Abstract
Plasminogen activator inhibitor-1 (PAI-1) is an important regulator of fibrinolysis. PAI-1 levels are elevated in type 2 diabetes, and this elevation correlates with macro- and microvascular complications of diabetes. However, the mechanistic link between insulin and up-regulation of PAI-1 is unclear. Here we demonstrate that overexpression of Forkhead-related transcription factor (Fox)O1, FoxO3a, and FoxC1 augment insulin’s ability to activate the PAI-1 promoter. In addition, insulin treatment promotes the phosphorylation of nuclear and cytoplasmic Fox03a and an increase of cytoplasmic Fox03a. In contrast, insulin treatment led to the accumulation of phospho-Fox01 only in the cytoplasm. Furthermore, insulin also increased the ability of chimeric LexA-FoxO1, LexA-FoxO3a, and LexA-FoxC1 proteins to increase the activity of a LexA reporter, suggesting that the effect of insulin on FoxO3a was direct. Using small interfering RNA to specifically deplete each of the Fox transcription factors tested, we demonstrate that only reduction of FoxO3a inhibits insulin-increased PAI-1-Luc expression and PAI-1 mRNA accumulation. Finally, chromatin immunoprecipitation assays confirm the presence of FoxO3a on the PAI-1 promoter. These results suggest that FoxO3a mediates insulin-increased PAI-1 gene expression.
Insulin increased-PAI-1 transcription in multiple cell types is mediated by FoxO3a and is independent of Akt-phosphorylation that increases FoxO3a phosphorylation in nuclear and cytoplasmic compartments.
Plasminogen activator inhibitor-1 (PAI-1) is a major regulator of fibrinolysis. It inhibits both tissue type and urokinase plasminogen activators and serves an essential role in wound healing where it is required to maintain the fibrin clot. Abnormal expression of PAI-1 is observed in obesity (1), inflammation (2), and diabetes (3), and increased PAI-1 has been correlated to the higher risk or cardiovascular disease seen in these syndromes (4).
PAI-1 is expressed in virtually all of the tissue types studied. PAI-1 transcription was increased by numerous factors, including platelet-derived growth factor (5), β-fibroblast growth factor (5), IL-1b, TGFβ (6), angiotensin II (7), TNFα (8), thrombin (9), and oxidation products (10) whereas interferon-γ (11) inhibited PAI-1 production. Several studies have also demonstrated that insulin increases PAI-1 expression. PAI-1 is increased in patients with type 2 diabetes (12) and insulin or proinsulin infusion can cause elevation of PAI-1 detected by analysis of PAI-1 protein levels (13,14,15) or by in situ hybridization (16). Insulin also increases expression of the endogenous PAI-1 gene in HepG2 cells (17) and the transcription of a luciferase reporter plasmid under control of the PAI-1 promoter in human umbilical vein endothelial cells (HUVECs) in culture (18,19).
Several specific response elements have been defined in the PAI-1 promoter. A paired Sp1 element at −73 and −42 mediated responses to glucose and angiotensin II (20,21). An activator protein 1 (AP-1)-like element at −59/−52 was reported to mediate the response of the PAI-1 promoter to D dimer, a proteolytic fragment of fibrin (22), and also to mediate effects from protein kinase C and protein kinase A (PKA) (23,24). TGFβ activation of the PAI-1 promoter is through sequences between −740/−528 (25,26). A glucocorticoid response element was identified at −1212 (27), and two hypoxia response elements were identified at −175/−158, the mutation of which eliminated the 3-fold response to hypoxia (28). We previously identified an insulin response element of the PAI-1 promoter at −52/−43 that was a Forkhead-related transcription factor (Fox)-like element, and insulin-increased PAI-1 expression was blocked by overexpression of the FoxO1 DNA-binding domain (29). This was confirmed in studies by others who suggested that the effect of insulin at this site was mediated by FoxC2 (30).
The Fox transcription factor family in humans is made up of approximately 40 members grouped into families (A to Q) that is characterized by a 100-amino acid DNA binding domain (http://biology.pomona.edu/fox/). They play important roles in differentiation, transformation, and metabolism and have been linked to diabetes (31,32,33,34,35). FoxA2 regulates liver lipid metabolism and ketogenesis in fasting and diabetes (36), it controls pancreatic Pdx expression (37), and it regulates insulin secretion (38). Mutation of FoxC2 is associated with lymphedema-distichiasis and diabetes (39). A C512T polymorphism of FoxC2 is associated with dyslipidemia and obesity (40) and may contribute to the incidence of obesity and diabetes in the Pima Indians (41). Some studies have suggested that mutation of the FoxP3 protein is responsible for X-linked enteropathy, endocrinopathy, and diabetes mellitus (42,43,44,45,46). Insulin has been shown to regulate several Fox-family members. Akt-dependent phosphorylation of FoxO1/3/4 at three sites resulted in binding to 14–3-3 proteins and nuclear export (47,48). It was also suggested that FoxA2 is regulated by phosphorylation and nuclear export (36). In contrast, transcription of FoxC2 is increased by insulin through phosphatidylinositol 3-kinase and ERK 1/2 (49,50,51).
Previous studies demonstrated that overexpression of murine FoxC2 in 3T3-L1 and bovine arterial endothelial (BAE) cells activated PAI-1-luciferase expression and the endogenous PAI-1 promoter. This overexpression suggested that FoxC2 mediated responses to insulin and TGFβ (30). However, these effects could have been secondary to FoxC2 stimulation/inhibition of other transcription factors or signaling components or it could be cell type dependent. Despite the important role of PAI-1 in diabetes and the clear association of Fox transcription factors with diabetes and PAI-1 transactivation, the mechanism of Fox transcriptional activation of the PAI-1 promoter and indeed the specific Fox family member(s) required for PAI up-regulation in response to insulin is unknown. We demonstrate using a variety of cell lines and molecular manipulations that the effect of insulin to increase PAI-1 is increased by the expression of FoxO3a. The knockdown of FoxO3a by siRNA impaired the effect of insulin on PAI-1-Luc reporter and on the endogenous PAI-1 mRNA. Finally, chromatin cross-linking and chromatin immunoprecipitation (ChIP) experiments show increased FoxO3a on the PAI-1 promoter in response to insulin treatment. Taken together, these experiments suggest that insulin increases PAI-1 through activation of FoxO3a.
Results
Fox transcription factors affect insulin-increased PAI-Luc expression in GH4 and T47D cells
Previous work from our laboratory and other have suggested that the insulin response of the PAI-1 promoter is mediated by a Fox transcription factor (30,52) that acts at a sequence −52/−43 in the PAI-1 promoter. Therefore, we reasoned that higher levels of Fox factors might increase the effect of insulin on PAI-1 expression if Fox factors were limiting. Alternately, the expression of Fox factors could squelch the effect of the endogenous factors by titrating essential transcription factors as was shown for Elk-1 (53). To determine which Fox transcription factors were most likely to mediate insulin’s effects, we screened human umbilical vein endothelial cells for Fox mRNA expression using RT-quantitative PCR (RT-qPCR) and comparing the transcripts to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). This screen (data not shown) indicated substantial expression of FoxO1 mRNA > FoxC1 > FoxO3a > FoxP1 (mRNAs for Fox D3, FoxD4, FoxO4, FoxJ2, FoxC2, and FoxH1, the levels of which were almost undetectable, were also seen). Thus, it seemed likely that a member of the FoxO, FoxC, or FoxP family mediated the response to insulin. GH4 cells were electroporated with a PAI-1-luciferase construct containing 900 bp of the PAI-1 promoter (Fig. 1A) along with an expression vector for either FoxO1, FoxO3a, FoxC1, FoxC2, and FoxP1 or plasmids encoding FoxO1 and FoxO3a with triple-alanine mutants of the Akt phosphorylation sites because FoxO1 and FoxO3a can be excluded from the nucleus in response to an insulin/ phosphatidylinositol 3-kinase/Akt triple phosphorylation (47). Western blot analysis confirmed that the Flag-tagged FoxO1 and FoxO3 were expressed and localized after transfection (Fig. 2C).
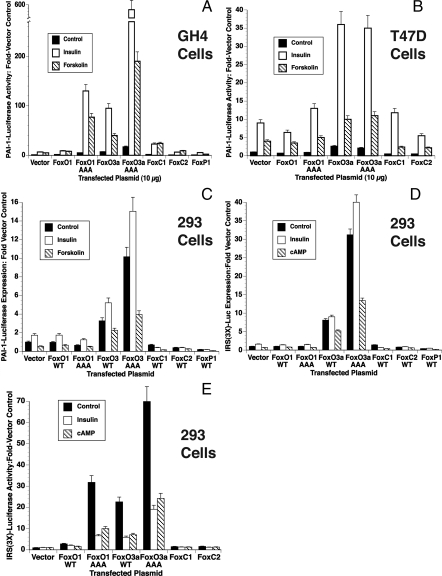
Figure 1.
Insulin-Increased PAI-1-Luc expression is affected by transfection with expression plasmids for Fox-family transcription factors: GH4 (A), T47D (B), and HEK293 (C) cells were transfected with PAI-1-Luc, human insulin receptor, and β-galactosidase and HEK293 cells (D and E) were transfected with IRS(3×)-Luc, and β-galactosidase as detailed in Materials and Methods. The electroporations also contained 10 μg of an expression vector for the indicated Fox transcription factor or 10 μg of vector control. The cultures were treated with 1 μg/ml insulin or 1 mm forskolin, and the plates were incubated for 20 h. The average relative light units/10 μg protein in control and insulin-treated cultures was determined, adjusted for β-galactosidase expression, and the relative light units from cells incubated with hormones were compared with control levels to determine the fold-stimulation (Fold-Basal). The results are from three separate experiments done in triplicate. WT, Wild type.
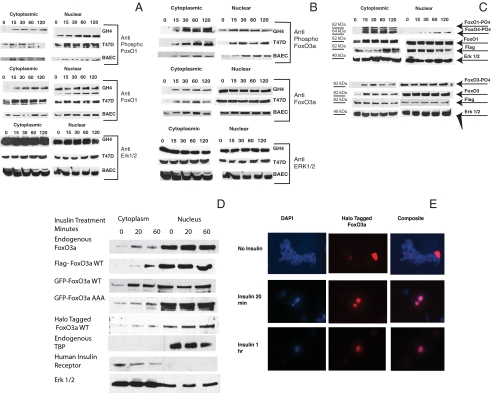
Figure 2.
Phosphorylation and compartmentalization of endogenous and transfected FoxO1 and FoxO3a: A and B, Endogenous FoxOs. GH4, T47D and BAE cells were plated in growth medium and switched to medium containing 10% charcoal-treated serum 24 h before the start of the experiment. Insulin, 1 μg/ml, was added for the indicated times, and the cells were rapidly washed with ice-cold saline and frozen at −75 C (<30 sec between 37 C and dry ice). Cytoplasmic and nuclear extracts were prepared as described in Materials and Methods, and the proteins were resolved by SDS-PAGE and transferred to nitrocellulose. A, The membranes were blocked and analyzed using antibodies to phospho-FoxO1 (Ser256 and 319) (top) and total FoxO1 (middle). The blots were also analyzed using antibody to ERK 1 and 2 as a loading control (bottom). B, The membranes were blocked and analyzed using antibodies to phospho-FoxO3a (Ser318/321) (top) and total FoxO3a (middle). The blots were also analyzed using antibody to ERK 1 and 2 as a loading control (bottom). C–E, Transfected FoxO1 and FoxO3a. C, GH4 cells were electroporated with 10 μg of Flag-FoxO1 (top) or 10 μg of Flag-FoxO3a (bottom). They were plated in growth medium and allowed to attach. The cells were switched to medium containing 10% charcoal-treated serum for 24 h. Insulin, 1 μg/ml, was then added for the indicated times. Cytoplasmic and nuclear extracts were prepared as described in Materials and Methods, and the proteins were resolved by SDS-PAGE and transferred to nitrocellulose. The membranes were blocked and analyzed using antibodies to phospho-FoxO1/O4 (Ser319/262) and total FoxO1 (top) or phospho-FoxO3a (Ser319/321) and total FoxO3a (bottom). The blots were also analyzed using antibody to Flag to show expression of exogenously expressed protein and with antibody to ERK 1 and 2 as a loading control. D, T47D cells were transfected with flag-FoxO3a wt, with GFP FoxO3a wt, GFP-FoxO3a AAA mutant, or HT-FoxO3a wt or untransfected for analysis of endogenous FoxO3a localization. After 24 h in medium containing charcoal-treated serum, the cultures were incubated with insulin for 20 min or 1 h or left untreated as controls. Cytoplasmic and nuclear extracts were prepared and analyzed by SDS-PAGE and blotted to nitrocellulose membrane. Anti-FoxO3a (Upstate), Anti-Flag (Sigma), and anti-GFP (Molecular Probes) were then used to show the distribution of FoxO3a. Extracts from HT-FoxO3a transfected cells were incubated for 30 min with HaloTMR ligand (Promega) that has a fluorescent label and covalently links to the HT-FoxO3a. Blots were stripped and blotted against ERK 1 and 2 as a loading control, TBP as an indicator of the specificity of the nuclear extract, or human insulin receptor as an indicator of the specificity of the cytoplasmic extract. E, T47D cells, grown on coverslips, were transfected using Lipofectamine 2000 (Invitrogen) with HT-FoxO3a wt and incubated with insulin for 20 min or 1 h or left untreated as a control. 4′,6-Diamidino-2-phenylindole fluorescence is in the left column, HT TMR ligand fluorescence is shown in the central column whereas the composite image is shown in the right column. Control cells are shown in the top row, whereas insulin treatment of 20 min is in the middle row and 1 h is in the bottom row. DAPI, 4′,6-Diamidino-2-phenylindole; WT, wild type.
FoxO1 expression did not have a significant effect on PAI-1-luciferase expression in GH4 cells. FoxO1AAA increased basal (2- to 3-fold) and insulin- and forskolin-increased PAI-1-luciferase expression 10-fold to approximately 100-fold. The increase in basal PAI-1-Luc mediated by this mutant was not remarkable because the AAA mutation disrupts the ability of insulin to promote nuclear exclusion of FoxO1. However, the insulin and forskolin responsiveness of this triple mutant was not expected because mutation of the Akt phosphorylation sites eliminated important insulin effector sites, and forskolin had not been shown to activate FoxOs. Thus, forskolin stimulation was expected to mirror any effect on basal transcription of the reporter and serve as a control for any increased insulin effects.
FoxO3a expression also induced basal and forskolin-increased PAI-1-Luc activity 5-fold and insulin-increased PAI-1-Luc 10-fold, which strongly suggested that FoxO3a was involved in PAI-1 gene transcription either directly or indirectly. FoxO3a AAA was an even more potent activator as would be expected because it cannot be excluded from the nucleus. The triple mutant remained insulin and forskolin sensitive despite mutation of the Akt phosphorylation sites. Insulin-increased PAI-1-luciferase expression was also increased 2-fold and forskolin-stimulated PAI-1-luciferase expression was increased 4-fold in cells expressing FoxC1. Neither insulin- nor forskolin-increased PAI-Luciferase expression was significantly different from controls in cells expressing FoxC2 or FoxP1.
To determine whether these results were cell type specific, these experiments were repeated in several other cell lines. T47D cells were derived from a human mammary gland ductal carcinoma and are an important model of hormone-sensitive breast cancer. Breast cancer cells make substantial PAI-1, which is a predictor of poor prognosis (54). In contrast to the GH4 cells, wild-type FoxO1 and FoxO1AAA had no effect on PAI-1-Luc expression (Fig. 1B). However, FoxO3a and FoxO3a AAA increased basal PAI-1-Luc expression 4-fold over control levels in T47D cells (Fig. 1B). Insulin further increased PAI-1-Luc expression to 35- to 40-fold in cells expressing either FoxO3a or FoxO3a AAA. Forskolin-increased PAI-1-Luc expression was 2-fold higher in cells in which FoxO3a and FoxO3aAAA were expressed. FoxC1 did not have a significant effect in this cell line whereas FoxC2 significantly inhibited both basal and stimulated PAI-1-luc expression.
The human embryonic kidney (HEK)293 cell line is derived from human kidney epithelial cells and has been used for studies of IGF-I- and serum-mediated FoxO1 and FoxO3a translocation from the nucleus (55,56). Thus, they should respond appropriately to insulin by exporting FoxO1/O3a/O4 from the nucleus. The previous results, showing that insulin activation of PAI-1 by insulin was enhanced by FoxO3a, are not consistent with reports using a reporter constructed from a 3× IRS (insulin receptor sequence) from the IGF-I binding protein 1 promoter in HepG2 cells. Those studies demonstrated that IRS-Luc expression was dependent on FoxOs and inhibited by insulin (47). These conflicting results could result from the reporter, the cell line, or both. First, we tested the PAI-1-Luc reporter in the HEK293 cells (Fig. 1C). PAI-1-Luc was highly expressed in HEK293 cells, and this was increased slightly by insulin treatment and decreased by forskolin (Fig. 1C). Neither FoxO1 nor FoxO1AAA affected these results. Basal expression of PAI-Luc was significantly increased by expression of FoxO3a or the triple-alanine mutations of FoxO3a, and this was increased by insulin and decreased by forskolin. Expression of Fox C1, FoxC2, or FoxP1 decreased PAI-1-Luc expression under all conditions in HEK293 cells.
These results were unexpected because previous reports consistently showed insulin inhibition of FoxO-mediated activation (47,48). Therefore, we tested whether insulin activated the Fox01-sensitive IRS(3×)-Luc reporter to determine whether the effect of FoxO3a on PAI-1 promoter in HEK293 cells was promoter specific (Fig. 1D). Preliminary experiments suggested that cell density was an important consideration in how these cells responded to transfection and insulin treatment. Therefore, cells were plated at low density, and the experiment was conducted during log phase (as for the PAI-1-Luc experiments in Fig. 1C). Under these conditions, the IRS(3×)-Luc responded almost identically with the PAI-1-Luc reporter except that the stimulation due to insulin treatment was only significant with the FoxO3a AAA construct. When the HEK293 cells are allowed to become confluent before transfection, results similar to those presented by others were obtained (Fig. 1E). The reporter had no activity in the absence of transfected FoxO expression plasmid, and both insulin and forskolin treatment inhibit the activity of the reporter. Surprisingly, the activity of the triple alanine mutants of both FoxO1 and FoxO3a was also significantly inhibited by insulin and forskolin. This suggests that insulin can repress transcription through mechanism other than nuclear export as was previously described (57,58). This suggests that, at least for this clone of HEK293 cells, the insulin-dependent inhibition of this promoter is independent of Akt-mediated phosphorylation of FoxOs. Foxc1, Foxc2, and FoxP1were also without effect under these conditions.
In summary, only FoxO3a and its triple-alanine mutant enhanced insulin-increased expression of PAI-1 in all of the cell lines tested, suggesting that it most likely mediated the effect of insulin. FoxO3a and FoxO3a AAA also increased basal PAI-1-Luc expression although not to the same degree as they increase insulin stimulation. The effect of FoxO3a on forskolin-increased PAI-1-Luc expression was enigmatic. Forskolin-increased PAI-1-Luc expression was stimulated more than basal transcription in GH4 cells by FoxO3a expression, but in HEK293 cells it inhibited PAI-1-Luc expression. FoxO1AAA and FoxC1 increased PAI-1-Luc expression under select conditions whereas Fox C2 and FoxP1 inhibited insulin-increased PAI-1 expression where they had any effect.
Phosphorylated FoxO3a accumulates in the nucleus for several hours after insulin treatment
Our findings that insulin-induced activation of the PAI-1 might be mediated by FoxOs are contrary to previous studies in which FoxO-mediated transcription was decreased by insulin activation of Akt that led to phosphorylation of FoxO and FoxO binding to 14-3-3 proteins and export to the cytoplasm (47). One possible explanation for this is that overexpressed FoxO proteins saturate the endogenous 14-3-3 proteins permitting FOXO to enter the nucleus and activate transcription. Insulin-sensitive phosphorylation at non-Akt sites or other modification could then mediate a further increase in PAI-1 transcription in response to insulin. This made it important to determine the distribution and phosphorylation of FoxO1 and FoxO3a in response to insulin in GH4 and T47D cells and how this is affected by overexpression of FoxO1 and FoxO3a.
Cytosolic and nuclear extracts from cells incubated for various times with insulin were resolved by SDS-PAGE, blotted to nitrocellulose, and probed with phosphorylation state-specific antibodies and antibodies to total FoxO1/O4 (Fig. 2A). The GH4 cells showed a pattern of FoxO1 compartmentalization that was expected from published studies (47). In GH4 cells, no phosphorylated FoxO1 was detected in the nucleus whereas it rapidly accumulated in the cytoplasm in response to insulin. Further, nonphosphorylated FoxO1 was constant in the nucleus of GH4 cells, despite the accumulation of the phosphorylated species in the cytoplasm. In T47D cells and BAE cells, FoxO1 was rapidly phosphorylated in insulin-treated cells, and phosphorylated species appear rapidly in both the cytoplasm and nucleus. Further, whereas phosphorylated FoxO1 levels increased in the cytoplasm in response to insulin, the level of total FoxO1 in the nucleus remained constant, despite insulin treatment. Although this may seem contradictory, Western blots are not strictly quantitative, but only show relative changes. Data from GH4, T47D, and BAE cells are shown in Fig. 2 because these cells were used in the functional studies reported here, but similar results were seen in HeLa, HUVEC, and HEK293 cells (data not shown).
We then examined the compartmentalization of other Fox transcription factors in response to insulin. The pattern of phosphorylation and compartmentalization of FoxO3 (Fig. 2B) was similar to that seen for FoxO1 in T47D and BAE cells. Phosphorylated FoxO3a appeared rapidly in both compartments (<10 min) and remained high for at least 2 h. This behavior was cell type independent because it was observed in a primary cell line (BAE) and in two transformed cell types (GH4 and T47D). Levels of unphosphorylated FoxO3a were high in the nucleus and did not change over the course of the experiment whereas cytoplasmic levels of FoxO3a were low in control cultures and increased rapidly with insulin treatment.
The pattern of phosphorylation and compartmentalization of transfected flag-tagged FoxO1 and FoxO3a was determined (Fig. 2C) and was similar to the patterns observed with endogenous FoxOs. The only remarkable difference was that there was significant unphosphorylated FoxO1 and FoxO3a in the cytoplasm of control cells. This is undoubtedly the result of overexpression.
The experiments in panels D and E of Fig. 2 were performed to further support the conclusion that FoxO3a is localized predominantly to the nucleus even in insulin-treated cells. Biochemical evidence of this conclusion is presented in Fig. 2D where the distribution of endogenous FoxO3a and FoxO3a labeled with Flag, green fluorescent protein (GFP), or HaloTag (HT) is examined. Insulin treatment for 20 min or 1 h increased the cytosolic concentration of the endogenous and labeled FoxO3a, but a substantial fraction, perhaps a majority, of the FoxO3a remains nuclear. FoxO3a with a triple-alanine mutation does not increase in the cytoplasm in response to insulin, but is also predominately nuclear. The specificity of the extraction process is verified by the presence of TATA-binding protein (TBP) only in the nuclear fraction and the presence of human insulin receptor only in the cytosolic fraction. Visual evidence is provided by Fig. 2E, which shows the nuclear localization of HT-FoxO3a in control and insulin-treated cells.
These experiments have shown that although FoxO1 phosphorylation results in the nuclear exclusion of phosphorylated FoxO1 in some cell lines (GH4 cells), this is never true of FoxO3a and that phosphorylated FoxO3a accumulates in both the nuclear and cytoplasmic fractions and total FoxO3a is predominately nuclear. Because the phospho-specific antibodies used in these experiments identify only Akt-modified FoxO3a, the FoxO3a remaining in the nucleus might be phosphorylated by other kinases or modified by acetylation, sumolation, ADP ribosylation, or other postsynthetic modification that might affect its activity.
The C terminus of FoxO1 and FoxO3a are insulin-activated transcription factors
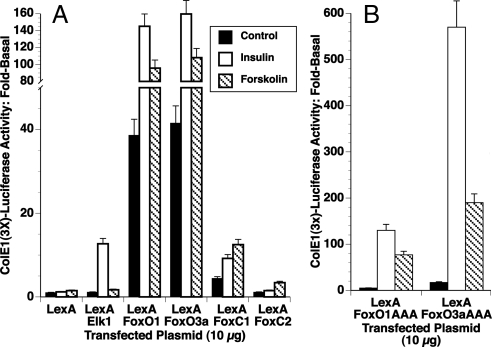
The expression of exogenous Fox proteins enhanced insulin-increased PAI-1-Luc expression and forskolin-increased expression in GH4 cells. This could result from a direct interaction of the Fox factors with the PAI promoter, but it is also possible that Fox proteins induce the expression of other factors that contribute to insulin/forskolin activation of PAI-1 transcription. To test whether Fox factors can directly activate the PAI-1 promoter, we made chimeric plasmids that link the DNA-binding domain of the bacterial LexA to the C terminus of several Fox transcription factors. The C terminus of FoxO1 (amino acids 236–655), FoxO3a (amino acids 233–673), and FoxC1 (amino acids 231–553) linked to LexA increased luciferase expression from a 3XLexA-luciferase reporter plasmid. Highest levels of basal expression were obtained using LexA-FoxO3a (45-fold) > LexA-FoxO1 (40-fold) ≫ LexA-FoxC1 (5-fold) (Fig. 3A). This high basal activity was further activated 3- to 4-fold by insulin and 2- to 3-fold by forskolin in cells expressing LexA-FoxO1 or LexA-FoxO3a, but was only minimally increased in the LexA-FoxC1-expressing cells. Conversely, the C terminus of FoxC2 (amino acids 231–493) decreased basal expression and was not insulin stimulated. The forskolin stimulation of 3X-LexA-Luc reporter through FoxO1 and FoxO3a was unexpected and suggested that FoxOs could be activated by PKA under some circumstances. This appears to be the first indication that FoxO3a can be activated by cAMP/PKA and that both the effect of insulin and the effect of cAMP on the PAI-1 promoter might be mediated through activation of FoxO3a. Further studies will be required to fully address this possibility.
Figure 3.
The C termini of FoxO1 and FoxO3a are insulin-activated transcriptional enhancers. GH4 cells were electroporated with 10 μg of a Luciferase reporter plasmid containing six copies of the LexA response element (ColE1-Luc), 5 μg of pRT3HIR2, and 0.5 μg of rous sarcoma virus-β-Gal. A, Each electroporation also contained 10 μg of an expression vector for a fusion protein containing the LexA DNA-binding domain and the indicated Fox transcription factor C terminus (FoxO1, aa 236–655; FoxO3a, aa 233–673; FoxC1, aa 231–553; FoxC2, aa 231–493; and FoxP1, aa 2–706). A previously described LexA-Elk1 (aa 105–428) was used as a control. The cultures were incubated for 20 h with insulin or forskolin. The luciferase activity was determined and corrected for β-Gal expression as described in Materials and Methods. B, Each electroporation also contained 10 μg of an expression vector for a fusion protein containing the LexA DNA-binding domain and FoxO1 AAA mutant (aa 236–255) or FoxO3a AAA mutant (aa 233–673). The cultures were incubated for 20 h with insulin or forskolin. The luciferase activity was determined and corrected for β-Gal expression as described in Materials and Methods.
Our previous studies with the PAI-1 promoter suggested that insulin stimulation was AKT independent. The LexA construct of the FoxC1 does not have an Akt phosphorylation site whereas the C-terminal construct of FoxO1 eliminates two of three Akt phosphorylation sites. The LexA-FoxO3a retains two of three sites. Thus, the high activity of the LEXA-FoxO3a could result from the phosphorylation of these sites in response to insulin. Therefore, we made LexA constructs with phosphorylation site mutants. These LexA-FoxO1AAA mutant (amino acids 236–655) and LexA-FoxO3a AAA mutant (amino acids 233–673) constructs had increased insulin and forskolin sensitivity, indicating that the Akt phosphorylation sites were not required for insulin or forskolin activation of transcription (Fig. 3B).
Fox transcription factors can be knocked down in T47D cells using siRNA
These experiments suggest that Fox factors could mediate the increase in PAI-1 gene expression that we have observed in several cell types. Although FoxO1 and FoxO3a most consistently induced PAI-1 in disparate cell lines, our data do not discriminate between the Fox proteins because we have observed some level of stimulation by all of them except for FoxC2 and FoxP1. Therefore, an RNA interference protocol was adopted to discriminate among these factors. Double-stranded RNA was obtained from Ambion (Austin, TX) or Dharmacon (Lafayette, CO) that targeted FoxO1, FoxO3a, FoxC1, FoxC2, and FoxP1, which were the most abundant Fox proteins found in endothelial cells. After 48 h with siRNA, the cells were harvested and total RNA was prepared and reverse transcribed. The relative mRNA levels of the various FOX proteins were then determined. The siRNA treatment knocked down each of the Fox mRNAs by 40–65% (Table 1). Because only a fraction of the cells are transiently transfected with siRNA, this likely represents a total block in transfected cells.
Table 1.
Fox mRNA levels in T47D cells treated with siRNA targeting Fox proteins
| FoxO1 mRNA | FoxO3a mRNA | FoxO4 mRNA | FoxC1 mRNA | FoxC2 mRNA | FoxP1 mRNA | |
|---|---|---|---|---|---|---|
| FoxO1 KD | 41 ± 3.4 | 84 ± 5 | 80 ± 6 | 105 ± 9 | 108 ± 12 | 99 ± 8.7 |
| FoxO3a KD | 86 ± 7.9 | 34 ± 4.2 | 95 ± 9.1 | 95 ± 10.7 | 117 ± 12.5 | 95 ± 9 |
| FoxO4 KD | 83 ± 9 | 93 ± 9.5 | 40 ± 3.6 | 104 ± 9.9 | 100 ± 11 | 117 ± 11 |
| FoxC1 KD | 93 ± 10.4 | 106 ± 10.9 | 106 ± 11.2 | 43 ± 3.9 | 83 ± 8.9 | 111 ± 10.7 |
| FoxC2 KD | 120 ± 13.1 | 110 ± 12.5 | 93 ± 8.7 | 89 ± 9.4 | 44 ± 4.4 | 101 ± 9.6 |
| FoxP1 KD | 95 ± 10.2 | 108 ± 11.7 | 114 ± 13 | 114 ± 10.3 | 123 ± 14 | 46 ± 5 |
T47D cells were treated with siRNA for the Fox factor indicated in the left column. RNA was prepared and reverse transcribed as described in Materials and Methods. The mRNA levels for the Fox transcription factors were quantified by qPCR. All reactions were standardized to the level of HPRT in the sample and are expressed as a % (±sem) of the level of that mRNA in cells treated with scrambled siRNA. KD, knockdown.
The level of Fox protein is also dependent on the half-life of the protein. Therefore, the effect of the siRNA treatment on the protein levels was determined by Western blot (Fig. 4A). All of the siRNAs were effective at reducing the respective protein levels. The siRNAs for FoxO1 and FoxO4, however, tended to be somewhat nonspecific in that they reduced the level of both proteins.
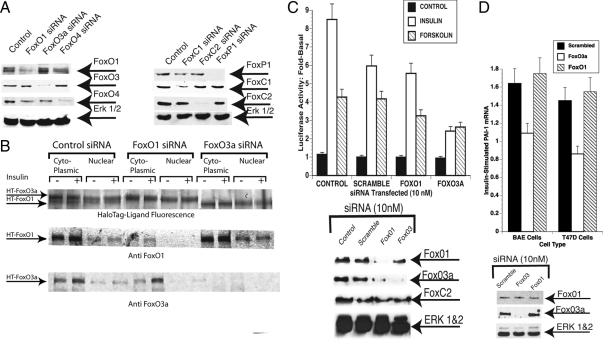
Figure 4.
Knockdown of Fox proteins with siRNA. A, T47D cells were treated with 10 nm siRNA to FoxO1, FoxO3a, or FoxO4 (Ambion; left) or with 20 nm smart pool siRNAs for FoxC1, FoxC2, or FoxP1 (Dharmacon; right) for 72 h. Whole-cell lysates prepared and analyzed by SDS-PAGE were blotted and probed with antibodies to FoxO1, FoxO3a, or FoxO4 (left) or with antibodies to FoxP1, FoxC1, and FoxC2. (right). B, T47D cells were transfected with HT-FoxO1 and HT-FoxO3a and simultaneously treated with 10 nm scrambled siRNA, FoxO1 siRNA, or FoxO3a siRNA. The cells were retreated with siRNA at 24 h and incubated for 20 min with 1 μg/ml insulin at 48 h. The plates were washed and nuclear and cytoplasmic extracts were prepared (Materials and Methods). Top row, The extracts were incubated for 30 min with HaloTMR Ligand, and proteins were resolved by SDS-PAGE and visualized using the Typhoon Trio laser scanner. (middle and bottom rows) Separate SDS-PAGE analysis of the same extracts using anti-FoxO1 (middle row) and anti-FoxO3a (bottom row). In this experiment, secondary antibody was infra red (800 nm) tagged and visualized using a Li-Cor Odyssey infrared scanner. C, Effect of knockdown of FoxO3a on PAI-1 promoter activity. T47D cells were treated with 10 nm siRNA for FoxO1, FoxO3a, or with a scrambled siRNA or were left untreated. After 48 h with siRNA, the cultures were retreated with siRNA and transfected with PAI-1-Luc using lipofectamine 2000. The cells were then treated with 1 μg/ml insulin or 1 mm forskolin for 20 h. Luciferase activity was determined and normalized with β-galactosidase. The fold-stimulation by insulin or forskolin was then determined. Top, Luciferase activity; bottom, Fox protein levels were determined by Western blot. D, Treatment of BAE and T47D cells with siRNA for FoxO3a inhibits insulin-increased PAI-1 mRNA. BAE or T47D cells were treated with 10 nm siRNA for FoxO1, FoxO3a, or scrambled siRNA as a control as in panel A. They were re-treated at 48 h, and 1 μg/ml of insulin was added to half of the cultures for an additional 24 h. The cells were lysed and total RNA was prepared and reverse transcribed as described in Materials and Methods. PAI-1 mRNA levels were then determined by RT-qPCR and normalized to GAPDH mRNA levels. The fold stimulation by insulin of PAI-1 mRNA in the cells treated with scrambled, FoxO1, and FoxO3a siRNA in BAE cells (left) and T47D cells (right) is shown. Bottom, Knockdown of FoxO3a in BAE cells was confirmed by Western blotting of whole-cell extracts from BAE cells treated with the different siRNAs (anti-FoxO1, top row; anti-FoxO3a, middle row; and anti-ERK 1 and 2, bottom row).
The distribution of FoxOs might also be affected by treatment with siRNA. To address this issue, we performed the experiment shown in Fig. 4B. Plasmids for expression of HT-FoxO1 and HT-FoxO3a were transfected into T47D cells and simultaneously treated with scrambled siRNA, FoxO1 siRNA, or FoxO3a siRNA. One plate from each group was insulin treated for 20 min, the other plate was kept as a control, and nuclear and cytoplasmic extracts were prepared. The extracts were incubated for 30 min with fluorescent HaloTMR Ligand, and the proteins were resolved on SDS-PAGE. The gels were then imaged using the Typhoon Trio laser scanner. In the lanes from the scrambled siRNA-treated cells, two HT bands are present in each lane. The top band is HT-Tagged FoxO3a, and the faster migrating band is HT-FoxO1. The HT-FoxO3a is equally distributed in the control and insulin-treated cytoplasmic lanes and in the control and insulin-treated nuclear lanes. The HT-FoxO1 is increased in the insulin-treated cytosol and decreased in the insulin-treated nuclei (more evident in the FoxO3a siRNA lanes where interference from HT-FoxO3a is not present). The FoxO1 siRNA completely eliminated the more rapidly migrating HT-FoxO1 band, and the FoxO3a siRNA completely eliminated the HT-FoxO3a band. Western blotting with specific antibodies to FoxO1 and FoxO3a give essentially the same results. Thus, knockdown seems to be specific and complete in those cells that are transiently transfected with the siRNA. Moreover, the relative abundance/distribution of one Fox protein does not seem to be significantly affected by knockdown of the other.
Knockdown of Fox with siRNA inhibits insulin-increased PAI-1-Luc expression
Next we tested whether the knockdown of Fox factors with siRNA could block insulin-increased PAI-1-Luc expression. T47D cells were treated with various siRNAs and incubated for 48 h. The cells were treated again with siRNA and then transfected with PAI-1-Luc 3 h later. Insulin or forskolin was added, and luciferase activity was determined 20 h later. Knockdown of FoxC1, FoxC2, and FoxP1 did not reduce insulin-increased PAI-1-luciferase activity (data not shown). Insulin-increased PAI-1-Luc was also indistinguishable from control (scrambled) in the FoxO1/O4 knockdown cells (Fig. 4C). The knockdown of FoxO3a was the only treatment that was effective in reducing the level of insulin-increased PAI-1-Luc expression (Fig. 4C). Western blotting verified that the level of FoxO3a was reduced by this treatment (Fig. 4C). In this experiment, the siRNA to FoxO3a also reduced the level of FoxO1, but this was not significant because the greater reduction of FOXO1 in FoxO1 knockdowns had no effect on PAI-1-Luc activity. The issue of whether FoxO3a can mediate the effect of cAMP is also unresolved in these studies. Although there was a slight reduction of forskolin-induced PAI-1-Luc expression, it was not significant because the stimulation by forskolin was not as great as that induced by insulin.
Knockdown of Fox with siRNA inhibits insulin-increased PAI mRNA accumulation
Analysis by qRT-PCR had demonstrated that insulin was able to activate the endogenous PAI-1 promoter in T47D and BAE cells (data not shown). BAE and T47D cells were treated with 10 nm siRNA to FoxO3a or scrambled siRNA using Hiperfect. After 48 h, they were treated again with siRNA, and 1 μg/ml insulin was added to half of the cultures. The siRNA to FoxO3a reduced FoxO3a protein levels substantially in BAE cells, whereas the siRNA to human FoxO1 did not reduce bovine FoxO1. Insulin increased PAI-1 mRNA 1.6-fold in BAE cells and 1.5-fold in T47D cells in cells treated with scrambled siRNA (Fig. 4D). There was no response to insulin in either BAE or T47D cells that had been treated with siRNA to FoxO3a. Western blotting of lysates from BAE cells treated with siRNA is shown (Fig. 4D, bottom). These data demonstrate that FoxO3a plays an important role in insulin’s up-regulation of endogenous PAI-1. Because there is some nonspecificity in knocking down FoxO3a in T47D cells and the ineffectiveness of the FoxO1 siRNA in BAE cells, it is not possible to completely rule out a role for FoxO1 in regulation of PAI-1 mRNA levels although it seems unlikely.
FoxO3a is associated with the PAI-1 promoter
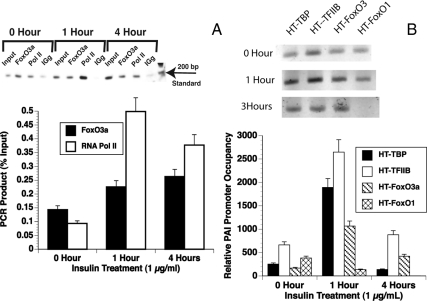
Previous studies showed that the Fox transcription factor FKHR (FoxO1) bound to a synthetic phosphoenol pyruvate carboxykinase/IGF-I binding protein-1 insulin response element (59). We showed that glutathione-S-transferase-FoxO1 bound to the region of the PAI-1 promoter containing the IRS (29). To determine whether FoxO3a associated with the PAI-1 promoter in vivo, ChIP assays were performed (Fig. 5). BAE cells were treated with insulin for 0, 1, or 4 h. The chromatin was cross-linked and precipitated overnight with antibody to FOXO3a (Upstate Biotechnology, Inc., Lake Placid, NY), antibody to RNA polymerase II (Santa Cruz Biotechnology, Inc.), and IgG served as positive and negative controls, respectively. PCR was then performed using primers to the proximal PAI-1 promoter that overlapped the TATA element and insulin response element. Insulin treatment recruits RNA polymerase II to the PAI-1 promoter as is seen in a representative gel image (Fig. 5A, top). Quantitation of three experiments show that RNA polymerase II occupancy of the promoter is increased more than 5-fold in 1 h and remained more than 3.5-fold at 4 h (Fig. 5A). Insulin treatment increased the FoxO3a occupancy of the promoter 2-fold during this time. PCR that used distal primers to a region 800 bp upstream of the TATA element that did not have a FoxO/insulin response element served as a control and had no significant signal (data not shown).
Figure 5.
FoxO3a is present on the PAI-1 promoter in BAE and T47D cells. A, BAE cells were treated with 1 μg/ml insulin for 0, 1, or 4 h. They were then treated with formaldehyde, and the nuclei were prepared and sonicated to produce fragments between 100 and 800 bp as described (Materials and Methods). The sonicated chromatin was precleared with BSA and herring sperm-DNA blocked protein A sepharose and incubated with antibody to FoxO3a (Upstate Biotechnology) or RNA polymerase II (Santa Cruz Biotechnology). The complexes were collected using BSA- and herring sperm-DNA-blocked protein A sepharose. After extensive washing, the samples were de-cross-linked and phenol-chloroform extracted and precipitated with ethanol. PCR with primers to the PAI-1 proximal promoter was performed. Top, SYBR green fluorescence was visualized using the Typhoon Trio laser scanner. Bottom, Quantitation of three experiments using ImageQuant software. B, T47D cells were transfected with plasmids expressing HT-TBP, TFIIB, FoxO1, or FoxO3a fusion proteins. The cells were treated with1 μg/ml insulin for 0, 1, or 4 h. They were then treated with formaldehyde, and the nuclei were prepared and sonicated to produce fragments between 100 and 800 bp as described (Materials and Methods). The sonicated chromatin was incubated with HaloLink resin and the complexes were collected by centrifugation and washed extensively. The samples were de-cross-linked and phenol-chloroform extracted and precipitated with ethanol. PCR with primers to the PAI-1 proximal promoter was performed. Top, SYBR green fluorescence of semiquantitative PCR was visualized using the TyphoonTrio laser scanner. Bottom, Analysis of three experiments using qPCR. The amount of DNA in each sample was determined in comparison with a standard curve. They were then compared with the value for the lowest point (HT-FoxO1 at 4 h) that was arbitrarily set equal to 1. Pol II, Polymerase II.
It is possible that the export of FoxO1 in response to insulin may allow its replacement by FoxO3a, which mediates the insulin activation of this promoter. Thus, the occupancy of the PAI-1 promoter by FoxO1 and FoxO3a in quiescent and insulin-stimulated states remains an important issue. We used the HaloChip system (Promega Corp.) for these experiments because it eliminates uncertainties due to having to use different antibodies that could give variable results depending on their affinity for the denatured and cross-linked proteins. In this system, cells are transfected with fusion proteins expressing a C-terminal HT. This HT can be linked covalently to a resin that has the HaloLigand attached. The covalent binding allows washes of great stringency and eliminates the high background that can be a problem with ChIP assays. T47D cells were transfected with vectors expressing HT-FoxO1 or HT-FoxO3a or HT-TBP or HT-TFIIB (transcription factor IIB) as controls. They were then treated with insulin for 1 or 4 h or left untreated as controls. They were then processed for ChIP as described in Materials and Methods. The cross-linked protein-DNA complexes were isolated by incubation with HaloLink resin overnight at 4 C. The resulting DNA was then subjected to semiquantitative PCR (Fig. 5B, top) but this necessitated using different numbers of cycles and gels for the different time points. Therefore, the samples were reanalyzed by qPCR (Fig. 4B, bottom). All of the proteins were present on the PAI-1 promoter at low levels in the quiescent state. Insulin incubation increased the levels of HT-TBP, HT-TFIIB, and HT-FoxO3a whereas levels of HT-FoxO1 were reduced. At 4 h, the level of HT-TBP had returned to basal levels whereas HT-TFIIB and HT-FoxO3a were still significantly elevated. However, HT-FoxO1 was reduced to background at 4 h. This is consistent with the hypothesis that the HT-FoxO1 has left the nucleus in response to insulin-mediated phosphorylation and has been replaced by the insulin-stimulated HT-FoxO3a. Separate experiments demonstrated that levels of FoxC1 (data not shown) associated with the PAI-1 promoter were not significantly higher than background and that insulin had no effect on this association. Thus, we considered it to be nonspecific.
Discussion
These results demonstrate that FoxO3a likely mediates insulin-increased transcription of PAI-1. Transfection experiments with Fox expression vectors (Fig. 1) demonstrated that the FoxO3a transcription factor was unique in the Fox family in activating PAI-1-Luc expression in response to insulin. FoxP3 (data not shown) and FoxC2 inhibited PAI-1 luciferase expression whereas FoxP1 and FoxP2 had no significant effect. This was confirmed in experiments with chimeric proteins composed of the LexA DNA-binding domain and the activation domain of the various Fox family members (Fig. 3). The siRNA-mediated FoxO3a knockdown (Fig. 4) reduced PAI-1-luc expression whereas knockdown of FoxO1, FoxO4, FoxC1, FoxC2, and FoxP1 had no effect. Lastly, ChIP analysis of the PAI-1 promoter (Fig. 5) demonstrated insulin-sensitive association of FoxO3a with the PAI-1 promoter. These results indicated that only FoxO3a, among the Fox family members tested, had the characteristics expected for the transcription factor that mediated insulin-increased PAI-1 gene expression.
The role of FoxO3a to mediate insulin-increased PAI-1 transcription surprised us because many studies have shown negative regulation of gene transcription by insulin mediated through FoxO1 (47,57,58,61) whereas insulin-mediated effects on transcription through FoxO3a are not well characterized. Further, phosphorylation of FoxOs by protein kinase B/Akt resulted in translocation of the phosphorylated form of FoxO1, FoxO3a, or FoxO4 from the nucleus into the cytoplasm where it was bound by 14–3-3 proteins (47,48). Our results generally support this analysis for FoxO1 (Figs. 2 and 4). However, significant amounts of phosphorylated FoxO3a were present in the nucleus. This suggested that FoxO3a could mediate the positive effects of insulin on the transcription of the PAI-1 promoter that we observed. It also suggests that differential modulation of FoxO proteins by insulin might be important to the regulation of many genes with insulin-mediated phosphorylation of FoxO1 producing an inhibition whereas FoxO3a could activate genes in response to insulin. Further investigation, to determine how the FoxOs regulate transcription and how they interact with other factors, will be needed to understand these important differences among the FoxO transcription factors.
These results might be expected in transformed cells such as the GH4 and T47D cells used in many of these experiments because aberrant retention of FoxO proteins in the nucleus has been proposed as one of the reasons for the survival of transformed cells (62). However, a similar pattern of FoxO1 and FoxO3a distribution was also observed in primary BAE cells (Fig. 2, A and B) and HUVECs (data not shown).
How insulin regulates PAI-1 through FoxO3a is not resolved by these studies. The experiments in Figs. 1 and 3 indicate that the Akt-dependent phosphorylation sites of FoxO3a are not important for insulin regulation of PAI-1. FoxO3a and LexA-FoxO3a proteins with mutations of the Akt phosphorylation sites were more effective at enhancing insulin-increased PAI-1 expression than were the wild-type proteins. This could be due to their higher concentrations in the nucleus because they do not translocate to the cytoplasm in response to insulin (Fig. 2). FoxO3a can be phosphorylated by many other kinases, including serum- and glucocorticoid-regulated kinase (63), Jun N-terminal kinase, ERK (64), p38 (64), dual specificity tyrosine-phosphorylated and regulated kinase (65), AMP-dependent kinase (66), cyclin-dependent kinase 2 (67), and Ikb kinase (68). Phosphorylation by one or more of these kinases in response to insulin is possible, and phosphorylation of FoxO3a by AMP-dependent kinase did not cause nuclear export (66) although FoxO1 was reported to behave differently in response to AMP-activated protein kinase phosphorylation (69).
FoxO3a can also be acetylated, and the acetylation alters the affinity of FoxO3a for some sites (70), increasing FoxO transactivation of some promoters (71). It is possible that acetylation shifts FoxO3a from one site to another or that it increases the affinity of FoxO3a for sites in the PAI-1 promoter. Previous studies suggested that at least two Fox-responsive sequences are present in the PAI-1 promoter (29,72), and our sequence analysis of the PAI-1 promoter revealed a third consensus site between −462/−445 (Stanley, F.M., unpublished analysis). Preliminary investigation of this site suggested that it could be an inhibitory site (Jag, U.R., and F.M. Stanley, unpublished). In this case, the acetylation-mediated shift of FoxO3a from one site to another on the promoter could activate it. Alternately, a model was proposed in Caenorhabitis elegans where Sir2.1 complexed with 14–3-3 and phosphorylated Daf-16 (73). This complex remained in the nucleus and activated transcription of a subset of genes. Thus, some models support the retention of FoxO3a in the nucleus and its increased activation of some promoters.
The PAI-1 promoter contains numerous response elements, and the aggregate response of the promoter is likely to result from the interplay of numerous transcription factors, both positive and negative. The insulin-response element is part of a complex that also includes an AP-1 response element, and this composite response element is sensitive to cAMP, phorbol esther, and oxidative stress (74). This element binds AP-1 in vitro (74), but it has not been determined what binds to this site in vivo. Other candidates for binding this element are cAMP response element binding protein, activating transcription factor, or CCAAT enhancer binding protein family of transcription factors as well as AP-1 (Fos/Jun). It is possible that the interaction between the Fox-related factor binding at the IRS and the factor binding at the AP-1 response element is critical. The regulation at this site would then depend on protein-protein interaction as well as protein-DNA interaction. The interaction of FoxA2 with pregnane X receptor and of FoxO1 with the peroxisome proliferator-activated receptor γ provides experimental evidence for the importance of protein-protein interactions in Fox biology (75,76). These types of interactions could account for the retention of FoxO3a in the nucleus and for the insulin activation of some promoters through FoxO3a.
The PAI-1 promoter also contains sites for SP-1 (77,78) and Ets (79) transcription factors that were shown to be important. Our previous studies in GH4 cells tended to rule out participation of these factors in insulin-increased PAI-1 expression (29), but they might be important for basal PAI-1 transcription or for other responses. Now that FoxO3a has been identified as the insulin-responsive transcription factor, its interaction with other transcription factors to mediate responses of the PAI-1 promoter can be determined.
Materials and Methods
Materials
Restriction enzymes were obtained from New England Biolabs (Beverly, MA) and were used as recommended. Oligonucleotides were from Operon Technologies (Huntsville, AL), and reagents for PCR were obtained from Roche (Indianapolis, IN). DMEM containing 4.5 g/liter glucose and iron-supplemented calf serum were obtained from Hyclone Laboratories (Logan, UT). All other reagents were of the highest purity available and were obtained from Sigma (St. Louis, MO), Bio-Rad Laboratories (Hercules, CA), Fisher Scientific (Pittsburgh, PA), or Calbiochem (La Jolla, CA).
Cell culture
GH4 pituitary tumor cells, T47D cells, HEK293 cells, and HeLa cells were maintained in DMEM with 10% iron-supplemented calf serum. Human umbilical vein endothelial cells (HUVEC) and human aortic endothelial cells were obtained from Lonza (Walkerville, MD) and were maintained in EGM2. BAE cells were the gift of Dr. M. Yorick (University of Iowa, Iowa City, IA) and were maintained in DMEM with 10% iron-supplemented calf serum. All experiments were done in medium containing charcoal-treated serum that was shown to be hormone and growth factor depleted.
Plasmids
The PAI-1 promoter reporter plasmid, p800neo-Luc, was the generous gift of Dr. D. Rifkin (New York University School of Medicine, New York, NY) (80). Flag-FoxO1 wild-type, Flag-FoxO1AAA, and IRS(3×)-Luc were the gift of T. Untermann (University of Chicago School of Medicine, Chicago, IL). The IRS(3×)-Luc plasmid has three repeats of the IGF-I binding protein 1 promoter and was previously shown to be activated by FoxO1 in HEK293 cells (81). Flag FoxO3a wild type and AAA were made by M. Greenberg and obtained from Addgene, Inc. (Cambridge, MA). Cytomegalovirus-FoxC1 and cytomegalovirus-FoxC2 were a gift of T. Kume (Vanderbilt University, Nashville, TN). FoxP1 and FoxP2 were provided by Dr. E. Morrisey (University of Pennsylvania, Philadelphia, PA) and FoxP3 was a gift of Dr. M. Greene (University of Pennsylvania). LexA chimeras of FoxO1, FoxO3a, FoxC1, FoxC2, and FoxP1 were made by cloning PCR fragments into pcDNA3-LexA as previously described (82). The human insulin receptor expression vector, pRT3HIR2, was the gift of Dr. J. Whittaker (Case Western Reserve University, Cleveland, OH).
Antibodies used
Phosphorylation state-specific antibodies were obtained from Cell Signaling Technology (Beverly, MA) and included antiphospho-FoxO1(Ser256) (no. 9461), antiphospho-FoxO1(Ser319) (no. 2486), antiphospho-FoxO3a(Ser318/321)(no. 9465), and antiphospho-FoxO1(Ser 319)/O4(Ser262) (no. 2487). Some studies also used anti-FoxO1 (no. 9462) that recognizes both FoxO1 and FoxO4 and anti-FoxO3a (no. 9467) from Cell Signaling Technology, but the anti-FoxO1 (sc11350) from Santa Cruz Biotechnology and anti FoxO3a (07–702) from Upstate Biotechnology were used in most of these studies. Anti-FoxC1 (ab5079), anti-FoxC2 (ab24340), and anti-FoxP1 (ab16645) were from Abcam, Inc. (Cambridge, MA) (as was the antibody to TBP (ab818). The antihuman insulin receptor was a gift from Dr. K. Siddle (Cambridge, UK), and the anti-ERK1 and ERK2 were from Santa Cruz (sc93 and sc154). Flag monoclonal antibody was from Sigma and anti-GFP polyclonal was from Molecular Probes (Eugene, OR).
Transient gene transfection facilitated by electroporation
Electroporation experiments and reporter assays were performed as described elsewhere (83). GH4 cells were harvested with an EDTA solution, and 6 × 106 cells were used for each electroporation. Trypan blue exclusion before electroporation ranged from 95–99%. The voltage of the electroporation was 1550 V. This resulted in trypan blue exclusion of 70–80% after electroporation. The transformed cells were plated in 96-well dishes (Falcon Plastics, Brookings, SD) at 1 × 105 cells per well in DMEM with 10% hormone-depleted serum. The cells were allowed to attach, and hormones were added for 24 h. The medium was replaced with assay buffer, and the plates were frozen at −80 C. Luciferase assays were performed using reagents and protocols from Promega.
Control of transfection efficiency was performed using an rous sarcoma virus-β-galactosidase expression plasmid (0.5 μg/electroporation) (84). The β-galactosidase activity in the cell lysates was determined using Beta Glo (Promega). Transfection efficiency did not vary significantly among transfections performed at the same time. The relative light units (RLU) of luciferase activity were then corrected for minor variations in β-galactosidase activity by converting the RLU to RLU/β-galactosidase activity/mg protein. The fold stimulation or inhibition was then determined.
Preparation of cytoplasmic and nuclear extracts and Western immunoblot analysis
GH4 cells were harvested in an hypotonic buffer consisting of 10 mm HEPES (pH 7.5), 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm Na3VO4, 1 mm (NH4)6Mo7O24, 10 mm NaF, 10 mm NaP2O7, 1 mm dithiothreitol, 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride, and 10 μg/ml aprotinin. They were allowed to swell on ice for 10 min and lysed by addition of Nonidet P40 to a final concentration of 0.5%. The nuclei were collected by centrifugation at 1000 × g for 5 min at 4 C and washed once. They were then extracted with a buffer containing 20 mm HEPES, pH 7.5, 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 1 mm (NH4)6Mo7O24, 10 mm NaF, 10 mm NaP2O7, 1 mm dithiothreitol, 1 mm AEBSF, and 10 μg/ml aprotinin. The samples were vortexed for 15 sec at the highest setting and then placed on ice. The process was repeated four times. The nuclear extract was collected after a 10 min centrifugation at 14,000 × g. Equal amounts of protein were analyzed by SDS-PAGE using 10% gels. The proteins were transferred to nitrocellulose membranes (Micron Separations, Westborough, MA) and immunoblotted using primary antibodies described in the figure legends and horseradish peroxidase-conjugated secondary antibodies (Pierce) and enhanced chemiluminescence (Pierce). Films were scanned using a Molecular Dynamics (Amersham) densitometer with ImageQuant software.
Cell staining and fluorescence microscopy
T47D cells were inoculated onto coverslips at a density of 100,000 cells per coverslip. The media was exchanged at 24 h, and the cells were transfected using cationic lipids in serum-free medium for 6 h. The serum-free medium was replaced with medium containing 10% charcoal-treated serum (hormone depleted). They were treated with insulin for 20 min or 1 h or left untreated as controls. They were fixed using 4% paraformaldehyde in PBS and permeabilized with 0.1% Triton X-100. The cells were then stained with 50 μm HT TMR ligand for 30 min at room temperature (RT) and washed with PBS to remove excess ligand. They were then stained with 4′,6-diamidino-2-phenylindole and mounted using Mowiol. The slides were photographed using a Zeiss Axio fluorescence microscope and Openlab software (Carl Zeiss, Thornwood, NY). Adobe Photoshop was used for postprocessing.
RT-qPCR of PAI-1
Total RNA was prepared using Purescript (Gentra Systems, Minneapolis, MN). The amount of RNA was estimated from the absorbance at 260/280 nm using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Total RNA was reverse transcribed using Superscript III (Invitrogen, Carlsbad, CA). Each reaction used 10 μg of total RNA and oligo dT primers. The cDNA was then amplified using the ABI7900 (ABI, Foster City, CA). Reactions for qPCR in 384-well plates typically contained 0.1 ng of cDNA, 200 nm each primer, and 5 μl SYBR Green Mix in 10 μl reaction. Primers for qPCR were designed using Primer 3 (Ref. 60; source code available at http://primer3.sourceforge.net) and in all cases spanned an intron/exon border. Controls included GAPDH, hypoxanthine phosphoribosyl transferase, and M2B. Primer sequences are available upon request.
Knockdown of FOX transcription factors with siRNA
T47D cells were seeded at 1 × 106 cells in 12-well plates. Duplex RNA was mixed with Hiperfect (QIAGEN, Valencia, CA) in serum- and antibiotic-free medium according to the instructions of the manufacturer. The liposome/RNA complexes were then added to the cells. T47D cells were treated with 10 nm siRNA to FoxO1, FoxO3a, or FoxO4 (Ambion) or with 20 nm smart pool siRNAs for FoxC1, FoxC2, or FoxP1 (Dharmacon). Treatment was typically for 72 h. Scrambled RNA and RNA interference for GAPDH constituted positive and negative controls. Sequences of siRNA were: FoxO3a, CCUGUCACUGCAUAGUCGAtt, UCGACUAUGCAGUGACAGGtt; FoxO1, GGCAUCUCAUAACAAAAUGtt, CAUUUUGUUAUGAGAUGCCtg; FoxO4, UCUCACCUCUUCCCAUUCCtt, GGAAUGGGAAGAGGUGAGAtt.
ChIP to determine Fox association with the PAI-1 promoter in vivo
ChIP experiments were performed as described elsewhere(85). Briefly, T47D cells were incubated with insulin, and the cells were fixed by adding formaldehyde to the medium at a final concentration of 1%. After 10 min at RT, the reaction was quenched with 0.125 m glycine. Nuclei were then prepared and sonicated 15 times for 15 sec each. The samples were made 1% Triton X-100, and the size and amount of the fragments were assessed on an agarose gel after de-cross-linking. The chromatin was precleared using protein A agarose that was blocked with 1 mg/ml sonicated salmon sperm DNA and 1 mg/ml BSA. The chromatin was incubated overnight with 2 μg of specific antibody at 4 C. The antibody-bound chromatin was then collected by incubating with blocked protein A/G beads for 3 h at 4 C. The beads were collected by centrifugation and washed once. The beads were then treated with RNase and Proteinase K for 3 h at 55 C. Formaldehyde cross-linking was then reversed by incubating the samples at 65 C overnight and then treating them again with RNase and Proteinase K. The samples were extracted twice with phenol chloroform and once with chloroform and precipitated with 70% ethanol using glycogen as a carrier. ChIP chromatin was then subjected to PCR analysis to determine whether PAI-1 promoter elements were isolated by this immunoprecipitation. Forward and reverse primers were designed using the Primer 3 program (see Acknowledgments). Primers were designed that would amplify a section of the PAI promoter that included the insulin response element at −52/−42 and to a distal region of the promoter that did not contain an IRS (control). PCRs were sampled at 20 and 25 cycles to ensure that all samples would be on a linear section of the curve. Sequences for the primers are as follows.
PAI (distal forward): GGGACCATCTAGTTGCAGGA; Pai (distal reverse), GGGACTGGTTTCATGGAAGA;
PAI (proximal forward), AGTCCCAGAGAGGGAGGTGT; Pai (proximal reverse), TCTTCTTGACAGCGCTCTTG.
HaloChip analysis of transcription factor binding
T47D cells in 100-mm plates were transfected with plasmids that expressed HT-TFIIB, FoxO3a, or FoxC1 fusion proteins. The plates were refed with hormone-depleted serum-containing medium for 20 h and incubated with insulin for 1 or 3 h or without insulin as controls. The plates were treated with formaldehyde, nuclei were prepared, and chromatin was sonicated as described above. The sonicated chromatin was then incubated with HaloLink resin for 2 h at RT. The HT protein is covalently bound to the resin during the incubation. The complexes were then washed four times with high-salt buffer (700 mm NaCl) and four times with H2O for 10 min each at RT. The cross-linked chromatin was recovered, purified, and analyzed by PCR essentially as described above for conventional ChIP. Some experiments were also repeated using qPCR (Fig. 5B). A SyberGreen master mix (ABI) was used with the same primers that were used as for semiquantitative PCR in a Bio-Rad iCycler. A total of 45 cycles were performed although all samples amplified between 22 and 35 cycles. A standard curve was created using sonicated T47D cell DNA, and curves from experimental samples were compared with this curve.
Acknowledgments
We thank Dr. R. Brent (Harvard University, Cambridge, MA), Dr. M. Greene (Univeristy of Pennsylvania), Dr. Kume (Vanderbilt University), Dr. B. Li (University of Pennsylvania), Dr. E. Morrisey (University of Pennsylvania), Dr. Rifkin (New York University School of Medicine), Dr. T. Untermann (University of Chicago), and Dr. J. Whittaker (Case Western Reserve) for plasmids used in these studies. We thank Dr. Lawrence B. Gardner (New York University) for his helpful suggestion on the manuscript. We thank the New York University Cancer Institute Genomics Facility for assistance with RT-qPCR analyses.
Footnotes
This work was supported by Grant DK067540 from the National Institutes of Health. Our preliminary studies were supported by The Diabetes Research and Education Foundation.
Disclosure Summary: F.M.S., J.Z., and U.J. have nothing to declare.
First Published Online July 16, 2009
Abbreviations: AP-1, Activator protein 1; BAE, bovine aortic endothelial; ChIP, chromatin immunoprecipitation; Fox, Forkhead-related transcription factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; HEK, human embryonic kidney; HT, Halo Tag; HUVEC, human umbilical vein endothelial cell; IRS, insulin response sequence; PAI-1, plasminogen activator inhibitor-1; qPCR, quantitative PCR; RLU, relative light units; RT, room temperature; siRNA, small interfering RNA; TBP, TATA-binding protein; TFIIB, transcription factor IIB.
References
- Samad F, Loskutoff DJ 1996 Tissue distribution and regulation of plasminogen activator inhibitor-1 in obese mice. Mol Med 2:568–582 [PMC free article] [PubMed] [Google Scholar]
- Agrenius V, Chmielewska J, Widström O, Blombäck M 1989 Pleural fibrinolytic activity is decreased in inflammation as demonstrated in quinacrine pleurodesis treatment of malignant pleural effusion. Am Rev Respir Dis 140:1381–1385 [DOI] [PubMed] [Google Scholar]
- Marutsuka K, Woodcock-Mitchell J, Sakamoto T, Sobel BE, Fujii S 1998 Pathogenetic implications of hyaluronan-induced modification of vascular smooth muscle cell fibrinolysis in diabetes. Coron Artery Dis 9:177–184 [DOI] [PubMed] [Google Scholar]
- Padró T, Emeis JJ, Steins M, Schmid KW, Kienast J 1995 Quantification of plasminogen activators and their inhibitors in the aortic vessel wall in relation to the presence and severity of atherosclerotic disease. Arterioscler Thromb Vasc Biol 15:893–902 [DOI] [PubMed] [Google Scholar]
- Lau HK 1999 Regulation of proteolytic enzymes and inhibitors in two smooth muscle cell phenotypes. Cardiovasc Res 43:1049–1059 [DOI] [PubMed] [Google Scholar]
- Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB 1990 Characterization of the activation of latent TGF-β by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol 111:757–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJ, Kim KS, Chen YQ, Blevins LS, Nadeau JH, Meranze SG, Vaughan DE 2000 Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab 85:336–344 [DOI] [PubMed] [Google Scholar]
- Samad F, Yamamoto K, Loskutoff DJ 1996 Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-α and lipopolysaccharide. J Clin Invest 97:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell KA, Ren S, Sun J, Angel A, Shen GX 1995 Effect of thrombin on release of plasminogen activator inhibitor-1 from cultured primate arterial smooth muscle cells. Thromb Res 77:119–131 [DOI] [PubMed] [Google Scholar]
- Dichtl W, Stiko A, Eriksson P, Goncalves I, Calara F, Banfi C, Ares MP, Hamsten A, Nilsson J 1999 Oxidized LDL and lysophosphatidylcholine stimulate plasminogen activator inhibitor-1 expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 19:3025–3032 [DOI] [PubMed] [Google Scholar]
- Gallicchio M, Hufnagl P, Wojta J, Tipping P 1996 IFN-gamma inhibits thrombin- and endotoxin-induced plasminogen activator inhibitor type 1 in human endothelial cells. J Immunol 157:2610–2617 [PubMed] [Google Scholar]
- Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H 1998 Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients. A potential factor predisposing to thrombosis and its persistence. Circulation 97:2213–2221 [DOI] [PubMed] [Google Scholar]
- Samad F, Pandey M, Bell PA, Loskutoff DJ 2000 Insulin continues to induce plasminogen activator inhibitor 1 gene expresssion in insulin-resistant mice and adipocytes. Mol Med 6:680–692 [PMC free article] [PubMed] [Google Scholar]
- Nordt TK, Sawa H, Fuji S, Sobel BE 1995 Induction of plasminogen activator inhibitor type-1 (PAI-1) by proinsulin and insulin in vivo. Circulation 91:764–770 [DOI] [PubMed] [Google Scholar]
- Carmassi F, Morale M, Ferrini L, Dell'Omo G, Ferdeghini M, Pedrinelli R, De Negri F 1999 Local insulin infusion stimulates expression of plasminogen activator inhibitor-1 and tissue-type plasminogen activator in normal subjects. Am J Med 107:344–350 [DOI] [PubMed] [Google Scholar]
- Nordt TK, Sawa H, Fujii S, Bode C, Sobel BE 1998 Augmentation of arterial endothelial cell expression of the plasminogen activator inhibitor type-1 (PAI-1) gene by proinsulin and insulin in vivo. J Mol Cell Cardiol 30:1535–1543 [DOI] [PubMed] [Google Scholar]
- Nordt TK, Schneider DJ, Sobel BE 1994 Augmentation of the synthesis of plasminogen activator inhibitor type-1 by precursors of insulin. A potential risk factor for vascular disease. Circulation 89:321–330 [DOI] [PubMed] [Google Scholar]
- Grenett HE, Benza RL, Fless GM, Li XN, Davis GC, Booyse FM 1998 Genotype-specific transcriptional regulation of PAI-1 gene by insulin, hypertriglyceridemic VLDL, and Lp(a) in transected, cultured human endothelial cells. Arterioscler Thromb Vasc Biol 18:1803–1809 [DOI] [PubMed] [Google Scholar]
- Grenett HE, Benza RL, Li XN, Akens ML, Grammer JR, Brown SL, Booyse FM 1999 Expression of plasminogen activator inhibitor type 1 in genotyped human endothelial cell cultures: genotype-specific regulation by insulin. Thromb Haemost 82:1504–1509 [PubMed] [Google Scholar]
- Chen YQ, Su M, Walia RR, Hao Q, Covington JW, Vaughan DE 1998 Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem 273:8225–8231 [DOI] [PubMed] [Google Scholar]
- Motojima M, Ando T, Yoshioka T 2000 Sp1-like activity mediates angiotensin-II-induced plasminogen-activator inhibitor type-1 (PAI-1) gene expression in mesangial cells. Biochem J 349:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman MA, Hagood JS, Simmons WL, Fuller GM, Vinson C, White KE 1999 Fibrin fragment induction of plasminogen activator inhibitor transcription is mediated by activator protein-1 through a highly conserved element. Blood 94:2029–2038 [PubMed] [Google Scholar]
- Arts J, Grimbergen J, Toet K, Kooistra T 1999 On the role of c-Jun in the induction of PAI-1 gene expression by phorbol ester, serum, and IL-1α in HepG2 cells. Arterioscler Thromb Vasc Biol 19:39–46 [DOI] [PubMed] [Google Scholar]
- Knudsen H, Olesen T, Riccio A, Ungaro P, Christensen L, Andreasen PA 1994 A common response element mediates differential effects of phorbol esters and forskolin on type-1 plasminogen activator inhibitor gene expression in human breast carcinoma cells. Eur J Biochem 220:63–74 [DOI] [PubMed] [Google Scholar]
- Song CZ, Siok TE, Gelehrter TD 1998 Smad4/DPC4 and Smad3 mediate transforming growth factor-β (TGF-β) signaling through direct binding to a novel TGF-β-responsive element in the human plasminogen activator inhibitor-1 promoter. J Biol Chem 273:29287–29290 [DOI] [PubMed] [Google Scholar]
- Hua X, Liu X, Ansari DO, Lodish HF 1998 Synergistic cooperation of TFE3 and smad proteins in TGF-β-induced transcription of the plasminogen activator inhibitor-1 gene. Genes Dev 12:3084–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzdzinski CJ, Johnson MR, Goble CA, Winograd SS, Gelehrter TD 1993 Mechanism of glucocorticoid induction of the rat plasminogen activator inhibitor-1 gene in HTC rat hepatoma cells: identification of cis-acting regulatory elements. Mol Endocrinol 7:1169–1177 [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Roth U, Jungermann K 1999 Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood 94:4177–4185 [PubMed] [Google Scholar]
- Vulin AI, Stanley FM 2002 A forkhead/winged helix-related transcription factor mediates insulin-increased plasminogen activator inhibitor-1 gene transcription. J Biol Chem 277:20169–20176 [DOI] [PubMed] [Google Scholar]
- Fujita H, Kang M, Eren M, Gleaves LA, Vaughan DE, Kume T 2006 Foxc2 is a common mediator of insulin and transforming growth factor β signaling to regulate plasminogen activator inhibitor type I gene expression. Circ Res 98:626–634 [DOI] [PubMed] [Google Scholar]
- Accili D 2004 Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes 53:1633–1642 [DOI] [PubMed] [Google Scholar]
- Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH 2004 Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest 114:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O 2004 Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279:41114–41123 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs III WH, Wright CV, White MF, Arden KC, Accili D 2002 The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Kitamura Y, Biggs 3rd WH, Arden KC, Accili D 2003 The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell 4:119–129 [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M 2004 Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature 432:1027–1032 [DOI] [PubMed] [Google Scholar]
- Lee CS, Sund NJ, Vatamaniuk MZ, Matschinsky FM, Stoffers DA, Kaestner KH 2002 Foxa2 controls Pdx1 gene expression in pancreatic β-cells in vivo. Diabetes 51:2546–2551 [DOI] [PubMed] [Google Scholar]
- Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH 2004 Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest 114:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim-Toruner C, Subramanian K, El Manjra L, Chen E, Goldstein S, Vitale E 2004 A novel frameshift mutation of FOXC2 gene in a family with hereditary lymphedema-distichiasis syndrome associated with renal disease and diabetes mellitus. Am J Med Genet 131A:281–286 [DOI] [PubMed] [Google Scholar]
- Carlsson E, Almgren P, Hoffstedt J, Groop L, Ridderstråle M 2004 The FOXC2 C-512T polymorphism is associated with obesity and dyslipidemia. Obes Res 12:1738–1743 [DOI] [PubMed] [Google Scholar]
- Carlsson E, Groop L, Ridderstrale M 2005 Role of the FOXC2–512C>T polymorphism in type 2 diabetes: possible association with the dysmetabolic syndrome. Int J Obes Relat Metab Disord 29:268–274 [DOI] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME 2001 X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27:18–20 [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F 2001 Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27:68–73 [DOI] [PubMed] [Google Scholar]
- Banerjee-Basu S, Baxevanis AD 2004 Structural analysis of disease-causing mutations in the P-subfamily of forkhead transcription factors. Proteins 54:639–647 [DOI] [PubMed] [Google Scholar]
- Bassuny WM, Ihara K, Sasaki Y, Kuromaru R, Kohno H, Matsuura N, Hara T 2003 A functional polymorphism in the promoter/enhancer region of the FOXP3/Scurfin gene associated with type 1 diabetes. Immunogenetics 55:149–156 [DOI] [PubMed] [Google Scholar]
- Gambineri E, Torgerson TR, Ochs HD 2003 Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol 15:430–435 [DOI] [PubMed] [Google Scholar]
- Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T 1999 Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem 274:17184–17192 [DOI] [PubMed] [Google Scholar]
- Cichy SB, Uddin S, Danilkovich A, Guo S, Klippel A, Unterman TG 1998 Protein kinase B/Akt mediates effects of insulin on hepatic insulin-like growth factor-binding protein-1 gene expression through a conserved insulin response sequence. J Biol Chem 273:6482–6487 [DOI] [PubMed] [Google Scholar]
- Dahle MK, Grønning LM, Cederberg A, Blomhoff HK, Miura N, Enerbäck S, Taskén KA, Taskén K 2002 Mechanisms of FOXC2- and FOXD1-mediated regulation of the RI α subunit of cAMP-dependent protein kinase include release of transcriptional repression and activation by protein kinase B α and cAMP. J Biol Chem 277:22902–22908 [DOI] [PubMed] [Google Scholar]
- Grønning LM, Cederberg A, Miura N, Enerbäck S, Taskén K 2002 Insulin and TNF α induce expression of the forkhead transcription factor gene Foxc2 in 3T3-L1 adipocytes via PI3K and ERK 1/2-dependent pathways. Mol Endocrinol 16:873–883 [DOI] [PubMed] [Google Scholar]
- Cederberg A, Grønning LM, Ahrén B, Taskén K, Carlsson P, Enerbäck S 2001 FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106:563–573 [DOI] [PubMed] [Google Scholar]
- Dimova EY, Kietzmann T 2006 The MAPK pathway and HIF-1 are involved in the induction of the human PAI-1 gene expression by insulin in the human hepatoma cell line HepG2. Ann NY Acad Sci 1090:355–367 [DOI] [PubMed] [Google Scholar]
- Marais R, Wynne J, Treisman R 1993 The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 73:381–393 [DOI] [PubMed] [Google Scholar]
- Beaulieu LM, Whitley BR, Wiesner TF, Rehault SM, Palmieri D, Elkahloun AG, Church FC 2007 Breast cancer and metabolic syndrome linked through the plasminogen activator inhibitor-1 cycle. Bioessays 29:1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rena G, Prescott AR, Guo S, Cohen P, Unterman TG 2001 Roles of the forkhead in rhabdomyosarcoma (FKHR) phosphorylation sites in regulating 14–3-3 binding, transactivation and nuclear targetting. Biochem J 354:605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, Dalal SN, DeCaprio JA, Greenberg ME, Yaffe MB 2002 14–3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol 156:817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V, Rechler MM 2003 Characterization of insulin inhibition of transactivation by a C-terminal fragment of the forkhead transcription factor Foxo1 in rat hepatoma cells. J Biol Chem 278:26111–26119 [DOI] [PubMed] [Google Scholar]
- Tsai WC, Bhattacharyya N, Han LY, Hanover JA, Rechler MM 2003 Insulin inhibition of transcription stimulated by the forkhead protein Foxo1 is not solely due to nuclear exclusion. Endocrinology 144:5615–5622 [DOI] [PubMed] [Google Scholar]
- Tang E, Nuñez G, Barr FG, Guan KL 1999 Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 274:16741–16746 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ 2000 Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, eds. Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana Press; 365–386 [DOI] [PubMed] [Google Scholar]
- Nakae J, Park BC, Acilli D 1999 Insulin stimulated phosphorylation of the forkhead transcription factor FKHR on serine 253 through a Wortmannin-sensitive pathway. J Biol Chem 274:15982–15985 [DOI] [PubMed] [Google Scholar]
- Jin GS, Kondo E, Miyake T, Shibata M, Takashima T, Liu YX, Hayashi K, Akagi T, Yoshino T 2004 Expression and intracellular localization of FKHRL1 in mammary gland neoplasms. Acta Med Okayama 58:197–205 [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME 2001 Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A 2007 Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal 19:519–527 [DOI] [PubMed] [Google Scholar]
- Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, Unterman TG, Cohen P 2001 The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J 355:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A 2007 The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282:30107–30119 [DOI] [PubMed] [Google Scholar]
- Huang H, Regan KM, Lou Z, Chen J, Tindall DJ 2006 CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science 314:294–297 [DOI] [PubMed] [Google Scholar]
- Hu MC, Hung MC 2005 Role of IκB kinase in tumorigenesis. Future Oncol 1:67–78 [DOI] [PubMed] [Google Scholar]
- Dixit M, Bess E, Fisslthaler B, Härtel FV, Noll T, Busse R, Fleming I 2008 Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res 77:160–168 [DOI] [PubMed] [Google Scholar]
- Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A 2004 Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA 101:10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME 2004 Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303:2011–2015 [DOI] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T 2006 The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol 294:458–470 [DOI] [PubMed] [Google Scholar]
- Berdichevsky A, Guarente L 2006 A stress response pathway involving sirtuins, forkheads and 14-3-3 proteins. Cell Cycle 5:2588–2591 [DOI] [PubMed] [Google Scholar]
- Vulin AI, Stanley FM 2004 Oxidative stress activates the plasminogen activator inhibitor type 1 (PAI-1) promoter through an AP-1 response element and cooperates with insulin for additive effects on PAI-1 transcription. J Biol Chem 279:25172–25178 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Moore R, Negishi M, Sueyoshi T 2007 Nuclear pregnane X receptor cross-talk with FoxA2 to mediate drug-induced regulation of lipid metabolism in fasting mouse liver. J Biol Chem 282:9768–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Imamura T, Sonoda N, Sears DD, Patsouris D, Kim JJ, Olefsky JM 2009 FOXO1 transrepresses peroxisome proliferator-activated receptor γ transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J Biol Chem 284:12188–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descheemaeker KA, Wyns S, Nelles L, Auwerx J, Ny T, Collen D 1992 Interaction of AP-1-, AP-2-, and Sp1-like proteins with two distinct sites in the upstream regulatory region of the plasminogen activator inhibitor-1 gene mediates the phorbol 12-myristate 13-acetate response. J Biol Chem 267:15086–15091 [PubMed] [Google Scholar]
- Banfi C, Eriksson P, Giandomenico G, Mussoni L, Sironi L, Hamsten A, Tremoli E 2001 Transcriptional regulation of plasminogen activator inhibitor type 1 gene by insulin: insights into the signaling pathway. Diabetes 50:1522–1530 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujii S, Matsumoto A, Goto D, Ishimori N, Watano K, Furumoto T, Sugawara T, Sobel BE, Kitabatake A 2002 Induction of plasminogen activator inhibitor-1 in endothelial cells by basic fibroblast growth factor and its modulation by fibric acid. Arterioscler Thromb Vasc Biol 22:855–860 [DOI] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB 1994 An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216:276–284 [DOI] [PubMed] [Google Scholar]
- Rena G, Guo S, Cichy SC, Unterman TG, Cohen P 1999 Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274:17179–17183 [DOI] [PubMed] [Google Scholar]
- Jacob KK, Stanley FM 2001 Elk-1, C/EBPa, and Pit-1 confer an insulin responsive phenotype on prolactin promoter expression in Chinese hamster ovary cells and define factors required for insulin-increased transcription. J Biol Chem 276:24931–24936 [DOI] [PubMed] [Google Scholar]
- Stanley FM 1989 Transcriptional regulation of prolactin gene expression by thyroid hormone: alternate suppression and stimulation in different GH cell lines. Mol Endocrinol 3:1627–1633 [DOI] [PubMed] [Google Scholar]
- Flug F, Copp RP, Casanova J, Horowitz ZD, Janocko L, Plotnick M, Samuels HH 1987 cis-Acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem 262:6372–6382 [PubMed] [Google Scholar]
- Ren B, Dynlacht BD 2004 Use of chromatin immunoprecipitation assays in genome-wide location analysis of mammalian transcription factors. Methods Enzymol 376:304–315 [DOI] [PubMed] [Google Scholar]