Abstract
Purpose
Some women with early-stage breast cancer are at higher risk of recurrence and can benefit from chemotherapy. We describe patterns of referral, receipt, and completion of chemotherapy among older women at high risk of recurrence.
Patients and Methods
A total of 2,124 women age 65 years or older who were diagnosed with early-stage breast cancer between 1990 and 1994 and 1996 to 1999 were included; 1,090 of these were at high risk of recurrence. We reviewed medical records to categorize chemotherapy outcomes as follows: did not discuss or were not referred to a medical oncologist (n = 133); discussed and/or referred to a medical oncologist but received no chemotherapy (n = 742); received an incomplete chemotherapy course (n = 29), or received a completed chemotherapy course (n = 186).
Results
Overall, 19.7% of high-risk women received any chemotherapy, and 86.5% of these women completed their chemotherapy courses. Just greater than 10% of high-risk women did not have a discussion about chemotherapy as part of breast cancer treatment documented in the medical record; these women also received fewer diagnostic assessments of their initial tumors.
Conclusion
Individuals who receive chemotherapy for early-stage breast cancer are a select subgroup of patients at high risk of recurrence. This study identifies characteristics of women who were referred for and who received chemotherapy, and this study plays an important role in understanding generalizability of studies that examine chemotherapy treatment effectiveness. Outcomes after breast cancer could continue to be improved with increased receipt of chemotherapy among older women at high risk of breast cancer recurrence.
INTRODUCTION
When diagnosed early, women with breast cancer have favorable long-term outcomes. Clinical trials and consensus guidelines identify subsets of women with early-stage breast cancer who can benefit from chemotherapy.1–4 Although chemotherapy treatment guidelines have evolved over time, the main criteria used to identify women who could benefit from chemotherapy have not significantly changed; nearly all criteria include women with stage II disease or women with poorly differentiated or undifferentiated tumors.5–7 Chemotherapy guidelines have been particularly unclear for older women because of the lack of clinical trial efficacy data and concerns about toxicities in older women.
Randomized, controlled trials address the efficacy of various therapies but are limited in generalizability because of restrictive enrollment criteria.8 Although observational studies offer advantages for examining the effectiveness of therapies in population-based settings, they too have limitations, including confounding by patient and tumor characteristics. Three recent articles that used Surveillance Epidemiology and End Results (SEER) – Medicare data examined chemotherapy treatment effectiveness in slightly different populations of women with breast cancer.9–11 An editorial that accompanied two of these papers highlighted the critical importance of understanding biases in observational studies that examine chemotherapy effectiveness, primarily because only a subgroup of patients with an indication for chemotherapy actually receive chemotherapy.12 It is important to understand reasons for not receiving chemotherapy among women with a clinical indication for receiving chemotherapy.
We report results from two observational, cohort studies conducted among women with early-stage breast cancer with tumor characteristics associated with high risk of recurrence.5–7 The purpose of this report is to describe patterns of referral, receipt, and completion of chemotherapy among older women in population-based settings at high risk of breast cancer recurrence.
PATIENTS AND METHODS
Study Populations
This report includes data from two observational, cohort studies. Both studies had institutional review board approval at all participating sites that included waivers of consent to access medical records. The first study, the Breast Cancer Treatment Effectiveness in Older Women (BOW) study,13 was conducted within the National Cancer Institute–funded Cancer Research Network (CRN).14 The CRN is a consortium of 14 integrated, health care–delivery systems with more than 10 million enrollees.14 The overall goal of the CRN is to assess and increase the effectiveness of preventive, curative, and supportive interventions for major cancers through a program of collaborative research among diverse populations and health systems. The BOW study included 1,859 women age 65 years or older who were diagnosed with incident, early-stage breast cancer (ie, American Joint Commission on Cancer stages I to II)6 from 1990 through 1994 within six health care organizations: Group Health Cooperative, Seattle, WA; Fallon Clinic, Worcester, MA; Henry Ford, Detroit, MI; HealthPartners, Minneapolis, MN; Kaiser Permanente Southern California, Pasadena, CA; and Lovelace, Albuquerque, NM. We included all eligible patients from all sites except Kaiser Permanente Southern California, where we sampled 10% of the subgroup of non-Hispanic white patients younger than 80 years with stage I breast cancer.
The second study included women diagnosed at Group Health Cooperative with incident, early-stage breast cancer from the American Cancer Society (ACS) –funded BOW sister study. With the exception of age and date of diagnosis, the same study entry criteria were applied to the ACS cohort for women age 18 years or older who were diagnosed from 1996 through 1999 (n = 908). The same abstraction instrument and methods as the BOW study were used. This report is limited to the ACS cohort of women 65 years or older for comparability to the BOW cohort (n = 431).
The total cohort included 2,290 women. We excluded 144 women diagnosed with adenocarcinoma of unspecified, mucinous, or tubular histology and 22 women who did not receive definitive surgery; thus, 2,124 women remained for consideration in this analysis.
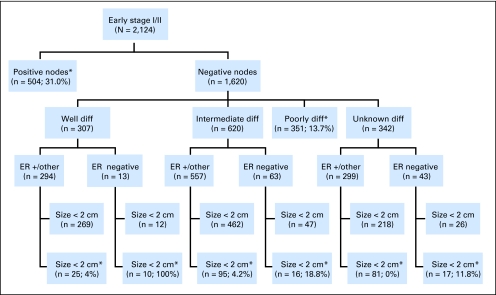
Because clinical guidelines that identify women who should be considered for chemotherapy have changed over time and have not been consistent for older women,1–4 we defined high risk of recurrence for this study by using American Joint Commission on Cancer staging guidelines,5–7 and we included all stage II disease and all poorly differentiated or undifferentiated tumors. Just greater than 50% of our sample, (n = 1,090; 51.3% of eligible) met the high-risk criteria (Fig 1) and comprised our study population for this analysis.
Fig 1.
Tumor characteristics of 2,124 women aged 65 years or older who were diagnosed between 1990 and 1994 and 1996 to 1999 with incident, invasive, early-stage (ie, stages I to II) breast cancer stratified by nodal status, differentiation (diff), estrogen receptor (ER) status, and tumor size. Of these women, 1,090 were in high-risk subgroups (8 boxes with the number of women in each subgroup and the percent of those who received any chemotherapy). (*) Women considered at high risk of recurrence, in whom chemotherapy could have been considered.5–7
Extensive details about this study methodology have been published previously13 and are summarized in this Patients and Methods section. Briefly, trained medical records abstractors completed standardized abstractions and directly entered information into a computerized data collection system that included a variety of preloaded automated data from cancer registry, administrative, and clinical databases.15 Women were ineligible if they had another clinically active malignancy, except nonmelanoma skin cancer diagnosed within 5 years or less, before or within 30 days after their breast cancer diagnosis; had bilateral breast cancer; or were enrolled in their health systems for less than 12 months before or after diagnosis. Women who died less than 12 months after diagnosis were not excluded from this study.
Data collection included date of diagnosis, stage at diagnosis, tumor size, lymph node evaluation, estrogen receptor (ER) and progesterone receptor (PR) protein positivity (ER positive or PR positive; ER negative and PR negative; or other [not done, ordered but no results, unknown]), histologic grade, stage (I, IIA, or IIB),6 age at diagnosis, and ethnicity (non-Hispanic white, Hispanic, African American, Asian, or other [includes unknown]). We used national consensus guidelines16 to define primary tumor therapy as follows: mastectomy, breast-conserving surgery (BCS) with radiation therapy, or BCS without radiation therapy. Medical records were used to collect comorbid conditions in the year before diagnosis to calculate a Charlson comorbidity index.17 We separately examined whether the presence of myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular event, hypertension (not included in the Charlson index), chronic obstructive pulmonary disease, connective tissue disease, ulcer disease, or diabetes mellitus differed by outcomes (described in the Results section); comorbid conditions were not mutually exclusive.
Referral for and Receipt of Chemotherapy
We recorded whether the medical record documented a discussion between the patient and surgeon or oncologist about receiving chemotherapy and whether patients were referred to a medical oncologist. These data were abstracted from medical records that included visits from providers seen inside and outside the integrated health plans. Women were categorized into mutually exclusive groups for chemotherapy receipt as follows: did not discuss or were not referred to a medical oncologist (not discussed/referred, n = 133), documented discussion and/or referral to a medical oncologist but did not receive chemotherapy (not received, n = 742), documented receipt of an incomplete course of chemotherapy (incomplete, n = 29), and documented receipt of a complete course of chemotherapy (completed, n = 186) on the basis of whether the patient completed the oncologist's recommended chemotherapy course. We examined tumor characteristics, primary therapy, and demographic and health characteristics by each chemotherapy group.
Documented reasons why patients did not discuss receiving chemotherapy or did not get referred to a medical oncologist were collected at the time of medical record review. All documented reasons for nonreferral, including logistical issues, concern about possible adverse effects, age, comorbidities, chemotherapy not indicated, or other reasons, were collected. Although reasons for nonreferral or nonreceipt were not mutually exclusive, only one source (ie, patient or family member v physician) could be recorded for each reason. We also collected any noted reasons why the chemotherapy was not completed, which included specific, documented adverse effects.
Sensitivity Analysis
We varied our definition of high risk of recurrence on the basis of varying clinical guidelines, including 19921 and 199518 St Gallen criteria, 19902 and 2000 National Institutes of Health3 consensus guidelines with and without inclusion of women age 70 years or older to demonstrate the influence of the high-risk inclusion criteria on chemotherapy referral and receipt. These varying clinical guidelines shifted the definition of high risk by varying criteria around tumor size, ER status, and lymph node status.
RESULTS
Among the 1,090 high-risk women (Fig 1), 12.2% did not discuss chemotherapy or were not referred to a medical oncologist; 68.1% had a documented discussion about chemotherapy but did not receive chemotherapy; 2.7% received an incomplete course of chemotherapy; and 17.1% received a full course of chemotherapy (Table 1). Having unmeasured tumor characteristics (grade unmeasured, unknown ER/PR status, or no nodal evaluation) was associated with not having a discussion or referral noted and not receiving chemotherapy. Most women (84.3%) who received BCS without radiation did not discuss, were not referred, or did not receive chemotherapy. Younger women were more likely to receive any chemotherapy (31.1% for age 65 to 69 years; 20.7% for age 70 to 74 years; 10.2% for age 75 to 79 years; and 2.7% for age 80 years or older). Less than 5% of women had Charlson comorbidity index scores ≥ 2 (and higher scores are indicative of more severe comorbidity); nearly all (97.9%) of these women were not referred or did not discuss chemotherapy. The prevalence of individual comorbid conditions, except for hypertension, was low. Women with cerebrovascular disease, hypertension, or dementia were less likely to receive chemotherapy. Receiving chemotherapy was more common in recent years. Among women who received any chemotherapy, women with nearly all comorbid conditions, except chronic obstructive pulmonary disease and connective tissue disease, were more likely to stop their chemotherapy before treatments were considered complete.
Table 1.
Discussion and Referrals for Chemotherapy Among 1,090 Women With Early-Stage, Incident-Invasive Breast Cancer Diagnosed Between 1990 to 1994 and 1996 to 1999
| Variable | Patients by Chemotherapy Referral and Discussion Category |
|||||||
|---|---|---|---|---|---|---|---|---|
| Not Discussed/Referred (n = 133) |
Not Received (n = 742) |
Incomplete (n = 29) |
Completed (n = 186) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Tumor characteristic | ||||||||
| Size, cm | ||||||||
| ≤ 1 | 73 | 54.9 | 388 | 52.3 | 21 | 72.4 | 92 | 49.5 |
| > 1-2 | 36 | 27.1 | 246 | 33.2 | 6 | 20.7 | 72 | 38.7 |
| > 2 | 24 | 18.0 | 108 | 14.6 | 2 | 6.9 | 22 | 11.8 |
| Histologic grade | ||||||||
| Well differentiated | 5 | 3.8 | 64 | 8.6 | 3 | 10.3 | 18 | 9.7 |
| Intermediate/moderately differentiated | 37 | 27.8 | 207 | 27.9 | 8 | 27.6 | 56 | 30.1 |
| Poorly or undifferentiated/anaplastic | 65 | 48.9 | 332 | 44.7 | 17 | 58.6 | 98 | 52.7 |
| Not determined/stated | 26 | 19.5 | 139 | 18.7 | 1 | 3.4 | 14 | 7.5 |
| ER/PR positivity | ||||||||
| ER or PR positive | 96 | 72.2 | 599 | 80.7 | 10 | 34.5 | 89 | 47.8 |
| ER and PR negative | 14 | 10.5 | 96 | 12.9 | 19 | 65.5 | 90 | 48.4 |
| Other* | 23 | 17.3 | 47 | 6.3 | 0 | 0.0 | 7 | 3.8 |
| No. of positive nodes | ||||||||
| Negative | 71 | 53.4 | 358 | 48.2 | 9 | 31.0 | 49 | 26.3 |
| 1 to 3 | 15 | 11.3 | 264 | 35.6 | 7 | 24.1 | 79 | 42.5 |
| ≥ 4 | 7 | 5.3 | 59 | 8.0 | 13 | 44.8 | 56 | 30.1 |
| Not done | 40 | 30.1 | 61 | 8.2 | 0 | 0.0 | 2 | 1.1 |
| Treatment type | ||||||||
| Primary therapy | ||||||||
| Mastectomy | 72 | 54.1 | 467 | 62.9 | 21 | 72.4 | 130 | 69.9 |
| BCS + RT | 34 | 25.6 | 223 | 30.1 | 6 | 20.7 | 53 | 28.5 |
| BCS only | 27 | 20.3 | 52 | 7.0 | 2 | 6.9 | 3 | 1.6 |
| Hormonal therapy | ||||||||
| No | 61 | 45.9 | 120 | 16.2 | 13 | 44.8 | 72 | 38.7 |
| Yes | 72 | 54.1 | 622 | 83.8 | 16 | 55.2 | 114 | 61.3 |
| Enrolled in randomized clinical trial for breast cancer treatment | 2 | 1.5 | 2 | 0.3 | 1 | 3.4 | 21 | 11.3 |
| Demographic and health characteristics | ||||||||
| Age at diagnosis, years | ||||||||
| 65-69 | 34 | 25.6 | 238 | 32.1 | 10 | 34.5 | 113 | 60.8 |
| 70-74 | 35 | 26.3 | 225 | 30.3 | 12 | 41.4 | 56 | 30.1 |
| 75-79 | 22 | 16.5 | 144 | 19.4 | 6 | 20.7 | 13 | 7.0 |
| ≥ 80 | 42 | 31.6 | 135 | 18.2 | 1 | 3.4 | 4 | 2.2 |
| Ethnicity | ||||||||
| White non-Hispanic | 113 | 85.0 | 629 | 84.8 | 26 | 89.7 | 163 | 87.6 |
| Hispanic | 3 | 2.3 | 28 | 3.8 | 1 | 3.4 | 6 | 3.2 |
| African American | 13 | 9.8 | 67 | 9.0 | 2 | 6.9 | 13 | 7.0 |
| Asian | 2 | 1.5 | 13 | 1.8 | 0 | 0.0 | 4 | 2.2 |
| Other† | 2 | 1.5 | 5 | 0.7 | 0 | 0.0 | 3 | 1.6 |
| Comorbidity information from diagnosis | ||||||||
| Charlson at diagnosis | ||||||||
| 0 | 83 | 62.4 | 481 | 64.8 | 17 | 58.6 | 151 | 81.2 |
| 1 | 35 | 26.3 | 230 | 31.0 | 12 | 41.4 | 34 | 18.3 |
| ≥ 2 | 15 | 11.3 | 31 | 4.2 | 0 | 0.0 | 1 | 0.5 |
| Myocardial infarction in past year or ever | 5 | 3.8 | 52 | 7.0 | 4 | 13.8 | 4 | 2.2 |
| Congestive heart failure | 9 | 6.8 | 37 | 5.0 | 1 | 3.4 | 3 | 1.6 |
| Peripheral vascular disease | 3 | 2.3 | 37 | 5.0 | 2 | 6.9 | 4 | 2.2 |
| Cerebrovascular history in past year or ever | 14 | 10.5 | 41 | 5.5 | 1 | 3.4 | 2 | 1.1 |
| Hypertension | 72 | 54.1 | 385 | 51.9 | 12 | 41.4 | 71 | 38.2 |
| Dementia | 13 | 9.8 | 9 | 1.2 | 0 | 0.0 | 0 | 0.0 |
| Chronic obstructive pulmonary disease | 12 | 9.0 | 59 | 8.0 | 1 | 3.4 | 9 | 4.8 |
| Connective tissue disease | 2 | 1.5 | 72 | 9.7 | 5 | 17.2 | 12 | 6.5 |
| Ulcer disease in past year or ever | 8 | 6.0 | 75 | 10.1 | 5 | 17.2 | 9 | 4.8 |
| Diabetes | 16 | 12.0 | 87 | 11.7 | 6 | 20.7 | 11 | 5.9 |
| Diagnosis year | ||||||||
| 1990 | 29 | 21.8 | 113 | 15.2 | 3 | 10.3 | 23 | 12.4 |
| 1991 | 25 | 18.8 | 123 | 16.6 | 5 | 17.2 | 19 | 10.2 |
| 1992 | 24 | 18.0 | 125 | 16.8 | 6 | 20.7 | 39 | 21.0 |
| 1993 | 28 | 21.1 | 130 | 17.5 | 3 | 10.3 | 34 | 18.3 |
| 1994 | 22 | 16.5 | 133 | 17.9 | 8 | 27.6 | 35 | 18.8 |
| 1996 | 1 | 0.8 | 33 | 4.4 | 1 | 3.4 | 4 | 2.2 |
| 1997 | 3 | 2.3 | 29 | 3.9 | 1 | 3.4 | 3 | 1.6 |
| 1998 | 1 | 0.8 | 23 | 3.1 | 0 | 0.0 | 14 | 7.5 |
| 1999 | 2 | 1.5 | 33 | 4.4 | 2 | 6.9 | 15 | 8.1 |
NOTE. Women met criteria for being at high recurrence risk for which chemotherapy should have been considered. Criteria used were American Joint Committee on Cancer staging guidelines5–7 and included all stage II disease and all poorly differentiated or undifferentiated tumors.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; BCS, breast-conserving surgery; RT, radiation therapy.
Other includes receptor test not done, ordered but no results, and unknown.
Other ethnicity includes American Indian/Alaskan native (n = 5; two not received and one completed); other (n = 2; not received), and unknown (n = 3; two not discussed, one not received).
Distribution of Chemotherapy Referral and Outcomes Among Women at High Risk
Figure 1 highlights the proportion of high-risk women by tumor subtype who received any chemotherapy. The greatest proportion of women who received chemotherapy (31.0%) was among node-positive women. A substantially smaller proportion of high-risk women with node-negative tumors received chemotherapy (59 [10.1%] of 586 women). The highest proportions were seen in poorly differentiated tumors (48 [13.7%] of 351 women); in intermediately differentiated tumors that were ER negative and greater than 2 cm (three [18.8%] of 16 women); and in tumors with unknown differentiation that were ER negative and greater than 2 cm (two [11.8%] of 17 women).
The distribution of chemotherapy referral and receipt differed when we varied our high-risk definition (Table 2). However, regardless of the high-risk definition used, the majority of women did not receive chemotherapy. The greatest proportion of women completed chemotherapy when the women age 70 years or older were excluded from the high-risk definitions, at which point just greater than 40% of women received chemotherapy.
Table 2.
Distribution of Chemotherapy Referral and Outcomes Among Women With Varying Definitions of High Risk
| Definition | No. of Patients | Patients by Chemotherapy Referral and Outcome Category (%) |
||||
|---|---|---|---|---|---|---|
| Not Discussed/Referred | Not Received | Incomplete | Completed | |||
| American Joint Committee on Cancer staging guidelines5–7* | 1,090 | 12.2 | 68.1 | 2.7 | 17.1 | |
| 1992 St Gallen criteria1 | ||||||
| All | 1,161 | 12.1 | 68.8 | 2.7 | 16.4 | |
| < 70 years of age; or ≥ 70 years of age with ER-negative disease | 659 | 10.6 | 62.1 | 3.6 | 23.7 | |
| 1995 St Gallen criteria18 | ||||||
| LN-positive disease; or LN-negative disease with either > 2 cm, ER-negative status, or grades 2 to 3 | 917 | 9.4 | 68.9 | 2.8 | 18.9 | |
| < 70 years of age with LN-positive disease; or LN-negative disease with either > 2 cm, ER-negative status, or grades 2 to 3 | 486 | 5.1 | 69.8 | 2.1 | 23.0 | |
| 1990 NIH consensus guidelines2 | ||||||
| LN-positive disease | 619 | 10.7 | 64.0 | 3.2 | 22.1 | |
| < 70 years of age with LN-positive disease | 224 | 4.9 | 54.9 | 3.1 | 37.1 | |
| 2000 NIH consensus guideines3 | ||||||
| LN-positive disease; or T > 1 cm regardless of nodal status | 1,211 | 11.0 | 71.0 | 2.5 | 15.5 | |
| < 70 years of age with LN-positive disease; or < 70 years of age with T > 1 cm regardless of nodal status | 419 | 6.7 | 63.5 | 2.4 | 27.4 | |
NOTE. Most of the clinical guidelines are equivocal about chemotherapy recommendations for older women.
Abbreviations: LN, lymph node; ER, estrogen receptor; NIH, National Institutes of Health.
Data presented are for this study.
Reasons for Nonreferral and Early Chemotherapy Termination
Half (n = 69) of the women who did not discuss or were not referred for chemotherapy had at least one reason documented in their medical record for nonreferral. The majority (84.6%) of reasons for nonreferral that were documented in the medical record were physician concerns. The four most common reasons for nonreferral were treatment was not indicated (n = 47), patient age (n = 21), other reason (not described, n = 17) and presence of comorbidities in the patient (n = 11).
Once chemotherapy was initiated, few women (29 [13.5%] of 215) stopped before completing a full course. Nineteen women had 47 documented reasons for not completing chemotherapy (data not shown). The most commonly recorded reasons were fatigue (10 of 19), cytopenia (seven of 19), mucositis (five of 19) and nausea/vomiting (four of 19).
DISCUSSION
Many factors, including access to care, ability to tolerate therapy, age, comorbidities, life expectancy, variation in provider recommendation, and risk of treatment complications, are associated with whether women at high risk of recurrence receive adjuvant chemotherapy. This article describes women age 65 years or older who met clinical criteria for being considered for chemotherapy and who had no documented discussion about chemotherapy or referral to a medical oncologist and who were referred for chemotherapy but did not receive or complete it. Our findings highlight that less than 20% of women at high risk of recurrence receive chemotherapy. Just greater than one in 10 high-risk women did not have a discussion about chemotherapy or a medical oncologist referral documented in their medical records as part of their breast cancer treatments. These data underscore that individuals who receive chemotherapy for early-stage breast cancer are a select subgroup of patients at high risk of recurrence. Importantly, all women in this study had access to health care and medical insurance, health maintenance organization (HMO)-Medicare and HMO-Medicaid, which reduces differences in receipt of chemotherapy that could arise because of socioeconomic status or other factors potentially associated with health insurance coverage and health care access. We expect the differences in referral and treatment patterns would have greater variability outside an integrated group practice.
Other studies support the findings of this study that chemotherapy is not consistently delivered to high-risk populations. The importance of understanding populations that do and do not receive therapies among indicated patients was highlighted recently, specifically in relation to chemotherapy receipt in older patients with breast cancer.12 In brief, two articles using the SEER-Medicare population examined factors associated with chemotherapy receipt and toxicity.9,10 The two studies reported different results regarding chemotherapy treatment effectiveness. The accompanying editorial12 underscored the importance of understanding unmeasured variables associated with chemotherapy receipt in clinical epidemiology and outcome studies; this understanding helps to ensure that improved survival in treatment groups is not misattributed to the treatment as opposed to the underlying confounder of receipt and nonreceipt of chemotherapy in these observational studies.
The women in this study received care for their breast cancer throughout the 1990s. We recognize that there have been changes in patterns of care with increasing use of BCS, radiation therapy after BCS, tamoxifen, and chemotherapy. Recent studies have shown increased chemotherapy use, even in older women19; however, chemotherapy receipt remains in the 30% range. We have no reason to believe that factors associated with referral have changed systematically since the 1990s. Our population-based cohort of women age 65 years or older included all women in our health plans with early-stage breast cancer, regardless of whether patients were referred to an oncologist; thus, the cohort represents an unbiased sample of patterns of referral for women with early-stage breast cancer with access to care in our respective communities.
Researching clinical outcomes over time in relation to clinical guidelines is a moving target that is impacted by changing clinical guidelines, changing definitions of high-risk groups, and differences in dissemination and diffusion of changing guidelines into practice. Because this study spanned many years, and because there continues to be controversy about chemotherapy for older women, we provided referral and outcome categories across a series of clinical guidelines classifications. There were differences in the proportion of chemotherapy referrals and receipt depending on the guidelines used and whether women age 70 years or older were considered; however, these results demonstrate that the majority of high-risk women do not receive chemotherapy.
Our report is important for understanding potential biases in observational studies that examine the effectiveness of chemotherapy receipt. Unfortunately, the characteristics of women who received chemotherapy varied in directions that could bias chemotherapy effectiveness study findings both toward and away from the null. For example, women who had fewer diagnostic assessments of their initial tumors (eg, incomplete staging, nodal dissection, radiation after BCS) were less likely to have a documented discussion about chemotherapy or to be referred to a medical oncologist. This finding is consistent with the recent review of 30 observational studies by Bouchardy.20 There is substantial evidence that women with fewer diagnostic work-ups and inadequate primary therapies have worse long-term outcomes, including increased likelihoods of having breast cancer recurrence and/or dying as a result of breast cancer.21,22 This study also highlights that women who received chemotherapy were younger, were healthier, and had more aggressive tumor characteristics; these characteristics could influence results from observational studies of chemotherapy effectiveness in different ways, toward and away from the null.
We were unable to systematically capture whether nonreferral for chemotherapy or nonreceipt of chemotherapy may have been reasonable because of considerations of life expectancy. However, there was strong suggestion through Charlson scores that life expectancy and overall comorbid conditions played a role in chemotherapy referral and receipt; of women who had Charlson scores of 2 or greater, 32% were not referred or did not discuss chemotherapy and 66% did not receive chemotherapy. The primary reason documented in the medical record was that the physician did not believe treatment was indicated; few documented reasons were patient driven. Logistic issues and adverse effect concerns were not commonly identified as reasons for not receiving a referral to a medical oncologist or having a discussion about chemotherapy. We were also unable to account for potential clustering of patients within physicians.
The review by Bouchardy20 identified increased adverse effects as one of the barriers to delivering chemotherapy in older women, and mucositis, neutropenia, and anemia were the most common adverse effects from chemotherapy.10 Adverse effects were documented as a reason for discontinuation in just greater than half of women in this study, and the most commonly reported adverse effects of this study were fatigue, cytopenia, mucositis, and nausea/vomiting. However, only approximately one in 10 women who started chemotherapy stopped their treatment early, which suggests a high degree of tolerance for chemotherapy among the select group of older women who received chemotherapy.
A strength of this study was that chemotherapy data were collected directly from the medical record and did not rely on tumor registry data, which are known to have limitations.23 Although reliance on the medical records for data is an important strength, there are noteworthy limitations. We did not have any patient-reported data on reasons for not receiving therapy or any details about patient discussions with their clinical teams. Medical records also often lack sufficient details for why providers feel treatment was not indicated. In addition, women diagnosed in later years were only diagnosed at one health plan, and we were unable to examine the effect of potential clustering by provider or health plan.
Just greater than 2.0% of women in our population-based study were enrolled on randomized clinical trials of breast cancer, and this number is consistent with adult enrollment in National Cancer Institute Cooperative Group clinical trials in the United States.24 Although randomized clinical trials provide gold-standard evidence for safety and efficacy data regarding cancer treatments, these studies are limited by select patient populations, protocol-dictated treatment regimens and evaluations, and limited duration of follow-up.8,25 Observational studies will continue to be important for examining treatment safety and effectiveness in subgroups of individuals in whom evidence from randomized trials is lacking (eg, older individuals and individuals with multiple comorbidities).25 This study identifies patient and tumor characteristics associated with not discussing chemotherapy or being referred to a medical oncologist and not receiving or completing chemotherapy among women who were likely to benefit from receiving chemotherapy. Clinically, there remains a group of women at high risk of breast cancer recurrence who do not receive guideline-recommended adjuvant therapy. There continues to be an opportunity to improve long-term outcomes of high-risk women by ensuring shared decision making between patients and providers about chemotherapy receipt.
Acknowledgment
The Acknowledgment is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
We thank Floyd J. Frost Jr, PhD, site principal investigator from Lovelace Clinic Foundation. We also thank our site project managers, programmers, and medical record abstractors: Group Health—Linda Shultz, Kristin Delaney, Margaret Farrell-Ross, Mary Sunderland, Millie Magner, and Beth Kirlin; Meyers Primary Care Institute and Fallon Community Health Plan—Jackie Fuller, Doris Hoyer, and Janet Guilbert; Henry Ford Health System—Sharon Hensley Alford, Karen Wells, Patricia Baker, and Rita Montague; HealthPartners—Maribet McCarty and Alex Kravchik; Kaiser Permanente Southern California—Julie Stern, Janis Yao, Michelle McGuire, and Erica Hnatek-Mitchell; and Lovelace Health Plan—Judith Hurley, Hans Petersen, and Melissa Roberts. We also thank Melissa Rabelhofer for her assistance with manuscript preparation.
Footnotes
Supported by Grant No. CRTG-03-024-01-CCE from the American Cancer Society (D.S.M.B.) and by Grant No. R01 CA093772 from the National Cancer Institute (R.A.S.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Diana S.M. Buist, Marianne Prout, Rebecca A. Silliman
Financial support: Diana S.M. Buist, Rebecca A. Silliman
Administrative support: Diana S.M. Buist, Virginia P. Quinn
Provision of study materials or patients: Diana S.M. Buist, Heather Taffet Gold, Terry S. Field, Virginia P. Quinn, Feifei Wei
Collection and assembly of data: Diana S.M. Buist, Jessica Chubak, Virginia P. Quinn, Feifei Wei, Rebecca A. Silliman
Data analysis and interpretation: Diana S.M. Buist, Jessica Chubak, Marianne Prout, Jaclyn L.F. Bosco, Soe Soe Thwin, Heather Taffet Gold, Cynthia Owusu, Terry S. Field, Virginia P. Quinn, Rebecca A. Silliman
Manuscript writing: Diana S.M. Buist, Jessica Chubak, Marianne Prout, Marianne Ulcickas Yood, Heather Taffet Gold, Virginia P. Quinn, Rebecca A. Silliman
Final approval of manuscript: Diana S.M. Buist, Jessica Chubak, Marianne Prout, Marianne Ulcickas Yood, Jaclyn L.F. Bosco, Soe Soe Thwin, Heather Taffet Gold, Cynthia Owusu, Terry S. Field, Virginia P. Quinn, Feifei Wei, Rebecca A. Silliman
REFERENCES
- 1.Glick JH, Gelber RD, Goldhirsch A, et al. Adjuvant therapy of primary breast cancer: 4th International Conference on Adjuvant Therapy of Primary Breast Cancer, St Gallen, Switzerland. Ann Oncol. 1992;3:801–807. doi: 10.1093/oxfordjournals.annonc.a058099. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Treatment of Early-Stage Breast Cancer. Bethesda, MD: National Institutes of Heath Consensus Statement online; 1990. pp. 1–19. [PubMed] [Google Scholar]
- 3.National Institutes of Health. Adjuvant Therapy for Breast Cancer. Bethesda, MD: National Institutes of Health Consensus Statement online; 2000. pp. 1–23. [Google Scholar]
- 4.Goldhirsch A, Wood W, Senn H, et al. Meeting highlights: International consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst. 1995;87:1441–1445. doi: 10.1093/jnci/87.19.1441. [DOI] [PubMed] [Google Scholar]
- 5.Beahrs O, Henson D, Hunter R, et al. AJCC Manual for Staging Cancer. ed 4. Philadelphia, PA: Lippincott; 1992. [Google Scholar]
- 6.Fleming ID, Cooper JS, Henson DE, et al. AJCC Cancer Staging Manual. ed 5. Philadelphia, PA: Lippincott; 1997. [Google Scholar]
- 7.Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual. ed 6. Philadelphia, PA: Lippincott; 2001. [Google Scholar]
- 8.Sorensen HT, Lash TL, Rothman KJ. Beyond randomized controlled trials: A critical comparison of trials with nonrandomized studies. Hepatology. 2006;44:1075–1082. doi: 10.1002/hep.21404. [DOI] [PubMed] [Google Scholar]
- 9.Giordano SH, Duan Z, Kuo YF, et al. Use and outcomes of adjuvant chemotherapy in older women with breast cancer. J Clin Oncol. 2006;24:2750–2756. doi: 10.1200/JCO.2005.02.3028. [DOI] [PubMed] [Google Scholar]
- 10.Elkin EB, Hurria A, Mitra N, et al. Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: Assessing outcome in a population-based, observational cohort. J Clin Oncol. 2006;24:2757–2764. doi: 10.1200/JCO.2005.03.6053. [DOI] [PubMed] [Google Scholar]
- 11.Du XL, Jones DV, Zhang D. Effectiveness of adjuvant chemotherapy for node-positive operable breast cancer in older women. J Gerontol A Biol Sci Med Sci. 2005;60:1137–1144. doi: 10.1093/gerona/60.9.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silliman RA, Ganz PA. Adjuvant chemotherapy use and outcomes in older women with breast cancer: What have we learned? J Clin Oncol. 2006;24:2697–2699. doi: 10.1200/JCO.2005.05.4742. [DOI] [PubMed] [Google Scholar]
- 13.Enger SM, Thwin SS, Buist DS, et al. Breast cancer treatment of older women in integrated health care settings. J Clin Oncol. 2006;24:4377–4383. doi: 10.1200/JCO.2006.06.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: The Cancer Research Network. J Natl Cancer Inst Monogr. 2005:3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 15.Thwin SS, Clough-Gorr KM, McCarty MC, et al. Automated inter-rater reliability assessment and electronic data collection in a multi-center breast cancer study. BMC Med Res Methodol. 2007;7:23. doi: 10.1186/1471-2288-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Goldhirsch A, Wood WC, Senn HJ, et al. Fifth International Conference on Adjuvant Therapy of Breast Cancer, St Gallen, March 1995: International Consensus Panel on the Treatment of Primary Breast Cancer. Eur J Cancer. 1995;31A:1754–1759. doi: 10.1016/0959-8049(95)00479-3. [DOI] [PubMed] [Google Scholar]
- 19.Harlan LC, Clegg LX, Abrams J, et al. Community-based use of chemotherapy and hormonal therapy for early-stage breast cancer: 1987-2000. J Clin Oncol. 2006;24:872–877. doi: 10.1200/JCO.2005.03.5840. [DOI] [PubMed] [Google Scholar]
- 20.Bouchardy C, Rapiti E, Blagojevic S, et al. Older female cancer patients: Importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25:1858–1869. doi: 10.1200/JCO.2006.10.4208. [DOI] [PubMed] [Google Scholar]
- 21.Geiger AM, Thwin SS, Lash TL, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–974. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 22.Yood MU, Owusu C, Buist DS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206:66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Bilimoria KY, Stewart AK, Tomlinson JS, et al. Impact of adjuvant radiation on survival: A note of caution when using cancer registry data to evaluate adjuvant treatments. Ann Surg Oncol. 2007;14:3321–3327. doi: 10.1245/s10434-007-9576-4. [DOI] [PubMed] [Google Scholar]
- 24.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 25.Avorn J. In defense of pharmacoepidemiology: Embracing the yin and yang of drug research. N Engl J Med. 2007;357:2219–2221. doi: 10.1056/NEJMp0706892. [DOI] [PubMed] [Google Scholar]