Abstract
Purpose
A randomized phase II trial of two novel treatment strategies in the first-line management of advanced non–small-cell lung cancer patients with performance status (PS) 2.
Patients and Methods
Patients were assigned to docetaxel 30 mg/m2 on days 1, 8, and 15 every 28 days in combination with either cetuximab 400 mg/m2 loading dose followed by 250 mg/m2 weekly (D + C) or bortezomib 1.6 mg/m2 on days 1, 8, and 15 every 28 days (D + B) for up to 4 cycles. Patients with responding or stable disease continued cetuximab or bortezomib until progression. The primary end point was progression-free survival (PFS) rate at 6 months.
Results
Sixty-four patients were enrolled and 59 were included in this analysis. Complete or partial response rates were 13.3% and 10.3% for D + C and D + B, respectively. Median PFS was 3.4 months in the D + C arm and 1.9 months in the D + B arm. Corresponding figures for 6-month PFS were 27.8% and 13.8% and 5.0 and 3.9 months for median survival, respectively. Grade 3/4 hematologic toxicity was 16% for D + C and 21% for D + B, whereas nonhematologic toxicities were observed in 63% and 44% of patients, respectively. There was one treatment-related death in each arm.
Conclusion
These results confirm the poor prognosis associated with a PS of 2 and the difficulty in translating recent advances in targeted therapy to this subset of patients. While the results in the D + C arm are numerically superior, neither combination met the prespecified PFS end point to justify further research in this setting.
INTRODUCTION
Performance status (PS) is the most important prognostic factor in advanced non–small-cell lung cancer (NSCLC). Prospective clinical trials and retrospective analyses in the 1980s suggested that patients with advanced NSCLC and compromised PS experienced substantial toxicity and derived no benefit from systemic chemotherapy.1 This observation led to the exclusion of PS 2 patients from cooperative group trials in the subsequent decade. As a consequence, the clinical management of PS 2 patients became largely empirical, ranging from supportive care alone to aggressive chemotherapy.
More recent trials have reestablished the role of chemotherapy in the management of PS 2 patients, but the optimal strategy remains unclear. Carboplatin-based combinations may offer an improvement in response rate and progression-free survival (PFS) over single-agent therapy,2,3 but a survival advantage has not been demonstrated, and current guidelines recommend single-agent therapy for the majority of PS 2 patients.4,5 One such option is weekly docetaxel, which generated promising results in a multicenter phase II trial and represents a reasonable template for newer combination regimens in this setting.6
Recent benefits from targeted therapy in advanced NSCLC have not yet translated to PS 2 patients. While the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors have shown benefit in the salvage setting, studies of gefitinib and erlotinib in the first-line therapy of PS 2 patients have yielded results that appear inferior to standard chemotherapy.7–9 The one exception, reported after our study had been closed, is the phase III FLEX trial of cetuximab in combination with chemotherapy, which demonstrated a survival advantage compared with chemotherapy alone that appeared to extend to the small PS 2 subset enrolled in the trial.10
Both cetuximab and bortezomib were tested in combination with docetaxel,11,12 yielding results that were felt to be of potential applicability to the PS 2 population. Therefore, we designed this trial to incorporate these new agents into the first-line management of PS 2 patients with advanced NSCLC.
PATIENTS AND METHODS
Eligibility
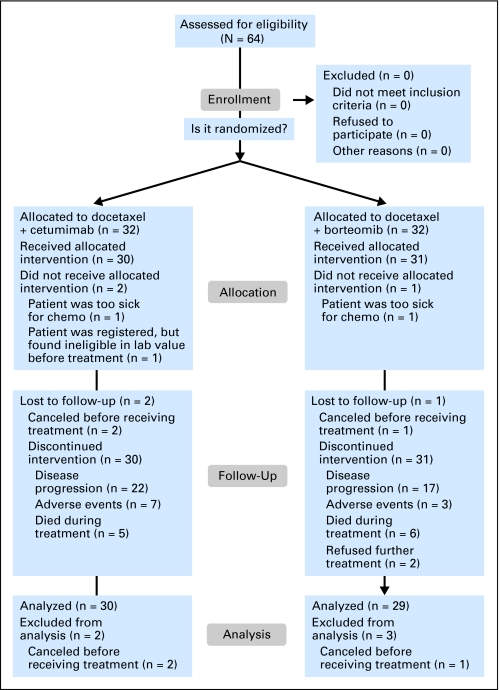
Patients with cytologic or histologic confirmation of stage IIIB (malignant effusion) and stage IV NSCLC were required to have measurable/nonmeasurable or evaluable disease and a PS of 2 by CALGB (Cancer and Leukemia Group B) criteria, as assigned by the treating physician. Patients with locally advanced disease amenable to combined-modality therapy were not eligible. Patients with brain metastases were eligible if neurologically stable and off steroids after appropriate therapy. No prior chemotherapy for advanced disease and no prior EGFR-directed therapy were allowed. Prior treatment for early-stage disease was permitted if completed at least 12 months before registration. Prior radiation was allowed and toxicities had to be resolved before study entry. Adequate organ function and less than grade 2 neuropathy were required. Patients with a currently active second malignancy except for nonmelanoma skin cancers were not eligible (Fig 1). Approval by the institutional review board at each participating institution was required. All patients signed informed consent. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. The statistical analyses were performed by CALGB statisticians.
Fig 1.
CONSORT diagram.
Treatment Plan
Patients were randomly assigned to docetaxel 30 mg/m2 IV over 30 minutes on days 1, 8, and 15 every 28 days in combination with cetuximab 400 mg/m2 IV over 120 minutes loading dose followed by 250 mg/m2 over 60 minutes weekly thereafter, or in combination with bortezomib 1.6 mg/m2 IV bolus on days 1, 8, and 15 every 28 days for up to 4 cycles. Patients who did not progress were allowed to continue cetuximab or bortezomib until documented progression or unacceptable toxicity.
Dose modifications were recommended for specific hematologic and nonhematologic toxicities outlined in the protocol. Dose level 1: docetaxel 25 mg/m2, cetuximab 200 mg/m2, and bortezomib 1.2 mg/m2; dose level 2: docetaxel 20 mg/m2, cetuximab 150 mg/m2, and bortezomib 0.9 mg/m2. Patients with persistent or recurrent toxicities despite dose reduction to level 2 or patients whose therapy was withheld for ≥ 3 weeks were removed from protocol therapy. Administration of myeloid growth factors was not routinely permitted. Response was assessed by imaging studies every two cycles and was evaluated by RECIST (Response Evaluation Criteria in Solid Tumors).5
Statistical Analysis
The primary objective was to determine the activity of two novel combinations as first-line therapy for PS 2 patients with advanced NSCLC. The primary end point was PFS at 6 months after registration/random assignment. For either arm of the study, if the true 6-month PFS was ≤ 20%, the treatment would be considered ineffective. If the true 6-month PFS was ≥ 42%, the treatment would be worthy of further evaluation. Using a one-sided test at the significance level of 0.10, 31 patients were needed in each arm to have 90% power to differentiate the null hypothesis (6-months PFS ≤ 20%) versus the alternative hypothesis (6-months PFS ≥ 42%). Of the 31 patients, 10 or more (≥ 32%) remaining progression free at 6 months was needed to conclude that the regimen was worthy of further evaluation. The study was not adequately powered to compare the two treatment arms.
Additional end points included response rate, overall survival, and toxicities. All eligible patients who received any protocol therapy were considered evaluable. Eligible patients who received at least two cycles of therapy and had disease reassessed were considered evaluable for response. Survival proportions were estimated from Kaplan-Meier curves.
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of nine patients (14%) of the 64 patients under this study.
RESULTS
Baseline Characteristics
A total of 64 patients were randomly assigned between July 2005 and September 2006 to D + C (n = 32) or D + B (n = 32). Two patients, one in each arm, were deemed ineligible. In addition, three patients, two in D + C and one in D + B, never received protocol therapy. The remaining 59 patients (30 in D + C and 29 in D + B) were included in this analysis. There were no major differences between the two arms in baseline characteristics (Table 1). Approximately 80% of patients had stage IV disease, and 51% were age 70 years or older. There were slightly more adenocarcinoma patients in the D + B arm (55%) than in the D + C arm (37%) but specific histologic type was not available in 24% of patients. There were five patients (17%) in the D + C arm and two patients (7%) in the D + B arm with treated brain metastases.
Table 1.
Patient Characteristics
| Characteristic | D + C(n = 30) |
D + B(n = 29) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Female | 9 | 30 | 11 | 38 |
| Male | 21 | 70 | 18 | 62 |
| Age, years | ||||
| < 70 | 15 | 50 | 14 | 48 |
| ≥ 70 | 15 | 50 | 15 | 52 |
| Race | ||||
| Caucasian | 29 | 97 | 26 | 90 |
| Other | 1 | 3 | 3 | 10 |
| Histology | ||||
| Adenocarcinoma | 11 | 37 | 16 | |
| Squamous carcinoma | 8 | 27 | 7 | 24 |
| Undifferentiated | 11 | 36 | 6 | 21 |
| Brain metastases | ||||
| Yes | 5 | 17 | 2 | 7 |
| No | 25 | 83 | 27 | 93 |
NOTE. No significant differences between D + C and D + B characteristics.
Abbreviations: D + C, docetaxel plus cetuximab; D + B, docetaxel plus bortezomib.
Patient Disposition
The median number of cycles received was two per patient. Only 29% of patients (30% in D + C and 28% in D + B) completed the prescribed four cycles of therapy. In the D + C arm, six patients (20%) went on to receive maintenance therapy with a median of four cycles. In the D + B arm, five patients (17%) received maintenance therapy with a median of two cycles. Principal reasons for removal or discontinuation from study in the D + C and D + B arms included disease progression (40% and 59%), adverse event/toxicity (23% and 7%), death (17% and 21%), patient request (17% and 7%), and others (3% and 7%), respectively. Data on second-line therapy were not collected.
Toxicity
Maximum hematologic toxicity in the D + C arm included one episode of grade 3 neutropenia (3%), one episode of grade 3 thrombocytopenia (3%), and three episodes of grade 3/4 anemia (10%). In the D + B arm, no grade 3/4 neutropenia was noted, whereas grade 3 thrombocytopenia and anemia occurred once each (Table 2). Selected grade 3 nonhematologic toxicities in the D + C arm included fatigue (17%), skin rash (17%), dehydration (13%), and stomatitis (10%). One patient had a hypersensitivity reaction. In the D + B arm, fatigue (20%) and dehydration (7%) were the most frequently noted toxicities. Grade 5 events included one patient with pulmonary infiltrates in the D + C arm and one patient with sudden death in the D + B arm, which was attributed as possibly related to treatment by the treating physicians.
Table 2.
Treatment-Related Adverse Events
| Adverse Events | D + C (n = 30) |
D + B (n = 29) |
||||||
|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4* |
Grade 3 |
Grade 4* |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Anemia | 2 | 7 | 1 | 3 | 1 | 3 | — | |
| Neutropenia | 1 | 3 | — | — | — | |||
| Thrombocytopenia | 1 | 3 | — | 1 | 3 | — | ||
| Fatigue | 5 | 17 | — | 5 | 17 | 1 | 3 | |
| Dehydration | 4 | 13 | 2 | 7 | ||||
| Hypersensitivity | 1 | 3 | ||||||
| Skin rash† | 5 | 17 | — | 1 | 3 | — | ||
| Nausea/vomiting | 1 | 3 | — | 1 | 3 | — | ||
| Diarrhea | 1 | 3 | — | 2 | 6 | — | ||
| Stomatitis | 3 | 10 | — | — | — | |||
| Pulmonary‡ | 2 | 7 | 1 | 3 | 1 | 3 | 2 | 7 |
| Neuropathy§ | 1 | 3 | — | 1 | 3 | — | ||
| Infection | 1 | 3 | — | 1 | 3 | — | ||
Abbreviations: D + C, docetaxel plus cetuximab; D + B, docetaxel plus bortezomib.
Two patients experienced grade 5 (fatal) treatment-related events: one lung infiltration in the D+ C arm and one sudden death in the D + B arm.
Pooled terms: dermatitis acneiform, dermatitis exfoliative, maculopapular rash, erythema, generalized rash, skin desquamation, skin disorder (other), dry skin, hand-foot skin reaction.
Pooled terms: dyspnea and pneumonitis/pulmonary infiltrates.
Pooled terms: peripheral motor and peripheral sensory neuropathy.
Efficacy
Response assessments were inadequate in seven patients (23%) in the D + C arm and four patients (14%) in the D + B arm. Reasons for lack of adequate response data are outlined in Table 3. Objective responses based on intention to treat were observed in four of a total of 30 patients in the D + C arm (13.3%; 95% CI, 3.8 to 30.7) and in three of 29 patients in the D + B arm (10.3%; 95% CI, 2.2 to 27.4).
Table 3.
Efficacy Results
| Variable | D + C (n = 30) | D + B (n = 29) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Response | ||||
| Complete response | 1 | 3 | 0 | 0 |
| Partial response | 3 | 10 | 3 | 10 |
| Stable disease | 11 | 37 | 9 | 31 |
| Progressive disease | 8 | 27 | 13 | 45 |
| Unable to determine | 7 | 23 | 4 | 14 |
| Median PFS, months | 3.4 | 1.9 | ||
| 95% CI | 2.0 to 4.7 | 1.7 to 3.0 | ||
| 6-month PFS, % | 27.8 | 13.8 | ||
| 95% CI | 13.2 to 44.5 | 4.3 to 28.6 | ||
| Median survival, months | 5.0 | 3.9 | ||
| 95% CI | 3.1 to 10.7 | 2.2 to 11.0 | ||
| 6-month survival, % | 48.4 | 48.3 | ||
| 95% CI | 29.6 to 64.9 | 29.5 to 64.8 | ||
NOTE. On D + C, two patients were taken off treatment due to adverse events during cycle 1, three patients refused further therapy during cycle 1, and two patients died within 1 month during cycle 2. On D + B, three patients died during cycle 1 or 2, and one patient received only one cycle and went on to nonprotocol therapy.
Abbreviations: D + C, docetaxel plus cetuximab; D + B, docetaxel plus bortezomib; PFS, progression-free survival.
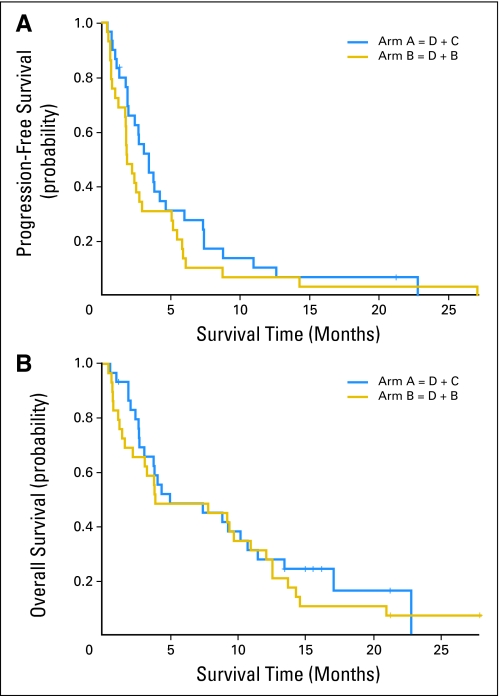
Median PFS was 3.4 months (95% CI, 2.0 to 4.7 months) for patients treated with D + C and 1.9 months (95% CI, 1.7 to 3.0 months) for patients treated with D + B (Fig 2A). The 6-month PFS rates were 28% (95% CI, 13.2% to 44.5%) and 13.8% (95% CI, 4.3% to 28.6%), respectively. Median survival times were 5.0 months (95% CI, 3.1 to 10.7 months) and 3.9 months (95% CI, 2.2 to 11.0 months), respectively, and 6-month survival rates were 48.4% (95% CI, 29.6% to 64.9%) and 48.3% (95% CI, 29.5% to 64.8%), respectively (Fig 2B).
Fig 2.
Kaplan-Meier plots. (A) Progression-free survival (PFS): patients treated with docetaxel plus cetuximab (D + C) had a median PFS of 3.4 months; patients treated with docetaxel plus bortezomib (D + B) had a median PFS of 1.9 months. (B) Overall survival: median survival times were 5.0 months in the D + C group and 3.9 months in the D + B group.
DISCUSSION
Recent progress in the first-line treatment of patients with advanced NSCLC, particularly the use of targeted and antiangiogenesis therapy, has not fully translated into the PS 2 population, which has been largely excluded from the pivotal trials. The primary goal of our study was to evaluate the safety and efficacy of new biologic regimens in PS 2 patients.
Our choice of weekly docetaxel as the chemotherapy template was based on a previous phase II trial in which this regimen was found to be active and well tolerated by PS 2 patients.6 Single-agent cetuximab produced a low response rate in previously treated patients,13 but the combination of docetaxel and cetuximab in a similar population led to a response rate of 28% with an acceptable toxicity profile.11 Bortezomib had also shown preliminary activity in NSCLC, and the weekly schedule, previously tested in prostate cancer,14 seemed particularly suitable for use in combination with weekly docetaxel in the front-line treatment of PS 2 patients.
The results in the D + B arm did not seem better than those reported for single-agent docetaxel. After our study was concluded, the results of a randomized phase II trial testing bortezomib alone or in combination with docetaxel in previously treated patients were published.12 Response rates were low in both arms (8% and 9%), and despite a nonsignificantly longer time to progression in the combination arm (4.0 v 1.5 months), no discernible differences in median or 1-year survival rates were noted between the two arms. Despite a subsequent SWOG (Southwest Oncology Group) single-arm phase II trial, which reported a median survival of 11 months for the combination of bortezomib with carboplatin and gemcitabine in the first-line setting,15 the overall experience with bortezomib in unselected patients with advanced NSCLC has not justified further exploration of this agent, and our experience in PS 2 patients corroborates this impression.
In contrast, while the results in the D + C arm also fell below the prespecified statistical threshold established in the protocol for further investigation, which in retrospect may have been overly ambitious, the outcome data for D + C seemed comparable to other treatment strategies in PS 2 patients. In our benchmark trial, CALGB 9730, which compared the combination of carboplatin and paclitaxel with paclitaxel alone, the PFS and median survival time (MST) in 99 eligible PS 2 patients were 1.3 and 2.5 months in the single-agent arm, and 2.6 and 4.4 months for the combination, similar to 3.4 and 5.0 months seen in the D + C arm of in this trial. Since the conclusion of our study, preliminary results of the phase III FLEX trial have been reported.10 A modest but significant survival advantage for cisplatin and vinorelbine with cetuximab compared with chemotherapy alone was observed in 1,125 patients whose tumors expressed EGFR by immunohistochemistry criteria. This benefit seemed to be maintained in 196 PS 2 patients enrolled in the trial.
Our study illustrates the challenges associated with the treatment of PS 2 patients in clinical practice. Despite a modest rate of actual treatment-related complications and a notable lack of severe hematologic toxicities, a substantial percentage of patients did not complete the prescribed treatment, and an even lower number received maintenance therapy. As reported in previous trials,16 treatment discontinuation in PS 2 patients is mostly due to disease-related rather than treatment-related complications. In contrast to patients with a better PS, for whom the paradigm of multiple lines of therapy tend to apply, the majority of PS 2 patients progress before completing two cycles of first-line treatment and do not have an opportunity to receive second-line therapy. While toxicity remains a fundamental concern and should continue to guide the selection of therapy in PS 2 patients, the lack of treatment efficacy remains the overwhelming reason for the poor prognosis associated with this patient subset. Further, a better understanding of the reasons for a low PS, as it relates to cancer or comorbidities, should lead to a more tailored treatment strategy.
In an era of personalized therapy, no data are yet available for selection of therapy on the basis of molecular features in PS 2 patients. The use of targeted agents as first-line therapy in unselected PS 2 patients does not seem appropriate.8 On the other hand, antiangiogenesis therapy, for which no specific predictor of benefit has been identified, may be more widely applicable in this patient population, provided that toxicities remain acceptable. There is scarce data on the use of bevacizumab in PS 2 patients and, at this time, its use cannot be recommended without additional safety data. A recent Canadian trial that tested the addition of cederanib (a potent vascular endothelial growth factor receptor inhibitor) to chemotherapy showed prohibitive toxicity and led to the subsequent exclusion of PS 2 patients.17 Studies of sunitinib and sorafenib, in different settings, also excluded PS 2 patients and cannot be recommended in this setting.
In summary, innovative treatments are urgently needed for patients with advanced NSCLC and PS 2. While D + C produced results that appear comparable to other current regimens with an acceptable toxicity profile, it did not meet our estimate for further pursuits. The role of EGFR inhibitors in unselected PS 2 patients remains unclear. Studies to incorporate antiangiogenesis agents in the treatment of PS 2 patients will be our next pursuit in CALGB.
Appendix
The following institutions participated in this study: Christiana Care Health Services, Community Clinical Oncology Program (CCOP), Wilmington, DE, Stephen Grubbs, MD, supported by CA45418; Georgetown University Medical Center, Washington, DC, Minetta C. Liu, MD, supported by CA77597; Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY, Jeffrey Kirshner, MD, supported by CA45389; Missouri Baptist Medical Center, St. Louis, MO, Alan P. Lyss, MD, supported by CA114558-02; Mount Sinai Medical Center, Miami, FL, Rogerio C. Lilenbaum, MD, supported by CA45564; Nevada Cancer Research Foundation CCOP, Las Vegas, NV, John A. Ellerton, MD, supported by CA35421; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN, Rafat Ansari, MD, supported by CA86726; Southeast Cancer Control Consortium CCOP, Goldsboro, NC, James N. Atkins, MD, supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, MD, supported by CA77658; University of California at San Diego, San Diego, CA, Barbara A. Parker, MD, supported by CA11789; University of Chicago, Chicago, IL, Gini Fleming, MD, supported by CA41287; University of Iowa, Iowa City, IA, Daniel A. Vaena, MD, supported by CA47642; University of Illinois Minority-Based CCOP (MBCCOP), Chicago, IL, Lawrence E. Feldman, MD, supported by CA74811; University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, MD, supported by CA31983; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO, Michael C. Perry, MD, supported by CA12046; University of Nebraska Medical Center, Omaha, NE, Anne Kessinger, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD, supported by CA47559; University of Vermont, Burlington, VT, Hyman B. Muss, MD, supported by CA77406; Washington University School of Medicine, St. Louis, MO, Nancy Bartlett, MD, supported by CA77440.
Footnotes
Written on behalf of the Cancer and Leukemia Group B (CALGB).
Supported in part by Grants No. CA31946 from the National Cancer Institute to the Cancer and Leukemia Group B (CALGB; R.L.S.) and CA33601 to the CALGB Statistical Center (S.G.); and by Grants No. CA45564, CA47577, CA45389, CA45808, and CA41287.
Presented in abstract form at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Rogerio Lilenbaum, sanofi-aventis (C), ImClone Systems (C); Everett Vokes, sanofi-aventis (C), ImClone Systems (C) Stock Ownership: None Honoraria: Rogerio Lilenbaum, sanofi-aventis, ImClone Systems; Everett Vokes, sanofi-aventis, ImClone Systems Research Funding: Rogerio Lilenbaum, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Rogerio Lilenbaum, Xiaofei Wang, Lin Gu, Everett Vokes
Administrative support: Xiaofei Wang, Lin Gu, Everett Vokes
Provision of study materials or patients: Rogerio Lilenbaum, Jeffrey Kirshner, Keith Lerro
Collection and assembly of data: Rogerio Lilenbaum, Xiaofei Wang, Lin Gu, Jeffrey Kirshner, Keith Lerro
Data analysis and interpretation: Rogerio Lilenbaum, Xiaofei Wang, Lin Gu, Everett Vokes
Manuscript writing: Rogerio Lilenbaum, Xiaofei Wang, Lin Gu, Jeffrey Kirshner, Keith Lerro, Everett Vokes
Final approval of manuscript: Rogerio Lilenbaum, Xiaofei Wang, Lin Gu, Jeffrey Kirshner, Keith Lerro, Everett Vokes
REFERENCES
- 1.Lilenbaum R. Treatment of advanced non-small-cell lung cancer in special populations. Oncology (Williston Park) . 2004;18:1321–1325. [PubMed] [Google Scholar]
- 2.Lilenbaum RC, Herndon JE, II, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: The Cancer and Leukemia Group B (Study 9730) J Clin Oncol . 2005;23:190–196. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 3.Obasaju CK, Conkling P, Richards D, et al. A randomized phase 3 trial of gemcitabine with or without carboplatin in performance status 2 (PS2) patients (pts) with advanced (stage IIIB with pleural effusion or stage IV) non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(suppl):392s. abstr 7533. [Google Scholar]
- 4.Gridelli C, Ardizzoni A, Le Chevalier T, et al. Treatment of advanced non-small-cell lung cancer patients with ECOG performance status 2: Results of a European Experts Panel. Ann Oncol . 2004;15:419–426. doi: 10.1093/annonc/mdh087. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Non-Small Cell Lung Cancer Clinical Practice Guidelines in Oncology (Version 2.2008) 2008. http://www.nccn.org. [DOI] [PubMed]
- 6.Lilenbaum R, Rubin M, Samuel J, et al. A randomized phase II trial of two schedules of docetaxel in elderly or poor performance status patients with advanced non-small cell lung cancer. J Thorac Oncol . 2007;2:306–311. doi: 10.1097/01.JTO.0000263713.38826.8e. [DOI] [PubMed] [Google Scholar]
- 7.Hesketh PJ, Chansky K, Wozniak AJ, et al. Erlotinib as initial therapy in patients with advanced non-small cell lung cancer patients and a performance status of 2: A SWOG phase II trial (S0341) J Clin Oncol. 2007;25(suppl):393s. abstr 7536. [Google Scholar]
- 8.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol . 2008;26:863–869. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 9.Morere J, Westeel V, Morin F, et al. Randomized phase II trial of first-line gefitinib, gemcitabine, or docetaxel in performance status 2 or 3 non-small-cell lung cancer patients (IFCT-0301) J Clin Oncol. 2008;26(suppl):445s. abstr 8086. [Google Scholar]
- 10.Pirker R, Szczesna A, von Pawel J, et al. FLEX: A randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first-line treatment of patients with advanced non-small cell lung cancer. J Clin Oncol. 2008;26(suppl):6s. abstr 3. [Google Scholar]
- 11.Kim ES, Mauer AM, William WN, Jr, et al. A phase 2 study of cetuximab in combination with docetaxel in chemotherapy-refractory/resistant patients with advanced non-small cell lung cancer. Cancer . 2009;115:1713–1722. doi: 10.1002/cncr.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol . 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 13.Hanna N, Lilenbaum R, Ansari R, et al. Phase II trial of cetuximab in patients with previously treated non-small cell lung cancer. J Clin Oncol . 2006;24:5253–5258. doi: 10.1200/JCO.2006.08.2263. [DOI] [PubMed] [Google Scholar]
- 14.Dreicer R, Petrylak D, Aqus D, et al. Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Cancer Res . 2007;13:1208–1215. doi: 10.1158/1078-0432.CCR-06-2046. [DOI] [PubMed] [Google Scholar]
- 15.Davies AM, McCoy J, Lara PN, et al. Bortezomib + gemcitabine (Gem)/carboplatin (Carbo) results in encouraging survival in advanced non-small cell lung cancer (NSCLC): Results of a phase II Southwest Oncology Group (SWOG) trial (S0339) J Clin Oncol. 2006;24(suppl):368s. abstr 7017. [Google Scholar]
- 16.Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: A phase II trial in patients with metastatic non-small cell lung carcinoma. Cancer . 2001;92:2639–2647. doi: 10.1002/1097-0142(20011115)92:10<2639::aid-cncr1617>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Wheatley-Price P, Shepherd F. Targeting angiogenesis in the treatment of lung cancer. J Thorac Oncol. 2008;10:1173–1184. doi: 10.1097/JTO.0b013e318187220f. [DOI] [PubMed] [Google Scholar]