Abstract
IGF-binding protein 3 (IGFBP-3) promotes apoptosis by both IGF-dependent and -independent mechanisms. We have previously reported that phosphorylation of IGFBP-3 (S156) by DNA-dependent protein kinase enhances its nuclear accumulation and is essential for its ability to interact with retinoid X receptor-α and induce apoptosis in cultured prostate cancer cells. Using specific chemical inhibitors and small interfering RNA, we demonstrate that preventing casein kinase 2 (CK2) activation enhanced the apoptotic potential of IGFBP-3. We mapped potential CK2 phosphosphorylation sites in IGFBP-3 to S167 and S175 and identified that wild-type IGFBP-3- and IGFBP-3-S175A-induced apoptosis to a comparable extent. In contrast, IGFBP-3-S167A was far more potently apoptosis inducing due to inability to undergo CK2 phosphorylation. Pretreatment of 22RV1 cells with IGFBP-3 small interfering RNA also limits the ability of high doses of CK2 inhibitor to induce apoptosis. These effects can be reversed by the addition of exogenous IGFBP-3 protein, suggesting reciprocal regulation of cell survival and apoptosis by IGFBP-3 and CK2. These studies reveal multisite phosphorylation of IGFBP-3 that both positively and negatively regulate its apoptotic potential. Understanding such intrinsic regulation of IGFBP-3 action may enhance the development of potential cancer therapies.
Phosphorylation of IGFBP-3 by the oncogenic kinase CK2 inhibits its nuclear localization and ability to induce apoptosis in prostate cancer.
IGF-binding protein 3 (IGFBP-3) is the most abundant of the IGFBPs in serum, where it forms a ternary complex with acid-labile subunit and IGF (1). In this way, it plays a key role in regulating the bioavailability of the IGFs and their ability to interact with the IGF type I receptor. In addition to its role in regulating IGF action, IGFBP-3 is known to exert IGF-independent effects to inhibit cell proliferation and enhance apoptosis in many cell types, including prostate (2) and breast (3,4) cancer.
Extracellular IGFBP-3 is rapidly internalized via transferrin and caveolin and is transported into the nucleus by importin-β (5,6) using its intrinsic nuclear localization signal (7). Once localized to the nucleus, IGFBP-3 can interact with several nuclear receptors including RXRα through which it promotes apoptosis (8). However, IGFBP-3 may function in additional ways to induce apoptosis, because IGFBP-3 lacking a functional NLS is reported to promote apoptosis in breast cancer cells (9), and retinoid X receptor-α (RXRα) is not required for IGFBP-3-induced apoptosis in PC-3 prostate cancer cells (10). However, little is understood about the cellular mechanisms regulating IGFBP-3 action in different tissues that may explain its differing actions.
IGFBP-3 is subject to posttranslational modifications such as glycosylation and proteolysis and also contains consensus phosphorylation sites for a variety of protein kinases. We recently demonstrated that IGFBP-3 can also be phosphorylated by DNA-dependent protein kinase (DNA-PK) and that this phosphorylation event is essential for IGFBP-3-induced apoptosis in human prostate cancer cells (11). In addition, Ser-111 and Ser-113 have been described as phospho-acceptor residues, possibly for CK2 (12,13). Phosphorylation of these sites may affect the ability of IGFBP-3 to become glycosylated, because the S111A/S113A double mutant showed a strongly reduced glycosylation pattern (12).
CK2, a potent suppressor of apoptosis, is a highly conserved and ubiquitously expressed protein kinase whose expression is frequently dysregulated in cancer (14). Because bioinformatic analysis on the IGFBP-3 amino acid sequence revealed multiple putative CK2 phosphorylation sites, we hypothesized that phosphorylation of IGFBP-3 by CK2 may modulate the cellular action of IGFBP-3 in prostate cancer. We identify that CK2-mediated phosphorylation partially inhibits the apoptosis-promoting actions of IGFBP-3. Low-level inhibition of CK2 activity results in reduced IGFBP-3 phosphorylation, enhanced nuclear accumulation, and increased IGFBP-3-mediated c-Jun N-terminal kinase (JNK) phosphorylation. We additionally identify Ser-167 as an IGFBP-3 residue phosphorylated by casein kinase 2 (CK2) and reveal that an S167A-IGFBP-3 mutant has enhanced potency as an apoptotic agent in prostate cancer cells.
Results
Inhibition of CK2 activity results in reduced phosphorylation and increased nuclear localization of IGFBP-3
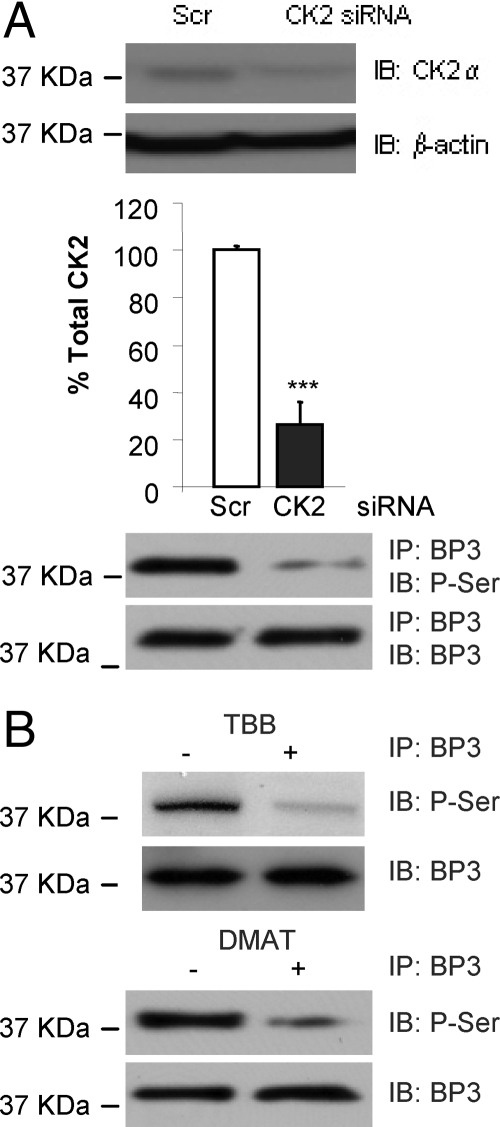
We recently demonstrated that IGFBP-3 can be phosphorylated by DNA-PK and that this phosphorylation event is essential for IGFBP-3-induced apoptosis in cultured human prostate cancer cells. In addition, previous studies have suggested phosphorylation at sites in IGFBP-3 consistent with a consensus CK2 phosphorylation motif (15) and have shown that CK2 can phosphorylate serum-derived IGFBP-3 in a cell-free system (13). To investigate whether IGFBP-3 can be phosphorylated by CK2 in vitro, we used two systems. First, 22RV1 prostate cancer cells were transfected with either scrambled or CK2α small interfering RNA (siRNA), and cell lysates were harvested. CK2α expression was reduced by approximately 50% after siRNA treatment (Fig. 1A, upper panel). Because CK2 is a potent survival factor, we did not want to achieve full knockdown of its activity, because this would have made analysis of any functional effect CK2 activity may have on the proapoptotic actions of IGFBP-3 difficult to interpret. IGFBP-3 was immunoprecipitated from the cell lysates, and its phosphorylation status was assessed by phospho-Ser/Thr immunoblotting. In the presence of reduced levels of CK2α, the phosphorylation of endogenous IGFBP-3 was reduced by approximately 50% (Fig. 1A, lower panel). To confirm these data, we used two highly specific, ATP-competitive chemical inhibitors against CK2α, 4, 5, 6, 7-tetrabromo-1H-benzimidazole (TBB), and 2-dimethylamino-4, 5, 6, 7-tetrabromo-1H-benzimidazole (DMAT). 22RV1 cells were incubated for 24 h with 900 nm TBB or 100 nm DMAT (16,17,18,19). Cell lysates were harvested, and the phosphorylation status of immunoprecipitated IGFBP-3 was assessed by immunoblotting. Consistent with Fig. 1A, we saw significantly reduced IGFBP-3 phosphorylation after treatment of cells with TBB or DMAT (Fig. 1B). These data demonstrate that inhibiting CK2 activity results in reduced phosphorylation of IGFBP-3 in prostate cancer cells in vitro.
Figure 1.
CK2 phosphorylates IGFBP-3 in vitro. A, 22RV1 cells were transfected with scrambled (Scr; control) or CK2α siRNA and incubated for 72 h. Immunoblots for CK2α and β-actin (upper panels, quantification of three separate experiments demonstrated; significance that mean is different from 100: ***, P < 0.001); immunoblots (IB) for phospho-Ser (P-Ser)/Thr and IGFBP-3 (BP3) after IGFBP-3 immunoprecipitation (IP) (lower panels). B, 22RV1 cells were incubated in SF media for 24 h followed by 24 h in the presence or absence of 900 nm TBB or 100 nm DMAT. Phospho-Ser/Thr and IGFBP-3 levels were detected by immunoblot after IGFBP-3 immunoprecipitation. Blots are representative of three independent experiments.
Inhibition of CK2 activity enhances the apoptotic potential of IGFBP-3
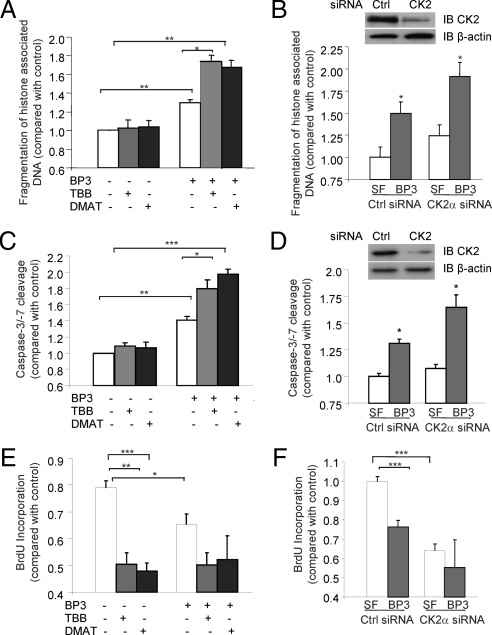
IGFBP-3 directly inhibits proliferation (20,21) and induces cell death in many tumor cell types, including prostate, lung, colon, and breast cancers (2,22,23,24), and we recently demonstrated that DNA-PK-mediated phosphorylation of IGFBP-3 at Ser-156 is essential for these actions in prostate cancer cells (11). We therefore decided to investigate the effect of CK2-catalyzed phosphorylation of IGFBP-3 on its proapoptotic actions in vitro. 22RV1 cells were incubated in serum-free (SF) media for 24 h, followed by 24 h incubation with or without 900 nm TBB or 100 nm DMAT and finally 24 h incubation with 1 μg/ml IGFBP-3. Apoptosis was assessed by ELISA for fragmentation of histone-associated DNA (Fig. 2A) and caspase-3/-7 activity (Fig. 2C). As expected, incubation with IGFBP-3 caused a significant induction of apoptosis (Fig. 2, A and C; P < 0.01). Surprisingly, the presence of either CK2 inhibitor amplified the apoptotic action of IGFBP-3, causing a 3-fold increase in apoptosis induction (P < 0.05). To confirm these data, we studied apoptosis levels in 22RV1 cells transfected with scrambled (control) or CK2α siRNA. We confirmed successful knockdown of CK2α by immunoblotting (upper panel). After 72 h, media were changed to SF, followed by 24 h incubation in the presence or absence of 1 μg/ml IGFBP-3. Treatment with IGFBP-3 caused significant apoptosis induction in 22RV1 cells treated with control siRNA. Consistent with our observations with the chemical inhibitors, IGFBP-3 was more potently apoptotic when CK2α levels were reduced (Fig. 2, B and D). Taken together, these data reveal that phosphorylation of IGFBP-3 by CK2 limits, but does not completely inhibit, the ability of IGFBP-3 to induce apoptosis. To test the effects of CK2 inhibition on IGFBP-3-mediated inhibition of proliferation, we assessed the effects of IGFBP-3 alone and in combination with CK2 inhibitors on cell proliferation using bromodeoxyuridine (BrdU) incorporation ELISA (Fig. 2E). However, our results demonstrated that treatment with either TBB or DMAT alone significantly inhibited cell proliferation, and we were unable to demonstrate additive effects with cotreatment with IGFBP-3. These data were confirmed using siRNA against CK2α (Fig. 2F).
Figure 2.
Inhibition of CK2 enhances IGFBP-3 (BP3)-induced apoptosis. 22RV1 cells were incubated in SF media for 24 h and then 24 h in the presence of 900 nm TBB or 100 nm DMAT followed by 24 h incubation in the presence or absence of 1 μg/ml IGFBP-3. A, C, and E, Apoptosis was assessed by ELISA for fragmentation of histone-associated DNA (A) or cleavage of a luminometric caspase-3 substrate (C), and cell proliferation was assessed by BrdU incorporation ELISA (E). B, D, and F, 22RV1 cells were transfected with control (Ctrl) or CK2α siRNA and incubated for 72 h, followed by 24 h in SF media and 24 h in the presence or absence of 1 μg/ml IGFBP-3. Demonstration of CK2 knockdown is shown. Apoptosis and proliferation were assessed as in A, C, and E. Results are shown as means ± sem. Significance that mean is different from 1: *, P < 0.05; **, P < 0.01; ***, P < 0.005; n = 3.
Inhibiting CK2 activity leads to enhanced nuclear accumulation of IGFBP-3
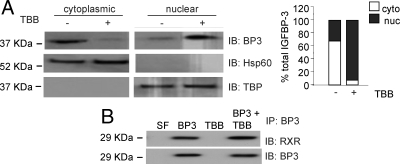
Previous studies in 22RV1 cells have revealed that the apoptosis-inducing actions of IGFBP-3 require its internalization, nuclear localization, and interaction with the nuclear receptor RXRα (6,8). In addition, we revealed that phosphorylation of IGFBP-3 by DNA-PK was associated with reduced nuclear accumulation and complete inhibition of interaction with RXRα. We therefore investigated whether the apoptosis-inhibitory phosphorylation by CK2 inhibited the nuclear localization of IGFBP-3. 22RV1 cells were incubated in SF medium for 24 h followed by 24 h incubation in the presence or absence of 900 nm TBB. Cell pellets were then harvested, and nuclear and cytoplasmic fractions were isolated. Immunoblotting revealed significantly enhanced nuclear accumulation of IGFBP-3 after CK2 activity was inhibited by incubation with TBB (Fig. 3A). Validity of fractionation was confirmed by immunoblotting for heat-shock protein 60 (Hsp60) (cytoplasmic fraction) and TATA-binding protein (TBP) (nuclear fraction). The ability of IGFBP-3 to interact with RXRα in conditions with reduced CK2 activity was assessed in 22RV1 cells incubated with 900 nm TBB in SF media for 24 h followed by incubation with 1 μg/ml IGFBP-3 for 24 h. The interaction between RXRα and IGFBP-3 was by immunoblotting for RXRα after immunoprecipitation of exogenously added IGFBP-3. We observed that IGFBP-3 and RXR were still able to interact under conditions of reduced CK2 activity. These data suggest that the enhanced apoptosis induction observed with IGFBP-3 treatment in the presence of reduced CK2α activity may be due to its enhanced nuclear accumulation.
Figure 3.
Preventing phosphorylation by CK2 enhances the nuclear localization of IGFBP-3 (BP3). A, 22RV1 cells were incubated in SF media for 24 h, followed by 24 h in the presence or absence of 900 nm TBB. The intracellular localization of endogenous IGFBP-3 was assessed by IGFBP-3 immunoblot after fractionation of nuclear and cytoplasmic fractions. Validity of fractionation was confirmed by blotting for Hsp60 [cytoplasmic (cyto)] and TBP [nuclear (nuc)]. Right panel shows quantitation of IGFBP-3. B, 22RV1 cells were incubated as in A, followed by 24 h ± 1 μg/ml IGFBP-3. The association of IGFBP-3 with RXR was assessed by immunoprecipitation (IP) with anti-RXR antibodies followed by immunoblotting (IB) for RXR-α and IGFBP-3. All blots are representative of three independent experiments.
Regulation of IGFBP-3 action by protein kinases is a cell-specific phenomenon
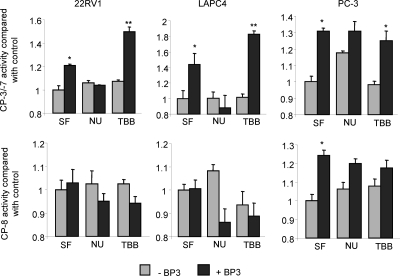
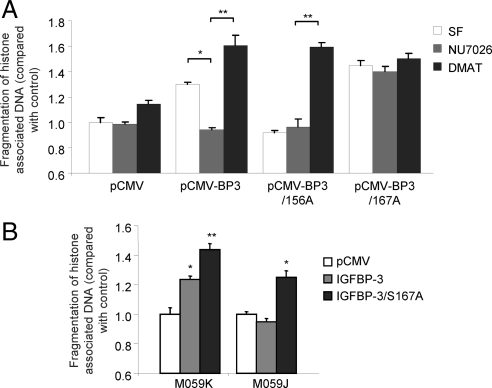
Recent data from our laboratory and others have revealed the importance of the nuclear localization of IGFBP-3 for its proapoptotic actions. However, seemingly contradictory studies carried out in PC-3 prostate cancer cells have suggested that neither the nuclear localization of IGFBP-3 nor its interaction with RXRα are required for the proapoptotic effects (10,25). To try and address this paradox, we investigated the ability of IGFBP-3 to induce apoptosis in the presence of the DNA-PK inhibitor NU7026 or the CK2 inhibitor TBB in 22RV1, LAPC4, and PC-3 prostate cancer cell lines. After 24 h incubation in SF media, cells were incubated for 24 h with 10 μm NU7026 or 900 nm TBB followed by 24 h treatment with 1 μg/ml IGFBP-3. Apoptosis was assessed by cleavage of luminometric substrates for both caspase-3/-7 and caspase-8 in an attempt to distinguish between intrinsic and extrinsic apoptotic pathways. In both LAPC4 and 22RV1 cells, IGFBP-3 induced caspase-3/-7 cleavage, but did not activate caspase-8, suggesting that apoptosis is induced via the intrinsic apoptotic pathway in these cell lines (Fig. 4). In contrast, IGFBP-3 activated caspase-8 in addition to -3/-7 in PC-3 cells, indicative of extrinsic pathway-mediated apoptosis. As we have previously demonstrated (11) (Fig. 2), apoptosis in 22RV1 and LAPC4 is inhibited by NU7026 and enhanced by incubation with TBB. In contrast, inhibition of DNA-PK (NU7026) or CK2 (TBB) had no effect on IGFBP-3-induced apoptosis in PC-3 cells. These data suggest that IGFBP-3 may use multiple mechanisms to induce apoptosis and may go some way to explaining the sometimes contradictory actions observed.
Figure 4.
Cell-specific apoptosis induction by IGFBP-3 (BP3). 22RV1, LAPC4, and PC-3 cells were incubated in SF media for 24 h, treated in SF ± 10 μm NU7026 (NU) or 900 nm TBB for 24 h, and then incubated in the presence or absence of 1 μg/ml IGFBP-3 for 24 h. Apoptosis via the extrinsic and intrinsic pathways was compared by using luminometric substrates for caspase-8 (CP-8) and -3/7 activity, respectively. Results are shown as means ± sem. Significance that mean is different from 1: *, P < 0.05; **, P < 0.01; n = 3.
Phosphorylation of Ser-167 partially inhibits IGFBP-3-induced apoptosis
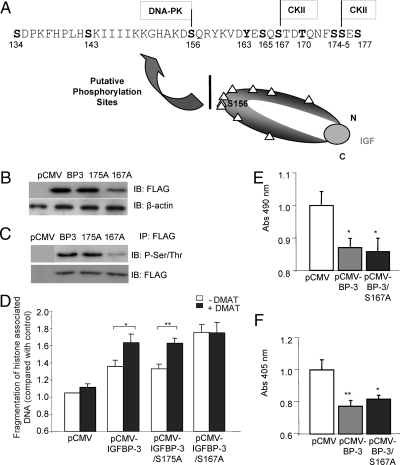
As the previously identified phosphorylation of IGFBP-3 at Ser-156 is required for its interaction with RXRα, we hypothesized that phosphorylation of IGFBP-3 in its central domain leads to a conformational change that may regulate the availability of important motifs in the N and C termini, such as nuclear localization or RXRα binding. We therefore used bioinformatic databases to search for CK2 consensus phosphorylation sites in the regions surrounding Ser-156 and identified two such sites at Ser-167 and Ser-175 (Fig. 5A). We mutated these sites to Ala by site-directed mutagenesis to prevent their phosphorylation and cloned the resulting cDNA into the pCMV-FLAG expression vector. Cells were transfected with pCMV control vector, pCMV-IGFBP-3, pCMV-IGFBP-3-S167A, or pCMV-IGFBP-3-S175A and incubated for 48 h in serum-containing media. To confirm that the constructs were expressed, we harvested cell lysates and immunoblotted for FLAG and β-actin (loading control; Fig. 5B). To assess which site of IGFBP-3 may be phosphorylated by CK2, we assessed the phosphorylation status of transfected wild-type IGFBP-3, IGFBP-3/S167A, and IGFBP-3/S175A by immunoprecipitation for FLAG followed by immunoblotting with phosphor-Ser/Thr. We demonstrated reduced phosphorylation of the S167A mutant but not S175A (Fig. 5C), suggesting that S167 of IGFBP-3 is phosphorylated in 22RV1 prostate cancer cells in vitro. To assess the functional significance of this phosphorylation event, transfected cells were switched to SF media for 24 h and incubated in the presence or absence of 100 nm DMAT for 24 h. Apoptosis was then assessed by ELISA for fragmentation of histone-associated DNA. The apoptosis induced by IGFBP-3 and IGFBP-3-S175A could be significantly enhanced by incubation with DMAT (Fig. 5D). However, IGFBP-3-S167A was potently more apoptotic, and its actions were unaffected by incubation with DMAT, suggesting that S167 can be phosphorylated by CK2 and that this phosphorylation event limits the ability of IGFBP-3 to induce apoptosis. To determine whether this phosphorylation event is also important for maximal growth inhibition by IGFBP-3, we tested the ability of IGFBP-3 and IGFBP-3-S167A to inhibit the growth of LAPC4 prostate cancer cells. LAPC4 cells were transfected with control vector, pCMV-IGFBP-3, or pCMV-IGFBP-3-S167A and incubated for 48 h in complete media. Media were transferred to SF for 72 h, and cell number was assessed by 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-z-(4-sulfo-phenyl)-2H-tetrazolium (MTS) assay. IGFBP-3 and IGFBP-3-S167A inhibited cell growth to a comparable extent (Fig. 5E), suggesting that CK2-mediated phosphorylation is important for IGFBP-3-induced apoptosis but not its growth-inhibitory properties. These data were confirmed by measuring cell proliferation by ELISA for BrdU incorporation (Fig. 5F). Together, these experiments reveal a novel phosphorylation of IGFBP-3 at S167 that is important for its apoptotic but not growth-inhibitory actions.
Figure 5.
Phosphorylation of S167 inhibits IGFBP-3-induced apoptosis. A, Demonstration of CK2 phosphorylation sites in IGFBP-3 in the vicinity of Ser-156 (S156), the site phosphorylated by DNA-PK. Phosphorylation sites are marked by triangles on the cartoon of the IGFBP-3 molecule, and the midregion sequence is shown. Panel B, 22RV1 cells were transiently transfected with wild-type IGFBP-3 or IGFBP-3 with putative CK2 phosphorylation sites mutated (S167A and S175A). The expression of the constructs was validated by FLAG and β-actin (loading control) immunoblotting (IB). Panel C, Cells were transfected as in panel B. The phosphorylation status of transfected IGFBP-3 was assessed by immunoprecipitation (IP) with anti-FLAG antibodies followed by phospho-Ser/Thr (P-Ser/Thr) and FLAG immunoblotting. Panel D, Histone-associated DNA fragmentation was assessed in 22RV1 cells transiently transfected as in panel B after 24 h in SF media followed by 24 h with or without 900 nm TBB. The ability of the mutant forms of IGFBP-3 to inhibit cell proliferation was assessed in LAPC4 cells by BrdU incorporation ELISA (panel E) and MTS assay (panel F). Cells were transfected as in panel B, and media were transferred to SF after 48 h. BrdU incorporation was assessed after 20 h incubation in SF, followed by 4 h BrdU treatment. MTS cleavage was assayed after 72 h in SF media. Results are shown as means ± sem. Significance that mean is different from 1: *, P < 0.05; **, P < 0.01; n = 3. Abs, Absorbance; C, C-terminal of IGFBP-3; N, N-terminal of IGFBP-3.
Enhanced apoptosis induction by IGFBP-3-S167A is associated with elevated JNK phosphorylation
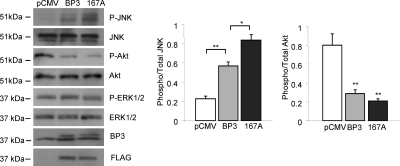
We previously revealed that IGFBP-3-induced apoptosis in 22RV1 cells is associated with reduced Akt activation and enhanced JNK phosphorylation (26). To determine whether IGFBP-3-S167A enhances these actions of wild-type IGFBP-3, we transfected 22RV1 cells with control vector, pCMV-IGFBP-3, or pCMV-IGFBP-3-S167A and harvested cell lysates after 24 h incubation in SF media. Levels of total and phospho-Akt (S473), total and phospho-JNK (T183/Y185), IGFBP-3, and FLAG were assessed by immunoblotting (Fig. 6). When compared with wild-type IGFBP-3, we observed a stronger induction of JNK phosphorylation by IGFBP-3-S167A but a comparable level of Akt activation, suggesting JNK may play a key role in the apoptosis induced by IGFBP-3 and inhibited by CK2.
Figure 6.
Enhanced JNK phosphorylation by IGFBP-3 (BP3)/S167A. 22RV1 cells were transfected with pCMV (control vector), pCMV-IGFBP-3, or pCMV-IGFBP-3/S167A and incubated for 72 h, followed by 24 h in SF media, and cell lysates were harvested. Levels of IGFBP-3, FLAG, and phospho-(P-JNK) and total JNK, Akt, and ERK were assessed by immunoblotting. All blots are representative of three independent experiments. Quantification of phospho-/total JNK and Akt is shown in the right panel. Results are shown as means ± sem. Significance that mean is different from control: *, P < 0.05; **, P < 0.01.
The stimulatory effects of inhibiting CK2 activity on IGFBP-3-induced apoptosis cannot be prevented by the inhibition of DNA-PK
Because we have identified both proapoptotic (DNA-PK) and antiapoptotic (CK2) phosphorylation of IGFBP-3, we set out to determine whether one event is dominant in terms of regulating IGFBP-3 function. 22RV1 cells were transfected with control vector, pCMV-IGFBP-3, pCMV-IGFBP-3-S156A or pCMV-S167A, followed by incubation with 0.1% dimethylsulfoxide (vehicle), 10 μm NU7026 (DNA-PK inhibitor), or 100 nm DMAT (CK2 inhibitor), and apoptosis was assessed by ELISA for fragmentation of histone-associated DNA. As we have previously described, wild-type IGFBP-3 induces apoptosis that is inhibited by NU7026 and enhanced by DMAT (Fig. 7A). IGFBP-3-S167A is unable to induce apoptosis and is unaffected by the presence of the DNA-PK inhibitor. However, in the presence of DMAT, it becomes proapoptotic (Fig. 7A). In contrast, IGFBP-3-S167A potently induces apoptosis and is unaffected by the presence of either inhibitor. To confirm these observations, we assessed the ability of transfected IGFBP-3 and IGFBP-3-S167A to induce apoptosis in M059J and M059K cells, a paired system of glioblastoma cells that either lack (M059J) or express (M059K) the catalytic subunit of DNA-PK (27). As we have previously shown, IGFBP-3 is able to induce apoptosis in M059K but not M059J cells (Fig. 7B). In contrast, IGFBP-3-S167A is apoptotic regardless of the presence of DNA-PK. Together these data suggest that the stimulatory effects of inhibiting CK2 activity on IGFBP-3-induced apoptosis cannot be prevented by the inhibition of DNA-PK.
Figure 7.
The prooncogenic phosphorylation event by CK2 is not inhibited by the tumor-suppressive phosphorylation by DNA-PK. A, 22RV1 cells were transfected with pCMV, pCMV-IGFBP-3, pCMV-IGFBP-3/S156A, or pCMV-IGFBP-3/S167A and incubated for 48 h. After 24 h in SF media, cells were incubated with or without 10 μm NU7026 or 100 nm DMAT. Apoptosis was assessed by histone-associated DNA fragmentation ELISA. B, M059K and M059J cells were transfected with pCMV, pCMV-IGFBP3, or pCMV-IGFBP-3/S167A and incubated for 48 h. Apoptosis was assessed as in A after incubation in SF media for 24 h. Results are shown as means ± sem. Significance that mean is different from 1: *, P < 0.05; **, P < 0.01; n = 3.
Inhibition of IGFBP-3 action is one mechanism by which CK2 promotes cell survival
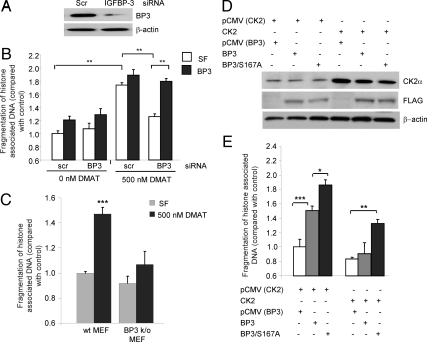
CK2 is a ubiquitously expressed protein kinase that functions as a critical survival factor; consequently, full inhibition of its actions results in apoptosis induction (14). To determine whether inhibition of IGFBP-3 is one of the mechanisms by which CK2 promotes cell survival, we assessed the ability of high-dose treatment with CK2 inhibitor to induce apoptosis in the presence and absence of IGFBP-3. 22RV1 cells were transfected with scrambled (control) or IGFBP-3 siRNA, and IGFBP-3 levels were assessed by immunoblotting to confirm successful knockdown of IGFBP-3 (Fig. 8A). Knockdown of endogenous IGFBP-3 had minimal effect on basal apoptosis levels, and the addition of 1 μg/ml exogenous IGFBP-3 induced apoptosis regardless of the presence of siRNA. As expected, incubation of cells with high-dose (500 nm) DMAT induced significant apoptosis in cells transfected with control siRNA, which could be slightly enhanced by the addition of exogenous IGFBP-3 (Fig. 8B). However, in the absence of endogenous IGFBP-3, inhibition of CK2 induced apoptosis but to a significantly lesser extent that in the presence of IGFBP-3. Indeed, full reactivity to CK2 inhibition could be restored by the addition of exogenous IGFBP-3 (Fig. 8B). These data were confirmed by assessing the ability of high-dose DMAT treatment to induce apoptosis in mouse embryonic fibroblasts (MEFs) generated from IGFBP-3 knockout mice. Again, high-dose DMAT treatment was more effective at inducing apoptosis in MEFs generated from wild-type mice, compared with IGFBP-3 knockout MEFs (Fig. 8C). In addition, we assessed the ability of IGFBP-3 and IGFBP-3-S167A to induce apoptosis in the presence of elevated levels of CK2α. First, we confirmed the expression of the transfected proteins by immunoblotting for CK2 and FLAG (Fig. 8D). As demonstrated (Fig. 2), IGFBP-3-S167A induces apoptosis to a greater extent than wild-type IGFBP-3. However, when CK2α is overexpressed, the ability of wild-type IGFBP-3 to induce apoptosis is completely inhibited (Fig. 8E). In contrast, IGFBP-3 S167A retains some ability, albeit reduced, to induce apoptosis despite the survival effects of CK2α. These data together reveal that, in addition to IGFBP-3 function being regulated by CK2, inhibition of IGFBP-3-induced apoptosis is one of the mechanisms by which CK2 promotes cell survival.
Figure 8.
A reciprocal relationship between IGFBP-3 (BP3) and CK2 regulates cell survival. 22RV1 cells were transfected with scrambled (scr) or IGFBP-3 siRNA and incubated for 72 h, and IGFBP-3 expression was verified by immunoblotting (A). Histone-associated DNA fragmentation was assessed after 24 h incubation in SF media followed by 24 h in the presence or absence of high-dose (500 nm DMAT) CK2 inhibitor and 24 h with or without 1 μg/ml IGFBP-3 (B). C, MEFs generated from wild-type (wt) and IGFBP-3 knockout mice were incubated in SF media for 24 h followed by 24 h incubation with or without 500 nm DMAT, and apoptosis was assessed as in B. D, 22RV1 cells were cotransfected with pCMV/pCMV-CK2α and pCMV, pCMV-IGFBP-3, or pCMV-IGFBP-3/S167A and incubated for 48 h. Protein expression was verified by immunoblotting. E, Apoptosis was assessed in cells transfected in D by histone-associated DNA fragmentation ELISA after 24 h incubation in SF media. Results are shown as means ± sem. Significance that mean is different from 1: *, P < 0.05; **, P < 0.01; ***, P < 0.001; n = 3. All blots are representative of three independent experiments.
Discussion
IGFBP-3 is the primary carrier of IGFs in the circulation, regulating IGF action by governing their ability to interact with and activate the IGF type I receptor (1), thus regulating IGF-mediated processes. In addition to its traditional IGF-regulatory role, many IGF-independent actions of IGFBP-3 have now been identified. For example, IGFBP-3 promotes apoptosis in numerous cancer models (2,22,23,24). In addition, several key intracellular binding partners for IGFBP-3 have been identified, including human papillomavirus type 16 E7 oncoprotein (28), RXRα (8), and RNA polymerase II binding subunit 3, Rpb3 (29). However, little is known about how these interactions or the IGF-dependent functions of IGFBP-3 are regulated. Previous reports have suggested that phosphorylation of IGFBPs may be important. For example, it is now well established that modification of IGFBP-1 by phosphorylation (30,31) can affect IGF binding. In addition, phosphorylation of IGFBP-3 has been suggested by others. Mishra and Murphy (32) demonstrated that IGFBP-3 could be phosphorylated by unidentified protein kinases located at the membrane of T47D cells and that this enhanced IGF-I binding. Hollowood et al. (15) identified two serine residues in the central domain of IGFBP-3 (S111 and S113) as phospho-acceptor sites for an unidentified kinase and suggested that phosphorylation of these amino acids could regulate the proapoptotic actions. Recently, we identified DNA-PK as an IGFBP-3 kinase (33) and showed it to be important for IGFBP-3-induced apoptosis. In addition to phosphorylation, IGFBP-3 action can be modified by many other posttranslational modifications, including glycosylation and proteolysis, whereas the function of other modifications such as ubiquitination remains unclear (34).
Although IGFBP-3 is well recognized as an apoptotic factor in cancer cells, its mechanism of action remains controversial. There have been reports of putative receptors on the cell surface in Hs578T breast cancer cells (35), but definite data regarding the identity and nature of the receptor have not yet been published. In contrast, our laboratory and others have suggested that internalization of IGFBP-3 to the nucleus and its specific interaction with nuclear receptors is the key to apoptosis induction in 22RV1 prostate cancer cells. In addition, others have demonstrated that neither secretion nor nuclear localization of IGFBP-3 is necessary for apoptosis induction in PC-3 prostate cancer cells (25). This suggests that IGFBP-3 has several potential mechanisms by which it can induce apoptosis and that the local environment within the circulation, or a specific cell or tissue, may play a role in regulating the precise actions of IGFBP-3. One such mechanism by which differential regulation may occur is the regulation of phosphorylation by the presence and activity of specific protein kinases, including DNA-PK and CK2. This may be important to consider when developing IGFBP-3 as a possible cancer therapeutic, because its effectiveness may be strongly regulated by the local tumor environment.
It has previously been suggested that the shuttling of CK2 from the cytoplasm to the nucleus may serve to protect against cell death and promote cell growth, whereas its shuttling from the nucleus to the cytoplasm may promote cell death and suppress growth (36,37). This would be consistent with a model whereby CK2 phosphorylates IGFBP-3 in the nucleus, suppressing its apoptotic effects. Indeed, among the literally hundreds of kinase substrates for CK2 that have been identified thus far, in addition to multiple transcription factors and signal transduction molecules, are many apoptotic and cancer-related proteins including BH3-interacting domain death agonist (BID), N-myc, double minute 2 protein (MDM2), p53, and phosphatase and tensin homolog (PTEN) (38), suggesting that this is a common mechanism of action for CK2.
Our findings have implications in terms of drug development as IGFBP-3-based therapies are now moving into clinical trials. Derivation of novel mutants that can yield higher antitumor apoptotic actions may serve to enhance the efficacy of this agent.
Thus, we have demonstrated here that IGFBP-3 represents a newly recognized target for CK2 that explains some of the effects of this kinase on proliferation and survival. Because IGFBP-3 also has actions related to angiogenesis and inflammatory processes (39), it remains to be determined what the full implication of this interaction entails. Conversely, the dramatic regulation of IGFBP-3 function by CK2 may explain how in certain situations IGFBP-3 has potent antiapoptotic activities, whereas in others, it does not (40).
In summary, the apoptotic actions of IGFBP-3 in prostate cancer cells are tightly regulated by site-specific phosphorylation and specific intracellular kinases. This provides valuable insight into the mechanism of action of IGFBP-3, which may in turn allow for the development of more targeted IGFBP-3-based therapeutics in cancer.
Materials and Methods
Reagents
Recombinant nonglycosylated IGFBP-3 was provided by Insmed (Mountain View, CA). pCMV-CK2α was a generous gift from Dr. David Litchfield (University of Western Ontario, London, Ontario, Canada). Goat antihuman IGFBP-3 antibody was purchased from Diagnostic Systems Laboratories (Webster, TX); rabbit anti-DYKDDDDK (FLAG tag), phospho-JNK (Thr-183/Tyr-185), total JNK, phospho-Akt (Ser-473), total Akt, phospho-ERK (Thr-202/Tyr-204), total ERK, and CK2α antibodies were from Cell Signaling Technology (Danvers, MA). The mouse anti-β-actin and mouse anti-Hsp60 antibodies, pCMV-FLAG expression vector, and the CelLytic NuCLEAR cell fractionation kit were purchased from Sigma Chemical Co. (St. Louis, MO). I-block was purchased from Applied Biosystems (Foster City, CA). The rabbit anti-RXRα antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-TBP (nuclear loading control) antibody was purchased from AbCam (Cambridge, MA). Pfx DNA polymerase, CK2α siRNA, T4 DNA ligase, Lipofectamine transfection reagent, and all cell culture reagents were purchased from Invitrogen (Carlsbad, CA). NU7026, TBB, DMAT, Gene Juice transfection reagent, and the BrdU cell proliferation assay were from EMD Biosciences (San Diego, CA). The rabbit anti-phospho-serine/threonine antibody was from Chemicon (Temecula, CA). SDS-PAGE precast gels and blotting equipment were purchased from Bio-Rad (Hercules, CA). FastDigest restriction enzymes were from Fermentas (Hanover, MD). CellTiter 96 AQueous One Solution cell proliferation assay, luminescent caspase-3/-7, and caspase-8 substrates were purchased from Promega (Madison, WI). The Cell Death Detection ELISAplus was from Roche Applied Science (Indianapolis, IN). ON-TARGETplus siRNA against IGFBP-3 and DharmaFECT transfection reagent were purchased from Dharmacon (Lafayette, CO).
Cloning and mutagenesis
Putative CK2 phosphorylation sites of IGFBP-3 (Ser-167 and Ser-175) in the region of the DNA-PK phosphorylation site (Ser-156) were identified using NetPhos program in the CBS prediction servers (41). The two putative phosphorylation sites were individually mutated to alanine to prevent their phosphorylation. IGFBP-3 in pCMV-FLAG was mutated using two-step PCR-based mutagenesis (forward primer 1, GTGTACGGTGGGAGGTCT; reverse primer 1, TCTGGGTATCTGTGGCCTGAGACTCGTAGT; forward primer 2, ACTTCTCCTCCGAGTCCAAGCGGGAGACAG; reverse primer 2, TCCTTGGTACCACTTGCTCTGC) using Pfu DNA polymerase (Invitrogen) as previously described (42). All mutants were verified by DNA sequencing (Laragen, Los Angeles, CA).
Cell culture
The LAPC4 prostate cancer cell line was a generous gift from Charles Sawyers (University of California, Los Angeles, Los Angeles, CA). LAPC4 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, and 10 nm R1881 (PerkinElmer Life Sciences, Wellesley, MA). The 22RV1 prostate carcinoma cell line [American Type Culture Collection (ATCC), Manassas, VA] was maintained in RPMI 1840 medium supplemented with 10% FBS and 1% penicillin/streptomycin. PC-3 cells (ATCC) were cultured in F-12K medium supplemented with 10% FBS and 1% penicillin/streptomycin. M059K and M059J glioblastoma cell lines (ATCC) were cultured in F-12/DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% nonessential amino acids. For individual experiments, cells seeded at a final density of 1 × 105 cells/cm2 in 96-well, 24-well, six-well, or 10 cm plates and grown to 80% confluence in a humidified atmosphere of 5% CO2 at 37 C before treatment. All treatments were carried out as indicated in serum-free media.
Generation of MEFs
MEFs were generated from 25-wk-old pregnant dams at d 15 postcoitum, as previously described (43).
Transient transfection
Cells in suspension were transfected using Gene Juice following the manufacturer’s instructions and were plated on 6-, 24-, or 96-well plates. Cells were incubated for 48 h in complete media before analysis.
siRNA transfection
Transfection of IGFBP-3 ON-TARGETplus siRNA was carried out using DharmaFECT transfection reagent (Dharmacon), and CK2α siRNA was transfected using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. All siRNA reactions were incubated for 72 h before analysis.
Immunoblotting
Cell lysates containing 20 μg protein were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Bio-Rad). Membranes were blocked in 0.2% I-block (Applied Biosystems) in PBS containing 0.1% Tween 20 for 3 h at room temperature and then probed with the appropriate primary and secondary antibodies. Antibody-antigen complexes were visualized by Chemilucent ECL detection system (Millipore, Billerica, MA) and autoradiography.
Cell fractionation
Cells on 10-cm plastic dishes were treated as indicated. Nuclear and cytoplasmic fractions were harvested using CelLytic NuCLEAR cell fractionation kit (Sigma) following the manufacturer’s instructions. Separated fractions were quantified and analyzed by SDS-PAGE. Validity of separation was determined by immunoblotting for TBP (nuclear fraction) and Hsp60 (cytoplasmic fraction).
Immunoprecipitation
Cell lysates (50 μg) were incubated with 5 μl goat antihuman IGFBP-3 antibody overnight at 4 C. Fifty microliters of 25% protein A/G Sepharose (Santa Cruz) was added, and samples were incubated at 4 C for 1 h. Bound protein was eluted in Laemmli sample buffer [60 mm Tris (pH 6.8), 2% SDS, 10% glycerol, 0.1% bromophenol blue], and the phosphorylation status of IGFBP-3 was assessed by immunoblotting with phosphospecific antibodies. For co-immunoprecipitation experiments, samples were immunoprecipitated as above and analyzed by SDS-PAGE followed by immunoblotting.
Analysis of apoptosis
Apoptosis was assessed in cells growing on 24-well plates using a cell death detection ELISA (Roche) following the manufacturer’s instructions. When apoptosis exerted via the intrinsic and extrinsic pathways had to be separated, apoptosis in cells growing on 96-well plates was assessed by cleavage of Apo-ONE luminometric caspase-3/-7 and caspase-8 substrates (Promega).
MTS cell proliferation assay
To assess cell viability/proliferation, cells growing in 96-well plates were treated as appropriate and analyzed by CellTiter 96 AQueous One Solution cell proliferation assay (Promega) following the manufacturer’s instructions.
Statistical analysis
Statistical analyses were analyzed using Student’s t test and are presented as means ± sem. Differences were considered statistically significant when P < 0.05.
BrdU incorporation assay
BrdU incorporation in to cells growing on 96-well plates and treated as appropriate was assessed using BrdU cell proliferation assay (EMD Biosciences) following the manufacturer’s instructions. The data were analyzed as an average of six samples in each experiment.
Acknowledgments
We thank Dr. David Litchfield (University of Western Ontario, Canada) for his generous gift of the pCMV-CK2α expression vector.
Footnotes
This work was supported by Grants P50CA92131 and Department of Defense (DOD) Grant DOD-PC050485 to P.C. and a DOD Fellowship Award for L.J.C.
Disclosure Summary: None of the authors has anything to declare.
First Published Online June 25, 2009
Abbreviations: BrdU, Bromodeoxyuridine; DMAT, 2-dimethylamino-4, 5, 6, 7-tetrabromo-1H-benzimidazole; DNA-PK, DNA-dependent protein kinase; FBS, fetal bovine serum; Hsp60, heat-shock protein 60; IGFBP-3, IGF-binding protein 3; JNK, c-Jun N-terminal kinase; MEF, mouse embryonic fibroblast; MTS, 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-z-(4-sulfophenyl)-2H-tetrazolium; RXRα, retinoid X receptor-α; SF, serum-free; siRNA, small interfering RNA; TBB, 4, 5, 6, 7-tetrabromo-1H-benzimidazole; TBP, TATA-binding protein.
References
- Firth SM, Baxter RC 2002 Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev 23:824–854 [DOI] [PubMed] [Google Scholar]
- Rajah R, Valentinis B, Cohen P 1997 Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-β1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem 272:12181–12188 [DOI] [PubMed] [Google Scholar]
- Kim HS, Ingermann AR, Tsubaki J, Twigg SM, Walker GE, Oh Y 2004 Insulin-like growth factor-binding protein 3 induces caspase-dependent apoptosis through a death receptor-mediated pathway in MCF-7 human breast cancer cells. Cancer Res 64:2229–2237 [DOI] [PubMed] [Google Scholar]
- Gucev Z, Oh Y, Kelley KM, Rosenfeld RG 1996 Insulin-like growth factor-binding protein 3 mediates retinoic acid- and transforming growth factor β2-induced growth inhibition in human breast cancer cells. Cancer Res 56:1545–1550 [PubMed] [Google Scholar]
- Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC 2000 Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin β subunit. J Biol Chem 275:23462–23470 [DOI] [PubMed] [Google Scholar]
- Lee KW, Liu B, Ma L, Li H, Bang P, Koeffler HP, Cohen P 2004 Cellular internalization of insulin-like growth factor-binding protein-3: distinct endocytic pathways facilitate re-uptake and nuclear localization. J Biol Chem 279:469–476 [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Young TF, Firth SM, Baxter RC 1998 Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem 273:18347–18352 [DOI] [PubMed] [Google Scholar]
- Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, Cohen P 2000 Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-α regulate transcriptional signaling and apoptosis. J Biol Chem 275:33607–33613 [DOI] [PubMed] [Google Scholar]
- Butt AJ, Fraley KA, Firth SM, Baxter RC 2002 IGF-binding protein-3-induced growth inhibition and apoptosis do not require cell surface binding and nuclear translocation in human breast cancer cells. Endocrinology 143:2693–2699 [DOI] [PubMed] [Google Scholar]
- Zappala G, Elbi C, Edwards J, Gorenstein J, Rechler MM, Bhattacharyya N 2008 Induction of apoptosis in human prostate cancer cells by insulin-like growth factor binding protein-3 does not require binding to retinoid X receptor-α. Endocrinology 149:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb LJ, Liu B, Lee KW, Cohen P 2006 Phosphorylation by DNA-dependent protein kinase is critical for apoptosis induction by insulin-like growth factor binding protein-3. Cancer Res 66:10878–10884 [DOI] [PubMed] [Google Scholar]
- Hoeck WG, Mukku VR 1994 Identification of the major sites of phosphorylation in IGF binding protein-3. J Cell Biochem 56:262–273 [DOI] [PubMed] [Google Scholar]
- Coverley JA, Martin JL, Baxter RC 2000 The effect of phosphorylation by casein kinase 2 on the activity of insulin-like growth factor-binding protein-3. Endocrinology 141:564–570 [DOI] [PubMed] [Google Scholar]
- Ahmed K, Gerber DA, Cochet C 2002 Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol 12:226–230 [DOI] [PubMed] [Google Scholar]
- Hollowood AD, Stewart CE, Perks CM, Pell JM, Lai T, Alderson D, Holly JM 2002 Evidence implicating a mid-region sequence of IGFBP-3 in its specific IGF-independent actions. J Cell Biochem 86:583–589 [DOI] [PubMed] [Google Scholar]
- Szyszka R, Grankowski N, Felczak K, Shugar D 1995 Halogenated benzimidazoles and benzotriazoles as selective inhibitors of protein kinases CK-I and CK-II from Saccharomyces cerevisiae and other sources. Biochem Biophys Res Commun 208:418–424 [DOI] [PubMed] [Google Scholar]
- Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, Pinna LA 2004 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun 321:1040–1044 [DOI] [PubMed] [Google Scholar]
- Wang G, Ahmad KA, Ahmed K 2006 Role of protein kinase CK2 in the regulation of tumor necrosis-factor-related apoptosis inducing ligand-induced apoptosis in prostate cancer cells. Cancer Res 66:2242–2249 [DOI] [PubMed] [Google Scholar]
- Götz C, Bachmann C, Montenarh M 2007 Inhibition of protein kinase CK2 leads to a modulation of androgen receptor dependent transcription in prostate cancer cells. Prostate 67:125–134 [DOI] [PubMed] [Google Scholar]
- Oh Y, Müller HL, Ng L, Rosenfeld RG 1995 Transforming growth factor-β-induced cell growth inhibition in human breast cancer cells is mediated through insulin-like growth factor-binding protein-3 action. J Biol Chem 270:13589–13592 [DOI] [PubMed] [Google Scholar]
- Valentinis B, Bhala A, DeAngelis T, Baserga R, Cohen P 1995 The human insulin-like growth factor (IGF) binding protein-3 inhibits the growth of fibroblasts with a targeted disruption of the IGF-I receptor gene. Mol Endocrinol 9:361–367 [DOI] [PubMed] [Google Scholar]
- Gill ZP, Perks CM, Newcomb PV, Holly JM 1997 Insulin-like growth factor-binding protein (IGFBP-3) predisposes breast cancer cells to programmed cell death in a non-IGF-dependent manner. J Biol Chem 272:25602–25607 [DOI] [PubMed] [Google Scholar]
- Lee HY, Chun KH, Liu B, Wiehle SA, Cristiano RJ, Hong WK, Cohen P, Kurie JM 2002 Insulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancer. Cancer Res 62:3530–3537 [PubMed] [Google Scholar]
- Williams AC, Collard TJ, Perks CM, Newcomb P, Moorghen M, Holly JMP, Paeaskeva C 2000 Increased p53-dependent apoptosis by the insulin-like growth factor binding protein IGFBP-3 in human colonic adenoma-derived cells. Cancer Res 60:20–27 [PubMed] [Google Scholar]
- Bhattacharyya N, Pechhold K, Shahjee H, Zappala G, Elbi C, Raaka B, Wiench M, Hong J, Rechler MM 2006 Nonsecreted insulin-like growth factor binding protein-3 (IGFBP-3) can induce apoptosis in human prostate cancer cells by IGF-independent mechanisms without being concentrated in the nucleus. J Biol Chem 281:24588–24601 [DOI] [PubMed] [Google Scholar]
- Lee KW, Cobb LJ, Paharkova-Vatchkova V, Liu B, Milbrandt J, Cohen P 2007 Contribution of the orphan nuclear receptor Nur77 to the apoptotic action of IGFBP-3. Carcinogenesis 28:1653–1658 [DOI] [PubMed] [Google Scholar]
- Lees-Miller SP, Godbout R, Chan DW, Weinfeld M, Day 3rd RS, Barron GM, Allalunis-Turner J 1995 Absence of p350 subunit of DNA-activated protein kinase from a radiosensitive human cell line. Science 267:1183–1185 [DOI] [PubMed] [Google Scholar]
- Mannhardt B, Weinzimer SA, Wagner M, Fiedler M, Cohen P, Jansen-Dürr P, Zwerschke W 2000 Human papillomavirus type 16 E7 oncoprotein binds and inactivates growth-inhibitory insulin-like growth factor binding protein 3. Mol Cell Biol 20:6483–6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oufattole M, Lin SW, Liu B, Mascarenhas D, Cohen P, Rodgers BD 2006 Ribonucleic acid polymerase II binding subunit 3 (Rpb3), a potential nuclear target of insulin-like growth factor binding protein-3. Endocrinology 147:2138–2146 [DOI] [PubMed] [Google Scholar]
- Jones JI, D'Ercole AJ, Camacho-Hubner C, Clemmons DR 1991 Phosphorylation of insulin-like growth factor (IGF)-binding protein-1 in cell culture and in vivo: effects on affinity for IGF. Proc Natl Acad Sci USA 88:7481–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, White A 1997 Purification and characterization of the insulin-like growth factor binding protein-1 phosphoform found in normal plasma. Endocrinology 138:1130–1136 [DOI] [PubMed] [Google Scholar]
- Mishra S, Murphy LJ 2003 Phosphorylation of insulin-like growth factor (IGF) binding protein-3 by breast cancer cell membranes enhances IGF-I binding. Endocrinology 144:4042–4050 [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Nilsen T, John AP, Jans DA, Baxter RC 2003 Phosphorylation of insulin-like growth factor binding protein-3 by deoxyribonucleic acid-dependent protein kinase reduces ligand binding and enhances nuclear accumulation. Endocrinology 144:1984–1993 [DOI] [PubMed] [Google Scholar]
- Santer FR, Moser B, Spoden GA, Jansen-Dürr P, Zwerschke W 2007 Human papillomavirus type 16 E7 oncoprotein inhibits apoptosis mediated by nuclear insulin-like growth factor-binding protein-3 by enhancing its ubiquitin/proteasome-dependent degradation. Carcinogenesis 28:2511–2520 [DOI] [PubMed] [Google Scholar]
- Oh Y, Müller HL, Pham H, Rosenfeld RG 1993 Demonstration of receptors for insulin-like growth factor-binding protein-3 on Hs578T human breast cancer cells. J Biol Chem 268:26045–26048 [PubMed] [Google Scholar]
- Ahmed K, Davis AT, Wang H, Faust RA, Yu S, Tawfic S 2000 Significance of protein kinase CK2 nuclear signaling in neoplasia. J Cell Biochem Suppl 35:130–135 [DOI] [PubMed] [Google Scholar]
- Yu S, Wang H, Davis A, Ahmed K 2001 Consequences of CK2 signaling to the nuclear matrix. Mol Cell Biochem 227:67–71 [PubMed] [Google Scholar]
- Meggio F, Pinna LA 2003 One-thousand-and one substrates of protein kinase CK2? FASEB J 17:349–368 [DOI] [PubMed] [Google Scholar]
- Liu B, Lee KW, Anzo M, Zhang B, Zi X, Tao Y, Shiry L, Pollak M, Lin S, Cohen P 2007 Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene 26:1811–1819 [DOI] [PubMed] [Google Scholar]
- Granata R, Trovato L, Lupia E, Sala G, Settanni F, Camussi G, Ghidonni R, Ghigo E 2007 Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J Thromb Haemost 5:835–845 [DOI] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S 1999 Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294:1351–1362 [DOI] [PubMed] [Google Scholar]
- Adereth Y, Champion KJ, Hsu T, Dammai V 2005 Site-directed mutagenesis using Pfu DNA polymerase and T4 DNA ligase. Biotechniques 38:864–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell C, Rubini M, Ribom R, Liu J, Efstratiadis A, Baserga R 1993 Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci USA 90:11217–11221 [DOI] [PMC free article] [PubMed] [Google Scholar]