aliquot of homogenate was removed for Pierce BCA Protein Assay (Thermo Scientific, Rockford, IL), and the remainder was acidified with a one-quarter volume of 5% sulfosalicylic acid. Homogenates were centrifuged for 10 min at 4°C at 15 800 × g. Supernatants were stored at −80°C until assay. Supernatants were assayed in triplicate for total and oxidized (GSSG) glutathione using an enzymatic recycling assay [25, 36, 38]. Reduced GSH concentrations were calculated as the total GSH concentration minus two times the GSSG concentration. The GSH concentrations were expressed as nanomoles per milligram of protein.
Immunoblotting
Granulosa cells were detached from culture plates by a rubber policeman and pelleted by centrifugation. Cell pellets or follicles were lysed in RIPA buffer (PBS, 0.5% sodium deoxycholate, 0.1% SDS, 1% Nonidet P-40) with protease inhibitors and were kept on ice. Protein concentrations were determined with the Pierce BCA Assay. A total of 20–40 μg of protein were separated by electrophoresis in 12% Tris-HCL polyacrylamide gels (BioRad, Hercules, CA), transferred to polyvinylidene difluoride membranes, and subjected to immunostaining for GCLC, GCLM, and β-actin as described previously [24]. Visualization was accomplished using ECL chemiluminescence (GE Healthcare Lifesciences, Piscataway, NJ). Films were subjected to densitometric analysis. Optical densities of GCLC and GCLM bands were normalized to β-actin. All normalized densities were then expressed as fold of the means of the control bands for the same blot for subsequent statistical analyses.
Quantitative Real-Time RT-PCR
Total RNA was extracted from granulosa cells or follicles using the Qiagen RNeasy Kit (Qiagen) according to the manufacturer's instructions. Fifty nanograms of RNA were reverse transcribed and subjected to PCR using the gene-specific forward and reverse primers shown in Table 1 and the Qiagen Quantitect SYBR Green RT-PCR reagent in 10-μl reaction volumes in duplicate in the Roche LightCycler. Standard curves derived from serial dilutions of rat kidney RNA (Gclc, Gclm, Gapdh) or rat ovary RNA (Lhr) were used to determine concentrations of Gclc, Gclm, and Lhr mRNAs normalized to Gapdh using the Roche LightCycler 4.0 software. Gclc, Gclm, and Gapdh primers were designed previously for the amplification of mouse and rat mRNA [25]. Lhr primer sequences were obtained from Bao et al. [39].
TABLE 1.
Primer pairs used in quantitative real-time PCR assays.
GCL Enzymatic Activity Assay
Cells were detached using a rubber policeman. Cells and their culture media were centrifuged at 400 × g for 5 min. Cell pellets (containing 2 × 106 to 3 × 106 cells) were homogenized in 30 μl of TES-SB buffer on ice and were centrifuged at 15 800 × g at 4°C for 10 min. The supernatants were stored at −80°C until assay. The supernatants were diluted to 3–5 mg protein/ml (determined by Pierce BCA assay) if necessary. GCL enzymatic activity was measured using a fluorimetric assay according to a modification of the method of White and coworkers [25, 40]. All samples were assayed in duplicate.
Progesterone and Estradiol Assays
Media from cultured granulosa cells and follicles were collected and stored at −20°C until the assays were performed. Progesterone concentrations were measured using the Progesterone ACTIVE radioimmunoassy kit (Diagnostic Systems Laboratories). Estradiol was measured in culture media using the Estradiol Double Antibody radioimmunoassay kit (Siemens Medical Solutions Diagnostics), except that a standard curve was generated using known concentrations of 17β-estradiol in tissue culture media.
Statistical Analyses
Data from two or three replicate experiments were pooled for statistical analyses. Homogeneity of variances was assessed by Levene test [41]. Comparisons of two groups were made using independent-samples t-test for homogeneous or nonhomogeneous variances as appropriate. The differences among multiple groups were analyzed by ANOVA followed by Fisher least significant difference (LSD) test. When variances were nonhomogeneous, the Kruskal-Wallis test for nonparametric data was used. If the Kruskal-Wallis test was statistically significant at P < 0.05, the Mann-Whitney test was then used for intergroup comparisons. Statistical analyses were carried out using SPSS 16.0 for Mac OS X.
RESULTS
Effects of FSH and Estradiol on GSH Concentrations, Progesterone Secretion, and Viability in Cultured Granulosa Cells
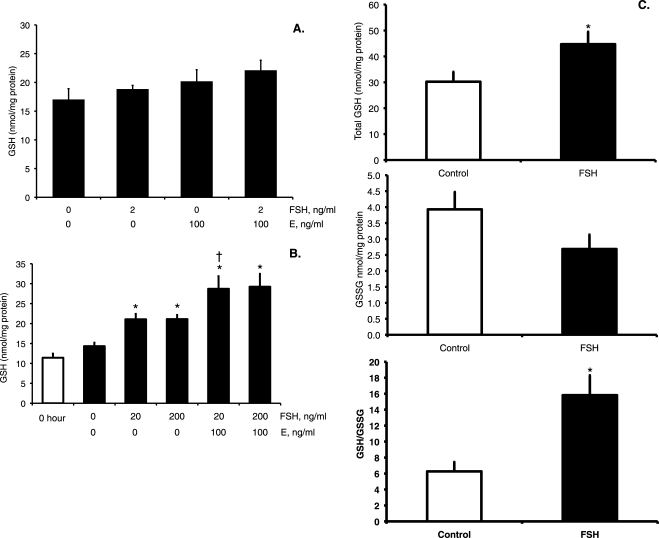
To test the independent and combined effects of FSH and estradiol on GSH synthesis in cultured granulosa cells, several experiments using a range of FSH concentrations were performed. Granulosa cells cultured for 48 h with 2 ng/ml FSH in the presence or absence of 100 ng/ml estradiol or cultured with estradiol alone did not have significantly increased concentrations of total intracellular GSH compared with control cells cultured without hormones for 48 h (Fig. 1A). Subsequent experiments with two higher concentrations of FSH (20 and 200 ng/ml) and the same concentration of estradiol demonstrated statistically significant increases in GSH concentrations at 48 h (Fig. 1B), but not at 24 h (data not shown), in all treated groups compared with untreated controls and 0-h controls (P < 0.001, effect of treatment by Kruskal-Wallis test at 48 h). The GSH concentrations were increased 1.5-fold in both FSH-treated groups (P < 0.005 FSH versus untreated control by Mann-Whitney test) and 2.0-fold in both FSH plus estradiol-treated groups relative to untreated controls (P < 0.003 versus untreated controls by Mann-Whitney test). The difference between FSH alone and FSH plus estradiol was statistically significant for the 20 ng/ml FSH alone compared with 20 ng/ml FSH plus estradiol (P < 0.05 Mann-Whitney test). Because the 2 ng/ml FSH concentration was ineffective in stimulating GSH and the 20 and 200 ng/ml concentrations were equally effective, we used 20 ng/ml FSH in subsequent experiments.
FIG. 1.
Effects of FSH and estradiol on GSH concentrations in granulosa cells and small antral follicles. A and B) Granulosa cells were cultured in DMEM-F12 serum-free media with ovine FSH and/or estradiol (E) at the indicated concentrations for 48 h prior to collection of cells for total intracellular GSH assay as described in Materials and Methods. The 0-h cells were immediately processed for GSH assay without culture. Data are expressed as means ± SEM (n = 7–9 per group). A) The effect of treatment was not statistically significant. B) The effect of treatment was statistically significant by Kruskal-Wallis test (P < 0.001) *Significantly different from untreated 48-h control and from 0-h by Mann-Whitney test, P < 0.01. †Significantly different from FSH alone at the same concentration by Mann-Whitney test, P < 0.05. C) Small antral follicles were cultured for 24 h with or without 20 ng/ml FSH and assayed for total (top graph) and oxidized (middle graph) GSH. The ratio of reduced GSH to GSSG was calculated and is presented in the bottom graph. Data are expressed as means ± SEM (n = 7–9 observations of eight follicles each per treatment group). *The effects of FSH treatment on total GSH and on the GSH:GSSG ratio (presented as GSH/GSSG) were statistically significant by t-test (P < 0.05).
We also measured the stimulation of progesterone production, a well-known effect of FSH in granulosa cells [42, 43], to verify that these cells respond normally to FSH in our hands. Concentrations of progesterone at 48 h in untreated controls and in the groups treated with 2 ng/ml FSH were near the limit of detection of the assay of 0.3 ng/ml. Treatment with 20 or 200 ng/ml FSH with or without estradiol significantly increased progesterone production to 15.5 ± 2.7 ng/ml in the 20-ng/ml FSH group, 27.2 ± 6.3 ng/ml in the 20-ng/ml FSH plus estradiol group, 29.8 ± 0.8 ng/ml in the 200-ng/ml FSH group, and 53.1 ± 0.6 ng/ml in the 200-ng/ml FSH plus estradiol group (P < 0.001, effect of treatment by Kruskal-Wallis test).
Trypan blue staining was used to test whether differences in cellular GSH concentrations were due to differences in cell viability. The fraction of viable cells did not differ between cells cultured for 48 h in untreated medium (0.71 ± 0.03 in the first set of experiments and 0.67 ± 0.02 in the second set of experiments); cells cultured for 48 h with 20 ng/ml (0.73 ± 0.02) or 200 ng/ml (0.69 ± 0.02) FSH alone, with 20 ng/ml FSH plus estradiol (0.66 ± 0.03), or with 200 ng/ml FSH plus estradiol (0.68 ± 0.03) in the second set of experiments; or cells cultured with 2 ng/ml FSH (0.72 ± 0.03) or estradiol alone (0.78 ± 0.03) in the first set of experiments. The fraction of viable cells was slightly, but statistically significantly, greater after 48 h of culture with 2 ng/ml FSH plus estradiol (0.79 ± 0.02) in the first set of experiments (P = 0.045 versus control by Fisher LSD test). However, the latter treatment group did not have statistically significant increases in GSH concentrations.
Effects of FSH on GSH Concentrations and Estradiol Secretion in Small Antral Follicles
To further explore the combined role of FSH and estradiol on ovarian GSH synthesis, we turned to the whole-cultured small antral follicle model, which preserves the intracellular interactions among granulosa cells, theca cells, and oocyte. Treatment of small antral follicles with 20 ng/ml FSH for 24 h resulted in a robust increase in estradiol concentrations in the treatment media (10.3 ± 1.1 pg/ml in controls versus 348.2 ± 18.3 pg/ml in FSH-treated group). Total intracellular GSH concentrations increased 1.5-fold after 24 h of FSH treatment (Fig. 1C; P = 0.032 by t-test). The GSSG concentrations were nonstatistically significantly lower by 0.7-fold in the FSH-treated group (Fig. 1C). The FSH treatment also resulted in a statistically significant 2.5-fold increase in the reduced GSH:GSSG ratio compared with untreated control follicles, indicating that the GSH redox state in FSH-treated follicles was less oxidized than in controls (Fig. 1C).
These data show that FSH treatment increases GSH concentrations in granulosa cells and follicles, whereas estradiol treatment alone has no effect on granulosa cell GSH concentrations. The fold increase in GSH concentrations was greater in granulosa cells treated with FSH plus estradiol than with FSH alone. The FSH treatment of follicles caused a robust increase in follicular estradiol production, which likely played a role in the stimulation of GSH synthesis by FSH in follicles.
Increased GCL Subunit Protein Levels in Response to FSH and Estradiol in Granulosa Cells and Small Antral Follicles
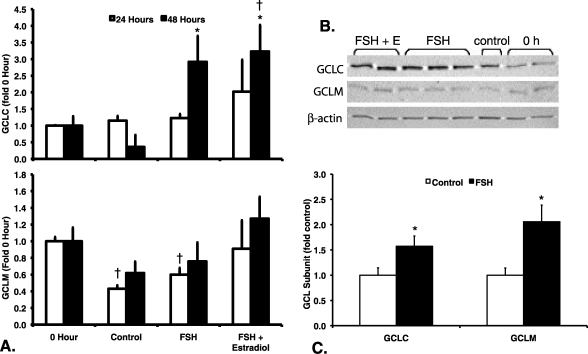
We performed immunoblotting to evaluate whether the increases in GSH concentrations after FSH and estradiol treatment of granulosa cells and follicles were due to increased levels of GCLC and GCLM protein. GCLC protein levels in granulosa cells (Fig. 2A) varied significantly by treatment at 48 h (P = 0.019 by Kruskal-Wallis test) but not at 24 h (P = 0.331 by Kruskal-Wallis test). GCLC protein levels in the FSH and the FSH plus estradiol groups were statistically significantly greater than levels in untreated controls at 48 h (P < 0.05 by Mann-Whitney test). GCLM protein levels (Fig. 2A) varied in a statistically significant manner with treatment at 24 h (P = 0.016 by Kruskal-Wallis test), but not at 48 h (P = 0.248). GCLM protein levels in the untreated controls and in the FSH-treated cells at 24 h were significantly lower than those of the 0-h controls (P < 0.05 by Mann-Whitney test). There was an apparent trend toward greater GCLM protein levels in the FSH and FSH plus estradiol groups compared with untreated controls at 48 h, but in these experiments the effect was not statistically significant.
FIG. 2.
Effects of FSH and estradiol on GCL subunit protein levels in granulosa cells and small antral follicles. Granulosa cells and follicles were cultured as described for Figure 1, except that only the 20-ng/ml concentration of FSH was used. GCLC, GCLM, and β-actin protein levels were measured by immunoblotting of protein extracts from granulosa cells (A and B) and antral follicles (C) as described in Materials and Methods. Relative protein levels normalized to β-actin were expressed as fold of 0-h controls (A) or untreated controls (C) for the same blot. Data are expressed as means ± SEM. A) GCLC protein levels (upper graph) varied significantly by treatment at 48 h (P = 0.019 by Kruskal-Wallis test) but not at 24 h (P = 0.331 by Kruskal Wallis test). GCLM protein levels (lower graph) varied significantly by treatment at 24 h (P = 0.016 by Kruskal-Wallis test) but not at 48 h (P = 0.248 by Kruskal-Wallis test). †Significantly different from 0-h controls at the same time point, P < 0.05 by Mann-Whitney test. *Significantly different from untreated controls at the same time point, P < 0.05 by Mann-Whitney test (n = 3–5 per treatment group and time point). B) Representative Western blot showing GCLC, GCLM, and β-actin levels in granulosa cells after 48 h of culture with the indicated treatments. C) *Treatment of antral follicles with FSH for 24 h resulted in statistically significant increases in protein levels of GCLC (P = 0.045 by t-test) and GCLM (P = 0.022) compared with untreated controls (n = 6 observations of eight follicles each per treatment group).
In antral follicles cultured for 24 h with FSH, there were statistically significant increases in both GCLC (P = 0.045 by t-test) and GCLM (P = 0.022) protein levels compared with untreated controls (Fig. 2B). GCLC increased 1.6-fold and GCLM increased 2.1-fold above control levels.
Effects of FSH and Estradiol on GCL Subunit mRNA Expression in Granulosa Cells and Small Antral Follicles
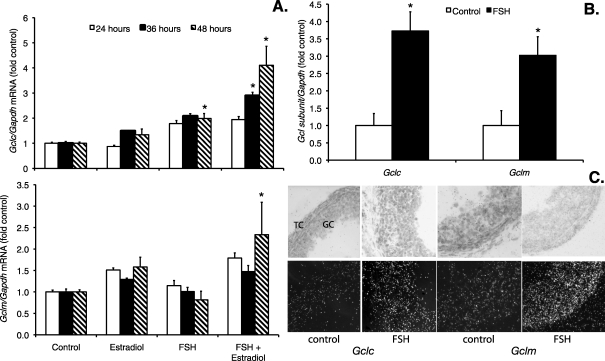
Gclc and Gclm mRNA levels in granulosa cells increased with FSH and with FSH plus estradiol treatment compared with untreated controls. For both Gclc and Gclm, the effect of FSH combined with estradiol was greater than the effect of FSH alone, and the effect was greatest at 48 h (Fig. 3A). Kruskal-Wallis tests at each time point showed statistically significant effects of treatment at 36 and 48 h for Gclc and at 48 h for Gclm (P < 0.05). The Gclc mRNA levels were significantly greater than control levels at 48 h after FSH alone and at both 36 and 48 h after FSH plus estradiol (P < 0.05 by Mann-Whitney test). The Gclm mRNA levels were significantly greater than control levels at 48 h after FSH plus estradiol (P < 0.05 by Mann-Whitney test).
FIG. 3.
Effects of FSH and estradiol on GCL subunit mRNA levels in granulosa cells and small antral follicles. Granulosa cells and follicles were cultured as described for Figures 1 and 2 and were harvested at the indicated times for analysis of Gclc, Gclm, and Gapdh mRNA by quantitative real-time RT-PCR as described in Materials and Methods. The graphs show mean ± SEM levels of Gclc and Gclm normalized to Gapdh and expressed as fold change from the untreated control levels. A) When each time point was analyzed separately by Kruskal-Wallis test, the effect of treatment was statistically significant for Gclc mRNA levels (upper graph) at 36 and 48 h and for Gclm mRNA levels (lower graph) at 48 h. *Significantly different from control at the same time point by Mann-Whitney test, P < 0.05 (n = 4–5 per treatment group). B) Gclc and Gclm mRNA levels in follicles cultured with FSH for 24 h were significantly increased compared with untreated control follicles (n = 4–5 observations of six follicles each per treatment group). *P = 0.003 and P = 0.020, respectively, by t-test. C) In situ hybridization localization of Gclc and Gclm mRNA in cultured follicles. Follicles were cultured as in B and were fixed and processed for in situ hybridization for Gclc and Gclm as described in Materials and Methods. Bright-field images are in the top row, and dark-field images are in the bottom row. GC, granulosa cells. TC, theca cells. Dark-field images show increased hybridization for both Gclc and Gclm in GC and TC after FSH treatment. Original magnification ×400.
Small antral follicles cultured with FSH for 24 h showed significantly increased Gclc and Gclm mRNA levels relative to controls (P = 0.003 and P = 0.020 by t-test, respectively; Fig. 3B). Gclc and Gclm were approximately 3.7- and 3.0-fold higher than controls, respectively. In situ hybridization showed that FSH treatment for 24 h increased Gclc and Gclm mRNA levels in both granulosa cells and theca cells of cultured follicles (Fig. 3C).
Together, the data from granulosa cells and small antral follicles suggest that the apparently greater capacity of follicles than granulosa cells to increase transcription of Gclc and Gclm in response to FSH stimulation may result from the stimulation of estradiol synthesis in whole follicles, but not in granulosa cells, by FSH.
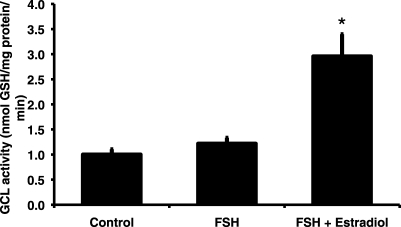
Effects of FSH and Estradiol on GCL Enzymatic Activity in Granulosa Cells
The effect of treatment group on GCL enzymatic activity in granulosa cells cultured for 48 h was statistically significant overall (P = 0.002 by Kruskall-Wallis test; Fig. 4). The FSH treatment resulted in a nonsignificant 1.2-fold increase in GCL enzymatic activity compared with controls. The combination of estradiol and FSH caused a statistically significant 3-fold increase in GCL activity compared with controls (P = 0.003 by Mann-Whitney test).
FIG. 4.
Follicle-stimulating hormone and estradiol are required for maximal induction of glutamate cysteine ligase enzymatic activity. Granulosa cells were cultured for 48 h in serum-free medium alone, with 20 ng/ml ovine FSH, or with FSH plus 100 ng/ml estradiol. The graph shows mean (±SEM) GCL enzymatic activity expressed as nanomoles of GSH produced per milligram of protein per minute (n = 6–8 per treatment group). The effect of treatment was statistically significant by Kruskal-Wallis test (P = 0.002). *Significantly different from control by Mann-Whitney test, P = 0.003.
The Stimulation of GSH Synthesis and GCL Subunit Expression by FSH and Estradiol in Granulosa Cells Is Associated with Luteinization of Granulosa Cells
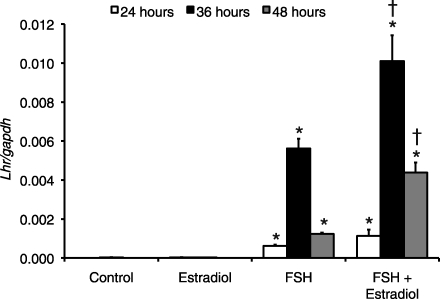
Granulosa cells undergo a process of differentiation called “luteinization” in response to high periovulatory concentrations of LH and FSH [42]. This process requires LH receptor expression on granulosa cell membranes, which occurs in response to FSH. Cultured granulosa cells have been shown to undergo this process after several days of gonadotropin stimulation [42]. We were therefore interested in determining whether the effects of FSH on GCL subunit expression were associated with luteinization. Quantitative real-time PCR revealed that LH receptor (Lhr) mRNA levels were essentially undetectable in control cells and in cells treated with estradiol alone (Fig. 5). There were statistically significant increases in Lhr mRNA in the FSH-treated and FSH plus estradiol-treated groups compared with controls at 24, 36, and 48 h (P < 0.01 for effect of treatment by Kruskal-Wallis test at each time point; P < 0.03 for intergroup comparisons by Mann-Whitney tests; Fig. 5). At 36 and 48 h, the levels of Lhr mRNA were significantly greater in FSH plus estradiol-treated cells than in those treated with FSH alone (P < 0.03 for intergroup comparisons by Mann-Whitney tests; Fig. 5).
FIG. 5.
Follicle-stimulating hormone and FSH plus estradiol increase LH receptor mRNA levels in cultured granulosa cells. Cells were cultured in 1) serum-free medium alone (control), 2) with 20 ng/ml ovine FSH, 3) 100 ng/ml estradiol, or 4) FSH plus estradiol. Cells were harvested at the indicated times for analysis of Lhr and Gapdh mRNA by quantitative real-time RT-PCR as described in Materials and Methods. Data are expressed as mean ratio (±SEM) of Lhr:Gapdh (presented as Lhr/Gapdh) for each treatment group (n = 4–5 per treatment group). At each time point, the effect of treatment group was statistically significant by Krukal-Wallis test (P < 0.01). *Significantly different from control at same time point by Mann-Whitney test, P < 0.03. †Significantly different from FSH at same time point by Mann-Whitney test, P < 0.03.
FSH Increased GCLC Protein Levels via the cAMP/PKA Signaling Pathway
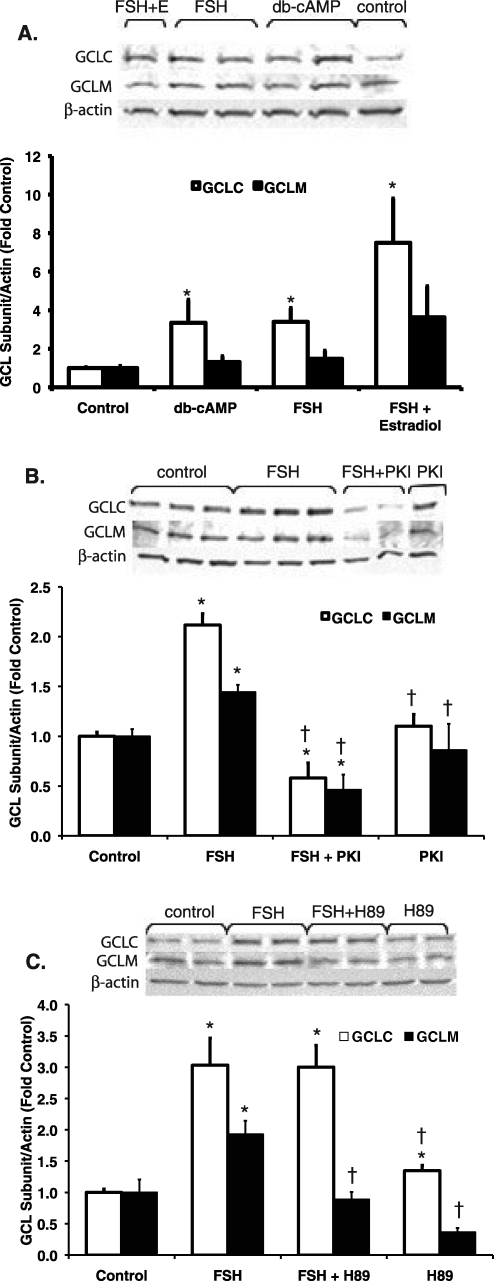
To test whether the observed FSH-stimulated increases in GCL subunit protein and mRNA in granulosa cells are mediated via cAMP/PKA signaling, we conducted experiments using dbcAMP, a cAMP analog, and two PKA inhibitors. When granulosa cells were cultured for 48 h with medium alone, FSH, dbcAMP, or FSH plus estradiol, the effect of treatment group was statistically significant for GCLC protein levels (P = 0.006 by Kruskal-Wallis test) but not for GCLM protein levels (Fig. 6). GCLC protein levels increased about 3-fold compared with untreated controls in both dbcAMP- and FSH-treated groups (P = 0.045 and P = 0.006, respectively, by Mann-Whitney test; Fig. 6A). GCLC protein levels were increased 7.5-fold in the FSH plus estradiol group, but the differences between this group and the FSH and dbcAMP groups were not statistically significant. GCLM protein levels were nonstatistically significantly increased by 1.3-, 1.5-, and 3.6-fold increases in dbcAMP, FSH, and FSH plus estradiol groups.
FIG. 6.
Involvement of cAMP/PKA signaling in the FSH-induced increase in GCL subunit protein levels in granulosa cells. Cells were cultured in serum-free medium alone (control), with 20 ng/ml FSH, with FSH plus 100 ng/ml estradiol, or with 1 mM dbcAMP (A); presented as db-cAMP or (B and C) FSH plus PKA inhibitor or inhibitor alone (50 μM PKI or 10 μM H89). Cells were pretreated with PKA inhibitors for 1 h before FSH was added. Protein was extracted and subjected to immunoblotting for GCLC, GCLM, and β-actin as described in Materials and Methods. Graphs show the mean ± SEM of the β-actin-normalized densitometry expressed as fold change from the control for the same blot. Representative Western blots are shown above each graph. A) The effect of treatment group was statistically significant for GCLC (P = 0.006 by Kruskal-Wallis test). Dibutyryl cAMP stimulated GCLC protein to a similar extent as FSH (n = 5–6 per treatment group). *Significantly different from control, P < 0.01 by Mann-Whitney test. B) The effects of treatment group on GCLC and GCLM were statistically significant (P < 0.001 by ANOVA). PKI prevented the stimulation of GCLC and GCLM protein by FSH (n = 5 per treatment group). *Significantly different from control, P < 0.02 by LSD test. †Significantly different from FSH, P < 0.02 by LSD test. C) The effects of treatment group on GCLC and GCLM were statistically significant (P = 0.005 by Kruskal-Wallis test for both subunits). H89 prevented the stimulation of GCLM, but not GCLC, protein by FSH (n = 4 per treatment group). *Significantly different from control, P < 0.03 by Mann-Whitney test. †Significantly different from FSH, P < 0.03 by Mann-Whitney test.
When granulosa cells were cultured for 48 h with medium alone, FSH, the PKA inhibitor PKI, or FSH plus PKI, the effect of treatment group on GCLC and GCLM protein levels was statistically significant (P < 0.001 by ANOVA for both subunits; Fig. 6B). The FSH treatment significantly increased GCLC and GCLM protein levels (P < 0.02, FSH versus control by LSD test), and cotreatment with FSH and PKI prevented the stimulation of GCLM and GCLC protein levels by FSH. PKI by itself had no effect on GCLC or GCLM protein levels.
When granulosa cells were cultured for 48 h with medium alone, FSH, the PKA inhibitor H89, or FSH plus H89, the effect of treatment group on GCLC and GCLM protein levels was statistically significant (P = 0.005 by Kruskal-Wallis test for both subunits; Fig. 6C). The FSH treatment significantly increased GCLC and GCLM protein levels (P < 0.03, FSH versus control by Mann-Whitney test), and cotreatment with FSH and H89 prevented the stimulation of GCLM, but not GCLC, protein levels by FSH. H89 by itself slightly increased GCLC levels and slightly decreased GCLM levels (P < 0.03, H89 versus control by Mann-Whitney test).
These data support the hypothesis that the cAMP/PKA pathway is involved in the FSH-induced upregulation of GCL subunit expression.
FSH Does Not Increase GCL Subunit Protein Stability
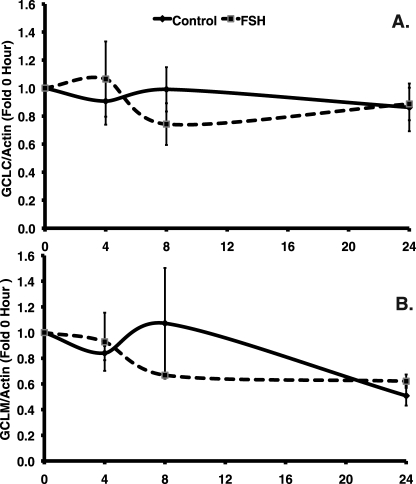
To examine whether treatment with FSH alone increases protein levels of GCL subunits, in part by increasing GCL subunit stability, we used CHX to inhibit protein synthesis in the presence and absence of FSH, and we measured protein levels of GCLC and GCLM at 4, 8, and 24 h. Granulosa cells were pretreated with 10 μg/ml CHX for 1 h before FSH was added, and control cells were treated only with CHX. The total protein content decreased at the same rate in both control and FSH-treated groups, reflecting the inhibition of protein synthesis by CHX (data not shown). However, cotreatment of granulosa cells with FSH and 10 μg/ml CHX revealed no statistically significant effect of FSH on GCLC (P = 0.881, effect of treatment by two-way ANOVA) and GCLM (P = 0.689) protein levels normalized to β-actin levels and expressed as fold above 0-h controls at 4, 8, and 24 h after initiation of treatment (Fig. 7). Moreover, there was no significant change in protein levels of either subunit during the time course of the experiment.
FIG. 7.
Follicle-stimulating hormone did not increase GCL subunit protein stability. Granulosa cells were cultured with 10 μg/ml CHX starting 1 h before 20 ng/ml FSH was added; control cells did not receive FSH. Cells were collected at 4, 8, and 24 h and were processed for immunoblotting for GCLC, GCLM, and β-actin. The 0-h baseline controls were not cultured and were processed immediately. A and B) The graphs show the mean ± SEM of the β-actin-normalized GCLC (A) or GCLM (B) densitometry values expressed as fold change from the 0-h control for the same blot. Neither the effect of FSH treatment nor the effect of time on GCLC or GCLM protein levels normalized to β-actin was statistically significant by two-way ANOVA.
DISCUSSION
Our results demonstrate that FSH regulates GSH synthesis in both granulosa cells and small antral follicles by increasing GCL subunit levels. The use of isolated granulosa cells, which are incapable of synthesizing estradiol in response to FSH because of the lack of the required substrate androstenedione from theca cells, allowed us to investigate the effects of FSH and estradiol alone and together on GCL subunit expression. In granulosa cells, FSH alone increased GSH concentrations and modestly increased GCL subunit expression. Estradiol alone had no effect on GSH synthesis. Follicle-stimulating hormone combined with estradiol increased expression of both GCL subunits, increased GCL enzymatic activity, and increased GSH concentrations in granulosa cells to a greater extent than FSH alone. We obtained further evidence that estradiol mediates optimal stimulation of GSH synthesis by FSH by showing that small antral follicles, which synthesized estradiol in response to FSH, rapidly increased GCL subunit mRNA and protein levels and GSH concentrations upon FSH treatment. We also showed that stimulation of GCL subunit expression by FSH alone involves activation of PKA and does not involve increasing GCL subunit protein stability.
Our data clearly show that the addition of estradiol to FSH enhanced the effects of FSH on GSH synthesis in isolated granulosa cells. At 48 h of treatment, GSH concentrations were increased about 1.4-fold, GCL enzymatic activity was increased about 2.4-fold, and GCLM protein levels were increased about 1.7-fold with estradiol plus FSH treatment relative to FSH treatment alone. Moreover, combined treatment led to robust increases in Gclc and Gclm mRNA levels, whereas no effect on Gclm mRNA and only a small effect on Gclc mRNA were seen with FSH alone. Notably, estradiol alone had no effect on GSH synthesis. The concentration of estradiol used in the granulosa cell experiments (2.6 × 10−7 M) is physiologically relevant because it is similar to concentrations reported in follicular fluid of rat preovulatory follicles (1.4 × 10−7 M) [35]. In parallel experiments, cultured small antral follicles synthesized estradiol in response to FSH, and FSH treatment significantly increased GCL subunit protein and mRNA levels and GSH concentrations within 24 h. It has been reported that estradiol increased transcription of GCLC in human breast cancer MCF-7 cells. The authors reported that ligand-bound estrogen receptor beta (ESR2) formed a complex with the transcription factor NFE2L2 (also known as NRF2 [nuclear factor-erythroid 2-related factor 2]), which bound to the antioxidant response element (ARE) in the promoter of the gene [44]. This mechanism is not likely to occur in rat granulosa cells because there are no AREs in the promoters of the rat Gclc and Gclm genes [45, 46]. However, more recent work by the same group showed that Activator Protein 1 (AP-1) and nuclear factor of kappa light polypeptide enhancer in B cells (NF-κB) sites in the promoter of rat Gclc are required for transcription of Gclc in rat fibroblasts, and that NRF2 increased the expression of AP-1 family members Jun and Fos, decreased expression of AP-1 member Fra1, and increased expression of NF-κB family members Nfkb1 and Rela to stimulate Gclc expression [47]. Cyclic AMP treatment and, by extension, FSH, increased protein levels of AP-1 family members FOS, JUNB, JUN, and FRA2 in cultured granulosa cells from small antral follicles of estradiol-primed rats within 40 min, with increases no longer apparent by 24 h [48]. Therefore, it is possible that the combined effects of FSH and estradiol to induce Gclc and Gclm transcription in rat granulosa cells and follicles are mediated by increased expression of AP-1 and/or NF-κB family members. Other effects of FSH on granulosa cells are also augmented by estradiol. For example, estradiol increased FSH stimulation of progesterone synthesis in cultured granulosa cells (Welsh et al. [43] and the present study) and enhanced FSH-induced aromatase activity [49].
The canonical signaling pathway activated by FSH in granulosa cells is cAMP/PKA [30]. In the present studies, dbcAMP treatment increased GCLC and GCLM protein in granulosa cells to the same degree as FSH treatment, implicating the cAMP/PKA signaling pathway. Further supporting a role for this pathway in mediating the effect of FSH to increase GCL protein levels, we showed that two different PKA inhibitors abolished the stimulatory effect of FSH on GCLM protein levels in granulosa cells. PKI, a specific peptide inhibitor of PKA, attenuated the effect of FSH on both GCLC and GCLM protein levels. On the other hand, a biochemical PKA inhibitor, H89, only blocked the effect of FSH on GCLM protein. These differences may be attributed to the specificity of each inhibitor. H89 inhibits the activity of several other kinases, including ribosomal protein S6 kinase (formerly MSK1), Rho-associated coiled-coil-containing protein kinase 2 (ROCK2), ribosomal protein S6 kinase, 70 kDa, polypeptide 1 (formerly S6K1), and protein kinase D1, at the 10-μM concentration used in our study [50]. PKI at a concentration of 1 μM was shown to inhibit PKA activity by 80% and to completely abolish PKA activity at 100 μM in pancreatic β cells, whereas it has no effect on PKC at concentrations up to 10 μM [51]. At the 50-μM concentration used in the present study, it is possible that some inhibition of PKC also occurred, and PKC signaling is important in granulosa cells [52]. Therefore, the divergent effects of H89 and PKI on GCLC protein observed in the present studies may have been due to inhibition of other signaling kinase pathways by either of these inhibitors. On the other hand, the observation that both of these compounds inhibited the FSH-induced increase in GCLM protein levels provides strong evidence that this effect of FSH is mediated via PKA signaling.
Granulosa cells undergo differentiation after 48 h in culture in the presence of FSH. The process of granulosa cell differentiation is gonadotropin dependent, is mediated by cAMP, and involves the production of steroid hormones and LH receptor expression [42]. In the present studies, increases in LH receptor expression confirmed that granulosa cells from small antral follicles of estradiol-primed rats differentiated during 48 h of FSH treatment. GCL subunit protein levels and GSH concentrations did not increase until 48 h in granulosa cells cultured with FSH alone, suggesting that events associated with granulosa cell differentiation may be required for FSH alone to stimulate GSH synthesis. Downstream targets of cAMP that have been implicated in granulosa cell differentiation are also involved in the regulation of GCL subunit expression in other cell types. Follicle-stimulating hormone activates adenylate cyclase, resulting in cAMP production, which can activate AKT via phosphoinositide-3 kinase (PIK3). Alam et al. [53] demonstrated that the PIK3/AKT/RHEB/FRAP1 pathway was required for expression of granulosa differentiation markers, including LH receptor. A study that used adenoviral vectors to constitutively express activated Akt1 or a dominant-negative Akt1 also showed that AKT signaling was required for FSH-induced LH receptor expression [54]. PIK3/AKT signaling was involved in the NFE2L2-ARE-mediated transcriptional response to hyperoxia in pulmonary epithelial cells [55]. Thus, PKA-induced activation of PIK3/AKT signaling is a candidate pathway by which FSH may regulate both granulosa cell differentiation and GSH synthesis. This remains to be explored in future studies.
Follicle-stimulating hormone alone increased granulosa cell GCLC and GCLM subunit protein levels and Gclc mRNA levels but did not increase Gclm mRNA levels. These data suggested that in addition to transcriptional effects, FSH may also exert translational or posttranslational effects on GCL subunits. We therefore tested the possibility that FSH stabilized GCL subunit proteins and prevented their degradation using CHX, a potent inhibitor of protein translation. During a 24-h period, we observed no changes in GCLC or GCLM protein levels normalized to β-actin levels. Our data clearly do not support the hypothesis that FSH increases GCLC or GCLM protein stability in granulosa cells. Franklin et al. [56, 57] have reported that GCLC and GCLM proteins, when equal amounts of total protein were loaded per lane, were highly stable in other cell systems in the presence of CHX for up to 48 h. Our data on GCLC and GCLM protein stability in granulosa cells are consistent with these reports.
In addition to increasing total intracellular GSH concentrations in small antral follicles, FSH treatment also nonsignificantly decreased GSSG and significantly increased the ratio of reduced GSH to GSSG. An increase in the ratio of reduced GSH to GSSG indicates that the cellular environment is less oxidized [58]. In addition to stimulating GSH synthesis, FSH has also been reported to upregulate other antioxidants in the ovary. Priming of immature rats with eCG increased ovarian mRNA levels of secreted (Sod3) and mitochondrial (Sod2) superoxide dismutase [59, 60] and increased ovarian levels of the antioxidant vitamin A [61]. Upregulation of other antioxidants in the FSH-treated follicles may have decreased reactive oxygen species in the follicles, resulting in the nonsignificant decrease in GSSG and significant increase in reduced GSH to GSSG ratio observed in the present study.
Regulation of de novo GSH synthesis via transcriptional and posttranscriptional regulation of GCL, the rate-limiting enzyme, is known to be important in maintenance of GSH concentrations. However, tissue-specific regulation of GSH synthesis is less well understood. This study demonstrated that maximal stimulation of GSH synthesis in granulosa cells and ovarian follicles by FSH requires estradiol. In the absence of estradiol, FSH alone increased GSH synthesis in granulosa cells by modestly upregulating GCL subunit expression. Estradiol enhanced the effects of FSH on granulosa cell GCL subunit expression and GSH synthesis but had no effects alone. Our results demonstrate that the independent effects of FSH required PKA signaling and appeared to require granulosa cell differentiation, which is PKA dependent. The combination of FSH and estradiol treatment of cultured granulosa cells or stimulation of estradiol synthesis by FSH in whole cultured follicles led to a more rapid and greater increase in intracellular GSH concentrations due to increased GCL subunit mRNA and protein expression and greatly increased GCL enzymatic activity. These findings are physiologically relevant. In a previous study, FSH-stimulated GSH synthesis in large antral (preovulatory) follicles partially mediated the antiapoptotic effects of FSH [36], and the present findings suggest that it may play a similar role in small antral follicles.
Acknowledgments
We thank Dr. Terrance Kavanagh (University of Washington) for the generous gift of the GCLC and GCLM antibodies.
Footnotes
1Supported by National Institutes of Health grant ES10963 to U.L., and by the University of California Irvine Office of Research and the University of California Irvine Center for Occupational and Environmental Health. Portions of this work were presented in abstract form at the 40th Annual Meeting of the Society for the Study of Reproduction, July 21–25, 2007, San Antonio, Texas, and at the 48th Annual Meeting of the Society of Toxicology, March 15–19, 2009, Baltimore, Maryland.
REFERENCES
- Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J.Fertility, family planning, and reproductive health of U.S. Women: data from the 2002 National Survey of Family Growth. Vital Health Stat 2005; 23(25):1–160.. [PubMed] [Google Scholar]
- Pal L, Santoro N.Premature ovarian failure (POF): discordance between somatic and reproductive aging. Ageing Res Rev 2002; 1: 413–423.. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Barton M, Jackson EK.Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res 2005; 66: 295–306.. [DOI] [PubMed] [Google Scholar]
- Silva I, Mor G, Naftolin F.Estrogen and the aging brain. Maturitas 2001; 38: 95–100.. [DOI] [PubMed] [Google Scholar]
- Molina JR, Barton DL, Loprinzi CL.Chemotherapy-induced ovarian failure. Manifestations and management. Drug Saf 2005; 28: 401–416.. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Themmen APN, Al-Qahtani A, Groome NP, Cameron DA.The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 2006; 21: 2583–2592.. [DOI] [PubMed] [Google Scholar]
- Howell S, Shalet S.Gonadal damage from chemotherapy and radiotherapy. Endocrinol Metab Clin North Am 1998; 27: 927–943.. [DOI] [PubMed] [Google Scholar]
- Lo Presti A, Ruvulo G, Gancitano RA, Cittadini E.Ovarian function following radiation and chemotherapy for cancer. Eur J Obstet Gynecol Reprod Biol 2004; 113: S33–S40.. [DOI] [PubMed] [Google Scholar]
- Meirow D, Nugent D.The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 2001; 7: 535–543.. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Sipes IG.Assessment of follicle destruction in chemical-induced ovarian toxicity. Annu Rev Pharmacol Toxicol 1996; 36: 307–331.. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Bearer CF, Perreault-Darney S, Daston GP, David RM, Luderer U, Olshan AF, Sanderson WT, Willhite CC, Woskie S.NTP-CERHR expert panel report on the reproductive and developmental toxicity of 2-bromopropane. Reprod Toxicol 2004; 18: 189–217.. [DOI] [PubMed] [Google Scholar]
- Borman SM, Christian PJ, Sipes IG, Hoyer PB.Ovotoxicity in female Fischer Rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic Index. Toxicol Appl Pharmacol 2000; 167: 191–198.. [DOI] [PubMed] [Google Scholar]
- Mattison DR.Difference in sensitivity of rat and mouse primordial oocytes to destruction by polycyclic aromatic hydrocarbons. Chem Biol Interact 1979; 28: 133–137.. [DOI] [PubMed] [Google Scholar]
- Mattison DR, White NB, Nightingale MR.The effect of benzo(a)pyrene on fertility, primordial oocyte number, and ovarian response to pregnant mare's serum gonadotropin. Ped Pharmacol 1980; 1: 143–151.. [PubMed] [Google Scholar]
- Flowers J, Ludeman SM, Gamcsik MP, Colvin OM, Shao KL, Boal JH, Springer JB, Adams DJ.Evidence for a role of chloroethylaziridine in the cytotoxicity of cyclophosphamide. Cancer Chemother Pharmacol 2000; 45: 335–344.. [DOI] [PubMed] [Google Scholar]
- Jernström B, Funk M, Frank H, Mannervik B, Seidel A.Glutathione-S-transferase A1–1-catalysed conjugation of bay and fjord region diol epoxides of polycyclic aromatic hydrocarbons with glutathione. Carcinogenesis 1996; 17: 1491–1498.. [DOI] [PubMed] [Google Scholar]
- Rubes J, Selevan SG, Sram RJ, Evenson DP, Perreault SD.GSTM1 Genotype influences the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res 2007; 625: 20–28.. [DOI] [PubMed] [Google Scholar]
- Seidel A, Friedberg T, Löllmann B, Schwierzok A, Funk M, Frank H, Holler R, Oesch F, Glatt H.Detoxification of optically active bay- and fjord-region polycyclic aromatic hydrocarbon dihydrodiol epoxides by human glutathione transferase P1–1 expressed in Chinese hamster V79 cells. Carcinogenesis 1998; 19: 1975–1981.. [DOI] [PubMed] [Google Scholar]
- Devine PJ, Sipes IG, Hoyer PB.Effect of 4-vinylcyclohexene diepoxide dosing in rats on GSH levels in liver and ovaries. Toxicol Sci 2001; 62: 315–320.. [DOI] [PubMed] [Google Scholar]
- Xue W, Warshawsky D.Metabolic activation of polycyclic aromatic hydrocarbon and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol 2005; 206: 73–93.. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Luo JL.Glutathione therapy: from prodrugs to genes. Sem Liver Dis 1998; 18: 415–424.. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luong BT, Tan Y, Luderer U.Cyclophosphamide-induced apoptosis in COV434 human granulosa cells involves oxidative stress and glutathione depletion. Toxicol Sci 2007; 98: 216–230.. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Nakamura BN, Luderer U.Induction of apoptosis by 9,10-dimethyl-1,2-benzanthracene (DMBA) in cultured preovulatory rat follicles is preceded by a rise in reactive oxygen species and is prevented by glutathione. Biol Reprod 2007; 77: 442–451.. [DOI] [PubMed] [Google Scholar]
- Luderer U, Kavanagh TJ, White CC, Faustman EM.Gonadotropin regulation of glutathione synthesis in the rat ovary. Reprod Toxicol 2001; 15: 495–504.. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luderer U.Gonadotropin regulation of glutamate cysteine ligase catalytic and modifier subunit expression in the rat ovary is subunit and follicle stage-specific. Am J Physiol 2005; 289: E391–E402.. [DOI] [PubMed] [Google Scholar]
- Mattison DR, Shiromizu K, Pendergrass JA, Thorgeirsson SS.Ontogeny of ovarian glutathione and sensitivity to primordial oocyte destruction by cyclophosphamide. Ped Pharmacol 1983; 3: 49–55.. [PubMed] [Google Scholar]
- Griffith OW, Mulcahy RT.The enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase. Advances in enzymology and related areas in molecular biology 1999; 73: 209–267.. [DOI] [PubMed] [Google Scholar]
- Griffith OW.Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 1999; 27: 922–935.. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ.Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 2009; 30: 86–98.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Maizels ET.FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal 2006; 18: 1351–1359.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, White CC, Krejsa CM, Diaz D, Woods JS, Eaton DL, Kavanagh TJ.Induction of glutamate-cysteine ligase (γ-glutamylcysteine synthetase) in the brains of adult female mice subchronically exposed to methylmercury. Toxicol Lett 1999; 110: 1–9.. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Alliston TN, Buse P, Firestone GL, Richards JS.Functional and subcellular changes in the A-kinase-signaling pathway: relation to aromatase and Sgk expression during the transition of granulosa cells to luteal cells. Mol Endocrinol 1999; 13: 1318–1337.. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW.Plasma concentrations of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 1974; 94: 1704–1708.. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB.Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod 1979; 20: 559–670.. [DOI] [PubMed] [Google Scholar]
- Goff AK, Henderson KM.Changes in follicular fluid and serum concentrations of steroids in PMS treated immature rats following LH administration. Biol Reprod 1979; 20: 1153–1157.. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luderer U.Opposing effects of glutathione depletion and FSH on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology 2006; 147: 1224–1236.. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Billig H, Kowalski KI, Hsueh AJW.Epidermal growth factor and basic fibroblast growth factor suppress the spontaneous onset of apoptosis in cultured rat ovarian granulosa cells and follicles by a tyrosine kinase-dependent mechanism. Mol Endocrinol 1992; 6: 1942–1950.. [DOI] [PubMed] [Google Scholar]
- Griffith OW.Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 1980; 106: 207–212.. [DOI] [PubMed] [Google Scholar]
- Bao B, Kumar N, Karp RM, Garverick HA, Sundaram K.Estrogen receptor-β expression in relation to the expression of luteinzing hormone receptor and cytochrome P450 enzymes in rat ovarian follicles. Biol Reprod 2000; 63: 1747–1755.. [DOI] [PubMed] [Google Scholar]
- White CC, Viernes H, Kresja CM, Botta D, Kavanagh TJ.Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem 2003; 318: 175–180.. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Johnson ME, Johnson MM.A comparative study of tests for homogeneity of variances, with applications to the outer continental shelf bidding data. Technometrics 1981; 23: 351–361.. [Google Scholar]
- Knecht M, Amsterdam A, Catt K.The regulatory role of cyclic AMP in hormone-induced of granulosa cell differentiation. J Biol Chem 1981; 25: 10628–10633.. [PubMed] [Google Scholar]
- Welsh THJ, Zhuang ZZ, Hsueh AJW.Estrogen augmentation of gonadotropin-stimulated progestin biosynthesis in cultured rat granulosa cells. Endocrinology 1983; 112: 1916–1924.. [DOI] [PubMed] [Google Scholar]
- Montano MM, Deng H, Liu M, Sun X, Singal R.Transcriptional regulation by the estrogen receptor of antioxidative stress enzymes and its functional implications. Oncogene 2004; 23: 2442–2453.. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang J, Huang ZZ, Ou X, Lu SC.Cloning and characterization of the 5′-flanking region of the rat glutamate-cysteine ligase catalytic subunit. Biochem J 2001; 285: 476–482.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang J, Ou X, Huang ZZ, Lu SC.Cloning and analysis of the rat glutamate-cysteine ligase modifier subunit promoter. Biochem Biophys Res Commun 2001; 285: 476–482.. [DOI] [PubMed] [Google Scholar]
- Yang H, Magilnick N, Lee C, Kalmaz D, Ou X, Chan JY, Lu SC.Nrf1 and Nrf2 regulate rat glutamate cysteine ligase catalytic subunit transcription indirectly via NF-κB and AP-1. Mol Cell Biol 2005; 25: 5933–5946.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SC, Richards JS.Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells. J Biol Chem 2000; 275: 33718–33728.. [DOI] [PubMed] [Google Scholar]
- Adashi EY, Hsueh AJW.Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimiulating hormone in cultured rat granulosa cells. J Biol Chem 1982; 257: 6077–6083.. [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P.Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351: 95–105.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TE, Persaud SJ, Jones PM.Pseudosubstrate peptide inhibitors of B-cell protein kinases: altered selectivity after myristoylation. Mol Cell Endocrinol 1999; 155: 61–68.. [DOI] [PubMed] [Google Scholar]
- Chamoun D, Choi D, Tavares AB, Udoff LC, Levitas E, Resnick CE, Rosenfeld RG, Adashi EY.Regulation of granulosa cell-derived insulin-like growth factor binding proteins (IGFBPs): role for protein kinase-C in the pre- and posttranslational modulation of IGFBP-4 and IGFBP-5. Biol Reprod 2002; 67: 1003–1012.. [DOI] [PubMed] [Google Scholar]
- Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M.Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem 2004; 279: 19431–19440.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik AJ, Saxena D, Little-Ihrig L.Protein kinase B is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 2003; 144: 3985–3994.. [DOI] [PubMed] [Google Scholar]
- Papaiahgari S, Zhang QG, Kleeberger SR, Cho HY, Reddy SP.Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3H-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal 2006; 8: 43–52.. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Kresja CM, Pierce RH, White CC, Fausto N, Kavanagh TJ.Caspase-3-dependent cleavage of the glutamate-L-cysteine ligase catalytic subunit during apoptotic cell death. Am J Pathol 2002; 160: 1887–1894.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Rosenfeld-Franklin ME, White CC, Kavanagh TJ, Fausto N.TGFβ1-induced suppression of glutathione antioxidant defenses in hepatocytes: caspase-dependent posttranslational and caspase-independent transcriptional regulatory mechanisms. FASEB J 2003; 17: 1535–1537.. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR.Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001; 30: 1191–1212.. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI.Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology 1995; 136: 242–252.. [DOI] [PubMed] [Google Scholar]
- Sasaki J, Sato EF, Nomura T, Mori H, Watanabe S, Kanda S, Watanabe H, Utsumi K, Inoue M.Detection of manganese superoxide dismutase mRNA in the theca interna cells of rat ovary during the ovulatory process by in situ hybridization. Histochemistry 1994; 102: 173–176.. [DOI] [PubMed] [Google Scholar]
- Aten RF, Duarte KM, Behrman HR.Regulation of ovarian antioxidant vitamins, reduced glutathione, and lipid peroxidation by luteinizing hormone and prostaglandin F2alpha. Biol Reprod 1992; 46: 401–407.. [DOI] [PubMed] [Google Scholar]