Abstract
Purpose
To assess the maximum-tolerated dosages (MTDs), and dose-limiting toxicities (DLTs) of the epidermal growth factor receptor inhibitor gefitinib and of intravenous (IV) irinotecan when administered together in children with refractory solid tumors. To assess the effect of gefitinib on the pharmacokinetics of IV irinotecan and on the bioavailability of a single oral dose of irinotecan.
Patients and Methods
IV irinotecan (15 or 20 mg/m2) was given daily for 5 days of 2 consecutive weeks. Oral gefitinib (150 or 112.5 mg/m2) was concomitantly given daily for 12 or 21 days. A single oral dose of irinotecan was given on day 9 of course 2 to allow pharmacokinetic analysis.
Results
The study enrolled 29 patients with recurrent solid tumors. The 21-day regimen of oral gefitinib with irinotecan was not tolerated. Diarrhea was the most common DLT. The MTD of the combination regimen was 15 mg/m2/d of IV irinotecan for 5 days of 2 consecutive weeks and 112.5 mg/m2/d of gefitinib given for 12 days. Gefitinib increased the bioavailability of oral irinotecan by four-fold over that observed in historical controls (median, 0.09 v 0.42; P < .000001), reducing the apparent clearance (an inverse measure of exposure) of irinotecan and SN-38 by 37% and 38%, respectively (P < .0001). A partial response was observed in a patient with refractory Ewing sarcoma.
Conclusion
IV irinotecan given with 12 days of oral gefitinib is well tolerated in children. We observed one partial response. Gefitinib significantly enhances the bioavailability of oral irinotecan. This combination warrants further investigation, particularly with orally administered irinotecan.
INTRODUCTION
The major therapeutic indication for irinotecan is in the treatment of colorectal carcinoma in adults. In the United States, the approved administration schedule is 125 mg/m2 intravenously over 90 minutes, weekly for 4 of 6 weeks.1 However, in pediatric xenograft models, protracted dosing appears to offer a therapeutic advantage, and a variety of childhood solid tumors have shown encouraging responses to protracted dosing.2–6 The next step in pediatric drug development is to determine how best to combine irinotecan with other agents.
As a single agent, the epidermal growth factor receptor (ERBB1) tyrosine kinase inhibitor gefitinib demonstrated minimal activity against a panel of pediatric xenografts, none of which express ERBB1. However, gefitinib and irinotecan exerted greater than additive activity in preclinical models of colorectal carcinoma7 and in several pediatric solid tumor models.8 We therefore combined intravenous irinotecan and gefitinib in a phase I clinical trial in children with recurrent or refractory solid tumors.
Oral administration is an attractive option for protracted chemotherapy; however, the use of oral irinotecan has been limited by its low bioavailability (only approximately 9% as a single agent), by dose-limiting diarrhea in most phase I studies,9–14 and by low systemic exposure to the active metabolite, SN-38.9 In clinical trials, coadministration of inhibitors of P-glycoprotein (ABCB1) or ABCG2 has increased the bioavailability of other oral anticancer drugs and has reduced interpatient variability in exposure.15–18 In a mouse model, oral coadministration of gefitinib dramatically increased the bioavailability of oral irinotecan.8 These results, plus the greater-than-additive antitumor activity in pediatric xenografts, suggest that oral coadministration of the two compounds may enhance the bioavailability of oral irinotecan, increase SN-38 systemic exposure, and possibly increase the antitumor effect.
To our knowledge, herein we report the first pediatric phase I trial of intravenous irinotecan given with gefitinib. We first determined the toxicity and maximum-tolerated doses (MTDs) of intravenous irinotecan and oral gefitinib. We then assessed the bioavailability and SN-38 systemic exposure of oral irinotecan (given on day 9) coadministered with gefitinib in children who received a second course of therapy.
PATIENTS AND METHODS
Eligibility
Patients younger than 22 years old with recurrent or refractory solid tumors for which conventional treatment had failed were eligible. Also required were: life expectancy ≥ 8 weeks, performance status (Eastern Cooperative Oncology Group [ECOG]) ≤ 2, recovery from toxicity of prior chemotherapy, hemoglobin ≥ 8 g/dL, absolute neutrophil count (ANC) ≥ 750/μL, platelet count ≥ 50,000/μL in the absence of marrow infiltration by tumor, adequate liver function (bilirubin ≤ 1.5 × normal, ALT ≤ 3 × normal), and adequate renal function (serum creatinine ≤ 2× normal for age). Exclusion criteria included active infection, incomplete healing from major surgery, evidence of clinically active interstitial lung disease, or current use of known CYP3A4 inhibitors such as phenytoin, carbamazepine, barbiturates, rifampin, or St John's wort. The study was approved by our institutional review board, and signed informed consent was obtained from patients, parents, or guardians, as appropriate.
Drug Formulation and Administration
Irinotecan (Camptosar; Pfizer Inc, New York, NY) was obtained commercially as a 20-mg/mL solution. Intact vials were stored at room temperature and protected from light. Irinotecan was diluted in 5% dextrose (final concentration, 0.12 to 1.1 mg/mL) and administered intravenously over 60 minutes daily for 5 days; a second 5-day cycle followed 2 days of rest (5 days of 2 consecutive weeks [qd × 5] × 2). Patients were instructed to begin loperamide hydrochloride (Imodium A-D; McNeil, Fort Washington, PA), supplied as a clear, cherry-flavored syrup (1 mg/5 mL) or a scored green caplet (2 mg) at the first change in bowel habits.
The intravenous irinotecan formulation was used for oral administration on day 9 of course 2. The dose was drawn up in a plastic oral syringe on the day of administration; the reconstituted solution remains stable for at least 21 days at 4°C in these syringes.9 The dose was mixed with cranberry-grape juice immediately before use and administered under a nurse's supervision.
Gefitinib (Iressa) was supplied by AstraZeneca (Wilmington, DE) as 25-mg and 100-mg tablets and was administered orally once daily, at least 1 hour before the start of the irinotecan infusion, for 12 or 21 consecutive days of each course, as described below.
Treatment and Dose Escalation
The starting irinotecan dosage (15 mg/m2/d) was 75% of the single-agent MTD given intravenously on the same schedule.2 In the absence of grade 3/4 toxicity, the dosage was to be escalated to 20 mg/m2/d in cohorts of three patients. The starting gefitinib dosage was 150 mg/m2/d (less than half the pediatric single-agent MTD of 400 mg/m2/d).19 Gefitinib was given on day −1 of the first course for single-dose pharmacokinetic studies, which are now of less interest and are not reported here. This dose is accounted for in the interest of accuracy but played no role in our analyses. No drug was given the next day (day 0). Irinotecan was given alone on day 1, and daily gefitinib was started on day 2. If dose-limiting gefitinib toxicity occurred, the gefitinib dosage was to be reduced by 25% in a subsequent cohort. No intrapatient dose escalation was permitted. Unacceptable or dose-limiting toxicity (DLT) was defined as grade 4 hematologic toxicity lasting more than 7 days, grade 4 thrombocytopenia requiring transfusion on more than 2 occasions within 7 days, any interruption of gefitinib treatment for toxicity, or grade 3/4 nonhematologic toxicity (with the exception of grade 3 nausea, vomiting, transient ALT/AST elevation, fever or infection) in two of a cohort of three to six patients. The MTD was defined as the dose immediately below that at which the DLT was identified. The first course of treatment (21 days) was used to assess DLT. Courses were repeated at 3-week intervals in the absence of disease progression or DLT. Patients with evidence of progressive disease after any cycle of treatment were removed from the study. A 25% reduction of the gefitinib dosage was permitted after reversible grade 3/4 toxicity in the absence of progressive disease.
With the first two cohorts of patients, four DLTs were seen, all on day 10 or later, requiring a study amendment administering concomitant oral gefitinib for 12 days rather than for 21 days of each course.
Patients who received a second course of therapy received 9 of 10 irinotecan doses intravenously but were administered irinotecan orally on day 9, and serial blood samples were obtained for pharmacokinetic analysis. After the MTDs of gefitinib and irinotecan were determined on the basis of toxicity during course 1, subsequent patients were to be enrolled at the MTD to assess the toxicity of two or more courses.
On the basis of our previous phase I study using cefixime to ameliorate the dose-limiting diarrhea of irinotecan,9 we amended this study a second time after the MTD was established to allow subsequent cohorts to receive prophylactic cefixime 8 mg/kg (maximum, 400 mg) once daily, beginning 1 day before irinotecan and continuing for 14 days, in an attempt to further escalate the irinotecan dosage. If cefixime was not commercially available, patients received cefpodoxime 10 mg/kg/d twice per day orally on the same schedule.
Patient Evaluation
Before enrollment, each patient underwent a complete history and physical examination. Measurable lesions were documented. A CBC, urinalysis, and complete metabolic profile were performed before treatment, at 3- to 4-week intervals, and at the end of the study. During the first course, serum blood urea nitrogen, creatinine, AST, and alkaline phosphatase were assayed weekly and complete blood counts were obtained at least twice weekly.
Toxicity was assessed according to the NCI Common Toxicity Criteria (version 3) and response, according to the Response Evaluation Criteria for Solid Tumors.20 Each patient's best response was recorded for the analysis of treatment effect. Complete response (CR), partial response (PR), and stable disease (SD) were confirmed by repeat imaging after at least 6 weeks.
Sampling and High-Performance Liquid Chromatography Analysis for Pharmacokinetic Studies
To evaluate the effect of gefitinib on intravenous irinotecan disposition, the pharmacokinetics of irinotecan, SN-38, and SN-38 glucuronide (SN-38G) were evaluated on day 1 of course 1 (without gefitinib coadministration) and on day 8 of course 1 (with gefitinib). To evaluate the effect of gefitinib on the bioavailability of oral irinotecan, the pharmacokinetics of irinotecan, SN-38, and SN-38G were evaluated on day 8 of course 2 (after intravenous irinotecan) and on day 9 of course 2 (after oral irinotecan). On days 1 and 8 of course 1 and day 8 of course 2, 2 mL of whole blood was obtained from a site contralateral to the infusion site, before and 0.25, 0.5, 1, 4, and 6 hours after the end of the irinotecan infusion. On day 9 of course 2, whole blood (2 mL) was collected before and 0.25, 1.5, 3, and 6 hours after the oral irinotecan dose. Plasma was immediately separated and the concentration of the lactone forms of irinotecan, SN-38, and SN-38G was assessed by high-performance liquid chromatography (HPLC) with fluorescence detection, as previously described.21
Pharmacokinetic Analysis
A multicompartment model (two compartments for irinotecan and one for each metabolite) was fit to irinotecan lactone, SN-38 lactone, and SN-38G lactone plasma concentrations by using nonlinear mixed-effects modeling via NONMEM software (version V, double precision, level 1.1) with the first-order conditional estimation method with INTERACTION.22 Posterior Bayesian individual estimates of irinotecan, SN-38, and SN-38G pharmacokinetic parameters were obtained with the POSTHOC option implemented in NONMEM. Model parameters estimated for irinotecan were absorption rate (ka), bioavailability (F), clearance (CL), volume (V), and the intercompartmental parameters. Model parameters estimated for SN-38 and SN-38G were apparent clearance (SN38 CL, SN38G CL) and apparent volume (SN38 V, SN38G V). The term “apparent” reflects the fact that the fraction of irinotecan converted to SN-38 was unidentifiable.
The area under the concentration time curve for hours 0 to 7 (AUC0→7) for each component was estimated from the model-estimated curves for individual patients. The metabolic and glucuronidation ratios were defined as the ratio of molar SN-38 AUC0→7/irinotecan AUC0→7 and the ratio of molar SN-38G AUC0→7/SN-38 AUC0→7, respectively.
Statistical Methods
The median bioavailability of irinotecan given with gefitinib (in this study) and without gefitinib (in a previous phase I study with a similar group of refractory solid tumor patients; n = 38)9 was compared by using the Mann-Whitney U-test. The effect of gefitinib on irinotecan clearance, SN-38 apparent clearance, and SN-38G apparent clearance was determined by the extent of reduction in the objective function of the nonlinear mixed-effect model (reduction of 3.84 units corresponds to P < .05, based on the χ2-test for the difference in −2 log-likelihood between two hierarchical models that differ by 1 df) and by whether the effect of gefitinib on any single parameter differed significantly from zero (two-tailed t-test).
RESULTS
Patients
Twenty-nine patients were enrolled on this study between June 2003 and March, 2006; 13 were male and 23 had a performance status of 0. The predominant diagnoses were neuroblastoma (n = 6) and osteosarcoma (n = 6; Table 1). The 29 patients received 71 courses of therapy (median, two; range, one to eight) at four different dosage levels without cefixime diarrheal prophylaxis and at one dosage level with cefixime (Table 2). All patients had been extensively pretreated with three or more multiagent chemotherapy regimens; 19 had received prior radiation therapy, and 12 had received prior autologous hematopoietic stem-cell transplants.
Table 1.
Patient Characteristics (n = 29)
| Characteristic | No. |
|---|---|
| Sex | |
| Male | 13 |
| Female | 16 |
| Median age, years | 9 |
| Range | 1-21 |
| Performance status | |
| 0 | 23 |
| 1 | 4 |
| 2 | 2 |
| Diagnosis | |
| Neuroblastoma | 6 |
| Osteosarcoma | 6 |
| Wilms' tumor | 4 |
| Brain tumor | 3 |
| Ewing sarcoma family of tumors | 3 |
| Other* | 7 |
| Median No. of prior chemotherapy agents | 8 |
| Range | 3-15 |
| No. of patients who had prior autologous BMT | 12 |
| No. of assessable courses | 71† |
| Median No. of courses per patient | 2 |
| Range | 1-8 |
Abbreviation: BMT, bone marrow transplantation.
Hepatoblastoma, hepatocellular carcinoma (n = 2), adrenocortical carcinoma (n = 2), undifferentiated sarcoma, polyphenotypic sarcoma.
One patient developed rapidly progressive disease after two doses of gefitinib and three doses of irinotecan and was taken off study.
Table 2.
Grade 3/4 Toxicity During Course 1
| No.of Days of Gefitinib | Dose of Gefitinib (mg/m2/d) | Irinotecan (mg/m2/d) | No. of Patients Enrolled/No. of Courses | No. of Patients Assessable for Toxicity | No. of Patients With DLT | DLT | Non-DLT |
|---|---|---|---|---|---|---|---|
| 21 | 150 | 15 | 5/19 | 5 | 2 | Diarrhea (2) | Anorexia, low Hg, infection |
| 112.5 | 15 | 3/5 | 3 | 2 | AST, ALT (1); anorexia (1) | Hypokalemia, hypoglycemia, hypophosphatemia | |
| 12 | 112.5 | 15 | 13/27 | 13 | 1 | Mucositis, anorexia, and dehydration | Nausea, infection |
| 112.5 | 20 | 4/15 | 4 | 2 | Diarrhea (2) | Grade 3 neutropenia | |
| 12 with cefixime | 112.5 | 20 | 4/5 | 4 | 2 | Diarrhea (2), abdominal pain, anorexia (1) | Grade 3 leukopenia, hypokalemia, low Hg, thrombocytopenia |
NOTE. Additional patients were enrolled at the maximum-tolerated dose to assess the toxicity of two or more courses.
Abbreviations: DLT, dose-limiting toxicity; Hg, hemoglobin.
Hematologic Toxicity
Clinically significant neutropenia (ANC < 500/μL) was seen in only two patients; it occurred after the first course and lasted fewer than 3 days. Only one patient had clinically significant thrombocytopenia (platelet count < 50,000/μL). This patient, with multiply recurrent neuroblastoma, was very heavily pretreated and had significant marrow involvement at the start of therapy.
Nonhematologic Toxicity
Diarrhea was a problem for two of five patients treated with gefitinib 150 mg/m2 for 21 days. To reduce toxicity and capitalize on drug synergy suggested by xenograft data, we altered the schedule to give gefitinib only during days 1 to 12 of each course (to coincide with irinotecan given on days 1 through 5 and 8 to 12). Even on this schedule, diarrhea was observed in two of four patients treated with 20 mg/m2/d of irinotecan, with or without cefixime (Table 2). Most other patients (22 of 29) had grade 1/2 diarrhea during course 1, some with associated cramping (n = 14). Grade 1/2 rash was seen in eight patients.
Antitumor Activity
A 14-year-old patient with recurrent pulmonary Ewing's sarcoma family of tumors had a PR after two cycles of therapy that was sustained for more than 2 months before she went on alternative therapy. Another patient with recurrent neuroblastoma had SD after one course; after two courses he had a 49% overall decrease in the size of target lesions but was taken off study and no further scans were done (best response, SD by Response Evaluation Criteria for Solid Tumors20). Eight others had disease stabilization during two to six courses (median two).
Pharmacokinetics
Pharmacokinetic parameters were assessable in 25 patients (319 samples, 66 studies; Table 3), including 13 patients who had an oral irinotecan dose during course 2. In the presence of gefitinib, irinotecan clearance decreased 37% (P < .00001), apparent SN-38 clearance decreased 38% (P < .0001), and SN-38G apparent clearance decreased 52% (P < .0001), increasing systemic exposure to all three components. Gefitinib had no effect on the metabolic and glucuronidation ratios.
Table 3.
Pharmacokinetic Parameters of Irinotecan and Its Metabolites in 25 Patients
| Parameter | IRN CL | IRN V | ka | F | SN38 CL | SN38G CL | SN38 V | SN38G V | IRN CL | IRN V | SN38 CL | SN38G CL | Residual Error (relative error CV%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average estimate | 40.6 | 160.5 | 0.46 | 0.38 | 209.0 | 123.0 | 114.6 | 2.9 | |||||
| SE estimate | 6.7 | 15.8 | 0.11 | 0.16 | 35.8 | 23.5 | 43.4 | 3.0 | |||||
| IIV (CV%) | 24 | 31 | 87 | 27 | 38 | 43 | 55 | 82 | |||||
| IOV (CV%) | 24 | — | — | — | 34 | 39 | — | — | |||||
| Change in parameter due to gefitinib | |||||||||||||
| Average estimate | −15.2 | −43.6 | −77.1 | −61.6 | |||||||||
| SE estimate | 6.2 | 10.8 | 20.4 | 14.3 | |||||||||
| IRN | 38 | ||||||||||||
| SN-38 | 23 | ||||||||||||
| SN-38G | 11 |
NOTE. Model parameters estimated for IRN, ka, F, CL, V; estimated for SN-38: apparent clearance (SN38 CL), apparent volume (SN38 V); and estimated for SN-38G: apparent clearance (SN38G CL), apparent volume (SN38G V).
Abbreviations: IRN, irinotecan; ka, absorption rate; F, bioavailability; CL, clearance; V, volume; CV%, coefficient of variation; IIV, interindividual variability; IOV, intraoccasion variability.
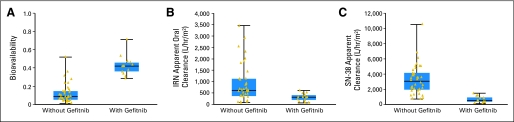
The median posthoc estimate of the bioavailability of oral irinotecan given with gefitinib was 0.42 (range, 0.29 to 0.71), which was significantly higher than that in a similar group of historical controls who did not receive concurrent gefitinib (median, 0.09; range, 0.01 to 0.52;Mann Whitney U-test, P < .000001).9 Therefore, the apparent oral irinotecan clearance (a normalized inverse measure of systemic exposure) was decreased by coadministration of gefitinib. Specifically, the median irinotecan apparent clearance after oral irinotecan plus gefitinib was reduced by a factor of 2, and that of SN-38 was reduced by a factor of 6, compared to those after oral irinotecan alone (Mann Whitney U-test, P < .0004).
DISCUSSION
After our extensive preclinical xenograft data demonstrated the tyrosine kinase inhibitor gefitinib potentiates the antitumor activity of irinotecan,8 we conducted the first clinical trial of gefitinib and irinotecan in children with cancer. The combination of oral gefitinib given for 12 days concurrently with a protracted schedule of intravenous irinotecan was safe and well tolerated in this group of children. One patient with a pulmonary recurrence of Ewing's sarcoma family of tumors had a PR; another with neuroblastoma had a 49% decrease in target lesions after two courses but was taken off study to pursue alternative therapy before the durability of this response could be confirmed with follow-up imaging. An additional eight patients had disease stabilization for two to six courses (median, two courses). By comparison, response rates (CR/PR) from other single agent pediatric phase I studies of irinotecan using various schedules have been 0 of 23,23 four of 81,24 two of 30,25 four of 28,26 and five of 232 assessable patients. Such responses in a very drug-resistant group of tumors warrant further investigation of this combination. We administered gefitinib with a single oral irinotecan dose during the second course of therapy and noted increased irinotecan bioavailability (P < .000001), which was associated with increased irinotecan and SN-38 systemic exposure (P < .0004).
Studies in our preclinical xenograft models of pediatric tumors have demonstrated compellingly that protracted irinotecan administration produces superior efficacy27; however, extensive clinical evaluation of a protracted regimen of irinotecan and gefitinib will be difficult if irinotecan must be given intravenously. A protracted oral irinotecan regimen has previously been precluded by dose-limiting diarrhea and poor irinotecan bioavailability. We have previously shown that concomitant administration of cefixime addresses the diarrhea and increases the maximum-tolerated oral irinotecan dosage by about one third.9
We tested the ability of gefitinib to improve the bioavailability of oral irinotecan because our preclinical data suggested that gefitinib inhibits ABCG28 and because another inhibitor of ABCG2, elacridar, increased the bioavailability of oral topotecan (a camptothecin analog) in humans.28 In general, the results of our pharmacokinetic studies corroborate the preclinical findings.8 When irinotecan was administered as a single oral dose in combination with gefitinib, the median irinotecan bioavailability was significantly higher,9 and systemic exposure to irinotecan and to SN-38 was greater, than that observed in patients not receiving gefitinib (Figs 1B and 1C).9 In fact, the SN-38 AUC after oral irinotecan with gefitinib was approximately half that observed after intravenous irinotecan with gefitinib (28 ng/mL × hr versus 47 ng/mL × hr). The increased bioavailability of oral irinotecan and the increased systemic exposure to SN-38 produced by coadministration of gefitinib suggests a promising way to address one of the limitations of oral irinotecan therapy. These intriguing findings warrant further evaluation.
Fig 1.
Comparison of the pharmacokinetics of oral irinotecan (IRN) and its active metabolite SN-38 in 13 patients who concurrently received gefitinib and in 38 patients who did not (comparison data from a previous study9). (A) Gefitinib increased the bioavailability of oral irinotecan. (B) Gefitinib decreased the apparent clearance of oral IRN. (C) Gefitinib decreased the apparent clearance of SN-38. The box indicates the 25th to 75th percentile, the horizontal line shows the median, and the vertical bars indicate the range.
To further emphasize the potential clinical relevance of our findings we compared the SN-38 systemic exposure (ie, AUC values) after oral irinotecan dosing with gefitinib in our study with SN-38 systemic exposure values reported associated with antitumor effects in phase II pediatric trials. The median SN-38 AUC value associated with the oral irinotecan MTD of 15 mg/m2 with gefitinib was 35 ng/mL × hr, which is similar to the median SN-38 achieved in other trials of irinotecan administered intravenously on the same schedule as used in the present clinical trial.29 This schedule and a similar dosage (ie, 20 mg/m2) have demonstrated significant antitumor activity in clinical trials,2,3,5 and the use of oral therapy should improve its convenience.
In conclusion, the combination of irinotecan and gefitinib was safe and tolerable and showed promising antitumor effects. Further, coadministration of gefitinib resulted in greater irinotecan bioavailability and SN-38 systemic exposure after a single oral dose. It remains to be determined whether this combination can produce SN-38 systemic exposures similar to those achieved with intravenous irinotecan. We are conducting a subsequent phase I study to answer this question.
Acknowledgment
The Acknowledgment is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
We thank Sharon Naron for editorial assistance and Dana Hawkins and Michelle Kunkel for facilitating data collection; Paula Condy, Margaret Edwards, Terri Kuehner, Sheri Ring, and Lisa Walters for assistance in obtaining plasma samples. We acknowledge the technical support of Geeta Nair, Feng Bai, and Thandra Owens, as well as the contributions of Lisa Iacono, PharmD, and Burgess B. Freeman, PharmD.
Footnotes
Supported by Grants No. CA23099 and CA21765 from the National Institutes of Health, by a grant from Astra Zeneca, and by the American Lebanese Syrian Associated Charities.
Presented in part at the 40th Annual Meeting of the American Society of Clinical Oncology, June 5-8, 2004.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00186979.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Wayne L. Furman, AstraZeneca; Najat C. Daw, AstraZeneca; Lisa M. McGregor, AstraZeneca; Clinton F. Stewart, AstraZeneca Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Wayne L. Furman, Najat C. Daw, Jianrong Wu, Peter J. Houghton, Victor M. Santana, Clinton F. Stewart
Administrative support: Wayne L. Furman
Provision of study materials or patients: Wayne L. Furman, Fariba Navid, Najat C. Daw, Lisa M. McGregor, Sheri L. Spunt, Carlos Rodriguez-Galindo, Amar J. Gajjar, Victor M. Santana, Clinton F. Stewart
Collection and assembly of data: Wayne L. Furman, Lisa M. McGregor, Kristine R. Crews, Clinton F. Stewart
Data analysis and interpretation: Wayne L. Furman, Najat C. Daw, M. Beth McCarville, Lisa M. McGregor, John C. Panetta, Kristine R. Crews, Jianrong Wu, Amar J. Gajjar, Clinton F. Stewart
Manuscript writing: Wayne L. Furman, Fariba Navid, Najat C. Daw, M. Beth McCarville, Lisa M. McGregor, Carlos Rodriguez-Galindo, John C. Panetta, Peter J. Houghton, Victor M. Santana, Clinton F. Stewart
Final approval of manuscript: Wayne L. Furman, Fariba Navid, Najat C. Daw, M. Beth McCarville, Lisa M. McGregor, Sheri L. Spunt, Carlos Rodriguez-Galindo, Kristine R. Crews, Peter J. Houghton, Victor M. Santana, Clinton F. Stewart
REFERENCES
- 1.Rothenberg ML, Kuhn JG, Burris HA, et al. Phase I and pharmacokinetic trial of weekly CPT-11. J Clin Oncol. 1993;11:2194–2204. doi: 10.1200/JCO.1993.11.11.2194. [DOI] [PubMed] [Google Scholar]
- 2.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;17:1815–1824. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 3.Cosetti M, Wexler LH, Calleja E, et al. Irinotecan for pediatric solid tumors: The Memorial Sloan-Kettering experience. J Pediatr Hematol Oncol. 2002;24:101–105. doi: 10.1097/00043426-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bisogno G, Riccardi R, Ruggiero A, et al. Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer. 2006;106:703–707. doi: 10.1002/cncr.21629. [DOI] [PubMed] [Google Scholar]
- 5.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: The Children's Oncology Group. J Clin Oncol. 2007;25:362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 6.Osone S, Hosoi H, Tsuchiya K, et al. Low-dose protracted irinotecan as a palliative chemotherapy for advanced neuroblastoma. J Pediatr Hematol Oncol. 2008;30:853–856. doi: 10.1097/MPH.0b013e318180bb71. [DOI] [PubMed] [Google Scholar]
- 7.Koizumi F, Kanzawa F, Ueda Y, et al. Synergistic interaction between the EGFR tyrosine kinase inhibitor gefitinib (“Iressa”) and the DNA topoisomerase I inhibitor CPT-11 (irinotecan) in human colorectal cancer cells. Int J Cancer. 2004;108:464–472. doi: 10.1002/ijc.11539. [DOI] [PubMed] [Google Scholar]
- 8.Stewart CF, Leggas M, Schuetz JD, et al. Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–7499. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 9.Furman WL, Crews KR, Billups C, et al. Cefixime allows greater dose escalation of oral irinotecan: A phase I study in pediatric patients with refractory solid tumors. J Clin Oncol. 2006;24:563–570. doi: 10.1200/JCO.2005.03.2847. [DOI] [PubMed] [Google Scholar]
- 10.Soepenberg O, Dumez H, Verweij J, et al. Phase I pharmacokinetic, food effect, and pharmacogenetic study of oral irinotecan given as semisolid matrix capsules in patients with solid tumors. Clin Cancer Res. 2005;11:1504–1511. doi: 10.1158/1078-0432.CCR-04-1758. [DOI] [PubMed] [Google Scholar]
- 11.Kuppens IE, Dansin E, Boot H, et al. Dose-finding phase I clinical and pharmacokinetic study of orally administered irinotecan in patients with advanced solid tumors. Clin Cancer Res. 2006;12:3774–3781. doi: 10.1158/1078-0432.CCR-05-2368. [DOI] [PubMed] [Google Scholar]
- 12.Schoemaker NE, Kuppens IE, Huinink WW, et al. Phase I study of an oral formulation of irinotecan administered daily for 14 days every 3 weeks in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2005;55:263–270. doi: 10.1007/s00280-004-0874-2. [DOI] [PubMed] [Google Scholar]
- 13.Drengler RL, Kuhn JG, Schaaf LJ, et al. Phase I and pharmacokinetic trial of oral irinotecan administered daily for 5 days every 3 weeks in patients with solid tumors. J Clin Oncol. 1999;17:685–696. doi: 10.1200/JCO.1999.17.2.685. [DOI] [PubMed] [Google Scholar]
- 14.Pitot HC, Adjei AA, Reid JM, et al. A phase I and pharmacokinetic study of a powder-filled capsule formulation of oral irinotecan (CPT-11) given daily for 5 days every 3 weeks in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2006;58:165–172. doi: 10.1007/s00280-005-0138-9. [DOI] [PubMed] [Google Scholar]
- 15.Kuppens IE, Witteveen EO, Jewell RC, et al. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res. 2007;13:3276–3285. doi: 10.1158/1078-0432.CCR-06-2414. [DOI] [PubMed] [Google Scholar]
- 16.Fracasso PM, Goldstein LJ, de Alwis DP, et al. Phase I study of docetaxel in combination with the P-glycoprotein inhibitor, zosuquidar, in resistant malignancies. Clin Cancer Res. 2004;10:7220–7228. doi: 10.1158/1078-0432.CCR-04-0452. [DOI] [PubMed] [Google Scholar]
- 17.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Kuppens IE, Breedveld P, Beijnen JH, et al. Modulation of oral drug bioavailability: From preclinical mechanism to therapeutic application. Cancer Invest. 2005;23:443–464. doi: 10.1081/cnv-58823. [DOI] [PubMed] [Google Scholar]
- 19.Daw NC, Furman WL, Stewart CF, et al. Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol. 2005;23:6172–6180. doi: 10.1200/JCO.2005.11.429. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Owens TS, Dodds H, Fricke K, et al. High-performance liquid chromatographic assay with fluorescence detection for the simultaneous measurement of carboxylate and lactone forms of irinotecan and three metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;788:65–74. doi: 10.1016/s1570-0232(02)01016-4. [DOI] [PubMed] [Google Scholar]
- 22.NONMEM Project Group UoCaSF. NONMEM Users' Guide Part I-VIII. San Francisco, CA: NONMEM Project Group, University of California at San Francisco; 1998. [Google Scholar]
- 23.Bomgaars L, Kerr J, Berg S, et al. A phase I study of irinotecan administered on a weekly schedule in pediatric patients. Pediatr Blood Cancer. 2006;46:50–55. doi: 10.1002/pbc.20355. [DOI] [PubMed] [Google Scholar]
- 24.Vassal G, Doz F, Frappaz D, et al. A phase I study of irinotecan as a 3-week schedule in children with refractory or recurrent solid tumors. J Clin Oncol. 2003;21:3844–3852. doi: 10.1200/JCO.2003.08.175. [DOI] [PubMed] [Google Scholar]
- 25.Blaney S, Berg SL, Pratt C, et al. A phase I study of irinotecan in pediatric patients: A pediatric oncology group study. Clin Cancer Res. 2001;7:32–37. [PubMed] [Google Scholar]
- 26.Mugishima H, Matsunaga T, Yagi K, et al. Phase I study of irinotecan in pediatric patients with malignant solid tumors. J Pediatr Hematol Oncol. 2002;24:94–100. doi: 10.1097/00043426-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Houghton PJ, Cheshire PJ, Hallman JD, et al. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol. 1995;36:393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- 28.Kruijtzer CM, Beijnen JH, Rosing H, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–2950. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]
- 29.Wagner LM, Crews KR, Iacono LC, et al. Phase I trial of temozolomide and protracted irinotecan in pediatric patients with refractory solid tumors. Clin Cancer Res. 2004;10:840–848. doi: 10.1158/1078-0432.ccr-03-0175. [DOI] [PubMed] [Google Scholar]