Abstract
The small ubiquitin-like modifier SUMO conjugates transcription factors and suppresses their respective activation of target genes. Although various SUMO-modified transcription factors have been isolated, mechanisms whereby sumoylated-substrates modulate transcription remain unknown. Here, we purified ARIP4 (AR interacting protein 4, a Rad54 family member and a SNF2 chromatin remodeling factor), which interacts with sumoylated Ad4BP/SF-1 through two SUMO-interacting motifs and one Ad4BP/SF-1–binding region. Remarkably, ARIP4 also interacts selectively with other sumoylated nuclear receptors including LRH-1, AR, and GR. Interestingly, the ATPase activity of ARIP4 was stimulated in the presence of sumoylated Ad4BP/SF-1 and the Ad4BP/SF-1–binding site containing double-stranded DNA. ChIP assays and siRNA studies strongly suggested that ARIP4 temporally suppresses Ad4BP/SF-1–mediated transcription through its transient recruitment to target genes. These findings suggest that ARIP4 may be a cofactor that modulates SUMO-mediated fine-tuning of transcriptional suppression.
INTRODUCTION
The small ubiquitin-like modifiers SUMOs (SUMO-1, -2, -3, and -4; Dohmen, 2004) are conjugated posttranslationally to target proteins through a series of enzymatic reactions. Similar to ubiquitination, sumoylation involves an E1 SUMO-activating enzyme (Aos1/Uba2), an E2 SUMO-conjugating enzyme (Ubc9), and multiple E3 ligases. The transfer of SUMO from Ubc9 to a target protein is promoted by a specific E3 ligase (Gill, 2004; Johnson, 2004; Hay, 2005). Because these components are highly conserved from yeast to higher organisms, it has been suggested that sumoylation is involved in essential cellular and nuclear events (Martin et al., 2007; Zhao, 2007). In terms of nuclear events, sumoylation has been linked to altered nuclear transport and subnuclear localization of the target proteins (Seeler and Dejean, 2003). Moreover, sumoylation has been implicated in the regulation of DNA repair, chromosome segregation, and transcriptional regulation (Marx, 2005; Geiss-Friedlander and Melchior, 2007; Zhao, 2007).
As for the transcriptional regulation, it has been shown that a number of transcription factors, cofactors, chromatin structural proteins, and chromatin modulators can be sumoylated, which commonly causes transcriptional suppression (Verger et al., 2003; Gill, 2005). These studies raised a possibility that SUMO regulates transcription through the recruitment of histone and chromatin modifying factors. In fact, proteins that inactivate the chromatin structure, such as histone deacetylase 2 (HDAC2; Yang and Sharrocks, 2004), HDAC6 (Girdwood et al., 2003), heterochromatin protein 1α (HP1α; Seeler and Dejean, 2001), and MBD (methyl-binding domain)-containing chromatin-associated factor 1 (MCAF1; Uchimura et al., 2006), were found to be recruited to sumoylated proteins to convert open chromatin into an inactive state. Furthermore, recent reports support the connection between sumoylation and transcriptional suppression through showing that HDAC4 and the polycomb group protein Pc2 can act as E3 SUMO ligases for the myocyte enhancer factor-2 (MEF2; Zhao et al., 2005) and the homeodomain interacting protein kinase 2 (Kagey et al., 2003), respectively.
The orphan nuclear receptor Ad4BP/SF-1 (Adrenal-4 Binding Protein/Steroidogenic Factor-1; NR5A1; Nuclear Receptors Nomenclature Committee, 1999) is essential for the development and function of steroidogenic tissues, and disruption of this gene in mice prevented the development of the adrenal glands, gonads, ventromedial hypothalamic nucleus, pituitary gonadotropes, and the spleen (Ingraham et al., 1994; Luo et al., 1994; Morohashi et al., 1999). The function of Ad4BP/SF-1 was shown to be modulated through interactions with other transcription factors, coactivators, and repressors/corepressors (Lund et al., 2002). Posttranslational modifications such as phosphorylation, acetylation (Val et al., 2003) and sumoylation (Chen et al., 2004; Komatsu et al., 2004; Lee et al., 2005) are also involved in its transcriptional regulation. The sumoylation of Ad4BP/SF-1 occurs at two lysine residues, which suppresses its transcriptional activity.
In the present study, we biochemically purified for the first time the proteins that interact with sumoylated Ad4BP/SF-1. One of these proteins has been shown to be the androgen receptor interacting protein 4 (ARIP4; Rouleau et al., 2002), which consists of an SNF2 (ATPase, DNA/RNA helicase) domain. Our structural and functional studies of ARIP4 directly interconnect sumoylation and temporal transcriptional suppression.
MATERIALS AND METHODS
Plasmids
The full-length cDNA for human ARIP4 was obtained as follows. The 5′ region of ARIP4 was amplified from human HeLa BD Marathon-Ready cDNA (BD Clontech, Palo Alto, CA) with the primers 5′-ATACGTCGACGAATGTCAGACGAATCTGCC-3′ (an SalI site is underlined) and 5′-GTAAAGGAACCGGATCCCGCCAA-3′ (a BamHI site is underlined). This PCR fragment was cloned into the SalI/BamHI sites of pBluescript SK+ (Stratagene, La Jolla, CA). Human KIAA0809 cDNA containing the remaining 3′ region of ARIP4 was purchased as a human IMAGE clone 4578867 (GenBank accession no. BC024298, Invitrogen, Carlsbad, CA), and was cloned into the BamHI/XhoI sites of the plasmid containing the 5′ region of ARIP4 (above). The resulting full-length ARIP4 cDNA was cloned into pFASTBAC and pcDNA3 (Invitrogen) for protein production by baculovirus and expression in mammalian cells, respectively.
Preparation of Anti-ARIP4 Antibody
His-ARIP4 (1194-1467 amino acids) corresponding to the C-terminal region of ARIP4 was cloned into pET28a (Stratagene) and expressed in Escherichia coli BL21 (DE3). The E. coli were lysed in G buffer containing 6 M guanidine-HCl, 20 mM Tris-HCl, pH 8.0, 500 mM KCl, 10% glycerol, 5 mM MgCl2, 0.1% Tween 20, and 20 mM imidazole. The lysates were mixed with Ni2+ resin (Qiagen, Chatsworth, CA) at room temperature for 1 h. The His-tagged proteins were eluted with G buffer containing 200 mM imidazole. Guanidine was removed by dialyzing against 50 mM Tris-HCl. pH 8.0, and 150 mM NaCl. Rabbits were immunized with the purified proteins using Ribi adjuvant (Corixa, Hamilton, MT). Anti-ARIP4 antiserum purified with an antigen column was used for the immunoblotting and chromatin immunoprecipitation (ChIP) studies.
Purification of Proteins Interacting with Sumoylated Ad4BP/SF-1
Nuclear extracts were prepared from HeLa, HEK293, and Y-1 cells (Dignam et al., 1983). In vitro sumoylation of FLAG-Ad4BP/SF-1 were performed as described (Komatsu et al., 2004). Unsumoylated or sumoylated Ad4BP/SF-1 was incubated for 1 h with anti-FLAG M2 agarose beads (Sigma, St. Louis, MO). After centrifugation and washing with a buffer (20 mM Tris-HCl, pH 8.0, 10% glycerol, 5 mM MgCl2, and 0.1% Tween 20) containing 400 mM KCl, the beads were incubated with the nuclear extracts for 3 h at 4°C. After the beads were washed five times with the same buffer containing 150 mM KCl, the proteins bound to the beads were eluted with 0.1 M glycine-HCl, pH 2.0, and subjected to SDS-PAGE. The proteins recovered from the gel were analyzed by mass spectrometry.
ATPase Assays
cDNAs encoding FLAG-tagged and hemagglutinin (HA)-tagged ARIP4 were cloned into pFASTBAC vector (Invitrogen). Recombinant baculoviruses were produced according to the manufacturer's protocol (Invitrogen). FLAG- and HA-tagged ARIP4 were expressed using the recombinant baculoviruses in Sf21 cells and purified as described previously (Komatsu et al., 2004). The ATPase activity of ARIP4 was determined using BIOMOL GREEN reagent (BIOMOL Research Laboratories, Plymouth Meeting, PA) according to the manufacturer's protocol. Recombinant FLAG-tagged ARIP4 was incubated in a 50-μl reaction mixture (containing 20 mM Tris-HCl, pH 8.0, 50 mM KCl, 5 mM MgCl2, and 200 μM ATP) in the presence of double-stranded DNA (dsDNA). pGL3-Basic was used as the dsDNA. The ATPase activity of ARIP4 was examined in the presence of sumoylated Ad4BP/SF-1 and 60 base pairs of dsDNA 5′-GAATTCCTGCAGCCCGGGGGATCGTGCATCCAAGGTCACTGATAGCGATCCACTAGTTCTAGAG-3′ (a binding sequence for Ad4BP/SF-1 is underlined). The CCA in the binding sequence was changed to ATC to generate the mutated dsDNA.
Cell Culture and Reporter Gene Assays
HEK 293 cells were grown in DMEM (Sigma) supplemented with 10% fetal bovine serum and 1× penicillin-streptomycin-glutamine (Invitrogen). MA-10 cells, which were derived from a mouse Leydig tumor (a generous gift of Dr. Mario Ascoli (University of Iowa); Ascoli, 1981), were grown in Waymouth medium (Invitrogen) containing 15% horse serum and 1× penicillin-streptomycin. Both cells were cultured at 5% CO2 and 37°C. pREP-hStAR-Luc, pREP-MIS380-Luc, and pREP-mScc-Luc were generated from pREP-CSF1Pr-Luc (Liu et al., 2001) by replacing the CSF1 promoter with the human StAR promoter (pGL2-human StAR; Sugawara et al., 2000), the mouse MIS promoter (pGL3-MIS380-Luc; Komatsu et al., 2004), and the mouse Cyp11A1(P450scc) promoter (pS2.3H-Luc; Suzuki et al., 2003), respectively. Transfections of these cells were performed with Lipofectamine 2000 reagent (Invitrogen). All transfection experiments were performed in triplicate.
Glutathione S-transferase Pulldown Assays
Recombinant FLAG-Ad4BP/SF-1 was sumoylated in vitro at 4°C for 16 h. Reaction mix was same as described (Komatsu et al., 2004), and glutathione S-transferase (GST)-E1 (a mouse SAE1/SAE2 fusion protein; Uchimura et al., 2004) was removed from the mixture with glutathione Sepharose (GE Healthcare Life Sciences, Piscataway, NJ). Various regions of ARIP4 were amplified by PCR and inserted into pGEX4T-1 or pGEX4T-3 (GE Healthcare Life Sciences). Next, these regions were expressed as GST fusion proteins in E. coli. The E. coli were sonicated in a buffer containing 20 mM Tris-HCl, pH 8.0, 150 mM KCl, 10% glycerol, 5 mM MgCl2, and 0.1% Tween 20. After centrifugation at 70,000 × g for 20 min, the supernatants were incubated with sumoylated Ad4BP/SF-1 and glutathione Sepharose at 4°C for 2 h. The beads were recovered by centrifugation and washed five times with the buffer above, and then the proteins bound to the beads were eluted by SDS-PAGE sample buffer and subjected to Western blotting with anti-FLAG M2 antibody.
Pulldown Assays with Sumoylated Nuclear Receptor
FLAG-tagged LRH-1, AR, Dax-1, Sox9, and PIAS1 constructed with cDNAs used in previous studies (Komatsu et al., 2004, Suzuki et al., 2003, Mukai et al., 2002) were cloned into pFASTBAC vector (Invitrogen). The expression and purification of the proteins were as described above. The baculoviruses of GST-fused VDR, GR, PPARγ, RXRα, FXRα, and SHP were provided by Phenex Pharmaceuticals (Ludwigshafen, Germany). The purified proteins were sumoylated in vitro and incubated with purified ARIP4-HA protein. The fractions that were pulled down by FLAG M2 antibody conjugated with agarose or glutathione Sepharose were immunoblotted with anti-ARIP4 antibody.
Small interfering RNA Treatments
MA-10 cells (n = 5 × 105) or HEK293 cells (n = 5 × 105) were seeded into six-well plates and 24 h later were transfected with 50 nM siRNA duplexes for Ad4BP/SF-1 and ARIP4 (Invitrogen) using 5 μl Lipofectamine 2000 (Invitrogen) in 2 ml of Opti-MEM (Invitrogen). The medium was replaced with fresh medium after 8 h, and total RNA was prepared from the cells 36 h later using ISOGEN reagent (Nippon Gene, Tokyo, Japan). A control small interfering RNA (siRNA; Stealth RNAi Negative Control Medium GC Duplex; Invitrogen) was used as a negative control.
Real-Time PCR
Total RNA was prepared from MA-10 cells or HEK293 cells and reverse-transcribed using a high-capacity cDNA reverse transcription kit (PE Applied Biosystems, Foster City, CA). Real-time PCR was performed with an ABI PRISM 7700 Sequence Detection System (Applied Biosystems). The fluorescent dye SYBR Green was used. Specific primers for the Cyp11A1, StAR, Inhibin α, and luciferase genes were as follows (5′ to 3′): Cyp11A1, forward: CGAAACTAAGACCTGGAAGGACCA; reverse: TGGGTGTACTCATCAGCTTTATTGAA; StAR, forward: CCGGAGCAGAGTGGTGTCA; reverse: GCCAGTGGATGAAGCACCAT; Inhibin α, forward: GCACAGGACCTCTGAACCAGA; reverse: TCACAGGTGGCACCTGTAGC; and luciferase, forward: GCCCGCGAACGACATTTA; reverse: TTTGCAACCCCTTTTTGGAA.
ChIP Assays
ChIP assays were performed basically with the procedure described by Winnay and coworkers (Shang et al., 2000; Winnay and Hammer, 2006). In brief, after cross-linking cells with 1% formaldehyde for 10 min at room temperature, they were rinsed twice with cold PBS, harvested in lysis buffer containing 20 mM Tris-HCl, pH 8.0, 10 mM EDTA, and 1% SDS and incubated for 5 min at 4°C. Next, their chromatin was sonicated to generate DNA fragments of 500-1000 base pairs. To reduce nonspecific background, the samples were precleared with normal rabbit IgG (1 μg/ml), salmon sperm DNA (100 mg/ml), BSA (10 mg/ml), and protein A agarose (50% slurry) for 60 min at 4°C. After centrifugation at 1000 × g for 20 min, the supernatants were incubated with normal rabbit IgG, anti-Ad4BP/SF-1, or anti-ARIP4 antibody overnight at 4°C. Next, protein A beads adsorbed to the immunocomplexes were collected by centrifugation, washed with 50 mM Tris-HCl, pH 8.0, 5 mM EDTA, 150 mM NaCl, 0.5% Nonidet P-40, 0.1% SDS, and Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN), and then washed again with 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 250 mM LiCl, and 0.1% Nonidet P-40. The beads were further washed three times with 10 mM Tris-HCl, pH 8.0, and 1 mM EDTA, and finally the chromatin fragments were eluted from the beads with 1% SDS in 0.1 M NaHCO3. After the cross-linking was reverted by heating at 65°C for 8 h, DNA fragments were recovered using the QIAquick PCR purification system (Qiagen). The purified DNAs were resuspended in 100 μl water and 5-μl aliquots were used for PCR. The PCR was carried out for 30 cycles, and the products were resolved on 2% agarose gels to visualize the SYBR Green staining.
Specific primers for the Mc2R proximal and distal region were described by Winnay and coworkers (Winnay and Hammer, 2006).
RESULTS
Purification of Proteins That Interact Specifically with Sumoylated Ad4BP/SF-1
Identification of proteins that specifically interact with sumoylated Ad4BP/SF-1 would provide valuable insights into the understanding of events that occur after sumoylation. To purify such the proteins, we prepared SUMO-1 modified Ad4BP/SF-1 in vitro (Komatsu et al., 2004). SUMO-1 modified or unmodified Ad4BP/SF-1 was conjugated onto beads and incubated with HeLa cell nuclear extracts, and the binding proteins were subsequently eluted with FLAG peptide (Figure 1A).
Figure 1.
Identification of proteins that preferentially interact with sumoylated Ad4BP/SF-1. (A) Affinity purification of proteins that interact with sumoylated Ad4BP/SF-1. FLAG-Ad4BP/SF-1 (lane 11) was conjugated with SUMO-1 in vitro (lane 12). The sumoylated and unsumoylated FLAG-Ad4BP/SF-1 were incubated with HeLa (lanes 1–3), HEK293 (lanes 4–6), or Y-1 (lanes 7–9) cell nuclear extracts. After recovery with anti-FLAG M2 agarose, the proteins were subjected to SDS-PAGE. The arrows in lanes 3, 6, and 9 indicate a protein that was specifically recovered by sumoylated Ad4BP/SF-1. (B) Affinity purification with SUMO-1– and -2–modified Ad4BP/SF-1. FLAG-Ad4BP/SF-1 (lane 6) was modified in vitro with either SUMO-1 (lane 7) or SUMO-2 (lane 8) and the products were incubated with HeLa nuclear extracts (NE) followed by pulldown with anti-FLAG M2 agarose (lanes 1–4). The recovered proteins were subjected to SDS-PAGE (top panel) and immunoblotting with anti-FLAG M2 antibody (bottom panel). Open and closed arrowheads indicate sumoylated and unsumoylated Ad4BP/SF-1, respectively. The arrows in lanes 3 and 4 indicate ARIP4. (C) Identification of ARIP4 by immunoblotting. The proteins recovered above were subjected to immunoblotting with anti-ARIP4 antibody. As indicated by the arrow, ARIP4 was recovered by both Ad4BP/SF-1 modified with SUMO-1 (lane 4) and SUMO-2 (lane 5), but not with unsumoylated Ad4BP/SF-1 (lane 3); 5% input is shown as a control (lane 1).
With this approach, we identified a protein (indicated by the arrows in Figure 1A) that specifically bound SUMO-Ad4BP/SF-1 but not unmodified Ad4BP/SF-1. This protein was subjected to MALDI–TOF MS analysis, which revealed the identity of this protein as a human homologue of AR interacting protein 4, ARIP4 (hARIP4/KIAA0809/SRISNF2L; Rouleau et al., 2002). ARIP4 belongs to the Rad54 family and consists of a SNF2 domain (Lusser and Kadonaga, 2003; Flaus et al., 2006). In addition to HeLa cells, ARIP4 was also found to bind Ad4BP/SF-1 in a sumoylation-dependent manner in both human HEK293 and mouse adrenocortical Y-1 cells (Figure 1A). These data reconcile with a previous study showing that ARIP4 was among the proteins that were pulled down by GST-SUMO (Rosendorff et al., 2006). Interestingly, neither HDACs, HP1s, DP103 (Lee et al., 2005), nor Daxx (Liu et al., 2006), which were previously reported to interact with sumoylated nuclear receptors or transcriptional factors, were purified in our assays. Importantly, the absence of HDACs in our eluates is consistent with a previous report showing that sumoylation-mediated suppression of Ad4BP/SF-1 is independent of HDACs (Lee et al., 2005).
Next we examined whether ARIP4 interacts differentially with Ad4BP/SF-1 conjugated with SUMO-1 or -2. When SUMO-1– or -2–modified Ad4BP/SF-1 was incubated with HeLa nuclear extracts, both forms successfully pulled down ARIP4 (Figure 1B). In contrast, unsumoylated Ad4BP/SF-1 failed to bind ARIP4. Western blotting with anti-hARIP4 antibody confirmed that ARIP4 bound both SUMO-1 and -2 modified Ad4BP/SF-1 (Figure 1C). Although higher amounts of ARIP4 were recovered by SUMO-1–modified Ad4BP/SF-1, this difference may be due to different stability against these two modified forms (Figure 1B, bottom panel, lanes 4 and 8), rather than different specificities of ARIP4. Because both forms were recognized by ARIP4, we used SUMO-1–modified Ad4BP/SF-1 in our subsequent studies. Of note, in vitro sumoylation at 30°C led to multi- or polysumoylation of Ad4BP/SF-1 (Figure 1A, lane 12). Because in vitro sumoylation at 4°C reduced multi- or polysumoylation of Ad4BP/SF-1, we used sumoylation at 4°C in our subsequent studies.
Regions of ARIP4 Responsible for Its Interaction with Sumoylated Ad4BP/SF-1
To further characterize the interaction between ARIP4 and sumoylated Ad4BP/SF-1, GST-tagged ARIP4 fragments were prepared (Figure 2A) and subjected to pulldown assays with sumoylated Ad4BP/SF-1. The N-terminal region 1 + 2 and the C-terminal regions 5 and 4 + 5 of ARIP4 bound SUMO-Ad4BP/SF-1, whereas regions 1, 3, and 4, and GST alone did not. Interestingly, region 4 + 5 bound unsumoylated Ad4BP/SF-1 with a higher affinity than the other regions, although the region 4 failed to bind the Ad4BP/SF-1. Taken together, these data indicate that regions 2 (259-600 amino acid) and 5 (1194-1467 amino acid) of ARIP4 are involved in its recognition of SUMO, whereas region 4 + 5 (894-1467 amino acid) is involved in its recognition of unmodified Ad4BP/SF-1.
Figure 2.
Localization of the SIMs in ARIP4. (A) Interaction domain mapping of ARIP4. Schematic indicates the truncated forms of ARIP4 that were fused to GST. These GST-ARIP4s (right bottom panel) were incubated with in vitro sumoylated (right top panel) FLAG-Ad4BP/SF-1. Precipitates with glutathione Sepharose were immunoblotted with anti-FLAG antibody. Closed arrowheads indicate unsumoylated Ad4BP/SF-1, and single and double open arrowheads indicate Ad4BP/SF-1 that was sumoylated at one or two sites, respectively; 2% input is indicated. (B and C) The amino acids of ARIP4 responsible for its interaction with sumoylated Ad4BP/SF-1. Amino acid substitutions were made in the candidate sequences for the SIMs in the ARIP4 domains 2 and 5. Muts A to F and Muts 1–7 carry indicated amino acid substitutions (underlined). These proteins were interacted with sumoylated Ad4BP/SF-1 (bottom panels). Glutathione Sepharose precipitates were immunoblotted with anti-FLAG M2 antibody. The single and double open arrowheads indicate Ad4BP/SF-1 that was sumoylated at one or two lysine residues, respectively; 2% input is indicated. (D) The cooperative interaction through the two SIMs of ARIP4. As illustrated on the left, HA-tagged wild-type (WT) ARIP4 and ARIP4 carrying a V431A/I432A (mN-SIM) mutation, an E1463A/V1464A (mC-SIM) mutation, or both mutations (mNC-SIM) were prepared. These proteins were subjected to binding assays with sumoylated Ad4BP/SF-1. As indicated on the right, the anti-FLAG M2 agarose precipitates were immunoblotted with anti-HA antibody; 2% input is indicated.
The amino acid sequences responsible for interactions with SUMO have been shown to be SUMO-interacting motifs (SIMs) Φ-Φ-X-Φ-D/E-D/E-D/E, where Φ indicates L, I, or V, and D/E-D/E-D/E-Φ-D/E-Φ-Φ (Kerscher, 2007). ARIP4 had two candidate SIM sequences, N- and C-SIM (in regions 2 and 5, respectively; Figure 2, B and C). To confirm function of SIMs in ARIP4, sequential amino acid substitutions of N- and C-SIM were performed (shown in Figure 2, B and C). These mutant recombinant proteins were then subjected to pulldown assays. Mutations in amino acids 431-439 (N-SIM) and 1458-1464 (C-SIM) of ARIP4 disrupted its interaction with sumoylated Ad4BP/SF-1. Moreover, functions of these SIMs were examined using the whole ARIP4 protein. ARIP4 mutants mN-SIM harboring V431A/I432A (amino acid substitutions of V to A and I to A at the positions of 431 and 432, respectively) and mC-SIM harboring E1463A/V1464A (amino acid substitutions of E to A and V to A at positions 1463 and 1464, respectively) displayed significantly reduced binding toward SUMO-Ad4BP/SF-1 (Figure 2D). Furthermore, ARIP4 harboring double substitutions (mNC-SIM) failed to interact with sumoylated Ad4BP/SF-1 despite an intact region 4 + 5 which binds unsumoylated Ad4BP/SF-1. Taken together, these results show that two SIMs cooperatively enhance these interactions.
Interaction of ARIP4 with Other Sumoylated Nuclear Receptors
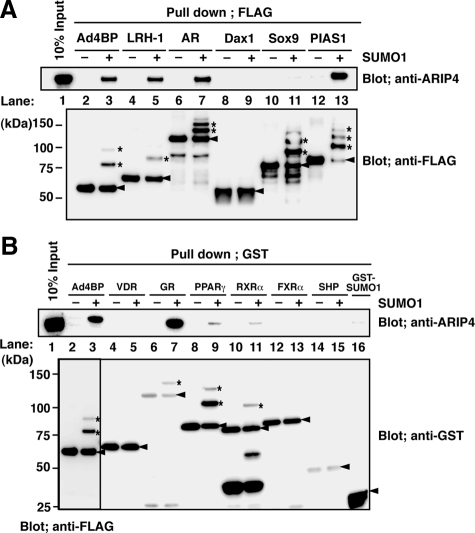
Because Ad4BP/SF-1 belongs to a nuclear receptor superfamily, we examined whether ARIP4 interacts with other sumoylated nuclear receptors. FLAG- or GST-tagged recombinant nuclear receptors were subjected to in vitro sumoylation reactions. Sox9 and PIAS1, two unrelated sumoylated proteins (Komatsu et al., 2004; Gocke et al., 2005), were used as controls. LRH-1, AR, GR, PPARγ, and RXRα were sumoylated along with Ad4BP/SF-1, whereas Dax-1, VDR, FXRα, and SHP were not. These GST-tagged proteins were incubated with ARIP4, and the recovered ARIP4 was detected by immunoblotting with anti-ARIP4 antibody (Figure 3, A and B). Similar to Ad4BP/SF-1, a sumoylation-dependent interaction was observed with LRH-1, AR, GR, and PIAS1. However, ARIP4 did not interact with sumoylated Sox9. The interaction with sumoylated PPARγ and RXRα was detectable albeit at a significantly lower level. As expected, Dax-1, FXRα, and SHP did not interact with ARIP4. Altogether, these data indicate that ARIP4 is capable of interacting with not only Ad4BP/SF-1, but also other sumoylated nuclear receptors.
Figure 3.
Interaction of ARIP4 with sumoylated nuclear receptors. (A and B) FLAG-tagged Ad4BP/SF-1, LRH-1, AR, Dax-1, Sox9, and PIAS1 (A), and GST-fused VDR, GR, PPARγ, RXRα, FXRα, and SHP (B) were purified and used for in vitro sumoylation with SUMO1. Ad4BP/SF-1, LRH-1, AR, Sox9, PIAS1, GR, PPARγ, and RXRα were sumoylated (indicated by asterisks), whereas the others were not. Arrowheads indicate the unsumoylated proteins. The sumoylated proteins were incubated with purified ARIP4-HA protein, and the fractions that were pulled down by FLAG M2 agarose or glutathione Sepharose were immunoblotted with anti-ARIP4 antibody. GST-SUMO1 is used as a control; 10% input is indicated.
The Double-stranded DNA-dependent ATPase Activity of ARIP4 Is Affected by Sumoylated Ad4BP/SF-1
ARIP4 contains a SNF2 domain that possesses an ATPase motif (Laurent et al., 1993; Auble et al., 1994). Indeed, recombinant ARIP4 exhibits ATPase activity (Rouleau et al., 2002; Domanskyi et al., 2006). Therefore, we examined whether the ATPase activity of ARIP4 is modulated through its interaction with sumoylated Ad4BP/SF-1. FLAG-ARIP4 and FLAG-Ad4BP/SF-1 were purified with anti-FLAG M2 resin (Figure 4A) for an ATPase assay. ARIP4 exhibited ATPase activity in the presence of dsDNA (Figure 4B), whereas control did not exhibit ATPase activity. In the presence of nonhydrolyzable ATP-γ-S, the ATPase activity of ARIP4 was not detected. As indicated previously (Domanskyi et al., 2006), the ATPase activity of ARIP4 was increased by the addition of increasing amounts of dsDNA, whereas K311A, an ATPase mutant form of ARIP4, had no ATPase activity (Figure 4B). Because Ad4BP/SF-1 binds to its target DNA sequence (Ad4 site) and its binding activity is unaffected by sumoylation (Komatsu et al., 2004), we assumed that ARIP4 is efficiently recruited to dsDNA by sumoylated Ad4BP/SF-1, and whereby its ATPase is activated. As shown in Figure 4C, although SUMO-Ad4BP/SF-1 itself did not stimulate ARIP4 ATPase activity, the dsDNA containing Ad4 site clearly stimulated its ATPase activity. Importantly, under the same conditions, neither unsumoylated Ad4BP/SF-1 nor SUMO-1 alone stimulated the ARIP4 ATPase. Moreover, dsDNA carrying a mutation in the Ad4 site failed to stimulate this ATPase.
Figure 4.
The ATPase activity of ARIP4 is enhanced in the presence of sumoylated Ad4BP/SF-1 and dsDNA containing an Ad4 site. (A) Purity of the ARIP4, Ad4BP/SF-1, and the in vitro sumoylation products used in the ATPase assay were shown. FLAG-tagged ARIP4 and Ad4BP/SF-1 were expressed in insect cells and purified with anti-FLAG M2 agarose. FLAG-Ad4BP/SF-1 attached to the agarose was subjected to in vitro sumoylation. Fractions eluted were subjected to SDS-PAGE/CBB staining. (B) The dsDNA-dependent ATPase activity of ARIP4. 200 ng of wild-type or ATPase mutant (K311A) ARIP4 was incubated with dsDNA (+1 μg). This assay was performed twice and the average values are indicated. Nonhydrolyzable ATP-γ-S was used as a control. (C) The ATPase activity of ARIP4 enhanced by sumoylated Ad4BP/SF-1 and dsDNA containing an Ad4 site. The ATPase activity of ARIP4 (150 ng) was analyzed in the presence of sumoylated or unsumoylated Ad4BP/SF-1 (100 ng) and 60 base pairs dsDNA containing a wild-type or mutated Ad4 site (40 ng). As a control, SUMO-1 (30 ng) was used. The assay was performed in triplicate, and data are shown as the means ± SD.
Effects of ARIP4 on Ad4BP/SF-1–mediated Transcription
Previous studies strongly suggested that transcriptional activity for the Ad4BP/SF-1 target gene is suppressed by sumoylation of Ad4BP/SF-1 (Chen et al., 2004; Komatsu et al., 2004; Lee et al., 2005; Yang et al., 2009). Considering the sumoylation-dependent interaction of ARIP4, we hypothesized the suppression of Ad4BP/SF-1 is mediated by ARIP4. To test this hypothesis, we performed loss-of-function studies with siRNAs to knock down endogenous ARIP4 and Ad4BP/SF-1 in mouse Leydig cell line (MA-10 cells). These siRNAs successfully reduced the amounts of ARIP4 and Ad4BP/SF-1 in the cells (Supplemental Figure S1). Transcription of the StAR reporter gene was activated by endogenous Ad4BP/SF-1 in response to cAMP, and the treatment with siRNA for Ad4BP/SF-1 decreased the activity to the basal level (Figure 5A). Interestingly, treatment with ARIP4 siRNA led to a 1.5-fold increase in activity. Although the stimulation ratio was weak, we consistently found this stimulation. Simultaneous transfection of both siRNAs failed to activate the StAR promoter, indicating that the endogenous ARIP4 exerts its suppressive function through Ad4BP/SF-1. Moreover, the effects of the siRNAs on the expression of three endogenous Ad4BP/SF-1 target genes (Cyp11A1, StAR, and Inhibin α) were examined in MA-10 cells. Consistent with the reporter assays, the expression of these genes was decreased by the treatment with the siRNAs for Ad4BP/SF-1 and enhanced by the treatment with the siRNAs for ARIP4 (Figure 5B).
Figure 5.
Suppression of Ad4BP/SF-1 mediated transcription by ARIP4. (A) The effect of Ad4BP/SF-1 or ARIP4 depletion on StAR promoter activity. MA-10 cells were transfected with siRNAs as indicated. pREP-hStAR-Luc was used as a reporter gene (200 ng), and 1 mM 8Br-cAMP was added 24 h later. The relative fold changes in the luciferase activities are plotted, with the activity of Ad4BP/SF-1 stimulated by 8Br-cAMP in the presence of control siRNA set at 100. Values are indicated as the means ± SD of at least three experiments. (B) The effect of Ad4BP/SF-1 or ARIP4 depletion on target gene expression. After MA-10 cells were treated with siRNA (as described above), the mRNA for Cyp11a1, StAR, and Inhibin α was examined with RT-PCR. As a control, Control siRNA was used. The relative fold changes are plotted, with the amounts of mRNA in the presence of the control siRNA set at 100. Values are indicated as the means ± SD of at least three experiments. (C) The effect of ARIP4 on Ad4BP/SF-1–mediated transcription of the Cyp11A1 (pREP-Cyp11A1-Luc) and StAR (pREP-hStAR-Luc) genes. HEK293 cells were transiently transfected with the indicated amounts (ng) of pCMX-Ad4BP/SF-1 (Ad4BP), pCMX-Ad4BP/SF-1 K119R/K194R mutant (KR), and pcDNA-ARIP4 (ARIP4). Luciferase reporter genes were cotransfected. After 24 h, 20 μM forskolin was added. After another 24 h, the total cell lysates were recovered. The relative fold changes in the luciferase activities are plotted, with the activity of Ad4BP/SF-1 or KR mutant in the absence of ARIP4 set at 100, respectively. Values are indicated as the means ± SD of at least three experiments. (D) The effect of hARIP4 ATPase mutation (K311A). pREP-hStAR-Luc was cotransfected into Y-1 cells with wild-type or ATPase mutant hARIP4. The cells were stimulated by 10 nM ACTH, and the luciferase activity was determined 24 h later. The relative fold changes in the luciferase activities are plotted, with the activity of Ad4BP/SF-1 in the absence of ARIP4 set at 100. Values are indicated as the means ± SD of at least three experiments. (E) Differential effect of ARIP4 on transcription mediated by wild-type and KR mutant form of Ad4BP/SF-1. HEK293 cells and pREP-hStAR-Luc were used for the study. pCMX-Ad4BP/SF-1 (solid and open circles) or pCMX-Ad4BP/SF-1 KR mutant (solid and open square) was transfected to HEK293 cells with siRNA for ARIP4 (open cycle and square) or control siRNA (solid circle and square). After 18 h incubation, the cells were treated with 2.5 μM α-amanitin for 2 h, and washed twice with PBS. The cells were incubated with serum-free medium containing 20 μM forskolin. Total RNAs were prepared from the cells 0, 30, 60, or 120 min after the forskolin treatment. Luciferase mRNAs were quantified with real time PCR. The relative mRNA levels are plotted when the amounts of the mRNAs at time 0 are fixed as basal level. Values are indicated as the means ± SD of at least three experiments. (F) Transient recruitment of ARIP4 to Ad4BP/SF-1 target gene promoter. HEK293 cells were transfected with pREP-hStAR-Luc and pCMX-Ad4BP/SF-1 (solid bar) or pCMX-Ad4BP/SF-1 KR (K119R/K194R) mutant (open bar). After α-amanitin treatment for 2 h and washing twice with PBS, the cells were incubated with serum-free medium containing 20 μM forskolin. Fixed chromatins were prepared from the cells at the time points indicated, and subjected to immunoprecipitation with anti-Ad4BP/SF-1 antibody. The recovered chromatin fraction was further subjected to immunoprecipitation with anti-ARIP4 antibody. The StAR gene promoter recovered in the two precipitated fractions was quantified by PCR. The amounts of StAR gene promoter recovered by ARIP4 antibody relative to those by Ad4BP/SF-1 antibody (ARIP/Ad4BP/SF-1 or ARIP4/KR) were calculated. The relative fold changes of the relative values above are plotted, with the amount in the presence of wild-type Ad4BP/SF-1 at 0 min incubation set at 1.0. Values are indicated as the means ± SD of at least three experiments. (G) Transient recruitment of ARIP4 to the endogenous Mc2R promoter. Y-1 cells were cultured in a serum-free medium for 48 h and treated with 2.5 μM α-amanitin for 2 h. After ACTH treatment, soluble chromatin was prepared from the cells at the time points indicated, and then subjected to ChIP assay with anti-Ad4BP/SF-1, anti-ARIP4 antibody, or control antibody. The recovered chromatin was amplified using primers for the proximal or distal Mc2R promoter.
To confirm the suppressive function of ARIP4, we examined whether overexpressed ARIP4 suppresses transcription mediated by Ad4BP/SF-1 using the reporter constructs containing Cyp11A1 or StAR promoter. Remarkably, Ad4BP/SF-1–mediated transcription was suppressed dose-dependently by ARIP4 in HEK293 human embryonic kidney cells (Figure 5C). Furthermore, this suppression was significantly weakened when ATPase-deficient ARIP4 (K311A) was used (Figure 5D). To rule out the possibility that ARIP4 protein level affects Ad4BP/SF-1 protein level, Western blotting was performed using whole cell extracts with or without overexpression of ARIP4. The Ad4BP/SF-1 level was not altered by overexpression of ARIP4 in HEK293 cells (Supplemental Figure S2).
Next, we examined whether the effect of ARIP4 depends on sumoylation of Ad4BP/SF-1 using lysine-to-arginine mutant (KR mutant) at the sumoylation sites (K119 and K194) of Ad4BP/SF-1. The transcriptional activity of the KR mutant was ∼1.5-fold higher than that of wild type (Supplemental Figure S3). The KR mutant was expected to be insensitive to suppressive action of overexpressed ARIP4, because ARIP4 is unable to interact with unsumoylated Ad4BP/SF-1. Unfortunately, however, ARIP4 suppressed the transcription mediated by the KR mutant (Figure 5C).
These results with the KR mutant suggested that the suppressive function of ARIP4 is unrelated to sumoylation. However, on the basis of the interaction between ARIP4 and sumoylated Ad4BP/SF-1, we assumed that ARIP4 could affect differentially an early phase of transcription mediated by the wild-type and KR mutant form of Ad4BP/SF-1. Therefore, we examined the accumulation of luciferase mRNA transcribed from the reporter gene up to 120 min after treatment with adenylate cyclase activator forskolin. This treatment is expected to simultaneously activate StAR promoter in most cells. The amounts of the mRNA accumulated by the wild-type Ad4BP/SF-1 increased steadily up to 120 min after the stimulation (Figure 5E). By contrast, the KR mutant activated transcription with a much faster kinetics, i.e., the amount of mRNA accumulated up to 60 min by the KR mutant was more than that up to 120 min by wild-type Ad4BP/SF-1. As expected, treatment with siRNA for ARIP4 increased the amount of mRNA transcribed by wild-type Ad4BP/SF-1 to the level similar to that by KR mutant. Importantly, the same siRNA treatment for ARIP4 did not enhance the amount of mRNA activated by the KR mutant. Interestingly, this suppression seemed to be transient and was released at 120 min. Therefore, these results strongly suggest that ARIP4 suppresses transcription at an early phase and its suppression is transient possibly due to transient recruitment of ARIP4 preferentially to the wild-type Ad4BP/SF-1.
We further examined the recruitment of ARIP4 with ChIP assays combined with quantitative PCR. As shown in Figure 5F, recruitment of ARIP4 was transiently increased at 30 min and returned to baseline by 60 min in the presence of wild-type Ad4BP/SF-1. Such increase was never seen with KR mutant. During this time frame, wild-type and KR mutant of Ad4BP/SF-1 had occupied the promoters (Supplemental Figure S4). Together, this differential recruitment of ARIP4 to the StAR gene promoter is consistent with the differential mRNA accumulation induced by wild-type and KR mutant of Ad4BP/SF-1. This transient recruitment of ARIP4 was further examined on an endogenous gene promoter, Mc2R (melanocortin 2 receptor), in Y-1 cells. The Mc2R gene is one of the targets of Ad4BP/SF-1, and its transcription is initiated by the stimulation of ACTH (Winnay and Hammer, 2006). Soluble chromatin was prepared after ACTH treatment and subjected to ChIP assay with anti-Ad4BP/SF-1 and anti-ARIP4 antibody, respectively. Proximal promoter harboring the site recognized by Ad4BP/SF-1 and distal upstream region lacking the site were amplified by PCR. ARIP4 was found to be recruited transiently to the proximal promoter 20 min after the treatment (Figure 5G). Such recruitment was never seen at the distal upstream region of the gene. Control antibody also produced no signals.
Although ARIP4 was originally shown to activate AR-mediated transcription (Rouleau et al., 2002), our results demonstrate that ARIP4 transiently suppresses Ad4BP/SF-1–mediated transcription. Although mechanism for ARIP4 bidirectional regulation is unclear, there are the multiple possibilities such as a cell type or gene promoter context–dependent mechanism. Otherwise, ARIP4 might form complex to interact with unidentified partner proteins (Table 1) rather than a canonical transactivation/repression pathway. So far, the functions of ARIP4 have been studied without evaluating its capacity to interact with a group of sumoylated proteins. Interestingly, our study demonstrated that the interaction between ARIP4 and AR is significantly enhanced upon the sumoylation of AR. Investigating the function of ARIP4 in light of its preferential binding to sumoylated AR will be required to understand the significance of this interaction.
Table 1.
Intimate correlation of SUMO and Rad54 family members
| SNF2 domain | Partner | Activity | SUMO interaction | SIM | Binding to sumoylated factors | References |
|---|---|---|---|---|---|---|
| Rad54 | Rad51 | D-loop formation, nucleosomesliding, cruciform formation | Rad51 | N.D. | N.D. | Shen et al. (1996); Van Komen et al. (2000); Alexeev et al. (2003); Jaskelioff et al. (2003); Bugreev et al. (2006) |
| ATRX | Daxx | Triple-helix displacement,mononucleosomedisplacement | Daxx | Daxx (1 site) | GR, AR | Xue et al. (2003); Lin et al. (2004, 2006); Tang et al. (2004) |
| ARIP4 | N.D. | Cruciform formation | ARIP4 | ARIP4 (2 sites) | Ad4BP/SF1, LRH-1,AR, GR | Rouleau et al. (2002); Domanskyi et al.(2006) |
N.D., not determined; SIM, SUMO interaction motif.
DISCUSSION
A variety of target proteins are sumoylated and sumoylation has been implicated in a variety of cellular events. To elucidate the mechanisms underlying the functions of SUMO, SUMO-interacting proteins have been identified by two-hybrid screenings using SUMO (Hannich et al., 2005; Hecker et al., 2006) or pulldown assays using GST-SUMO as the baits (Rosendorff et al., 2006). Because these screens merely dependent on an interaction with SUMO, the isolated proteins could not specify the proteins that were targeted for sumoylation, and thus these studies failed to differentially and selectively delineate the events downstream of sumoylation.
To directly link sumoylation to specific downstream events and render specificity of these interactions, factors capable of recognizing individual proteins upon sumoylation would be required to simultaneously recognize both target proteins and SUMO. On the basis of this concept, we attempted to purify proteins that specifically interact with sumoylated Ad4BP/SF-1. Consequently, we successfully purified ARIP4, which possesses regions required for interaction with SUMO and Ad4BP/SF-1.
ARIP4 as a Member of the Rad54 Family
Considering the possibility that sumoylation regulates Ad4BP/SF-1–mediated transcription by recruiting ARIP4, it is interesting to note that ARIP4 contains SNF2 domain that functions as a motor protein in chromatin remodeling complexes (Becker and Horz, 2002). Indeed, some SNF2 family members (such as Swi2/Snf2) are ATPase components residing in chromatin-remodeling complexes (Aalfs and Kingston, 2000). Among the SNF2 domain containing proteins, ARIP4 is classified in the Rad54 subfamily, which included Rad54 and ATRX (α-thalassemia, mental retardation, and X-linked; Lusser and Kadonaga, 2003; Flaus et al., 2006). These proteins have been implicated to have chromatin-remodeling activity like other SNF2 members. As summarized in Table 1, Rad54 and ATRX have the ability to replace/displace nucleosomes and to make conformational changes in DNA (D-loop and cruciform formation). Although cruciform DNA formation was accelerated by ARIP4 (Rouleau et al., 2002), it is still unknown whether ARIP4 has nucleosomal replacement/displacement activity.
Because the SNF2 family proteins were purified as complexes, its chromatin-remodeling activity has been usually evaluated as a complex. This would suggest that the assembly with other proteins is required for the intrinsic activity of ARIP4. In fact, Rad54 interacts with Rad51 and thereby enhances its ability to form cruciforms and D-loops. Moreover, biochemical studies identified Daxx as an interacting partner for ATRX (Xue et al., 2003; Tang et al., 2004). Therefore, finding of new partners of ARIP4 would be responsible for the specific recognition of a specific sumoylated transcription factor and would provide further insight into its functions.
SUMO-dependent Transcriptional Suppression by Chromatin Remodeling Complexes
As to the biological functions of SUMO, it is interesting to note that in addition to ARIP4, Rad51 and Daxx also interact with SUMO (Table 1; Shen et al., 1996; Li et al., 2000; Lin et al., 2006). These interactions suggest that these family members are also functionally related in that sumoylation affects their chromatin-remodeling activity. In fact, a recent study by Lin and coworkers demonstrated that Daxx is capable of recognizing sumoylated AR and GR, and thereby it suppresses their ability to activate transcription (Li et al., 2000; Lin et al., 2006). Considering that sumoylated AR and GR are recognized by ARIP4, selective and concerted recognition by ARIP4 and Daxx could precisely control a variety of events downstream to the sumoylation. Moreover, possible recognition by ARIP4 and Daxx strongly suggests that heterodimeric complexes of Rad54 family members are able to recognize particular sets of sumoylated factors and thereby induce conformational changes in chromatin and DNA to regulate downstream events. In addition to this family, recent siRNA screening successfully isolated a new protein complex, containing the chromatin remodeler Mi-2, the polycomb protein Sfmbt, and MEP-2, which interacts with the sumoylated transcription factor Sp3 and strongly suppresses its activity (Stielow et al., 2008). Based on these data and present study, it is conceivable that individual chromatin remodeling complexes may each contain a unique component that is responsible for the specific recognition of a particular sumoylated transcription factor and that these complexes can selectively and cooperatively regulate SUMO-dependent transcription.
Possible Regulation of Transcriptionally Active versus Suppressive State of Chromatin by SUMO
Transcriptional regulation by nuclear receptors consists of two alternative states, namely, active versus suppressive states. The former state is achieved with recruitment of coactivator such as p160 family members, p300/CBP, and other coactivator complexes, whereas the latter is achieved with recruitment of corepressors such as SMRT and NcoR. Because the molecules recruited to the active or suppressive state are different, these two states seemed to be unrelated and in some cases even mutually exclusive. However, recent studies using ChIP assays revealed that transcriptionally active nuclear receptors continuously bind and release from the binding sites of target promoters (Shang et al., 2000; Metivier et al., 2003; Winnay and Hammer, 2006; Dammer et al., 2007), which is accompanied by the assembly and disassembly of multiple cofactors. Consistent with these observations, ligand-dependent corepressors such as LCoR, RIP140, and PRAME have been identified (Cavailles et al., 1995; Fernandes et al., 2003; Christian et al., 2004; Epping et al., 2005). Moreover, HDAC and other suppressive cofactors have been demonstrated to be recruited even during the transcriptional activation process. Taken together, these data strongly suggested that the active and suppressive transcriptional processes are not mutually exclusive and the presence of the suppressive complex during the transcriptional active target chromatin region is essential for swift switching of transcriptional states.
As for the chromatin structure during the repetitive binding-release cycle of transcriptionally active nuclear receptors, it is critical to know whether histone modification leading to the chromatin structural change would repeatedly occur during the cycling process. This issue was examined with ChIP assays and demonstrated that the repeated recruitment of HDAC leads to repeated deacetylation of histones (Winnay and Hammer, 2006; Dammer et al., 2007). Therefore, the chromatin structure is thought to be changed between active and suppressed states even at transcriptionally active loci. In the case of Ad4BP/SF-1 target genes, the chromatin structures of Mc2R and Cyp11A1 have been thought to be activated by chromatin-remodeling complexes such as SWI/SNF complex (Winnay and Hammer, 2006; Dammer et al., 2007).
In addition to this known regulation, our current study demonstrated that a suppressive factor, ARIP4, is transiently recruited to the active promoters possibly through the recognition of sumoylated Ad4BP/SF-1, resulting in transcriptional suppression of the transcriptionally active genes (Figure 6B). Unexpectedly, however, our present study showed that ARIP4 suppresses transcription mediated by the KR mutant of Ad4BP/SF-1, suggesting that the suppressive action of ARIP4 is unrelated to SUMO. Although we do not have any reasonable explanation for this discrepancy, there are some points to be considered. Although both K119 and K194 of Ad4BP/SF-1 are the major sumoylation sites in vivo and in vitro, the KR mutant might be potentially sumoylated at minor sumoylation sites such as K100, K106, and K460 (predicted by SUMOsp2.0; http://sumosp.biocuckoo.org/). Sumoylation of these sites might be critical for interaction with ARIP4. Another possibility is that ARIP4 forms a complex with an unidentified protein(s) that link ARIP4 with unsumoylated Ad4BP/SF-1 (Figure 6C). In addition to these, two recent studies investigating molecular mechanisms of transcriptional suppression by SUMO should be considered (Campbell et al., 2008; Yang et al., 2009). One of the studies demonstrated that sumoylation affects DNA binding of Ad4BP/SF-1 when the target sequence is not a canonical (strong) but weak sequence. The other demonstrated that SUMO suppresses phosphorylation of Ser203 of Ad4BP/SF-1. Because the phosphorylation by CDK7 activates transcription by Ad4BP/SF-1 (Lewis et al., 2008), sumoylation of the protein leads to transcriptional suppression. Although factors involved in these processes remain to be identified, it is likely that SUMO potentially regulates transcription through multiple and complexed mechanisms. Therefore, to investigate whether ARIP4 is involved in the regulatory processes above would provide direct evidence supporting the functional correlation between SUMO and ARIP4.
Figure 6.
Model of transcriptional suppression by SUMO through transient recruitment of ARIP4 to sumoylated or unsumoylated Ad4BP/SF-1. (A) The transcriptional active state of Ad4BP/SF-1 is achieved with recruitment of cofactors such as p160 family members, p300/CBP, and other cofactor complexes. (B) Once Ad4BP/SF-1 is sumoylated, ARIP4 is transiently recruited to the active promoters through the recognition of sumoylated Ad4BP/SF-1, resulting in temporal transcriptional suppression of the transcriptionally active genes. (C) Unidentified factor(s) is recruited to the target promoter to tether ARIP4 and unsumoylated Ad4BP/SF-1, which leads to transcriptional suppression.
The presence of suppressive regulatory system working simultaneously with the active regulatory system has been known in a variety of biological responses including transcriptional regulation. In this present study, we identified ARIP4, a member of the Rad54 chromatin remodeling family, as a factor that interacts with sumoylated nuclear receptors. Although it remains to be clarified whether the process of ARIP4-dependent regulation is accompanied by recruitment of previously identified or unidentified factors, our current study highlights a novel mechanism for SUMO-dependent transcriptional regulation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. H. Taniguchi and M. Tsuchiya for preliminary experiments; J. Hsieh, Y. Nakatani, T. Kokubo, S. Kato, T. Haraguchi, and S. Hirose for useful comments; M. Shirakawa (Kyoto University) for sumoylation enzymes; and M. Ascoli for MA-10 cells. We also thank the National Institute for Basic Biology Center for analytical instruments for the MALDI-TOF MS analyses. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.O., T.K., and K.M.) and by the Fumi Yamamura Memorial Foundation for Female Natural Scientists (T.K.).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/[[insertDOI]]) on August 19, 2009.
REFERENCES
- Aalfs J. D., Kingston R. E. What does ‘chromatin remodeling’ mean? Trends Biochem. Sci. 2000;25:548–555. doi: 10.1016/s0968-0004(00)01689-3. [DOI] [PubMed] [Google Scholar]
- Alexeev A., Mazin A., Kowalczykowski S. C. Rad54 protein possesses chromatin-remodeling activity stimulated by the Rad51-ssDNA nucleoprotein filament. Nat. Struct. Biol. 2003;10:182–186. doi: 10.1038/nsb901. [DOI] [PubMed] [Google Scholar]
- Ascoli M. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology. 1981;108:88–95. doi: 10.1210/endo-108-1-88. [DOI] [PubMed] [Google Scholar]
- Auble D. T., Hansen K. E., Mueller C. G., Lane W. S., Thorner J., Hahn S. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 1994;8:1920–1934. doi: 10.1101/gad.8.16.1920. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Horz W. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 2002;71:247–273. doi: 10.1146/annurev.biochem.71.110601.135400. [DOI] [PubMed] [Google Scholar]
- Bugreev D. V., Mazina O. M., Mazin A. V. Rad54 protein promotes branch migration of Holliday junctions. Nature. 2006;442:590–593. doi: 10.1038/nature04889. [DOI] [PubMed] [Google Scholar]
- Campbell L. A., Faivre E. J., Show M. D., Ingraham J. G., Flinders J., Gross J. D., Ingraham H. A. Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1) Mol. Cell. Biol. 2008;28:7476–7486. doi: 10.1128/MCB.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles V., Dauvois S., L'Horset F., Lopez G., Hoare S., Kushner P. J., Parker M. G. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Y., Lee W. C., Hsu N. C., Huang F., Chung B. C. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1) J. Biol. Chem. 2004;279:38730–38735. doi: 10.1074/jbc.M405006200. [DOI] [PubMed] [Google Scholar]
- Christian M., Tullet J. M., Parker M. G. Characterization of four autonomous repression domains in the corepressor receptor interacting protein 140. J. Biol. Chem. 2004;279:15645–15651. doi: 10.1074/jbc.M313906200. [DOI] [PubMed] [Google Scholar]
- Dammer E. B., Leon A., Sewer M. B. Coregulator exchange and sphingosine-sensitive cooperativity of steroidogenic factor-1, general control nonderepressed 5, p54, and p160 coactivators regulate cyclic adenosine 3′,5′-monophosphate-dependent cytochrome P450c17 transcription rate. Mol. Endocrinol. 2007;21:415–438. doi: 10.1210/me.2006-0361. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen R. J. SUMO protein modification. Biochim. Biophys. Acta. 2004;1695:113–131. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Domanskyi A., Virtanen K. T., Palvimo J. J., Janne O. A. Biochemical characterization of androgen receptor-interacting protein 4. Biochem. J. 2006;393:789–795. doi: 10.1042/BJ20050823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping M. T., Wang L., Edel M. J., Carlee L., Hernandez M., Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Fernandes I. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol. Cell. 2003;11:139–150. doi: 10.1016/s1097-2765(03)00014-5. [DOI] [PubMed] [Google Scholar]
- Flaus A., Martin D. M., Barton G. J., Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Friedlander R., Melchior F. Concepts in sumoylation: a decade on Nat. Rev. Mol. Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- Gill G. Something about SUMO inhibits transcription. Curr. Opin. Genet Dev. 2005;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Girdwood D., Bumpass D., Vaughan O. A., Thain A., Anderson L. A., Snowden A. W., Garcia-Wilson E., Perkins N. D., Hay R. T. P300 transcriptional repression is mediated by SUMO modification. Mol. Cell. 2003;11:1043–1054. doi: 10.1016/s1097-2765(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Gocke C. B., Yu H., Kang J. Systematic identification and analysis of mammalian small ubiquitin-like modifier substrates. J. Biol. Chem. 2005;280:5004–5012. doi: 10.1074/jbc.M411718200. [DOI] [PubMed] [Google Scholar]
- Hannich J. T., Lewis A., Kroetz M. B., Li S. J., Heide H., Emili A., Hochstrasser M. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:4102–4110. doi: 10.1074/jbc.M413209200. [DOI] [PubMed] [Google Scholar]
- Hay R. T. SUMO: a history of modification. Mol. Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Hecker C. M., Rabiller M., Haglund K., Bayer P., Dikic I. Specification of SUMO1- and SUMO2-interacting motifs. J. Biol. Chem. 2006;281:16117–16127. doi: 10.1074/jbc.M512757200. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Lala D. S., Ikeda Y., Luo X., Shen W. H., Nachtigal M. W., Abbud R., Nilson J. H., Parker K. L. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M., Van Komen S., Krebs J. E., Sung P., Peterson C. L. Rad54p is a chromatin remodeling enzyme required for heteroduplex DNA joint formation with chromatin. J. Biol. Chem. 2003;278:9212–9218. doi: 10.1074/jbc.M211545200. [DOI] [PubMed] [Google Scholar]
- Johnson E. S. Protein modification by SUMO. Annu. Rev. Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Kagey M. H., Melhuish T. A., Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell. 2003;113:127–137. doi: 10.1016/s0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Kerscher O. SUMO junction-what's your function? New insights through SUMO-interacting motifs. EMBO Rep. 2007;8:550–555. doi: 10.1038/sj.embor.7400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol. Endocrinol. 2004;18:2451–2462. doi: 10.1210/me.2004-0173. [DOI] [PubMed] [Google Scholar]
- Laurent B. C., Treich I., Carlson M. The yeast SNF2/SWI2 protein has DNA-stimulated ATPase activity required for transcriptional activation. Genes Dev. 1993;7:583–591. doi: 10.1101/gad.7.4.583. [DOI] [PubMed] [Google Scholar]
- Lee M. B., Lebedeva L. A., Suzawa M., Wadekar S. A., Desclozeaux M., Ingraham H. A. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol. Cell. Biol. 2005;25:1879–1890. doi: 10.1128/MCB.25.5.1879-1890.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. E., Rusten M., Hoivik E. A., Vikse E. L., Hansson M. L., Wallberg A. E., Bakke M. Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol. Endocrinol. 2008;22:91–104. doi: 10.1210/me.2006-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Hesabi B., Babbo A., Pacione C., Liu J., Chen D. J., Nickoloff J. A., Shen Z. Regulation of double-strand break-induced mammalian homologous recombination by UBL1, a RAD51-interacting protein. Nucleic Acids Res. 2000;28:1145–1153. doi: 10.1093/nar/28.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. Y., Fang H. I., Ma A. H., Huang Y. S., Pu Y. S., Jenster G., Kung H. J., Shih H. M. Negative modulation of androgen receptor transcriptional activity by Daxx. Mol. Cell. Biol. 2004;24:10529–10541. doi: 10.1128/MCB.24.24.10529-10541.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. Y. Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol. Cell. 2006;24:341–354. doi: 10.1016/j.molcel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Liu H., Mulholland N., Fu H., Zhao K. Cooperative activity of BRG1 and Z-DNA formation in chromatin remodeling. Mol. Cell. Biol. 2006;26:2550–2559. doi: 10.1128/MCB.26.7.2550-2559.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Liu R., Liu H., Chen X., Kirby M., Brown P. O., Zhao K. Regulation of CSF1 promoter by the SWI/SNF-like BAF complex. Cell. 2001;106:309–318. doi: 10.1016/s0092-8674(01)00446-9. [DOI] [PubMed] [Google Scholar]
- Lund J., Borud B., Mellgren G., Aesoy R., Hoang T., Jacob A. L., Bakke M. Differential regulation of SF-1-cofactor interactions. Endocr. Res. 2002;28:505–513. doi: 10.1081/erc-120016830. [DOI] [PubMed] [Google Scholar]
- Luo X., Ikeda Y., Parker K. L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Lusser A., Kadonaga J. T. Chromatin remodeling by ATP-dependent molecular machines. Bioessays. 2003;25:1192–1200. doi: 10.1002/bies.10359. [DOI] [PubMed] [Google Scholar]
- Martin S., Wilkinson K. A., Nishimune A., Henley J. M. Emerging extranuclear roles of protein SUMOylation in neuronal function and dysfunction. Nat. Rev. Neurosci. 2007;8:948–959. doi: 10.1038/nrn2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. Cell biology. SUMO wrestles its way to prominence in the cell. Science. 2005;307:836–839. doi: 10.1126/science.307.5711.836. [DOI] [PubMed] [Google Scholar]
- Metivier R., Penot G., Hubner M. R., Reid G., Brand H., Kos M., Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- Morohashi K. Structural and functional abnormalities in the spleen of an mFtz-F1 gene-disrupted mouse. Blood. 1999;93:1586–1594. [PubMed] [Google Scholar]
- Mukai T., Kusaka M., Kawabe K., Goto K., Nawata H., Fujieda K., Morohashi K. Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells. 2002;7:717–729. doi: 10.1046/j.1365-2443.2002.00556.x. [DOI] [PubMed] [Google Scholar]
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Rosendorff A., Sakakibara S., Lu S., Kieff E., Xuan Y., DiBacco A., Shi Y., Shi Y., Gill G. NXP-2 association with SUMO-2 depends on lysines required for transcriptional repression. Proc. Natl. Acad. Sci. USA. 2006;103:5308–5313. doi: 10.1073/pnas.0601066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau N., Domans'kyi A., Reeben M., Moilanen A. M., Havas K., Kang Z., Owen-Hughes T., Palvimo J. J., Janne O. A. Novel ATPase of SNF2-like protein family interacts with androgen receptor and modulates androgen-dependent transcription. Mol. Biol. Cell. 2002;13:2106–2119. doi: 10.1091/mbc.01-10-0484.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler J. S., Dejean A. SUMO: of branched proteins and nuclear bodies. Oncogene. 2001;20:7243–7249. doi: 10.1038/sj.onc.1204758. [DOI] [PubMed] [Google Scholar]
- Seeler J. S., Dejean A. Nuclear and unclear functions of SUMO. Nat. Rev. Mol. Cell Biol. 2003;4:690–699. doi: 10.1038/nrm1200. [DOI] [PubMed] [Google Scholar]
- Shang Y., Hu X., DiRenzo J., Lazar M. A., Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- Shen Z., Pardington-Purtymun P. E., Comeaux J. C., Moyzis R. K., Chen D. J. UBL1, a human ubiquitin-like protein associating with human RAD51/RAD52 proteins. Genomics. 1996;36:271–279. doi: 10.1006/geno.1996.0462. [DOI] [PubMed] [Google Scholar]
- Stielow B., Sapetschnig A., Kruger I., Kunert N., Brehm A., Boutros M., Suske G. Identification of SUMO-dependent chromatin-associated transcriptional repression components by a genome-wide RNAi screen. Mol. Cell. 2008;29:742–754. doi: 10.1016/j.molcel.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Sugawara T., Saito M., Fujimoto S. Sp1 and SF-1 interact and cooperate in the regulation of human steroidogenic acute regulatory protein gene expression. Endocrinology. 2000;141:2895–2903. doi: 10.1210/endo.141.8.7602. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kasahara M., Yoshioka H., Morohashi K., Umesono K. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol. Cell. Biol. 2003;23:238–249. doi: 10.1128/MCB.23.1.238-249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Wu S., Liu H., Stratt R., Barak O. G., Shiekhattar R., Picketts D. J., Yang X. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 2004;279:20369–20377. doi: 10.1074/jbc.M401321200. [DOI] [PubMed] [Google Scholar]
- Uchimura Y., Ichimura T., Uwada J., Tachibana T., Sugahara S., Nakao M., Saitoh H. Involvement of SUMO modification in MBD1- and MCAF1-mediated heterochromatin formation. J. Biol. Chem. 2006;281:23180–23190. doi: 10.1074/jbc.M602280200. [DOI] [PubMed] [Google Scholar]
- Uchimura Y., Nakamura M., Sugasawa K., Nakao M., Saitoh H. Overproduction of eukaryotic SUMO-1- and SUMO-2-conjugated proteins in Escherichia coli. Anal. Biochem. 2004;331:204–206. doi: 10.1016/j.ab.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Val P., Lefrancois-Martinez A. M., Veyssiere G., Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl. Recept. 2003;1:8. doi: 10.1186/1478-1336-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Komen S., Petukhova G., Sigurdsson S., Stratton S., Sung P. Superhelicity-driven homologous DNA pairing by yeast recombination factors Rad51 and Rad54. Mol. Cell. 2000;6:563–572. doi: 10.1016/s1097-2765(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Verger A., Perdomo J., Crossley M. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 2003;4:137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnay J. N., Hammer G. D. Adrenocorticotropic hormone-mediated signaling cascades coordinate a cyclic pattern of steroidogenic factor 1-dependent transcriptional activation. Mol. Endocrinol. 2006;20:147–166. doi: 10.1210/me.2005-0215. [DOI] [PubMed] [Google Scholar]
- Xue Y., Gibbons R., Yan Z., Yang D., McDowell T. L., Sechi S., Qin J., Zhou S., Higgs D., Wang W. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA. 2003;100:10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. H., Sharrocks A. D. SUMO promotes HDAC-mediated transcriptional repression. Mol. Cell. 2004;13:611–617. doi: 10.1016/s1097-2765(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Yang W. H., Heaton J. H., Brevig H., Mukherjee S., Iniguez-Lluhi J. A., Hammer G. D. SUMOylation inhibits SF-1 activity by reducing CDK7-mediated serine 203 phosphorylation. Mol. Cell. Biol. 2009;29:613–625. doi: 10.1128/MCB.00295-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Sumoylation regulates diverse biological processes. Cell Mol. Life Sci. 2007;64:3017–3033. doi: 10.1007/s00018-007-7137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Sternsdorf T., Bolger T. A., Evans R. M., Yao T. P. Regulation of MEF2 by histone deacetylase 4- and SIRT1 deacetylase-mediated lysine modifications. Mol. Cell. Biol. 2005;25:8456–8464. doi: 10.1128/MCB.25.19.8456-8464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.