Abstract
Purpose
We sought to improve outcomes for patients with high-risk head and neck squamous cell cancer (HNSCC) after surgical resection by testing the feasibility and safety of early postoperative chemotherapy followed by concurrent chemoradiotherapy.
Patients and Methods
Eligible patients had resected, stages III to IV HNSCC with positive margins, extracapsular nodal extension, or multiple positive nodes. Paclitaxel (80 mg/m2) was given once weekly during postoperative weeks 2, 3, and 4 and was given before radiation therapy (RT). Paclitaxel (30 mg/m2) and cisplatin (20 mg/m2) were given once weekly during the last 3 weeks of RT (60 Gy over 6 weeks, beginning 4 to 5 weeks after surgery). The primary end points were treatment safety and tolerability compared with concurrent cisplatin (100 mg/m2 every 3 weeks) and RT, as tested in Radiation Therapy Oncology Group trial RTOG 9501.
Results
The median follow-up time for the 70 patients enrolled was 3.3 years (range, 0.6 to 4.4 years) for surviving patients. Tolerability of all treatment components was comparable to that of RTOG 9501 treatment, which is the current standard of care (compliance rate, 75%; 95% CI, 63% to 85%). One patient died, and seven patients experienced grade 4 nonhematologic toxicities. Rates of locoregional control, disease-free survival, and overall survival exceeded those of RTOG 9501 after adjustment for important prognostic variables (ie, positive margins, extracapsular extension, primary site, and performance status).
Conclusion
Chemotherapy soon after surgery followed by concurrent chemoradiotherapy therapy was feasible; tolerance was in line with standard postoperative chemoradiotherapy; and this regimen led to excellent rates of locoregional control and disease-free survival.
INTRODUCTION
The standard therapy for many advanced head and neck squamous cell carcinomas (HNSCCs)— surgery followed by postoperative radiation therapy (PORT)—is associated with high rates of locoregional (30%) and distant (25%) failure and with a 5-year survival rate of only approximately 40%.1–3 Locoregional failure, and perhaps survival rates, can be improved by adding cisplatin, as indicated by the results of two recent, phase III clinical trials, Radiation Therapy Oncology Group (RTOG) 9501 and European Organization for Research and Treatment of Cancer (EORTC) 22931.4,5 However, approximately 20% of patients receiving concurrent chemoradiotherapy at maximum-tolerated doses still experience locoregional failure.
Known risk factors for locoregional failure after surgery include adverse pathologic findings (ie, positive margins, extracapsular nodal extension, multiple involved lymph nodes, lymph node laterality and levels, and perineural invasion) and treatment factors (ie, radiation therapy [RT] dose, fractionation, chemotherapy, and timing of therapies).3,6 A combined analysis of findings from RTOG 9501 and EORTC 22931,7 both of which involved patients randomly assigned to receive PORT (60 to 66 Gy/30 to 33 fractions) with or without high-dose cisplatin, confirmed the benefit of chemotherapy-enhanced RT for patients with positive margins or extracapsular nodal extension. RTOG 9501 had a 2-year locoregional failure rate of 19% for chemoradiotherapy compared with 28% in the RT-only group. Significantly, neither trial addressed the effects of treatment timing on cancer outcomes.
Locoregional failure after surgery and PORT is a direct result of residual tumor cells that were neither extirpated by surgery nor expunged by irradiation. The potential for postoperative proliferation of residual tumor cells is real because of the growth factor–rich surgical bed, which has been shown to stimulate tumors,8,9 angiogenesis, and the progression of micrometastases.10–12 The postoperative recovery interval is also an opportunity for tumor cells to become radiation refractory, because resistance is proportional to the number of surviving cells.13 Studies have shown that prolonged treatment package time, defined as the interval from surgery to the completion of PORT, leads to poorer survival.3,6 Similarly, preclinical and clinical data demonstrate that tumor cells undergo accelerated repopulation during RT.9,14 RT time factors are, thus, important determinants of outcome for both the definitive and postoperative settings.
To address these challenges, the RTOG launched protocol RTOG 0024 to test the feasibility and safety of administering chemotherapy shortly after surgery and continuing until the start of PORT. The overall goal in biologic terms was to prevent the proliferation of residual tumor cells in a growth factor–rich milieu; in clinical terms, it was to test the tolerability of this regimen as a way to improve the therapy without enhancing chemoradiotherapy-related toxicity.
PATIENTS AND METHODS
Patient Characteristics
All patients gave written informed consent in accordance with each center's institutional review board guidelines. Eligible patients had Zubrod performance scores of 0 to 1; adequate hematologic, renal, cardiac, and hepatic function; and macroscopically complete resection of American Joint Committee on Cancer stage III or IV HNSCC with high-risk characteristics—involvement of two or more regional lymph nodes, extracapsular nodal extension, and/or microscopically involved mucosal or deep resection margins.
Evaluations included a medical history and physical examination, blood counts, serum chemistry profile, urinalysis, chest radiography, neck computed tomography (CT) or magnetic resonance imaging (MRI) scan, and standard dental care.
Exclusion criteria included pregnancy or lactation; peripheral neuropathy; unhealed wound infection, fistula, or dehiscence; prior head and neck irradiation; and prior malignancy unless the patient was considered disease free for a minimum of 3 years, had low-risk, nonmelanoma skin cancer, had carcinoma in situ, or had stage T1-2, low- to moderate-grade prostate cancer.
Treatment
The planned treatment consisted of up to six cycles of chemotherapy, three in the early-adjuvant period (ie, postoperative, before the start of PORT), and three concurrent with PORT. Early-adjuvant chemotherapy consisted of single-agent paclitaxel 80 mg/m2 administered intravenously (IV) for three weekly cycles (ie, cycles 1 through 3). Concurrent chemotherapy was paclitaxel 30 mg/m2 IV followed immediately by cisplatin 20 mg/m2 IV once weekly for three cycles (ie, cycles 4 through 6) during PORT.
Early-adjuvant paclitaxel was to begin in the first or second week after surgery (ie, postoperative day 7 through 14) if there were no wound complications. RT was to begin 4 to 6 weeks after surgery (ie, postoperative day 28 through 42). Concurrent chemotherapy was to be given in the last 3 weeks of PORT, which was treatment weeks 7 through 9 (ie, on postoperative days 49, 56, and 63).
PORT was delivered according to the conformal standards established by the RTOG and consisted of 60 Gy given in 30 fractions over 6 weeks. A high-risk site-boost dose of 6 Gy was permitted.
Interval and Follow-Up Evaluation
All acute toxic effects were scored according to the National Cancer Institute Common Toxicity Criteria version 2.0. Late effects were scored by RTOG/EORTC criteria.15 Patients were seen before each cycle of chemotherapy, weekly during the RT, 6 to 10 weeks after treatment, then every 3 months during the first 2 years, every 6 months during years 3 to 5, and then annually. Follow-up was to include CT or MRI and office head-and-neck examination.
Statistical Considerations
The primary purposes of this study were to determine the safety and tolerability of this new regimen, defined in terms of the percentage of patients who were able to complete it. A patient was considered compliant if early-adjuvant paclitaxel was begun no later than postoperative day 18 and was continued for all three cycles, if at least 90% of the protocol RT dose was given, and if at least two of the three cycles of chemotherapy concurrent with RT were given. On the basis of the results from the chemoradiotherapy arm of RTOG 9501,5 in which 79% of patients received 90% of the prescribed radiation dose and at least two of three planned doses of cisplatin, the treatment was considered tolerable if 75% of the eligible patients were compliant (as described above in the Treatment section). A patient had to receive a dose of chemotherapy for all three cycles during the induction phase.
An important secondary end point was the rate of acute toxicity, defined as acute nonhematologic grade 4 toxicity or any grade 5 (ie, fatal) toxicity. In RTOG 9501, 13% of patients experienced such toxicity, including four patients (2%) who experienced treatment-related deaths. This end point was used to calculate the sample size. By using Fleming's one-stage, multiple-testing procedure with target rate 15% and an unacceptable rate 30%, and by setting both type I and II errors at .10, 49 patients were required. To allow a 15% rate of ineligibility, the required sample size was 60 patients. With 49 analyzable patients, we could estimate the tolerance rate within 12%.
Other secondary end points were locoregional control, disease-free survival (defined as local, regional, or distant disease recurrence; development of a second primary tumor; or death as a result of any cause), overall survival (defined as death as a result of any cause), and types and rates of acute and chronic toxicities.
Locoregional failure was estimated by the cumulative incidence method to account for the competing risk of death without locoregional failure.16 Disease-free and overall survival rates were calculated by the Kaplan-Meier method.17 All time-to-failure end points were measured from the date of registration until the date of failure, competing risk, or last follow-up; the 2-year rates were estimated along with 95% CIs.
Overall survival was compared with that of the historical control, RTOG 9501, by using a one-sided, log-rank test with alpha set as .05, as described by Dixon and Simon.18 The RT-only and chemoradiotherapy groups from RTOG 9501 were combined, because chemoradiotherapy showed no survival advantage in that study.
When RTOG 0024 was designed, the results of the joint RTOG-EORTC analysis7 had not been reported. As such, the differences in outcome according to specific risk-factor groupings (ie, positive margins v extracapsular nodal extension v multiple positive lymph nodes) were not known. In addition, the effect of human papillomavirus on a subset of oropharyngeal cancers was not appreciated then. To account for these differences in prognosis, Cox proportional hazards models19 were used to estimate hazard ratios and 95% CIs to compare RTOG 0024 to each regimen of RTOG 9501 after adjustment for risk (ie, positive margin and/or extracapsular extension v multiple positive lymph nodes only), primary site (oropharynx v others), and Zubrod performance status (0 v 1). Adjusted survival curves were generated by using the corrected group prognosis method.20 These analyses were not part of the study design but were used to suggest additional hypotheses.
RESULTS
Seventy patients were entered on the study between March 2001 and May 2003. Four of these patients were retrospectively declared ineligible, and a fifth patient never started the protocol therapy, which left 65 patients who could be evaluated for the protocol end points Fifty-one were men, and 14 were women (median age, 54 years; range, 31 to 70 years). Fifty-three were white, three were Hispanic/Latino, eight were African American, and one was Native Hawaiian. Sixty-two of these 65 patients had undergone surgery before registration. Pretreatment patient and tumor characteristics are as follows: Zubrod performance status was 0 for 27 patients and 1 for 38 patients; 23 tumors (35%) were in the oral cavity, 22 (34%) were in the oropharynx, five (8%) were in the hypopharynx, and 15 (23%) were in the larynx. Six tumors (9%) were stage T1, 17 (26%) were T2, 12 (18%) were T3, and 26 (40%) were T4. Forty-five patients (69%) had positive margins or extracapsular nodal extension (with or without multiple positive nodes), and 20 patients (31%) had multiple positive nodes only. Disease in 62 patients (95%) was pathologic stage IV. The median follow-up time was 3.3 years (range, 0.6 to 4.4 years) for surviving patients. Only six surviving patients have been observed for less than 2 years.
Tolerability
The tolerability of all three components of the treatment—early-adjuvant chemotherapy, concurrent chemotherapy, and RT—was as follows: Fifty-four patients (83%) began early-adjuvant chemotherapy on postoperative day 7 through 14, nine patients (14%) began on day 15 through 18, and two patients (3%) began after 18 days. Fifty-seven patients (88%) received three doses of early-adjuvant chemotherapy, and two patients (3%) received four doses. Fifty-two patients (80%) received three doses of concurrent chemotherapy, six patients (9%) received two doses, and four patients (6%) received one dose as concurrent chemotherapy. The overall compliance rate was 75.4% (95% CI, 63.3% to 85.2%).
Toxicity
During the early-adjuvant chemotherapy phase, three patients (4.6%) experienced grade 4 nonhematologic toxicity (n = 1 each of fistula, mucositis, and hyponatremia; Table 1). Three patients had grade 3 mucocutaneous fistulae, and three others had more superficial cases of skin dehiscence. During the concurrent chemoradiotherapy phase, one patient (1.5%) experienced grade 5 (ie, fatal) toxicity (myocardial ischemia), and five additional patients (7.7%) experienced grade 4 nonhematologic toxicity (n = 1 each of hypercalcemia, radiation dermatitis, dysphagia/esophageal spasm, radiation mucositis, and stomatitis; Table 2). In all, eight patients experienced acute nonhematologic grade 4 or 5 toxicity, which provided an estimated rate of 12.3% (95% CI, 5.5% to 22.8%). Among the 62 patients evaluated for late RT-related toxicities, nine patients (14.5%) experienced grade 3 toxicities, and only one patient (1.6%) experienced grade 4 toxicity (bone; Table 3).
Table 1.
Early Adjuvant Chemotherapy Toxicities for Evaluable Patients
| Toxicity | No. of Patients by Toxicity Grade (N = 65) |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Blood/bone marrow | 19 | 17 | 1 | 1 |
| Cardiovascular, general | 1 | 0 | 0 | 0 |
| Constitutional | 7 | 2 | 0 | 0 |
| Dermatologic/skin | 6 | 0 | 0 | 0 |
| Gastrointestinal | 12 | 5 | 4 | 1 |
| Hemorrhage | 0 | 0 | 0 | 1 |
| Hepatic | 8 | 3 | 1 | 0 |
| Infection, febrile neutropenia | 2 | 3 | 0 | 0 |
| Metabolic/laboratory | 6 | 2 | 1 | 1 |
| Neurology | 0 | 1 | 0 | 0 |
| Pain | 2 | 5 | 0 | 0 |
| Renal/genitourinary | 1 | 0 | 0 | 0 |
| Worst nonhematologic | 19 | 15 | 6 | 3 |
| % of patients | 29.2 | 23.1 | 9.2 | 4.6 |
| Worst overall | 22 | 21 | 7 | 4 |
| % of patients | 33.8 | 32.3 | 10.8 | 6.2 |
Table 2.
Acute Toxicities Associated With Concurrent CRT for Evaluable Patients
| Toxicity | No. of Patients by Toxicity Grade (N = 65) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Allergy/immunology | 1 | 1 | 0 | 0 | 0 |
| Auditory/hearing | 1 | 4 | 0 | 0 | 0 |
| Blood/bone marrow | 30 | 10 | 5 | 1 | 0 |
| Cardiovascular, arrhythmia | 2 | 0 | 0 | 0 | 0 |
| Cardiovascular, general | 5 | 0 | 2 | 0 | 1 |
| Coagulation | 1 | 0 | 0 | 0 | 0 |
| Constitutional | 11 | 12 | 4 | 0 | 0 |
| Dermatologic/skin | 8 | 25 | 18 | 1 | 0 |
| Endocrine | 0 | 1 | 0 | 0 | 0 |
| Gastrointestinal | 4 | 26 | 31 | 3 | 0 |
| Hepatic | 7 | 5 | 0 | 0 | 0 |
| Infection febrile neutropenia | 2 | 3 | 2 | 0 | 0 |
| Metabolic/laboratory | 10 | 5 | 1 | 1 | 0 |
| Musculoskeletal | 1 | 2 | 2 | 0 | 0 |
| Neurology | 8 | 2 | 2 | 0 | 0 |
| Pain | 9 | 26 | 13 | 0 | 0 |
| Pulmonary | 15 | 5 | 2 | 0 | 0 |
| Renal/genitourinary | 4 | 4 | 0 | 0 | 0 |
| Sexual/reproductive function | 0 | 1 | 0 | 0 | 0 |
| Worst nonhematologic | 3 | 15 | 40 | 5 | 1 |
| % of patients | 4.6 | 23.1 | 61.5 | 7.7 | 1.5 |
| Worst overall | 3 | 14 | 41 | 5 | 1 |
| % of patients | 4.6 | 21.5 | 63.1 | 7.7 | 1.5 |
Abbreviation: CRT, chemoradiotherapy.
Table 3.
Late RT-Related Toxicities for Evaluable Patients Who Survived More Than 90 Days
| Toxicity | No. of Patients by Toxicity Grade (N = 62) |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Skin within RT field | 25 | 4 | 0 | 0 |
| Mucous membrane | 16 | 9 | 1 | 0 |
| Subcutaneous tissue within RT field | 19 | 11 | 2 | 0 |
| Salivary gland xerostomia or taste impairment | 21 | 18 | 2 | 0 |
| Esophagus | 12 | 12 | 7 | 0 |
| Larynx | 8 | 4 | 1 | 0 |
| Spinal cord | 3 | 1 | 0 | 0 |
| Brain | 3 | 0 | 0 | 0 |
| Bone, including osteonecrosis | 4 | 2 | 2 | 1 |
| Joint | 6 | 3 | 0 | 0 |
| Other | 7 | 12 | 1 | 0 |
| Worst overall | 13 | 25 | 9 | 1 |
| % of patients | 21.0 | 40.3 | 14.5 | 1.6 |
Abbreviation: RT, radiotherapy.
Survival and Patterns of Failure
Eight patients experienced locoregional failure within the first 2 years (9 overall). The estimated 2-year locoregional failure rate was 12.4% (95% CI, 4.3% to 20.5%).
First failure was local, regional, or both in nine patients, distant in nine patients, new primary in nine patients, and death without disease progression in six patients. Four of the patients with second primaries died, three as a result of the study cancer and one as a result of a second primary. The estimated 2-year, disease-free survival was 59.5% (95% CI, 47.5% to 71.5%).
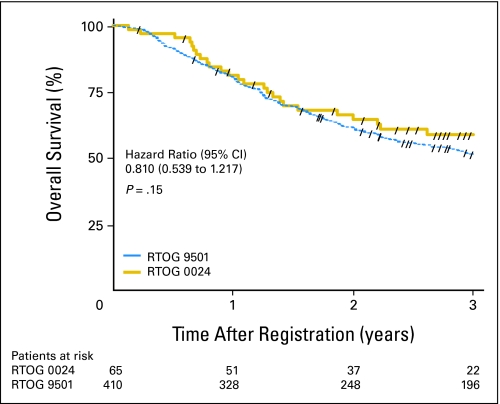
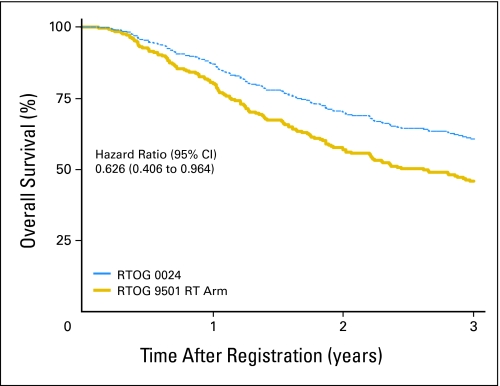
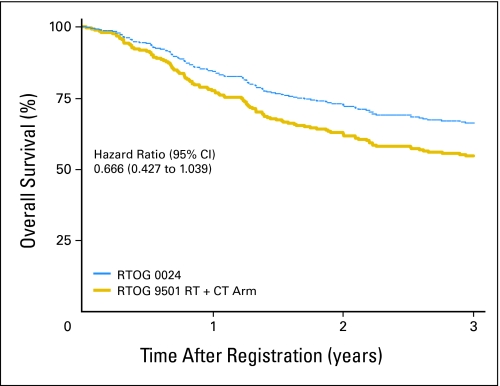
At a median follow-up time of 3.3 years, a new primary tumor was the first failure in 13.8% of patients (compared with 13.4% at 6.1 years in RTOG 9501), and 26 patients had died. The estimated 2-year overall survival rate was 64.7% (95% CI, 52.8% to 76.6%). A comparison of overall survival in this study (ie, RTOG 0024) and that in RTOG 9501 is shown in Figure 1. The estimated hazard ratio was 0.810 (95% CI, 0.539 to 1.217), with a one-sided log-rank P = .15. Overall survival estimates adjusted for Zubrod performance status, high-risk group (ie, extracapsular extension, positive margins), and primary tumor site among patients in this study (RTOG 0024) compared with those in the RT-alone and chemoradiotherapy groups of RTOG 9501 appear in Figures 2 and 3, respectively. Adjusted hazard ratios for overall survival compared with RTOG 9501 RT and chemoradiotherapy were 0.626 (95% CI, 0.406 to 0.964) and 0.666 (95% CI, 0.427 to 1.039), respectively. The hazard ratios for disease-free survival in the same comparisons were 0.631 (95% CI, 0.429 to 0.930) and 0.698 (95% CI, 0.468 to 1.040), respectively, and for locoregional control were 0.395 (95% CI, 0.194 to 0.803) and 0.590 (95% CI, 0.282 to 1.236), respectively.
Fig 1.
Kaplan-Meier estimates of overall survival among patients in this study (ie, Radiation Therapy Oncology Group [RTOG] 0024) compared with the historical control group from RTOG 9501. Forward slashes, censored patients.
Fig 2.
Overall survival estimates adjusted for Zubrod performance status, high-risk group (ie, extracapsular extension, positive margins), and primary tumor site among patients in this study (ie, Radiation Therapy Oncology Group [RTOG] 0024) compared with those in the radiation therapy (RT) –alone group of RTOG 9501.
Fig 3.
Overall survival estimates adjusted for Zubrod performance status, high-risk group (ie, extracapsular extension, positive margins), and primary tumor site among patients in this study (ie, Radiation Therapy Oncology Group [RTOG] 0024) compared with those in the radiation therapy (RT) plus chemotherapy (CT) group of RTOG 9501.
DISCUSSION
The early postoperative chemotherapy used in RTOG 0024 was designed to address the repopulation of residual tumor cells in a growth factor–rich postoperative environment. This was well tolerated and did not compromise the concurrent phase. The overall treatment program was associated with better risk-adjusted locoregional control,disease-free survival, and overall survival compared with RTOG 9501, the historic control selected in study design for prospective comparison. All patients enrolled on RTOG 9501 were allowed to recover from surgery without active treatment for up to 8 weeks before RT or chemoradiotherapy was begun.
Chemotherapy has been used previously before PORT. The Intergroup 0034 trial randomly assigned patients with resected HNSCC to receive PORT or PORT after three cycles of cisplatin plus fluorouracil.2 No differences were found for locoregional control, disease-free survival, or overall survival. The findings can be interpreted as provocative, however, because the chemotherapy group had no decline in locoregional control or survival, despite a 4-month delay in starting RT; high-dose, multiagent, postoperative chemotherapy did not compromise the delivery of subsequent RT; and the chemoradiotherapy group had fewer regional-node recurrences and distant metastases. These results suggest that postoperative chemotherapy prevented tumor regrowth during the 4-month postoperative interval.
We selected the safety and tolerability findings from RTOG 9501, which used the regimen representing the current standard of care, as the benchmark to which we would compare RTOG 0024 findings. The CIs of the compliance rate in RTOG 0024 overlap those of RTOG 9501, in which 79% of patients received within 10% of the prescribed RT dose and at least two cycles of high-dose cisplatin. The worst-grade toxicity rates in RTOG 0024 were similar to those observed in RTOG 9501, which had 13% acute nonhematologic grades 4 to 5 and 2% grade 5 (ie, fatal) toxicities. Thus, the tolerance of the investigational regimen used in RTOG 0024 can be considered similar to the current postoperative standard of care.
PORT for HNSCC is generally begun when surgical wounds are judged to be adequately healed and patients are sufficiently recovered. RT has been started as soon as 2 weeks after neck dissection alone.21 Commonly, 4 to 8 weeks are allowed for healing if the mucosa is breached through external skin incisions that create a nonsterile wound. In those circumstances, rates of pharyngocutaneous fistulae are reported to range from 8% to 29% in patients treated with surgery and PORT.22 The 10% rate of fistula plus skin dehiscence in the RTOG 0024 trial is at the lower end of the expected range, perhaps because patients with early wound-healing complications were ineligible.
A limitation of this study is that currently recognized risk variables had not been identified when the study was designed. As such, this trial was designed to test simple, unadjusted, efficacy outcomes against those of historical controls in RTOG 9501. According to those criteria, this regimen was not superior to that delivered to historical, control patients. However, the patients in RTOG 0024 had less favorable disease characteristics than did those in RTOG 9501. Notably, RTOG 0024 had more patients with positive margins and/or extracapsular extension (69% v 59% in RTOG 9501) and had fewer patients with oropharyngeal tumors (34% v 42%). After adjustment for these prognostic factors, the regimen in RTOG 0024 may be superior to either RT alone (Fig 2) or to RT with concurrent chemotherapy (Fig 3), with an estimated reduction in risk for disease-free survival and death of approximately 30% to 40%. The locoregional control benefit is greater, with estimated reductions in risk of 41% versus chemoradiotherapy and 60% versus RT alone. Other patterns of failure must be addressed separately. The 2-year rate of second primary tumors in RTOG 0024—14% at a median 3.3 years of follow-up—was actuarially higher than the 13% seen in RTOG 9501 at a median 6.1 years of follow-up. Second primaries are expected to occur at a rate of approximately 3% per year, including in the first year.23 Second tumors and distant metastases are also important targets to improve survival.
Another limitation in the interpretation of this study is that two parameters are changed from the comparison study RTOG 9501: early-adjuvant chemotherapy and only three weekly doses of chemotherapy (ie, paclitaxel plus cisplatin). If the results of RTOG 0024 are truly better than RTOG 9501, it would not be absolutely clear which component or if the combination of components led to the change. This truncated, concurrent course used in RTOG 0024 is, in fact, less intense than if given weekly for 6 weeks, and it is most likely less toxic than the high-dose platinum given in RTOG 9501. The use of a more intense, concurrent regimen after early postoperative chemotherapy might produce even better cancer control rates, perhaps at the expense of greater toxicity.
In summary, the repopulation of residual tumor cells in the growth factor–rich postoperative bed is likely to contribute to cancer recurrence. The investigational component of RTOG 0024 redistributed therapy into a previously unexplored disease-risk interval by starting the first phase of chemotherapy soon after surgery. This program was well tolerated and was associated with excellent rates of locoregional control and disease-free survival. The strategy used here could be pursued in future trials, perhaps by starting the systemic therapy earlier (ie, preoperatively) or by incorporating other cytotoxic or biologic agents in novel sequences/combinations.
Footnotes
Supported by Grants No. RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 from the National Cancer Institute.
This manuscript's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00011999.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: David I. Rosenthal, Arlene A. Forastiere, Andy Trotti
Collection and assembly of data: David I. Rosenthal, Jonathan Harris
Data analysis and interpretation: David I. Rosenthal, Jonathan Harris, Arlene A. Forastiere, K. Kian Ang
Manuscript writing: David I. Rosenthal
Final approval of manuscript: David I. Rosenthal, Jonathan Harris, Arlene A. Forastiere, Randal S. Weber, John A. Ridge, Jeffrey N. Myers, Adam S. Garden, Michael R. Kuettel, Kulbir Sidhu, Christopher J. Schultz, Andy Trotti, K. Kian Ang
REFERENCES
- 1.Fu KK, Pajak TF, Trotti A, et al. A radiation therapy oncology group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 2.Laramore G, Scott C, al-Sarraf M, et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck: Report on Intergroup Study 0034. Int J Radiat Oncol Biol Phys. 1992;23:705–713. doi: 10.1016/0360-3016(92)90642-u. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal D, Liu L, Lee J, et al. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24:115–126. doi: 10.1002/hed.10038. [DOI] [PubMed] [Google Scholar]
- 4.Bernier J, Domenge C, Ozsahin M, et al. Head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 5.Cooper J, Pajak TJ, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 7.Bernier J, Cooper J, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Licitra LF, Perrone F, Tamborini E, et al. Effect of antityrosyne kinase agents on in vitro tumor cell proliferation induced by wound drainage fluids (WDFs) of head and neck cancer (HNSCC) patients. J Clin Oncol. 2008;26(suppl):335s. abstr 6077. [Google Scholar]
- 10.O'Reilly M. The interaction of radiation therapy and antiangiogenic therapy. Cancer J. 2008;14:207–213. doi: 10.1097/PPO.0b013e3181836af3. [DOI] [PubMed] [Google Scholar]
- 11.Poulsen MG, Denham JW, Peters LJ, et al. A randomised trial of accelerated and conventional radiotherapy for stage III and IV squamous carcinoma of the head and neck: A Trans-Tasman radiation oncology group study. Radiother Oncol. 2001;60:113–122. doi: 10.1016/s0167-8140(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 12.Retsky MW, Demicheli R, Swartzendruber DE, et al. Computer simulation of a breast cancer metastasis model. Breast Cancer Res Treat. 1997;45:193–202. doi: 10.1023/a:1005849301420. [DOI] [PubMed] [Google Scholar]
- 13.Devita V. The James Ewing lecture: The relationship between tumor mass and resistance to chemotherapy—Implications for surgical adjuvant treatment of cancer. Cancer. 1983;51:1209–1220. doi: 10.1002/1097-0142(19830401)51:7<1209::aid-cncr2820510707>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Peters LJ, Withers HR. Applying radiobiological principles to combined modality treatment of head and neck cancer: The time factor. Int J Radiat Oncol Biol Phys. 1997;39:831–836. doi: 10.1016/s0360-3016(97)00466-5. [DOI] [PubMed] [Google Scholar]
- 15.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 16.Kalbfleisch J, Prentice R. New York, NY: John Wiley; 1980. The Statistical Analysis of Failure Time Data. [Google Scholar]
- 17.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Dixon D, Simon R. Sample size considerations for studies comparing curves using historical controls. J Clin Epidemiol. 1988;41:1209–1213. doi: 10.1016/0895-4356(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 19.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–229. [Google Scholar]
- 20.Chang I, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35:669–674. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 21.Byers RM, Clayman GL, Guillamondequi OM, et al. Resection of advanced cervical metastasis prior to definitive radiotherapy for primary squamous carcinomas of the upper aerodigestive tract. Head Neck. 1992;14:133–138. doi: 10.1002/hed.2880140210. [DOI] [PubMed] [Google Scholar]
- 22.Yu P, Robb G. Pharyngoesophageal reconstruction with the anterolateral thigh flap: A clinical and functional outcomes study. Plast Reconstr Surg. 2005;116:1845–1855. doi: 10.1097/01.prs.0000191179.58054.80. [DOI] [PubMed] [Google Scholar]
- 23.Khuri F, Lee J, Lippman S, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]