Abstract
Calcitonin gene-related peptide (CALCB), amylin, and adrenomedullin (ADM) belong to a unique group of calcitonin (CALCA)/CALCB family peptides that have overlapping biological effects owing to their structure and cross-reactivity between receptors. CALCB and ADM are expressed in fetoplacental tissues and are important in maintaining normal placental function. Recently, ADM 2 (ADM2)/intermedin was identified as a novel CALCA/CALCB family peptide that functions through CALCB and ADM receptors. ADM2 is expressed in the pituitary, digestive tract, and other organs of vertebrates and reduces blood pressure in both normal and hypertensive rats. We recently reported that the level of immunoreactive ADM2 is significantly upregulated in pregnant rats and that its hypotensive effects are also increased during rat pregnancy. Furthermore, infusion of ADM2 antagonist in pregnant rats causes fetoplacental growth restriction. The objective of this study was to analyze the expression and possible role of ADM2 in human placenta. We show that ADM2 mRNA is expressed in human placenta and that immunoreactive ADM2 is localized in syncytiotrophoblasts, cytotrophoblasts, and endothelial cells throughout human pregnancy. This study also demonstrates that ADM2 enhances the invasion and migration of first-trimester HTR-8SV/neo cells. ADM2 increases the invasive index of HTR-8SV/neo cells by 2.2-fold compared with controls. Taken together, the findings from this study suggest that ADM2 may have a role in the physiology of human pregnancy via regulation of trophoblast invasion and migration.
Keywords: early development, placenta, pregnancy, syncytiotrophoblast, trophoblast
Adrenomedullin 2 enhances human trophoblast cell invasion and migration.
INTRODUCTION
The calcitonin (CALCA)/calcitonin gene-related peptide (CALCB) family peptide adrenomedullin 2 (ADM2) (also called intermedin [IMD]) is a 47-amino acid peptide with ∼28% structural homology to ADM and with <20% to CALCB. ADM2 is abundantly expressed in rat ovary, uterus, stomach, and kidney, as well as in other tissues such as brain, heart, and pituitary gland [1, 2]. Previous investigations showed that ADM2 has vasodilatory and hypotensive actions that were similar to or more potent than those of ADM and CALCB [1]. Pharmacological analysis showed that ADM2 exerts its effects through a seven-transmembrane G protein-coupled receptor(GPCR) calcitonin receptor-like receptor (CALCRL) in combination with one of the receptor activity-modifying proteins RAMP1, RAMP2, or RAMP3 [1]. Unlike ADM and CALCB, ADM2 is a nonselective agonist for RAMPs but exhibits greater potency with CALCRL/RAMP1 and CALCRL/RAMP3 [1]. Thus, ADM2 is a novel ligand for CALCRL/RAMP1 and CALCRL/RAMP3 receptors and could be important for regulation of diverse physiological processes that have been attributed to CALCB and ADM [1]. The ADM2 gene has an estrogen response element sequence and is an estrogen-dependent regulator of prolactin [3]. Recently, we reported that plasma levels of immunoreactive ADM2 are elevated during pregnancy and that ADM2-induced vasodilation is greater during pregnancy in rats [4]. The C-terminal receptor binding domain ADM217–47 is a functional antagonist of AMD2 [1]. We have shown that blocking the endogenous effects of ADM2 in pregnant rats causes fetoplacental growth restriction [5].
Findings from these studies suggest a role for this novel peptide in female reproductive function and pregnancy. However, to date there are no studies on the expression and function of this peptide during human pregnancy. It is unknown if ADM2 is expressed in the human placenta. In this study, we show for the first time (to our knowledge) that ADM2 mRNA is expressed in human placenta and that ADM2-specific immunoreactivity is localized in syncytiotrophoblasts, cytotrophoblasts, and endothelial cells throughout human pregnancy. In addition, our data show that ADM2 has a functional role in the invasion and migration of trophoblast cells. The results of our study suggest that ADM2 may have an important role in the regulation of trophoblast invasion and migration in human placenta.
MATERIALS AND METHODS
All human subjects were patients admitted to the University of Texas Medical Branch. Placental tissues from first-, second-, and third-trimester gestations (n = 6) were collected from clinically normal pregnancies that were either voluntarily terminated by dilation and curettage or delivered by cesarean section before the initiation of labor for obstetric reasons. Written informed consent was obtained from each woman before surgery using consent forms, and protocols were approved by the institutional review board of the University of Texas Medical Branch. Placental tissue was fixed in 10% buffered formalin and embedded into paraffin for immunohistochemistry or was snap frozen for RNA extraction. Thereafter, tissues were cut at 5- to 7-μm thickness and mounted on gelatin-coated slides.
Isolation of Total RNA and RT
Total RNA was isolated from placental tissue and HTR-8SV/neo cells using TRIzol (Life Technologies, Grand Island, NY). RNA extraction was followed by DNase 1 (Ambion, Austin, TX) treatment to remove DNA contamination. The quality and quantity of the RNA were assessed at 260/280 A, and all samples showed absorbency ratios ranging from 1.8 to 2.0. For the RT, 2 μg of total RNA was mixed with 3.0 nmol of random primer (Invitrogen, Carlsbad, CA), 200 μM deoxyribonucleotide triphosphate solution (Sigma-Aldrich, St. Louis, MO), and 10 U of Avian Myeloblastosis Virus RT (Promega, Madison, WI) in the presence of 5 U of RNase inhibitor (Invitrogen) and placed in a thermal cycler for one cycle at 28°C for 15 min, 42°C for 30 min, 99°C for 5 min, and 4°C for 5 min.
PCR of cDNA
The PCR of the cDNA was initiated by the published primers for human ADM2 (GenBank/European Bioinformatics Institute accession number AF529213). The primer sequences for ADM2 PCR analysis are 5′-AGGGAGGGGAACTCAGCAGTTCAGGAG-3′ (forward) and 5′-GTTCTTGTTCTTGCTGTCACTTGGGCCT-3′ (reverse). The PCRs were performed using 2 μl of cDNA. The cDNA was mixed with Red Tag mix (Sigma-Aldrich). For 18S, 0.75 μl of primer pair (Ambion) was used according to the supplier's specifications. The PCRs for ADM2 and 18S were carried out in a GeneAmp PCR system 9700 (Perkin-Elmer, Norwalk, CT) with the following conditions: An initial denaturation step at 95°C for 5 min was followed by 30 cycles of 30 sec at 95°C, 30 sec at 60°C, and 45 sec at 72°C. All reactions were terminated by a 7-min elongation step at 72°C. The total cycle number was chosen for each gene from the linear portion of its respective curve. The PCR product obtained was sequenced for its identity by DNA sequence analysis with forward and reverse gene-specific primers.
Electrophoresis and Gel Imaging
The PCR products were visualized on 1.4% agarose gels containing 0.5 μg/ml of ethidium bromide and run for 1.5 h at 100 V in 0.5× Tris-borate-edetic acid buffer. The DNA signals on the gel were imaged under UV light using a Polaroid camera (Photodyne, Inc., New Berlin, WI). The identity of the amplified sequences was verified by sequencing the gel-extracted PCR product, which showed 100% homology with published sequences of human ADM2 (data not shown). Negative controls were run in PCR using total RNA in place of cDNA, and no signal was detectable when run on agarose gel (data not shown).
Immunofluorescent Confocal Imaging
Buffered formalin-fixed paraffin-embedded tissue sections (4 μm) were deparaffinized and rehydrated by passage through xylene and graded ethanol solutions. Slides were incubated in 0.05% casein (Sigma-Aldrich)/0.05% Tween-20 (DAKO, Glostrup, Denmark)/PBS for 30 min to block nonspecific protein binding. The slides were then treated with Image-It Enhancer (Molecular Probes, Eugene, OR). The first primary antibody, rabbit anti-ADM2 antibody (Alpha Diagnostics, San Antonio, TX), was applied at 1:150 dilution, followed by donkey anti-goat IgG Alexa Fluor 488 (Molecular Probes). For double immunostaining, the slides stained with the first primary antibody were thoroughly rinsed with Tween/PBS, followed by the application of casein. The second primary antibody, monoclonal anti-cytokeratin 7 antibody (DAKO) or anti-CD34 antibody (DAKO), was applied to sections at 1:750 dilution for 60 min. Mouse IgG1 (DAKO) was applied as a negative control. Chicken anti-mouse IgG Alexa Fluor 594 (Molecular Probes) was used for detection of the first primary antibody. Preimmune serum or rabbit IgG (ready to use) (DAKO) was used as a negative control. ADM2 antibody neutralized by ADM2 peptide and secondary antibody alone was also used as a negative control (data not shown). Slides were then counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA) after treatment with Autofluorescence Eliminator Reagent (Chemicon International, Temecula, CA). They were viewed under an Olympus BX51 microscope, and images were recorded using a DP70 digital camera (Olympus Optical Co., Ltd., Tokyo, Japan).
Matrigel Invasion Assay
Invasion was measured using 24-well Matrigel invasion chamber plates (35–4480; Becton Dickinson Labware, Bedford, MA) according to published methods [6]. Cells (2.5 × 104 cells/well) in 500 μl of 0.5% bovine serum albumin were seeded in the upper compartment of the Matrigel-coated insert in the presence of the following: ADM2 (10−8–10−9 M), ADM2 (10−8 M) + ADM2 17–47 (10−6 M), ADM2 (10−8 M) + ADM217–47 (10−5 M), ADM2 (10−8 M) + ADM217–47 (10−4 M), or ADM2 17–47 (10−5 M). RPMI 1640 (750 μl) with 5% serum was added to the lower chambers. After 48 h of incubation, noninvasive cells on the upper surface of the insert were wiped away with a cotton applicator. Invasive cells that penetrated to the lower surface of the insert were cultured with methylthiotetrazole (MTT) for 4 h. MTT-formazan crystals were resolved by adding 200 μl of dimethyl sulfoxide. The lysates were then transferred to 96-well plates. The optical absorbance at 550 nm was measured in a microplate reader. For morphological study, Matrigel-coated insert membranes were stained with Diff-Quick stain (Biochemical Science, Inc., Swedesboro, NJ) and viewed under a microscope. The percentage of invasion and the invasive index were calculated as follows: % of Invasion = (Absorbance of Invasive Cells/Absorbance of Total Cells) × 100. Invasive Index = % of Invasion/% of Invasion of Control Nontreated Cells.
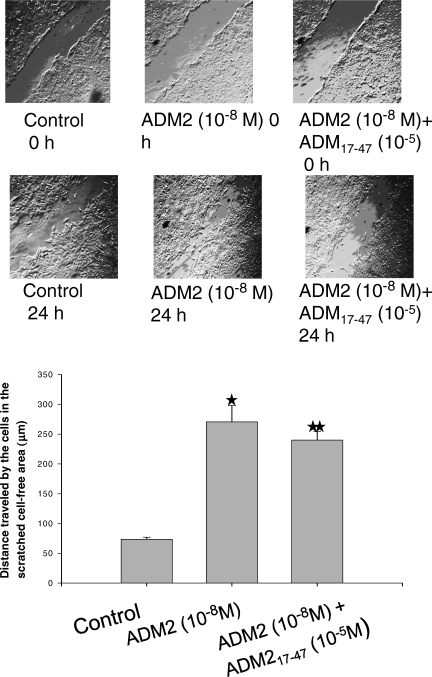
Scratch Wound Assay for Migration
The first-trimester cytotrophoblast HTR-8SV/neo cells (a gift from Dr. C.H. Graham, Queen University, Kingston, ON, Canada) were cultured in RPMI 1640 containing 10% fetal bovine serum in a humidified 5% CO2 incubator at 37°C. Cells were seeded in 24-well plates until confluent and were subsequently wounded by scratching with a pipette tip across the well according to the published protocol [7]. The cells were then washed with medium to remove the unattached cells, followed by treatment with ADM2 (10−8 M) or with ADM2 (10−8 M) + ADM217–47 (10−5 M). Cells were cultured for an additional 24 h, followed by visualization with phase-contrast microscopy and photography of the scratched area. In vitro migration of cells was documented by photography, and the amount of migration was quantitated by computer-assisted image analysis using NIH Image 1.6 (National Institutes of Health, Bethesda, MD). The distance (in micrometers) traveled by the cells to fill up the cell-free gap created by the scratch wound was calculated and plotted. Data obtained are representative of five different experiments.
Statistical Analysis
Data are given as the mean ± SEM and are compared using Bonferroni t-test. Unless specified, we used six replicates per group for the invasion assay and three replicates for RT-PCR as determined by the power analysis for each parameter.
RESULTS
Expression of ADM2 Transcript in Human Placenta
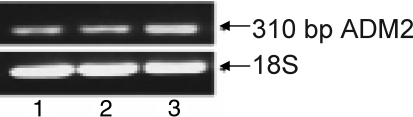
To analyze if ADM2 is expressed in human placenta, we performed RT-PCR of the total RNA isolated from first-trimester HTR-8SV/neo cells and from first- and third-trimester placental villi. Gene sequence analysis of the purified PCR product confirmed the expression of ADM2 in placenta. Figure 1 shows that the ADM2 transcript is expressed in all the samples analyzed, indicating that human placenta expresses ADM2 mRNA.
FIG. 1.
The RT-PCR demonstrating expression of ADM2 in human placenta. ADM2 is expressed in human villous tissue collected from 1) first-trimester placenta, 2) third-trimester placenta, and 3) first-trimester HTR-8SV/neo cells.
ADM2 Immunoreactivity in Human Placental Villi
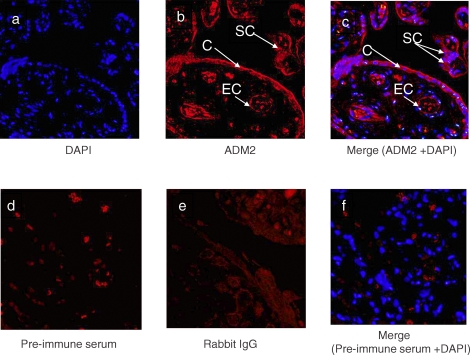
The cellular localization of ADM2 in first-trimester villous tissue was determined by immunofluorescent confocal imaging. The primary antibody, rabbit anti-ADM2 polyclonal antibody, was applied to sections and detected by Alexa Fluor 488 with the color red. As shown in Figure 2, ADM2 staining could be recognized in the cytoplasm of syncytiotrophoblasts, cytotrophoblasts, and endothelial cells in the placental villi, suggesting that ADM2 protein is present in the human placental villous tissue.
FIG. 2.
Single immunofluorescent staining for ADM2 in first-trimester villous trophoblastic tissue, followed by counterstaining with DAPI. a) Nuclei are stained blue when counterstained with DAPI. b) Red staining indicates ADM2 localization in cytotrophoblasts (C), syncytiotrophoblasts (SC), and endothelial cells (EC). c) Red staining indicates ADM2, and nuclei are stained blue when counterstained with DAPI in cytotrophoblasts, syncytiotrophoblasts, and endothelial cells. d) Negative control using preimmune serum. e) Negative control using rabbit IgG. f) Negative control staining using preimmune serum and counterstained with DAPI. Original magnification ×200.
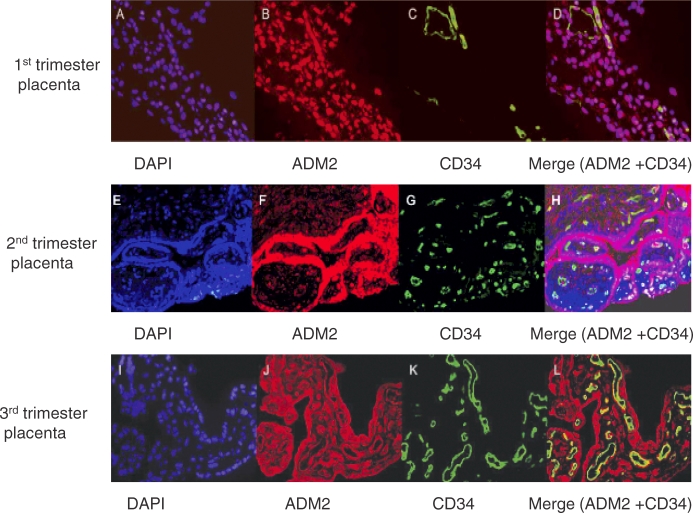
Colocalization of ADM2 Immunoreactivity with KRT7 (CK7) and CD34 Protein in the Placenta from the First, Second, and Third Trimesters of Pregnancy
The cellular colocalization of ADM2 with the trophoblast cell marker cytokeratin 7 (KRT7, also known as CK7) and the endothelial cell marker CD34 in first-, second-, and third-trimester villous tissue was determined by immunofluorescent confocal imaging. The first primary antibody, rabbit anti-ADM2 polyclonal antibody, was applied to sections and detected by Alexa Fluor 488 with the color green. The second primary antibody, monoclonal anti-KRT7 or anti-CD34, was applied to sections and detected by Alexa Fluor 594 with the color red. As shown in Figure 3, ADM2 immunoreactivity is colocalized with KRT7 immunostaining, indicating that placental trophoblast cells express ADM2 protein and that this is consistent throughout gestation. However, colocalization of ADM2 immunoreactivity with CD34 (Fig. 4) was not consistent in all the vessels and throughout gestation. We believe that this may be owing to heterogeneity of the origin of placental endothelial cells [8, 9].
FIG. 3.
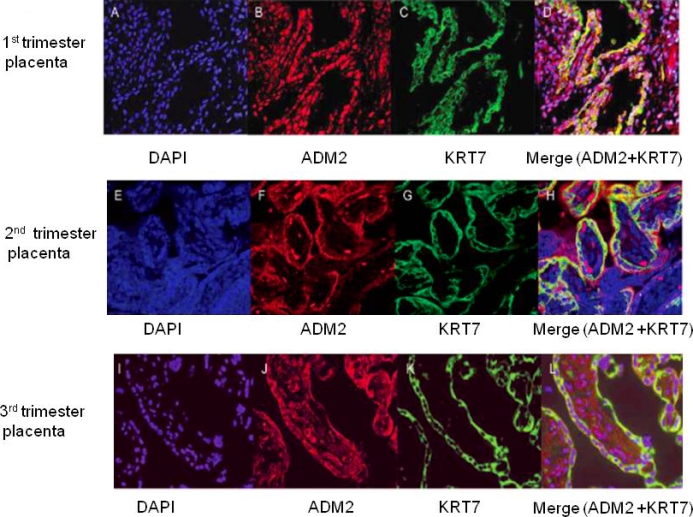
Double immunofluorescent staining for ADM2 and cytokeratin 7 in villous trophoblastic tissue, followed by counterstaining with DAPI. (D, H, and L). Double staining for ADM2 and cytokeratin 7 are shown in yellow in first-, second-, and third-trimester placental villi counterstained with DAPI in blue (C, G, and K). Single staining for KRT7 is shown in green in first-, second-, and third-trimester placental villi (B, F, and J). Staining for ADM2 is shown in red in first-, second-, and third-trimester placental villi (A, E, and I). Negative control using rabbit IgG and counterstained with DAPI. Original magnification ×200.
FIG. 4.
Double immunofluorescent staining for ADM2 and CD34 in villous tissue, followed by counterstaining with DAPI (D, H, and L). Double staining for ADM2 and CD34 is shown in yellow in first-, second-, and third-trimester placental villi counterstained with DAPI in blue (C, G, and K). Single staining for CD34 is shown in green in first-, second-, and third-trimester placental villi (B, F, and J). Staining for ADM2 is shown in red in first-, second-, and third-trimester placental villi (A, E, and I). Negative control using rabbit IgG and counterstained with DAPI. Original magnification ×200.
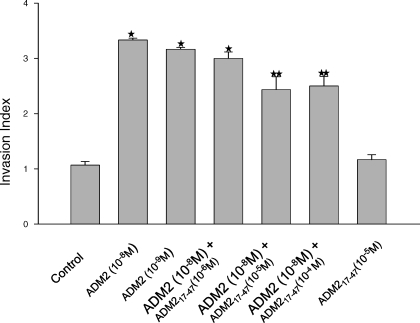
Effect of ADM2 on HTR-8SV/Neo Cell Invasion
The effect of ADM2 on the invasion of HTR-8SV/neo cells was assessed using the Matrigel-based assay. As shown in Figure 5, the addition of ADM2 substantially increased HTR-8SV/neo cell invasion. The cell invasive index was significantly higher (2.2-fold) in ADM2-treated cells than in untreated controls (P < 0.05), and this effect was inhibited by ADM217–47. However, ADM217–47 alone had no effect on HTR-8SV/neo cell invasion. These data suggest a role of ADM2 in the invasion of first-trimester trophoblast cells.
FIG. 5.
Invasive index of ADM2-treated HTR-8SV/neo cells. Cells were cultured on Matrigel-coated transwell filters and treated with or without ADM2 (10−8–10−9 M) in the presence or absence of ADM2(17–47) 10−6 M, ADM2(17–47) 10−5 M, ADM2(17–47) 10−4 M, or ADM2(17–47) 10−5 M for 48 h. Invasive cells that penetrated to the downward surface of the insert were incubated with MTT, and the optical absorbance at 550 nm was measured using a microplate reader. The invasive index was calculated as the ratio of the percentage of invasion in ADM2-treated cells to the percentage of invasion of control nontreated cells. Data are expressed as the mean ± SEM (n = 6, P < 0.05). Asterisk (*) indicates significant difference compared with the control cells, and two asterisks (**) indicate significant difference compared with the cells treated with ADM2 10−8 M.
Effect of ADM2 on HTR-8SV/Neo Cell Migration
The migration of HTR-8SV/neo cells was examined by means of an in vitro migration assay as described in Materials and Methods. As shown in Figure 6, ADM2 increased the migration of the cells toward the scratched area more rapidly compared with untreated controls. The addition of ADM217–47 to the incubation medium greatly suppressed the ADM2-induced increase in trophoblast cell migration.
FIG. 6.
ADM2 promotes migration of HTR-8SV/neo cells in the in vitro scratch assay. An in vitro wound was introduced in the confluent cultures of first-trimester HTR-SV/neo cells. The cells were then incubated with or without ADM2 (10−8 M) in the presence or absence of ADM2(17–47) 10−5 M for 24 h. The top panel shows the cells photographed under the phase-contrast microscope. Original magnification ×4. The bottom panel shows bars representing the mean ± SEM of the distance covered by the cells. The figure represents the results of three different experiments (P < 0.05). Asterisk (*) indicates significant difference compared with the control cells, and two asterisks (**) indicate significant difference compared with the cells treated with ADM2 10−8 M.
DISCUSSION
The present study was undertaken to demonstrate the expression and role of ADM2/IMD in human placenta. We show that ADM2 mRNA is expressed in human placental villi and first-trimester trophoblast cells (HTR-8SV/neo). ADM2 mRNA was also detected in other trophoblast cells such as human choriocarcinoma JAr and JEG-3 trophoblastic cell lines (data not shown). Immunoreactive ADM2 is expressed predominantly in trophoblast cells in human placenta at all stages of pregnancy. However, the ADM2 immunoreactivity in endothelial cells of the placental vessels was not consistent in all cells and at all stages of gestation. Furthermore, invasion and migration of HTR-8SV/neo cells were also increased by ADM2. Therefore, we suggest that ADM2 may have a role in the physiology of human pregnancy via regulation of trophoblast invasion and migration.
ADM2 is a novel CALCA/CALCB family peptide, which was identified as IMD in 2004 by Roh et al. [1] using a phylogenetic profiling approach. Simultaneously, Takei et al. [2] also identified a novel member of the ADM family (ADM2) in mammals (mice, rats, and humans) with the same nucleotide and amino acid sequence as IMD. Therefore, IMD and ADM2 were considered the same peptide [1]. The amino acid sequence of ADM2 is conserved among species, which suggests that ADM2 has an important regulatory role in homeostasis. CALCB and ADM are expressed in fetoplacental tissues and are important in maintaining normal placental function [10–14]. Altered expression of these peptides has been implicated in pathological pregnancies diagnosed in conditions such as hypertension and preeclampsia [10, 12, 15]. We recently reported that plasma levels of ADM2 are elevated in rats during pregnancy and that infusion of ADM2 antagonist to pregnant rats caused fetoplacental growth restriction [5]. In addition, we demonstrated that ADM2-induced relaxation of mesenteric arteries in rats is more pronounced in pregnancy, suggesting that ADM2 may be involved in vascular adaptations during pregnancy and that ADM2 may be involved in maintaining normal fetoplacental growth in pregnant rats [4]. However, to date there are no studies on the expression and possible functional role for this novel CALCA/CALCB family peptide in human placenta. In this study, we demonstrate for the first time (to our knowledge) that ADM2 is expressed in human placenta (Figs. 1 and 2) and that ADM2 immunoreactivity is abundantly present in trophoblast cells (Fig. 3) throughout pregnancy. However, as shown in Figure 4, colocalization of ADM2 immunoreactivity with the endothelial cell marker CD34 was not observed in all of the endothelial cells. Evidence of different phenotypes of endothelial cells in human placental blood vessels has been reported [8, 9]. Heterogeneity of placental vascular endothelial cells may be the cause of the observed uneven staining pattern of ADM2 antibody. Further studies using specific markers for fetal and maternal origins of placental endothelial cells are needed to characterize ADM2-specific staining patterns in human placental vessels. The present study further demonstrates that ADM2 is involved in the invasion and migration of trophoblast cells.
Implantation in humans begins with invasion of uterine epithelium and underlying stroma by extraembryonic trophoblast cells. Villous cytotrophoblast cells at the tips of some anchoring villi proliferate outward from the underlying basement membrane to form columns from which individual cells migrate into the decidual tissue. These interstitial trophoblast cells invade as far as the superficial layer of the myometrium [16]. During this process, the walls of the maternal spiral arteries are destroyed and are converted from muscular vessels into flaccid sinusoidal sacs [17]. Failure of successful invasion leads to clinicopathological conditions such as intrauterine growth retardation, preeclampsia, and others [18]. CALCB and ADM have been reported to function as angiogenic agents in placental development [6, 19]. The ADM-null mutation (ADM−/−) is embryonically lethal, and no embryos survive beyond the midterm of gestation, indicating an indispensable role of ADM in early placental development and fetal morphogenesis [20]. Therefore, to elucidate the involvement of ADM2 in human placental function, we analyzed its effects on the regulation of migration and invasion of HTR-8SV/neo cells (representative of first-trimester placental trophoblast cells). An in vitro Matrigel-based invasion chamber was used to determine the influence of ADM2 on trophoblast invasion in HTR cells. As shown in Figure 5, ADM2 increases the invasive capacity of HTR cells by 2.2-fold in ADM2-treated cells compared with the untreated control cells, and this increase is reduced in the presence of antagonist ADM217–47, suggesting the specificity of ADM2 action. Therefore, we suggest that ADM2 may be involved in the regulation of invasion of maternal arteries by extravillous trophoblasts in early pregnancy.
Despite extensive studies on trophoblast migration, which is a necessary step in trophoblast invasion, what controls its mechanism is as yet unknown. Evidence suggests that this is a complex process tightly regulated by factors produced within the trophoblast-endometrial microenvironment [16]. We recently demonstrated restricted fetoplacental growth and placental apoptosis in pregnant rats treated with ADM2 antagonist [5]. Mechanisms that limit extravillous trophoblast invasion and migration include apoptosis, reduced expression of integrin α1β1 (ITGA1B1) [21], decreased secretion of metalloproteinases (MMP9) [22], low cell surface plasminogen activator activity, and reduced expression of human leukocyte antigen G [23]. Therefore, to assess the effect of ADM2 on trophoblast migration, we used an in vitro scratch assay on HTR cells. As shown in Figure 6, the addition of exogenous ADM2 significantly increases the migrating capability of the HTR cells, and this was inhibited by ADM217–47, suggesting the specificity of action of ADM2 on trophoblast migration.
CALCB and ADM are expressed by the placenta, along with the complete receptor system for these peptides [19, 24]. ADM2 has the highest structural homology with the ADM peptide [1]. Our recent studies [4, 5] and other reports [25, 26] suggest that ADM and ADM2 have similar biological functions. Because all three ligands, CALCB, ADM, and ADM2, are capable of interacting with the same CALCRL, optimal regulation by the GPCR signaling pathway likely depends on an integrated release of different endocrine-paracrine ligands in a tissue-specific, cell-specific, and time-coordinated manner. Activation of the same receptor by CALCB, ADM, and ADM2 indicates the complexity of the mechanism of action involved in their downstream signaling cascade. Our studies provide preliminary evidence of a role for this novel peptide in trophoblast invasion and migration in early human placental function. Additional studies are required to identify the mechanisms underlying these processes.
Footnotes
1Supported by NIH grants R03 HD054867 (to M.C.) and R01 HL58144 (to C.Y.).
REFERENCES
- Roh J, Chang CL, Bhalla A, Klein C, Hsu SYT.Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J Biol Chem 2004; 279: 7264–7274.. [DOI] [PubMed] [Google Scholar]
- Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S.Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett 2004; 556: 53–58.. [DOI] [PubMed] [Google Scholar]
- Lin CC, Roh J, Park JI, Klein C, Cushman N, Haberberger RV, Hsu SY.Intermedin functions as a pituitary paracrine factor regulating prolactin release. Mol Endocrinol 2005; 19: 2824–2838.. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Ross GR, Yallampalli U, Yallampalli C.Adrenomedullin-2, a novel calcitonin/calcitonin-gene-related peptide family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology 2007; 148: 1727–1735.. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Yallampalli U, Reed L, Yallampalli C.Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod 2006; 75: 940–947.. [DOI] [PubMed] [Google Scholar]
- Zhang X, Green KE, Yallampalli C, Dong YL.Adrenomedullin enhances invasion by trophoblast cell lines. Biol Reprod 2005; 73: 619–626.. [DOI] [PubMed] [Google Scholar]
- Liang CC, Park AY, Guan J.In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007; 2(2):329–333.. [DOI] [PubMed] [Google Scholar]
- Hsi BL, Yeh CJG.Monoclonal antibodies to placental vascular structures. Trophoblast Res 1988; 3: 139–148.. [Google Scholar]
- Lang I, Hartmann M, Blaschitz A, Dohr G, Skofitsch G, Desoye G.Immunohistochemical evidence for the heterogeneity of maternal and fetal vascular endothelial cells in human full-term placenta. Cell Tissue Res 1993; 274: 211–218.. [DOI] [PubMed] [Google Scholar]
- Stevenson JC, MacDonald DWR, Warren RC, Booker MW, Whitehead MI.Increased concentration of circulating calcitonin gene-related peptide during normal human pregnancy. BMJ 1986; 293: 1329–1330.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio R, Marinoni E, Cosmi EV.New peptides, hormones and parturition. Gynecol Endocrinol 1998; 12: 1–6.. [DOI] [PubMed] [Google Scholar]
- Di Iorio R, Marinoni E, Letizia C, Villaccio B, Alberini A, Cosmi EV.Adrenomedullin production is increased in normal human pregnancy. Eur J Endocrinol 1999; 140: 201–206.. [DOI] [PubMed] [Google Scholar]
- Kobayashi KKT, Aso T, Hirata Y, Imai TMF.Immunoreactive adrenomedullin (AM) concentration in maternal plasma during human pregnancy and AM expression in placenta. Eur J Endocrinol 2000; 142: 683–687.. [DOI] [PubMed] [Google Scholar]
- Dong YL, Vegiraju S, Chauhan M, Yallampalli C.Expression of calcitonin gene-related peptide receptor components, calcitonin receptor-like receptor and receptor activity modifying protein 1, in the rat placenta during pregnancy and their cellular localization [published correction appears in Mol Hum Reprod 2003;9(11):737]. Mol Hum Reprod 2003; 9: 481–490.. [DOI] [PubMed] [Google Scholar]
- Knerr I, Dachert C, Beinder E, Metzler M, Dotsch J, Repp R, Rascher W.Adrenomedullin, calcitonin gene-related peptide and their receptors: evidence for a decreased placental mRNA content in preeclampsia and HELLP syndrome. Eur J Obstet Gynecol Reprod Biol 2002; 101: 47–53.. [DOI] [PubMed] [Google Scholar]
- Burrows TD, King A, Loke YW.Trophoblast migration during human placental implantation. Hum Reprod Update 1996; 2: 307–321.. [DOI] [PubMed] [Google Scholar]
- Boyd JD, Hamilton WJ. The Human Placenta. Cambridge, England:: W Heffer & Sons Ltd;; 1970. [Google Scholar]
- King A, Loke YW.Unexplained fetal growth retardation; what is the cause? Arch Dis Child 1994; 70: 225–227.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong YL, Reddy DM, Green KE, Chauhan MS, Wang HQ, Nagamani M, Hankins GD, Yallampalli C.Calcitonin gene-related peptide (CALCA) is a proangiogenic growth factor in the human placental development. Biol Reprod 2007; 76: 892–899.. [DOI] [PubMed] [Google Scholar]
- Imai Y, Shindo T, Maemura K, Kurihara Y, Nagai R, Kurihara H.Evidence for physiological and pathological roles of adrenomedullin from genetic engineering in mice. Ann N Y Acad Sci 2001; 947: 26–34.. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ.Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest 1993; 91: 950–960.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, McCrare KR.Altered expression of gelatinase and surface-associated plasminogen activator activity by trophoblast cells isolated from placenta of preeclampsia patients. Am J Obstet Gynecol 1996; 175: 555–562.. [DOI] [PubMed] [Google Scholar]
- Hara N, Fujii T, Yamashita T, Kozuma S, Okai T, Taketani Y.Altered expression of human leukocyte antigen G (HLA-G) on extravillous trophoblasts in preeclampsia: immunohistological demonstration with anti-HLA-G specific antibody “87G” and anti-cytokeratin antibody “CAM5.2.” Am J Reprod Immunol 1996; 36: 349–358.. [DOI] [PubMed] [Google Scholar]
- Dong YL, Green KE, Vegiraju S, Hankins GD, Martin E, Chauhan M, Thota C, Yallampalli C.Evidence for decreased CGRP receptors and compromised responsiveness to CGRP of fetoplacental vessels in preeclamptic pregnancies. J Clin Endocrinol Metab 2005; 90: 2336–2343.. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK.Intermedin/adrenomedullin-2 acts within central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol Regul Integr Comp Physiol 2005; 288: R919–R927.. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK.Intermedin/adrenomedullin-2 inhibits growth hormone release from cultured, primary anterior pituitary cells. Endocrinology 2006; 147: 859–864.. [DOI] [PubMed] [Google Scholar]