Abstract
Purpose
The primary objectives of this phase I/II study were to evaluate the safety and immunogenicity of combination therapy consisting of concurrent trastuzumab and human epidermal growth factor receptor 2 (HER2)/neu-specific vaccination in patients with HER2/neu-overexpressing metastatic breast cancer.
Patients and Methods
Twenty-two patients with stage IV HER2/neu-positive breast cancer receiving trastuzumab therapy were vaccinated with an HER2/neu T-helper peptide-based vaccine. Toxicity was graded according to National Cancer Institute criteria, and antigen specific T-cell immunity was assessed by interferon gamma enzyme-linked immunosorbent spot assay. Data on progression-free and overall survival were collected.
Results
Concurrent trastuzumab and HER2/neu vaccinations were well tolerated, with 15% of patients experiencing an asymptomatic decline in left ventricular ejection fraction below the normal range during combination therapy. Although many patients had pre-existing immunity specific for HER2/neu and other breast cancer antigens while treated with trastuzumab alone, that immunity could be significantly boosted and maintained with vaccination. Epitope spreading within HER2/neu and to additional tumor-related proteins was stimulated by immunization, and the magnitude of the T-cell response generated was significantly inversely correlated with serum transforming growth factor beta levels. At a median follow-up of 36 months from the first vaccine, the median overall survival in the study population has not been reached.
Conclusion
Combination therapy with trastuzumab and a HER2/neu vaccine is associated with minimal toxicity and results in prolonged, robust, antigen-specific immune responses in treated patients.
INTRODUCTION
A potential application of cancer vaccines is in the prevention of tumor recurrence or progression in patients with minimal residual disease. Vaccines will need to be coadministered with primary therapy or given after optimal treatment. Human epidermal growth factor receptor 2 (HER2)/neu is a tumor antigen and vaccine target in breast cancer. With the prolonged use of trastuzumab in the treatment of most HER2/neu-positive breast cancers, evaluation of the potential additive toxicity of the combination of trastuzumab and HER2/neu vaccination is warranted.
The vaccine used in this trial was designed to elicit HER2/neu-specific T-helper (Th) immunity.1 Vaccine-induced CD4+ Th1 cells may traffic to the tumor, secrete inflammatory cytokines such as interferon gamma (IFN-γ), and activate local antigen-presenting cells (APC) enhancing cross-priming at the tumor site.2 Via cross-priming, tumor-specific CD8+ T cells can be elicited.3 Finally, antigen-specific CD4+ T cells can enhance and sustain tumor-specific T-cell immunity over time.
Data presented here suggest that combination trastuzumab and HER2/neu vaccine therapy is safe and generates robust and persistent tumor-specific T-cell immunity.
PATIENTS AND METHODS
Patient Population
After informed consent, patients were enrolled in this trial approved by the United States Food and Drug Administration and the University of Washington Human Subjects Division. Enrollment criteria were as follows: stage IV breast cancer in complete remission (CR) or stable disease (SD) on trastuzumab; documented HER2/neu overexpression via immunohistochemistry or fluorescent in situ hybridization; HLA-A2+; and a left ventricular ejection fraction (LVEF) in the normal range (Table 1). Twenty-two patients were enrolled, and 21 patients received vaccinations. Fourteen of 21 patients completed all six immunizations; five of 21 patients completed at least three immunizations, a sufficient number to immunize; and two of 21 patients received fewer than three immunizations.4 Clinical data are presented on 21 patients. Immunologic data for at least one immunizing peptide and protein are presented on patients who had baseline and at least one additional evaluation of immunity assessed (n = 19).
Table 1.
Patient Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Intent to treat | 22 | |
| Received treatment | 21 | |
| Age, years | ||
| Median | 49 | |
| Range | 33-76 | |
| Time from metastatic diagnosis, months | ||
| Median | 18 | |
| Range | 7-76 | |
| Disease status | ||
| Complete remission | 11 | 52 |
| Stable | 10 | 48 |
| Time from last chemotherapy, months | ||
| Median | 4 | |
| Range | 1-61 | |
| No. of prior chemotherapy regimens | ||
| < 2 | 6 | 29 |
| 2-3 | 12 | 57 |
| ≥ 4 | 3 | 14 |
| Time on trastuzumab before study entry, months | ||
| Median | 13 | |
| Range | 3-85 | |
| LVEF at time of study entry | ||
| Median | 61 | |
| Range | 46-72 | |
| ER status | ||
| Positive | 13 | 62 |
| Negative | 8 | 38 |
| PR status | ||
| Positive | 9 | 43 |
| Negative | 12 | 57 |
| Prior hormonal therapy | ||
| Yes | 15 | 71 |
| No | 6 | 29 |
| Concurrent hormonal therapy with vaccination | ||
| Yes | 7 | 33 |
| No | 14 | 67 |
| HER2 status by IHC | ||
| 2+ | 5 | 24 |
| 3+ | 15 | 71 |
| Unknown | 1 | 5 |
| HER2 status by FISH, n = 12 | ||
| Median | 5.18 | |
| Range | 2.05-12.34 | |
Abbreviations: LVEF, left ventricular ejection fraction; ER, estrogen receptor; PR, progesterone receptor; IHC, immunohistochemistry; FISH, fluorescent in situ hybridization.
Study Design
Serum and peripheral-blood mononuclear cells (PBMCs) were collected before, at midpoint, and at 1, 3, 6, and 12 months after immunization. The sample size was chosen based on the following: if no toxicities were seen, the probability of such an occurrence would be at least 90% if the true toxicity rate was 10% or less.
T-Cell Responses
IFN-γ enzyme-linked immunosorbent spot assay was performed as previously described.5 Ten μg/mL of immunizing peptides were used: p369 through 384 (KIFGSLAFLPESFDGDPA) derived from the extracellular domain (ECD), p688 through 703 (RRLLQETELVEPLTPS) from the transmembrane domain, and the intracellular domain (ICD) derived p971 through 984 (ELVSEFSRMARDPQ; denoted as 15 [eg, “X.”15] or 9 [eg, “X.”9] amino acids in length).1,6 Each antigen was assessed in six replicates of 2 × 105/well. One μg/mL of overlapping peptide pools (15 amino acids overlapping by 11 amino acids) for the HER2/neu ICD or ECD, 1 μg/mL of recombinant human p53, insulin-like growth factor binding protein 2 (IGFBP-2; Sigma-Aldrich, St Louis, MO), and topoisomerase II-α (Topogen, Columbus, OH) were also used. Tetanus toxoid (0.5 U/mL) and phytohemaglutinin (2.5 ug/mL) were positive controls. All samples for each patient were cryopreserved, then thawed and analyzed simultaneously to ensure comparability.7,8 Validation studies demonstrated that the assay is linear and precise between 2.0 and 3.5 × 105 PBMCs/well, with a detection limit of 1:100,000. Data are calculated estimates, and some results are considered below the level of reproducible detection. Ten age-matched volunteer female donors were evaluated as controls. Data are presented on individuals as a calculated 1/frequency of IFN- γ–secreting cells in 106 PBMCs and discussed as the ratio of responding cells to PBMCs. In summary analyses, data are presented as IFN-γ spots per well (SPW) corrected for background or described as the number of spots per 106 PBMCs postvaccination minus the number of spots prevaccination.
Patients were considered to have pre-existing immunity if, at baseline, the mean antigen-specific SPWs were statistically different (P < .05) from no antigen wells. Patients were considered to have increased response if the current SPW was greater than 2 standard deviations (SD) above the previous value, remained the same if the mean SPW was within 2 SD of the previous value, and decreased if the mean SPW was greater than 2 SD below the previous value. Two SD is equivalent to a P value of .05 in that there is a 95% probability that the values are statistically significant.9
Cytolytic function of generated T-cell lines5 was evaluated (Cytotox 96, Promega, Madison, WI). The HLA-A2 transfected human HER2/neu-expressing breast cancer cell line, SKBR3-A2, was plated at 104 cells/well in triplicate. T cells, expanded after stimulation with immunizing peptide, were added in an effector/target ratio of 40:1. Nontransfected SKBR3 cells were controls. After incubation, supernatants were analyzed per manufacturer's specifications. Percent specific lysis was calculated as: ([experimental release − spontaneous releases of cytotoxic T-lymphocyte cells and target cells]/[maximum release − spontaneous release of target cells]) × 100.
Serum Transforming Growth Factor Beta Levels
Levels were measured, in triplicates, by enzyme-linked immunosorbent assay (eBioscience, San Diego, CA). The concentration of human transforming growth factor beta (TGF-ß) was calculated from a curve of serially diluted human recombinant TGF-ß. The change in TGF-ß levels is described as the value of TGF-ß postvaccination minus the value of TGF-ß prevaccination in picograms per milliliter.
T-Regulatory Cell Levels
Evaluation was performed as previously described.10 Data are expressed as the percentage of FOXP3+CD4+ cells among all CD4+CD3+ T cells.
Statistical Analysis
Differences in median immune responses were assessed using a two-tailed Mann-Whitney test, with a level significance set at .05. The relationship between magnitude of immunity and serum TGF-ß levels was assessed using Pearson's product moment correlation. Pre- versus postimmunization data were compared using a paired t test (two-tailed). Kaplan-Meier curves were generated to show the probability of overall survival (OS) and progression-free survival (PFS), where OS was defined as the time elapsed between beginning vaccinations and death or last follow-up, and PFS was defined as the time elapsed between first vaccine and the earliest of death, disease progression as reported by the patients' primary physicians, or last contact. Data for patients without death (for OS) or death as a result of progression (for PFS) were censored at the date of last known status. Differences in survival curves based on immunologic response were assessed by the Gehan-Breslow-Wilcoxon test. Analyses were performed with GraphPad InStat v.5.01 (GraphPad Software, San Diego, CA).
RESULTS
HER2/neu Th Peptide Vaccine Administered Concurrently With Trastuzumab Was Well Tolerated and Did Not Result in Additional Cardiac Toxicity
Table 2 details 573 adverse events. The majority of toxicities were grade 1 or 2 (99%). There were four grade 3 toxicities, three possibly related to the treatment: injection-site reaction, fainting, and ulceration. There was one nonrelated grade 4 event, a stroke. The median LVEF before treatment was 61% (range, 46% to 72%) and post-treatment was 61% (range, 45% to 66%). Three patients (15%) had a decrease in LVEF to less than normal values on study. None developed symptoms of left ventricular dysfunction. Cardiac toxicities were grade 1 and 2.
Table 2.
Adverse Events
| Adverse Event | AE |
Possibly, Probably, or Definitely Related |
||
|---|---|---|---|---|
| No. | % of All AEs | No. | % of All Related AEs | |
| Most common | ||||
| Injection site reaction | 64 | 11 | 64 | 17 |
| Fatigue | 42 | 7 | 38 | 10 |
| Myalgias | 42 | 7 | 33 | 9 |
| Headache | 41 | 7 | 27 | 7 |
| Lymphopenia | 33 | 6 | 28 | 7 |
| Leukocytes, total WBCs | 29 | 5 | 27 | 7 |
| Pruritus/itching | 21 | 4 | 19 | 5 |
| Nausea | 20 | 3 | 13 | 3 |
| Rigors/chills | 20 | 3 | 16 | 4 |
| Diarrhea | 16 | 3 | 5 | 1 |
| AE grading* | ||||
| 1 | 508 | 89 | 337 | 88 |
| 2 | 60 | 10 | 43 | 11 |
| 3 | 4 | 1 | 3 | 1 |
| 4 | 1 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
| Cardiac AEs | ||||
| Palpitations | 9 | 45 | 2 | 29 |
| Hypertension | 5 | 25 | 0 | 0 |
| Left ventricular systolic dysfunction | 3 | 15 | 3 | 43 |
| Chest tightness | 1 | 5 | 0 | 0 |
| Supraventricular and nodal arrhythmia | 1 | 5 | 1 | 14 |
| Other | 1 | 5 | 1 | 14 |
| AE grading* | ||||
| 1 | 12 | 60 | 1 | 14 |
| 2 | 8 | 40 | 6 | 86 |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
Abbreviation: AE, adverse event.
For AE grading, percent is shown (not % of all AEs or % of all related AEs).
HER2/neu Th Peptide Vaccine Administered Concurrently With Trastuzumab Stimulates or Boosts HER2/neu-Specific Immunity in the Majority of Patients
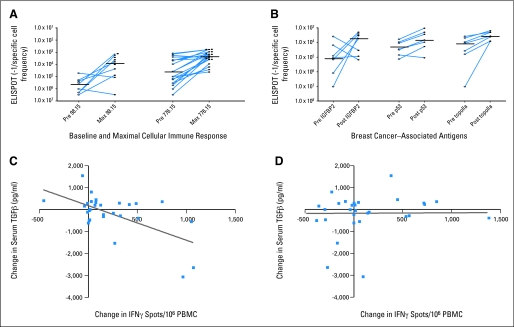
The median peptide-specific T-cell response before the first vaccine was a frequency of less than 1 antigen-specific cell in 75,000 PBMCs (range, 1:400,000 to 1:77,000; Fig 1A). Ninety percent of patients developed new or augmented immunity. The maximal response to p369.15 was a median frequency of one in 4,121 PBMCs (range, 1:323 to 1:2,000,000; P = .0015 compared with prevaccination), the maximal response to p688.15 was one in 4,152 PBMCs (range, 1:307 to 1:3,000,000; P = .0012), and the maximal response to p971.15 was one in 2,086 PBMCs (range, 1:266 to 1:86,960; P = .0066; Fig 1A). Ten (53%) of 19 patients had pre-existing immunity to any of these peptides. Sixteen patients (84%) significantly augmented immunity, three (16%) did not augment, and none had a decrease in peptide-specific immunity with immunization. The percentage of responding patients for the peptides is shown (Fig 1B).
Fig 1.
A human epidermal growth factor receptor 2 (HER2)/neu T-helper peptide vaccine administered concurrently with trastuzumab stimulates or boosts HER2/neu-specific immunity in the majority of patients. (A) Prevaccine (Pre) and maximal (Max) responses 1/frequency (Y axis) tested antigens (X axis). Connected points: mean and SE of six replicates with median bar. Data derived from 10 controls are shown for extracellular domain (ECD) and intracellular domain (ICD). (B) Percent HER2/neu-specific immunity after vaccination. Blue, percent increased; yellow, percent unchanged. ELISPOT, enzyme-linked immunosorbent spot assay.
Peptides were derived from both the HER2/neu ECD and ICD. Of note, seven (64%) of 11 patients had significant pre-existing immunity to the ECD (median, 1:17,729; range, 1:660 to 1:3,000,000), and six (32%) of 19 patients had significant pre-existing immunity to the ICD (median, 1:309,524; range, 1:226 to 1:3,000,000; Fig 1A). Sixty-nine percent of patients developed new or augmented immunity: 37% to ECD, 53% to ICD, and 21% to both. The maximal response to the ECD was a median of one cell in 2,312 PBMCs (range, 1:678 to 1:1,000,000; P = .3017 compared with baseline) and to the ICD was one cell in 9,677 PBMCs (range, 1:232 to 1:3,000,000; P = .0894; Fig 1A). Five patients did not augment, and none had a significant decrease in domain immunity with immunization. The percentage of responding patients is shown (Fig 1B).
Vaccination Can Elicit Tumor-Specific Cytotoxic T Cells
Embedded within the native sequence of the Th peptides are HLA-A2 binding motifs: p369.9, p688.9, and p972.9.1,5 The maximal response to class I peptides was a median frequency to p369.9 of 1:10,200 (range, 1:1,032 to 1:3,000,000; P = .0030 compared with baseline), to p689.9 was 1:10,050 (range, 1:1,039 to 1:3,000,000; P = .1720), and to p971.9 was 1:5,659 (range, 1:845 to 1:3,000,000; P = .0126; Fig 2A). T-cell cultures were established for five patients who had excess PBMCs available. The resultant T-cell lines could specifically lyse HER2/neu-positive/HLA-A2–positive breast cancer cells (range, 7% to 70% lysis; Fig 2B).
Fig 2.
Vaccination can elicit tumor-specific cytotoxic T cells. (A) Prevaccine (Pre) and maximal (Max) responses 1/frequency (Y axis) HLA-A2 peptides (X axis). Connected points: mean and SE of six replicates with median bar. (B) Percent lysis: SKBR3 (blue), SKBR3-A2 (yellow) with SE of four replicates. (C) Percent human epidermal growth factor receptor 2 (HER2)/neu peptide-specific immunity. Blue, percent increased; Yellow, percent unchanged. ELISPOT, enzyme-linked immunosorbent spot assay.
Seven (37%) of 19 patients had pre-existing immunity to these HLA-A2 peptides. Overall, 14 patients (74%) significantly augmented the class I HER2/neu peptide-specific immune response, four patients (21%) did not augment, and one patient had a decrease in immunity to the peptides with immunization. Percentage of responding patients for the HLA-A2 peptides is shown (Fig 2C).
Vaccination-Induced or Enhanced Epitope Spreading Was Observed in the Majority of Patients and Was Associated With a Decrease in Serum TGF-ß
p98.15 and p776.15 are native epitopes of HER2/neu, immunogenic, and not included in the vaccine formulation.11 Development of immunity to these epitopes demonstrates intramolecular epitope spreading, which was elicited or augmented in the majority of patients (Fig 3A).6 The median maximal T-cell response to nonimmunizing epitopes was a frequency to p98.15 of one in 7,558 PBMCs (range, 1:1,205 to 1:3,000,000; P = .0055 compared with prevaccination) and to p776.15 of one in 2,183 PBMCs (range, 1:527 to 1:40,000; P = .0006; Fig 2A). Nine (47%) of 19 patients had pre-existing immunity to these peptides. Fourteen patients (74%) significantly augmented the immune response, five patients (26%) did not augment, and none significantly decreased immunity to these peptides with immunization.
Fig 3.
Vaccination-induced epitope spreading occurred in the majority of patients and was associated with a decrease in serum transforming growth factor beta (TGF-β) levels. (A) Prevaccine (Pre) and maximal (Max) intramolecular epitope spreading (IMS) 1/frequency (Y axis) peptides (X axis). (B) Pre-Max intermolecular epitope spreading (Y axis) antigens (X axis). Connected points: mean and standard deviation of six replicates with median bar. (C) X axis, change in magnitude of IMS response (interferon gamma [IFN-γ] spots per well/106 peripheral-blood mononuclear cells [PBMCs]). (D) X axis, change in magnitude of tetanus toxoid response. Y-axis; change in TGF-ß. ELISPOT, enzyme-linked immunosorbent spot assay.
We have identified several immunogenic breast cancer–associated proteins and questioned whether new or augmented immunity to IGFBP-2, p53, and topoisomerase II-α were stimulated with vaccination (ie, intermolecular epitope spreading).10,12 Seven patients had sufficient PBMCs available for this analysis. All patients had a pre-existing immune response to at least one antigen, and all seven developed new or augmented immunity to at least one of the antigens (Fig 2B). The postvaccination median response to IGFBP-2 was a frequency of one in 5,405 PBMCs (range, 1:1,993 to 1:150,000; P = .0973 compared with prevaccination), postvaccination median response to p53 was one in 6,793 PBMCs (range, 1:1,061 to 1:109,091; P = .1282), and postvaccination median response to topoisomerase II-α was one in 3,659 PBMCs (range, 1:1,575-1:8,219; P = .0111; Fig 2B). Five patients (71%) augmented immunity to IGFBP-2, six patients (86%) augmented immunity to p53, and all patients tested augmented immunity to topoisomerase II-α. Two patients had a significant decrease in a pre-existing immune response to IGFBP-2 with immunization.
The multiple specificities of IFN-γ secreting T cells induced by vaccination led us to question whether these T cells, which could potentially traffic to tumor, might impact the immunosuppressive environment that has been described in breast cancer.13,14 TGF-ß has been shown to be elevated in the serum of patients with breast cancer and is associated with T-cell dysfunction.15–17 The greater the magnitude of the intramolecular epitope spreading T-cell response, the greater the decrease in serum TGF-ß (r = 0.614; P = .0003; Fig 3C). There was weak correlation between the magnitude of the tetanus toxoid response, evaluated as a control, and change in TGF-ß levels (r = 0.016; P = .93; Fig 3D).
Treg levels were measured before and after immunization in eight patients. The median percent Treg was 1.64% before immunization (range, 0.33% to 7.33%) and 1.32% 1 month after vaccines (range, 0.41% to 5.08%; P = .60). At 1 year after immunization, the median Treg level was 1.11% (range, 0.24% to 4.91%; P = .61 compared with preimmunization).
HER2/neu-Specific Immunity Can Persist and Even Increase After Active Immunizations Have Ended
Figure 4 shows the OS and PFS of the study population from the time of first vaccination. The median follow-up among survivors was 36 months (range, 21 to 49 months). The median PFS was 17.7 months, and the Kaplan-Meier estimate of PFS was 33% at 3 years. The median OS has not been reached; the Kaplan-Meier estimate of OS is 86% at 4 years.
Fig 4.
Overall survival (OS) and progression-free survival (PFS) of immunized patients. Kaplan-Meier curves of the percent OS and PFS in months from the time of first vaccine (n = 21).
Although the generation of a new or augmentation of a pre-existing immune response to either immunizing peptides or protein (P = .11) or to epitope spreading peptides (P = .47) was not associated with survival, the magnitude of immunity generated tended to be higher in surviving patients. All 10 patients who had T-cell responses greater than the median to HER2/neu immunizing peptides and associated protein (P = .08) or to peptides associated with intramolecular epitope spreading (P = .09) were survivors as compared with patients with responses less than the median.
We monitored immunity to HER2/neu-related antigens after the end of immunizations in 11 patients (ECD, n = 6). Five patients (46%) maintained the same level of immunity to p369.15 in long-term follow-up as compared with the maximal response achieved during active immunization. Two (18%) decreased immunity and four (36%) continued to augment immunity (median, 1:4,121; range, 1:788 to 1:3,000,000). Seven patients (64%) maintained the same level of immunity to p688.15. One patient (9%) decreased compared with the maximal response achieved, and three patients (27%) continued to augment immunity to p688.15 (median, 1:12,876; range, 1:307 to 1:3,000,000). Five patients (46%) maintained the same level of immunity to p971.15. Two patients (18%) decreased and four patients (36%) continued to augment immunity (median, 1:5,236; range, 1:266 to 1:222,222).
Persistent immunity to the HER2/neu ECD and ICD was assessed. Five (83%) of six patients maintained the same level of immunity to the ECD in follow-up. One patient (17%) had decreased and none continued to augment immunity to the ECD (median, 1:4,625; range, 1:803 to 1:11,321). Six (55%) of 11 patients maintained immunity to the ICD. Two (18%) decreased and three (27%) continued to augment immunity (median, 1:6,024; range, 1:232 to 1:3,000,000). Finally, intramolecular epitope spreading was maintained in five patients (46%) as evidenced by immunity to p776.15. Two patients (18%) significantly decreased and four (36%) continued to augment immunity (median, 1:2,667; range, 1:527 to 1:300,000).
DISCUSSION
Concurrent administration of trastuzumab and a HER2/neu-specific vaccine is tolerated in patients with metastatic breast cancer (MBC), and significant pre-existing immunity to HER2/neu can be boosted and maintained with immunization. Moreover, epitope spreading elicited with vaccination may modulate systemic mediators of tumor-induced immune suppression.
Trastuzumab-related cardiac damage is generally reversible once the drug is stopped.18 After immunization, however, the T-cell response is not as easily “turned off.” As long as antigen is present, T-cells will clonally expand, further augmenting immunity, as occurred in a third of our patients. A study of long-term trastuzumab use in MBC reported that approximately 25% of patients experience some cardiac event.18 In our trial, 15% of patients had an asymptomatic drop in left ventricular function below the normal range, although given the limited sample size, we cannot rule out the possibility that the true rate is higher than the historical rate.
An unexpected finding was the number of patients treated with trastuzumab who had significant pre-existing immunity to HER2/neu and other antigens. Although we do not have an assessment of HER2/neu immunity before starting trastuzumab, investigations by our group suggest only 10% of trastuzumab-naïve patients would have measurable cellular immunity.19 Only one study has shown that endogenous HER2/neu-specific humoral and T-cell immunity could be elicited with trastuzumab treatment.20 Despite the presence of immunity to HER-2/neu at the start of immunization, most responses could be boosted to greater levels with vaccination, and in patients with no pre-existing immunity, robust T-cell responses could be generated.
Priming with trastuzumab and boosting with an HER2/neu vaccine may generate levels of immunity more robust than vaccination alone. Indeed, the magnitude of the response achieved after immunization in this trial seemed to be greater than what we have historically observed. The patients in this study were comparable to the patients in our initial study both demographically and clinically.1 There were no statistically significant differences in the two populations in age, disease status at time of vaccination, hormone receptor status, or number of chemotherapy regimens before vaccination. Our initial study did not include trastuzumab, as the drug was not in widespread use at the time, and the median peptide-specific T-cell response after vaccination was one in 16,129 PBMCs.1 In this current study, the median T-cell response to all HER2/neu antigens was 1:1,838 PBMCs, a log-fold increase. Moreover, in our initial study, none of the patients continued to augment immunity after vaccinations had ended.1 Here, nearly one third of patients augmented immunity, only a minority demonstrated a diminution in response, and the remainder maintained the same level of HER2/neu immunity long term as during active immunization.
Vaccination induced epitope spreading. Epitope spreading is associated with autoimmune disease and enhances tissue destruction.21,22 Some studies suggest that antibody-dependent cell-mediated cytotoxicity, by which APC presentation of new antigens is increased, is responsible for epitope spreading.23,24 Others suggest that antigen-homing Th1-cells deliver cytokines, such as interferon, to the tumor, which activate local APC-enhancing cross-priming.2,22 The presence of immunity to HER2/neu and other antigens before vaccination suggests that antibody-dependent cell-mediated cytotoxicity mediated by trastuzumab may have initiated cross-priming at the tumor site. The observation that increasing numbers of Th1 antigen-specific T cells, associated with epitope spreading and elicited via vaccination, decrease serum TGF-ß indicate that trafficking T cells may also be effectively modulating the tumor microenvironment. Theoretically, a decrease in serum TGF-ß could facilitate the continued augmentation and persistence of tumor-specific immunity.
Is HER2/neu-specific immunity induced by vaccination related to clinical outcome? Although the study was not designed to address a clinical end point, OS was assessed to gather additional data on the potential therapeutic efficacy of the combination.25 The current observed results are encouraging in light of the historical results for patients with pretreated HER2/neu-positive MBC. Recent phase II studies using trastuzumab and various chemotherapy agents in the salvage setting have demonstrated that 40% to 60% of patients are able to achieve either complete or partial responses with second- or third-line trastuzumab-containing regimens. The median PFS and OS ranged from 7 to 12 and 18 to 23 months, respectively.26–28 Combination therapy with trastuzumab and an HER2/neu-specific vaccine warrants further evaluation as a therapeutic regimen.
Acknowledgment
We thank Devon J. Webster, MD, for the care she provided to many of the patients on the study; Molly Boettcher for assistance in manuscript preparation; and the women who enrolled onto this study.
Footnotes
Supported by grants from Gateway Foundation (Cancer Research Treatment Foundation; M.L.D.) and National Institutes of Health (NIH) Grant No. K23CA100691 (to L.G.S.). Patient care was conducted through the Clinical Research Center Facility at the University of Washington (NIH Grant No. UL1 RR025014).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00194714.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Mary L. Disis, VentiRx Pharmaceuticals (C) Stock Ownership: None Honoraria: None Research Funding: Mary L. Disis, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Mary L. Disis, Theodore A. Gooley, Lupe G. Salazar
Financial support: Mary L. Disis
Administrative support: Mary L. Disis, Jennifer S. Childs, Patricia A. Fintak, Kathleen Tietje
Provision of study materials or patients: Mary L. Disis, Jennifer S. Childs, Patricia A. Fintak, John Link, James Waisman, Lupe G. Salazar
Collection and assembly of data: Mary L. Disis, Danelle R. Wallace, Yushe Dang, Meredith Slota, Hailing Lu, Andrew L. Coveler, Jennifer S. Childs, Doreen M. Higgins, Patricia A. Fintak, Corazon dela Rosa
Data analysis and interpretation: Mary L. Disis, Danelle R. Wallace, Theodore A. Gooley, Yushe Dang, Meredith Slota, Hailing Lu, Lupe G. Salazar
Manuscript writing: Mary L. Disis, Danelle R. Wallace, Lupe G. Salazar
Final approval of manuscript: Mary L. Disis, Danelle R. Wallace, Kathleen Tietje, John Link, James Waisman, Lupe G. Salazar
REFERENCES
- 1.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 3.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: When, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 4.Salazar LG, Coveler AL, Swensen RE, et al. Kinetics of tumor-specific T-cell response development after active immunization in patients with HER-2/neu overexpressing cancers. Clin Immunol. 2007;125:275–280. doi: 10.1016/j.clim.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Dang Y, Knutson KL, Goodell V, et al. Tumor antigen-specific T-cell expansion is greatly facilitated by in vivo priming. Clin Cancer Res. 2007;13:1883–1891. doi: 10.1158/1078-0432.CCR-06-2083. [DOI] [PubMed] [Google Scholar]
- 6.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 7.Disis ML, dela Rosa C, Goodell V, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–18. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Maecker HT, Moon J, Bhatia S, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005;6:17. doi: 10.1186/1471-2172-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagano M, Gauvreau K. ed 2. Pacific Grove, CA: Duxbury Press; 2000. Principles of Biostatistics. [Google Scholar]
- 10.Park KH, Goodell V, Higgins D, et al. Insulin-like growth factor binding protein 2 is an essential target for the immunomodulation of breast cancer. Cancer Res. 2008;68:8400–8409. doi: 10.1158/0008-5472.CAN-07-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar LG, Fikes J, Southwood S, et al. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res. 2003;9:5559–5565. [PubMed] [Google Scholar]
- 12.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–1394. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 13.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 14.Tan AR, Alexe G, Reiss M. Transforming growth factor-beta signaling: Emerging stem cell target in metastatic breast cancer? Breast Cancer Res Treat. doi: 10.1007/s10549-008-0184-1. [epub ahead of print on October 9, 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grau AM, Wen W, Ramroopsingh DS, et al. Circulating transforming growth factor-beta-1 and breast cancer prognosis: Results from the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2008;112:335–341. doi: 10.1007/s10549-007-9845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moutsopoulos NM, Wen J, Wahl SM. TGF-beta and tumors: An ill-fated alliance. Curr Opin Immunol. 2008;20:234–240. doi: 10.1016/j.coi.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei S, Shreiner AB, Takeshita N, et al. Tumor-induced immune suppression of in vivo effector T-cell priming is mediated by the B7–H1/PD-1 axis and transforming growth factor beta. Cancer Res. 2008;68:5432–5438. doi: 10.1158/0008-5472.CAN-07-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M. D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 19.Disis ML, Knutson KL, Schiffman K, et al. Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer. Breast Cancer Res Treat. 2000;62:245–252. doi: 10.1023/a:1006438507898. [DOI] [PubMed] [Google Scholar]
- 20.Taylor C, Hershman D, Shah N, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 21.Hueber W, Utz PJ, Steinman L, et al. Autoantibody profiling for the study and treatment of autoimmune disease. Arthritis Res. 2002;4:290–295. doi: 10.1186/ar426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 23.Dai YD, Carayanniotis G, Sercarz E. Antigen processing by autoreactive B cells promotes determinant spreading. Cell Mol Immunol. 2005;2:169–175. [PubMed] [Google Scholar]
- 24.Harbers SO, Crocker A, Catalano G, et al. Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest. 2007;117:1361–1369. doi: 10.1172/JCI29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 26.Jackisch C. HER-2-positive metastatic breast cancer: Optimizing trastuzumab-based therapy. Oncologist. 2006;11(suppl 1):34–41. doi: 10.1634/theoncologist.11-90001-34. [DOI] [PubMed] [Google Scholar]
- 27.Schaller G, Bangemann N, Weber J, et al. Efficacy and safety of trastuzumab plus capecitabine in a German multicentre phase II study of pre-treated metastatic breast cancer. J Clin Oncol. 2005;23(suppl):57s. abstr 717. [Google Scholar]
- 28.Yamamoto D, Iwase S, Kitamura K, et al. A phase II study of trastuzumab and capecitabine for patients with HER2-overexpressing metastatic breast cancer: Japan Breast Cancer Res Network (JBCRN) 00 Trial. Cancer Chemother Pharmacol. 2008;61:509–514. doi: 10.1007/s00280-007-0497-5. [DOI] [PubMed] [Google Scholar]