Abstract
An in vitro real-time single turnover assay for the Escherichia coli Sec transport system was developed based on fluorescence dequenching. This assay corrects for the fluorescence quenching that occurs when fluorescent precursor proteins are transported into the lumen of inverted membrane vesicles. We found that 1) the kinetics were well fit by a single exponential, even when the ATP concentration was rate-limiting; 2) ATP hydrolysis occurred during most of the observable reaction period; and 3) longer precursor proteins transported more slowly than shorter precursor proteins. If protein transport through the SecYEG pore is the rate-limiting step of transport, which seems likely, these conclusions argue against a model in which precursor movement through the SecYEG translocon is mechanically driven by a series of rate-limiting, discrete translocation steps that result from conformational cycling of the SecA ATPase. Instead, we propose that precursor movement results predominantly from Brownian motion and that the SecA ATPase regulates pore accessibility.
INTRODUCTION
The SecYEG complex forms the core structure of the transport machinery through which the majority of secreted proteins cross the bacterial cytoplasmic membrane. The SecA ATPase binds to the N-terminal signal sequence and helps guide an extended “linearized” form of the preprotein through the SecYEG pore (N- to C-terminus) from the cytoplasm to the periplasm. ATP hydrolysis by SecA is essential for transport activity. The proton motive force (PMF) increases protein transport efficiency and reduces, but does not eliminate, the reliance on ATP. The cytoplasmic chaperone SecB maintains the precursor protein in a transport competent configuration and thereby delays folding to the active functional form until after the protein has been translocated to the periplasm (see de Keyzer et al., 2003; Dalbey and Chen, 2004; Vrontou and Economou, 2004; Gold et al., 2007; Xie and Dalbey, 2008 for reviews).
A mechanistic model of precursor translocation through the SecYEG complex must explain the movement of the precursor through the pore. Because ATP is essential for transport and SecA is the only ATPase in a minimal transport system, it is natural to assume a direct coupling between ATP hydrolysis and precursor movement. Numerous groups have investigated the properties of SecA in its various conformational states. When bound to the SecYEG complex, large domains of SecA are inaccessible to cytoplasmic proteases and accessible to periplasmic reagents: consequently, this state has been called the “membrane inserted” state. ATP hydrolysis promotes increased accessibility to cytoplasmic proteases, consistent with a conformational change: this process has been called “deinsertion” (Economou and Wickner, 1994, 1997; Kim et al., 1994; Ramamurthy and Oliver, 1997; Nishiyama et al., 1999; Jilaveanu and Oliver, 2007). At low ATP concentrations, protease digestion of the precursor protein yields discrete molecular-weight bands that differ by ∼5 kDa. Nonhydrolyzable ATP analogs shift the molecular weight (MW) spacing to ∼2–2.5 kDa, suggesting that both the “membrane-insertion” and “membrane-deinsertion” conformational changes each translocate ∼20–30 residues of the precursor protein (Schiebel et al., 1991; van der Wolk et al., 1997). Translocation intermediates are formed when a disulfide loop of at least ∼20 residues is formed near the C-terminus of the proOmpA precursor protein. The MW of protease-generated fragments is strikingly dependent on loop size, consistent with the hypothesis that the precursor is translocated in discrete steps (Uchida et al., 1995). However, protease digestion experiments have inherently low time resolution, and thus, a precursor protein stuck within the translocon could seek out thermodynamic minima, i.e., preferred binding arrangements within the channel. Consistent with this hypothesis, movement of one of the two hydrophobic domains in proOmpA changes the apparent MW of the protease-digested precursor protein (Sato et al., 1997). The transmembrane domains of membrane proteins are laterally secreted from the SecYEG channel, presumably due to recognition of the hydrophobic domains (Dalbey and Chen, 2004; Xie and Dalbey, 2008). Therefore, it would not be surprising if a weak translocation arrest could occur at a hydrophobic region of a fully translocated precursor, and, upon inhibition of translocation by a low ATP concentration or a loop structure, that protease protection assays would report these interactions. The SecA insertion/deinsertion (“piston”) model has been challenged by x-ray structural data (van den Berg et al., 2004), which reveal a channel too narrow to accommodate a large domain of SecA that fully penetrates to the periplasm. An alternative hypothesis is that SecA conformational changes occurring entirely on the cytoplasmic side of the SecYEG complex directly feed the precursor into the translocation channel (Erlandson et al., 2008a; Zimmer et al., 2008). Such conformational changes could lead to protease protected domains and periplasmic accessibility through the translocation channel.
According to a model in which precursor translocation is driven by SecA conformational cycling in discrete steps of ∼5 kDa/step, a full kinetic scheme for a single translocation cycle of proOmpA would minimally require a series of ∼6–7 first-order reactions, each driven by an ATP molecule:
In Equation 1, the precursor/SecA/SecYEG complex is denoted simply by P (which may or may not include SecB), the subscripts on P denote the MW (in kDa) that has been translocated through the SecYEG pore, and full translocation is denoted by M (the precursor may or may not have been processed to the mature length protein by signal peptidase). To probe the validity of this reaction mechanism, a transport assay with improved time resolution (1–2 s) is required. A real-time fluorescence-based transport assay utilizing a fluorescently tagged precursor protein has been reported earlier (de Keyzer et al., 2002). However, quenching of the fluorescent dye occurs upon transport into inverted membrane vesicles, and this quenching is nonlinear with respect to concentration, as we show here. Therefore, we have developed a modified assay based on dequenching a His-tagged precursor protein under dilute conditions where the lumenal quenching is accounted for in the kinetic analysis. We present here a detailed kinetic analysis of Sec precursor transport. We find that the proOmpA transport kinetics do not support the stepwise translocation model summarized in Equation 1, and we propose an alternative model.
MATERIALS AND METHODS
Precursor Proteins
The gene for wild-type proOmpA was cloned from the ptrcOmp9 plasmid (gift of Timothy Yahr [University of Iowa] and William Wickner [Dartmouth Medical School]; Crooke et al., 1988) into pET28a (Novagen, Madison, WI). The two wild-type cysteines were mutagenized to serine, and a –LEHHHHHHC C-terminal tag was added with the QuikChange protocol (Stratagene, La Jolla, CA) to yield proOmpA-HisC. The different length precursor constructs outlined in Figure 1A contain the following fragments of OmpA: 0.53XproOmpA-HisC, residues 1-171; 0.77XproOmpA-HisC, residues 1-249; 1.34XproOmpA-HisC, residues 215-325 with a LEVVKVL linker; and 1.51XproOmpA-HisC, residues 161-325 with a LEVVKVG linker. The coding region of all plasmid constructs was confirmed by DNA sequencing.
Figure 1.
The model precursor proteins and the lumenal quenching observed when Atto565-labeled proOmpA was transported into inverted membrane vesicles. (A) Precursor protein constructs used in this study. Each construct contains a single cysteine at the end of a C-terminal 6xHis tag. The signal sequence (SS) and OmpA domains/fragments are indicated. An alanine was added after the N-terminal methionine for cloning purposes. (B) Import reactions with different concentrations of proOmpA-HisC-Atto565, as indicated. Kinetic traces were started ∼3 min after IMVs were added to the SecA/SecB/precursor mixture. Succinate (5 mM) was added to induce a PMF, and ATP (1 mM) was added 30 s later to initiate precursor translocation. [IMV] (A280) = 0.25. (C) Concentration dependence of the total observed fluorescence quenching for different IMV concentrations. The total quenching (n = 3) was determined from experiments like those described in B. [IMV] (A280) = 0.25 (blue), 1.0 (red). (D) Exponential fit of the import kinetics. The ATP-induced fluorescence quenching data (red) from an experiment in B were fit with a single exponential (black). [proOmpA-HisC] = 20 nM.
Precursor proteins were overexpressed in BL21 Star (λDE3; Invitrogen, Carlsbad, CA) by 0.5 mM IPTG induction at 37° when the A600 reached ∼1. Cells (∼2 g) were lysed in 35 ml 20 mM Tris, 5 mM MgCl2, 1 mM β-mercaptoethanol (β-ME), and 1 mM EDTA, pH 7.6, plus protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2 μg/ml pepstatin, 2 μg/ml leupeptin, and 20 μg/ml soybean trypsin inhibitor) by 3× passage through a French press (∼15,000–16,000 psi). The pellet (15,000 × g, 15 min, 4°CC) was resuspended in 10 ml buffer A (8 M urea, 100 mM NaHEPES, and 50 mM sodium citrate, pH 8.0) plus 10 mM imidazole, 10 mM β-ME, and protease inhibitors for 30 min at room temperature (RT). The supernatant (15,000 × g, 15 min, RT) was equilibrated with 12 ml NiNTA resin (Qiagen, Chatsworth, CA) for 45 min at RT. The resin was washed with 60 ml buffer A plus 1% Triton X-100, 1 M NaCl, and 20 mM imidazole, and the protein was eluted with 40 ml buffer A, pH 4.5. Fractions (2 ml each) containing protein (by A280) were pooled and precipitated with an equal volume of cold 30% trichloroacetic acid (TCA) on ice for 30 min. The pellet (16,100 × g, 15 min, 4°CC) was resuspended with 1–2 ml buffer B (8 M urea, 200 mM NaHEPES, pH 8.0); the pH was adjusted to pH 7–8 with 10 N NaOH and pH paper.
Purification of Atto565-labeled Precursor Proteins
Because the precursor protein preparations obtained from the Ni-NTA resin contained significant amounts of mature protein (30–50%), precursor proteins were labeled and purified as follows. Proteins were reacted with a 10-fold molar excess of tris-[2-carboxyethylphosphine] hydrochloride for 10 min to reduce disulfides and then with a 10-fold molar excess of Atto565 maleimide (Sigma) for 15 min (dark, RT). The reaction was quenched with 10 mM β-ME and then diluted with 800 μl SDS sample buffer (500 mM Tris-HCl, 10% SDS, 25% glycerol, 0.1% bromophenol blue, and 1% β-ME, pH 6.8). The proteins were resolved by 12% SDS-PAGE with no wells (200 μl per gel). The precursor bands were visually identified and cut out of the gels with a razor blade. The gel strips were cut into small pieces, and the protein was electroeluted with an Electro-Eluter (Bio-Rad Laboratories, Richmond, CA). Protein was precipitated from the eluate with 1.5 volumes of 30% TCA (ice, 30 min, 4°CC). The pellet (16,100 × g, 15 min) was resuspended in 150 μl buffer B and reprecipitated with an equal volume of 30% TCA (ice, 30 min, 4°CC) to ensure complete removal of SDS. The pellet (16,100 × g, 15 min) was resuspended in 120 μl buffer B, and the pH was adjusted to pH 7–8 with 10 N NaOH and pH paper.
IMV Preparation
Inverted membrane vesicles (IMVs) were prepared from Escherichia coli strain MC4100 using pET610 (gift of Arnold Driessen [University of Groningen]; Kaufmann et al., 1999) to overexpress SecYEG by 0.5 mM IPTG induction at 37° when the A600 reached ∼2. Cells (∼10 g) were suspended in 30 ml lysis buffer (50 mM NaHEPES, 40 mM NaOAc, 1 mM KOAc, 5 mM MgCl2, 250 mM sucrose, and 1 mM β-ME, pH 7.5) and treated with 0.5 mg/ml lysozyme (ice, 10 min). The pellet (15,000 × g, 10 min, 4°CC) was resuspended in 30 ml lysis buffer plus 10 mM EDTA. Cells were lysed by 2× passage through a French press (∼8000–11,000 psi). The supernatant (15,000 × g, 10 min, 4°CC) was loaded onto a 3 ml 1.8 M sucrose cushion (1.8 M sucrose in IMV buffer: 10 mM KH2PO4, 10 mM NaCl, and 5 mM MgSO4, pH 7.5) and ultracentrifuged (235,000 × g, 2 h, 4°CC). The dense brown band at the top of the 1.8 M sucrose cushion was diluted threefold with IMV buffer (no sucrose). The pellet (144,000 × g, 1 h, 4°C) was resuspended in 2 ml IMV buffer with 250 mM sucrose. IMVs were stored at −80°CC in 100-μl aliquots.
Other Proteins
SecB was overexpressed with 0.5 mM IPTG in E. coli strain JM109 containing plasmid pHKSB366 (gift of Arnold Driessen; Fekkes et al., 1998). It was purified with NiNTA resin and dialyzed against buffer C (50 mM NaHEPES, 30 mM NaCl, pH 7.5). SecA was purified from BL21.19 (λDE3) pT7secA2 (gift of Donald Oliver, Wesleyan University) using Cibacron Blue Agarose (Sigma) as described (Mitchell and Oliver, 1993). The SecA was further purified by fast protein liquid chromatography size-exclusion chromatography (Amersham, Piscataway, NJ; Superdex 200 column) using buffer C.
Concentration Estimates
The total protein in IMV preparations was estimated as the A280 in 2% SDS. The SecY concentration was estimated by quantitative Western blotting using NiNTA-purified 6xHis-SecY (encoded by the pET610 plasmid) as a standard. The 6xHis-SecY standard concentration was determined by SDS-PAGE and Coomassie staining using bovine serum albumin (BSA) as a standard. SecB, SecA, and unlabeled precursor concentrations were determined by the BCA method (Pierce, Rockford, IL) using BSA as a standard. For Atto565-labeled precursors, protein concentrations were estimated from the Atto565 extinction coefficient (ε561 = 120,000 M−1 cm−1), assuming that the labeling reaction was quantitative. ATP stock concentrations were estimated from the ATP extinction coefficient (ε259 = 15,400 M−1 cm−1).
Transport Reactions
Import reactions consisted of precursor protein, IMVs, 8 μM SecB and 200 nM SecA (concentrations based on monomeric forms) in import buffer (10 mM KH2PO4, 5 mM MgSO4, 10 mM NaCl, 250 mM sucrose, 0.4 mg/ml BSA, and 1 mM β-ME, pH 7.5).
Fluorescence Measurements
Steady-state and time-resolved fluorescence measurements were made with an SLM-8100 spectrofluorometer. All measurements (800 μl) were performed at 37°CC using a 4 × 10-mm (internal dimensions) cuvette with stir bar. For transport reactions with Atto565-labeled precursors, excitation and emission wavelengths were 565 nm (4-nm slits) and 590 nm (16-nm slits), respectively. Oxonol VI, 100 nM (EX = 610 nm, EM = 645 nm), and 200 nM quinacrine (EX = 420 nm, EM = 510 nm) were used to monitor the presence of Δψ and ΔpH gradients (Kawasaki et al., 1993).
Gel-based Transport Assay
Transport reactions were initiated by addition of IMVs or ATP (as indicated) and were incubated at 37°CC. Reactions were quenched with 1 mg/ml proteinase K, 5 μM valinomycin, and 5 μM nigericin (ice, 15 min). Protease activity was then quenched with 2.5 mM phenylmethylsulfonyl fluoride (ice, 5 min). Samples were boiled (5 min), and proteins were resolved by 12% SDS-PAGE. Bands were detected in wet gels by Atto565 fluorescence using a model FX PhosphorImager (Bio-Rad Laboratories).
Membrane-binding Assay
The number of binding sites on the IMVs was estimated by Scatchard plot analysis after determining the amount of proOmpA-HisC-Atto565 that sedimented with IMVs at various precursor concentrations (2.36–236 nM). The pellet (16,100 × g, 15 min, 4°CC) was resuspended in import buffer. The amount of precursor protein in the supernatant, and the resolubilized pellet was determined via Atto565 fluorescence.
Errors
Error bars are reported as SEMs. Transport times and efficiencies were variable for different IMV preparations. Comparisons of different conditions within each figure panel utilized the same IMV preparation(s). Values compared between panels may be different due to different IMV preparations.
RESULTS
Fluorescence Quenching of Transported proOmpA
The series of proOmpA derivatives used in this study are summarized in Figure 1A. We mutagenized the two cysteines in wild-type proOmpA to serine (C311S, C323S), and added a −HHHHHHC tag to the C-terminus to yield proOmpA-HisC. Precursor proteins of different lengths were constructed by either removing residues from the C-terminus of the proOmpA sequence or by adding part of a second OmpA mature domain. In all cases, the precursor proteins had a single cysteine residue located at the end of a C-terminal 6xHis-tag. The His-tag was used for purification of the protein from urea-solubilized inclusion bodies, and the C-terminal cysteine was used for attachment of the fluorescent dye Atto565 through maleimide chemistry. For most of the experiments reported here, we used the full-length precursor proOmpA-HisC; the other precursors were used solely for determining the relationship between transport time and precursor length.
de Keyzer et al. (2002) demonstrated that fluorescently tagged proOmpA becomes less fluorescent after transport into IMVs from E. coli. Because they did not determine the mechanism of fluorescence quenching, we examined the characteristics of the quenching in greater detail. Incubation of Atto565-labeled proOmpA-HisC with IMVs, ATP, and succinate resulted in a decrease in fluorescence with time (Figure 1B), consistent with the transport of labeled proOmpA into the vesicle lumen (de Keyzer et al., 2002). The initial fluorescence of the bulk solution was linear with precursor concentration, indicating that inner filter effects were not present under the initial conditions. The magnitude of the total observed quenching was greater at higher initial precursor concentrations and for lower IMV concentrations (Figure 1C). Because transport efficiency was largely independent of the precursor concentration (shown later) and the IMV concentration (Supplemental Figure S4A), these data indicate that larger lumenal dye concentrations resulted in greater quenching. This concentration-dependent quenching is inconsistent with the hypothesis that quenching magnitude was dominated by an intrinsic property of the transported protein or by the presence of an intrinsic quencher in the IMVs. Both of these explanations predict that the percent quenching would be relatively invariant with lumenal dye concentration. On the other hand, the concentration dependence of quenching magnitude is consistent with self-quenching, defined here as quenching due to an interaction between identical molecules. The greater quenching observed at the lower IMV concentration (Figure 1C) is consistent with increased self-quenching when a lower lumenal volume was available to the transported precursor protein. Thus, we conclude that the fluorescence decrease observed when precursor proteins were transported into IMVs is consistent with a concentration-dependent self-quenching mechanism.
A Fluorescence Dequenching-based Transport Assay
A reliable fluorescence-based transport assay requires not only that the fluorescence signal is different on the two sides of the membrane, but also that this change in signal is linearly dependent on the dye concentration. The nonzero slopes of the fits to the data in Figure 1C indicate that the lumenal fluorescence emission intensity was not linearly dependent on the lumenal dye concentration. Thus, to obtain accurate kinetic information, the transport kinetics must be decoupled from the concentration-dependent quenching effects. Specifically, we sought to determine whether the short initial lag in the fluorescence change that was observed upon import initiation (Figure 1D) was a consequence of the quenching mechanism or whether it reflected a fundamental property of the transport mechanism.
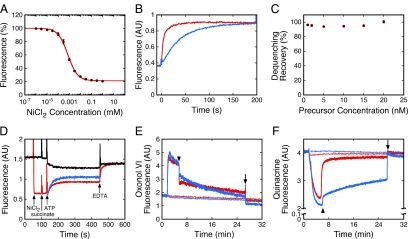
The fluorescence of proOmpA-HisC-Atto565 was significantly quenched by free Ni2+ ion, as expected (Richmond et al., 2000). This fluorescence quenching is consistent with the binding of one or more Ni2+ ions to the 6xHis-tag adjacent to the labeled cysteine residue with a dissociation constant of 0.7 ± 0.1 μM (Figure 2A) (Kapanidis et al., 2001). The bound Ni2+ could be removed from the 6xHis-tag by the addition of EDTA, a strong metal chelator, with recovery of 95–100% of the initial fluorescence (Figure 2, B and C). In addition, reducing the Ni2+ concentration by dilution led to an increased fluorescence, consistent with dissociation of bound Ni2+ (Supplemental Figure S3C). Thus, the Ni2+ binding reaction was reversible.
Figure 2.
Transport assay based on fluorescence dequenching of proOmpA-HisC-Atto565(Ni2+). (A) Quenching of proOmpA-HisC-Atto565 fluorescence by Ni2+. The proOmpA-HisC-Atto565 (30 nM) fluorescence was measured at various concentrations of NiCl2 in import buffer with 8 μM SecB. Data were fit with a single binding site model using Ftotal = (100 + Fbound[NiCl2]/KD)/(1 + [NiCl2]/KD), where Ftotal and Fbound are the observed (total) fluorescence and the fluorescence when Ni2+ was bound to all the 6xHis-tagged precursor molecules, respectively (KD = 0.7 ± 0.1 μM, n = 3). (B) Dequenching of proOmpA-HisC-Atto565(Ni2+) by EDTA. ProOmpA-HisC-Atto565 (7.5 nM) in import buffer with 8 μM SecB was preincubated with NiCl2 (5 μM), quenching the fluorescence as shown in A. Addition of EDTA (3 mM, blue; 5 mM, red) at time t = 0 resulted in dequenching with single exponential kinetics (fits not shown). The reaction order was ∼3–4 with respect to EDTA concentration (not shown). Thus, the dequenching rate is not linear with respect to EDTA concentration. (C) Percent fluorescence dequenching recovery at different proOmpA-HisC-Atto565 concentrations. Dequenching recovery was performed as in B using 10 mM EDTA (n = 3). (D) Fluorescence-based import assay. ProOmpA-HisC-Atto565 (10 nM) was incubated with IMVs (A280 = 1.0). NiCl2 (5 μM), succinate (5 mM), ATP (1 mM), and EDTA (10 mM) were added where indicated. Precursor import was initiated with ATP and was observed as fluorescence dequenching when Ni2+ was present (red), and fluorescence quenching when Ni2+ was absent (black). The true transport kinetics, FN→D (blue), was obtained by subtracting the ATP-induced kinetics without NiCl2 (FD→Q1, black) from that with NiCl2 (FN→Q2, red). (E and F) Δψ (E) and ΔpH (F) gradients produced under various conditions. The gradients produced by succinate alone (5 mM, thick red curves) exhibited a sudden decrease in magnitude when the solutions became anaerobic (arrowheads). The gradients produced by ATP (1 mM) + succinate (thick blue curves) also exhibited a sudden decrease in magnitude when the solutions became anaerobic, although the ΔpH decrease was significantly reduced. Both gradients fully collapsed when ionophores were added (5 μM valinomycin + 5 μM nigericin, arrows). No gradients were obtained if the ionophores were added at t = 0 min (thin curves).
When proOmpA-HisC-Atto565 was preincubated with SecA, SecB, IMVs, succinate, and NiCl2, ATP addition caused fluorescence dequenching (Figure 2D). This dequenching required SecA, SecB, and IMVs. It also required the full-length precursor protein, as dequenching was not observed with fluorescent OmpA (Supplemental Figure S1). Reduced SecYEG levels yielded substantially reduced dequenching (Supplemental Figure S2), indicating that the dequenching did not arise through an interaction with the membrane lipids or with other non-Sec proteins. Together, these control experiments indicate that the observed fluorescence dequenching results from precursor transport through the SecYEG complex.
The time-dependent fluorescence observed when proOmpA-HisC-Atto565(Ni2+) was transported into IMVs was a convolution of dye quenching due to transport of the fluorescent precursor into IMVs and dequenching due to loss of the bound Ni2+. The true transport kinetics were extracted as follows. The ATP-induced kinetics in the absence of Ni2+ (Figure 2D, black curve; same conditions as Figure 1B) is consistent with the transfer of the Atto565 dye from a dilute state (fluorescence = FD) to a more concentrated, quenched state (fluorescence = FQ1), a process that can be represented by the time-dependent function FD→Q1. The increase in fluorescence observed upon ATP addition in the presence of external Ni2+ (Figure 2D, red curve) is consistent with the loss of the bound Ni2+ when the precursor was transported into the IMV lumen (Ni2+ was only added to the external solution). In this case, the Atto565 dye was transferred from a highly quenched state in the presence of Ni2+ (FN) to a higher concentration, quenched state in the absence of Ni2+ (FQ2), a process that can be represented by the time-dependent function FN→Q2. A time-dependent function describing the transfer of the Atto565 dye from the Ni2+-quenched state to a dilute, Ni2+-free state (FN→D) can be obtained by the difference of these two functions, i.e., FN→D = FN→Q2 − FD→Q1 (Figure 2D, blue curve). This resultant function reports the true transport kinetics if the following three conditions hold: 1) the precursor transport rate was identical in the presence and absence of Ni2+; 2) the dequenching reaction (loss of the Ni2+ bound to the precursor protein) was rapid relative to the transport rate; and 3) the two quenched states in the IMV lumen were identical in terms of fluorescence emission intensity (i.e., FQ1 = FQ2). The validity of each of these assumptions is discussed in detail in the Supplemental Material. For simplicity, we shall refer to the time constant obtained from the fluorescence-based kinetics as the transport time. We recognize, however, that the Ni2+ ion might be lost before complete protein translocation and therefore that the observed τ is shorter than the true transport time. This issue is discussed in greater detail below.
Electrical and pH Gradients
The transmembrane Δψ and ΔpH gradients were monitored in independent experiments to confirm that these gradients were established and maintained during the entire period in which the transport kinetics were measured (Figure 2, E and F). In the presence of succinate alone, a sudden spontaneous decrease in both of these gradients was observed ∼3 min after succinate addition because of the consumption of O2 in the solution (Bageshwar and Musser, 2007). When ATP was added 30 s after succinate, as in a typical experiment (e.g., Figure 2D), the ΔpH was significantly higher after O2 depletion (Figure 2F), because of catalytic reversal of the F0F1 ATPase. All of the transport kinetics reported here were >93% completed within ∼2.5 min after ATP addition (i.e., during the high gradient period); the kinetics for shorter precursors were entirely completed within this time period.
Effect of Ni2+ on Transport Time and Transport Efficiency
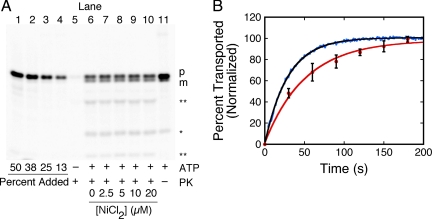
The precursor transport efficiency is independent of Ni2+ concentration, as demonstrated by a gel-based transport assay (Figure 3A). The fluorescence-based assay consistently yielded a slightly faster transport rate than the gel-based transport assay. The ratio of the rates averaged over three IMV preparations was 65 ± 9% (Figure 3B). Because 5 μM NiCl2 was present in both the gel- and fluorescence-based assays, the observed difference in transport rates cannot be explained by an effect of NiCl2 on transport activity. Likely explanations include the loss of the bound Ni2+ before complete precursor translocation (discussed in detail in the Supplementary Material and Discussion), and/or backsliding of the precursor through the translocation channel during work-up for gel analysis (Erlandson et al., 2008b). According to the gel-based assay, the presence of 5 μM NiCl2 caused a slight (∼27%) increase in translocation time compared with that observed in the absence of NiCl2 (Supplemental Figure S3A). These data are consistent with a model in which the bound Ni2+ is removed by unraveling the chelation structure during translocation through the SecYEG pore, and the energy required for this process results in a small reduction in the transport rate. The proOmpA-HisC transport time was independent of NiCl2 concentration at concentrations above the KD for the His-tag (Figure 4A), suggesting a lack of secondary effects. The proOmpA-HisC transport time was about twofold slower in the absence of succinate (Figure 4A).
Figure 3.
Comparison of fluorescence- and gel-based transport assays. (A) Gel-based import assay. The proOmpA-HisC-Atto565 (50 nM) translocation yield, as measured with a protease (proteinase K = PK) protection assay analyzed by SDS-PAGE, was unaffected by NiCl2. Quantification standards (lanes 1–4) contain 13–50% of the precursor added to the import reactions (lanes 5–11). Transport reactions were quenched 5 min after IMV addition. Precursor (p) and mature (m) length protein bands are identified. The starred (*) band of lower MW is a digestion product that was included in the quantification of transport because: 1) it was not present in the precursor stock solution (lanes 1–4); 2) its appearance was ATP-dependent (cf. lane 5 with lane 6); 3) it was protease protected (lanes 6–10) and was generated in the absence of protease addition (lane 11); and 4) it was observed for the unpurified precursor protein (not shown), consistent with formation by an in vivo process. However, the double starred bands (**) were not observed in the absence of protease, suggesting that they were produced by the protease treatment. Bands were identified by Atto565 fluorescence emission. [IMV] (A280) = 1; [succinate] = 5 mM; [ATP] = 1 mM. (B) Comparison of the kinetics obtained from fluorescence- and gel-based transport assays. Shown are typical results obtained as in Figure 2D (blue, fluorescence assay) and Figure 3A (red, gel assay). Single exponential fits to the averaged data from three different IMV preparations yielded τ = 29 ± 4 s and τ = 46 ± 12 s, respectively. The proOmpA-HisC-Atto565 concentration (50 nM) was higher here than for most fluorescence-based assays to directly compare the fluorescence-based assay with translocation rates obtained from the lower sensitivity gel-based assay. Both transport assays were initiated by ATP addition and included 5 μM NiCl2.
Figure 4.
Membrane-binding efficiency of proOmpA-HisC and transport efficiencies and transport times for different NiCl2 and precursor concentrations. (A) Transport time (τ = 1/k) dependence on NiCl2 concentration in the presence (blue) and absence (red) of succinate (5 mM), based on fluorescence-based assays. (B) Transport efficiency dependence on NiCl2 concentration. The transport efficiency of proOmpA-HisC-Atto565 (50 nM) was estimated for different NiCl2 concentrations from gel assays as in Figure 3A (red) and from fluorescence assays as in Figure 2D (blue; n = 3 for each). (C) Sedimentation assay. The amount of proOmpA-HisC-Atto565 that sedimented with IMVs (A280 = 1) was determined at various precursor concentrations (n = 3). [SecB] = 8 μM, [SecA] = 200 nM. (D) Scatchard analysis. The data from C were replotted to estimate the number of proOmpA binding sites on the IMVs. The fit equation is [bound]/[unbound] = KA[V] − KA[bound], where [V] is the IMV binding site concentration. From this analysis, the x-intercept yields an estimate of the number of IMV binding sites (140 ± 40 nM) and the negative inverse of the slope yields the KD (510 ± 60 nM). (E and F) Transport efficiency (E) and transport time (F) dependencies on precursor concentration, according to fluorescence-based assays. Conditions of Figure 2D (n = 3).
We next compared the effect of Ni2+ concentration on the precursor transport efficiency using both gel- and fluorescence-based assays (Figure 4B). The magnitude of the ATP- and EDTA-induced fluorescence changes in our transport experiments (Figure 2D) were measures of the amount of precursor inside and outside the IMVs, respectively. Therefore, these values were quantified and used to estimate the transport efficiency of the fluorescence-based assay. At 5 μM NiCl2, both assays yielded similar precursor transport efficiencies (Figure 4B). According to the gel-based assay, the transport efficiency was independent of Ni2+ concentration (Figures 3A and 4B). However, according to the fluorescence-based assay, the transport efficiency was reduced somewhat at higher Ni2+ concentrations (Figure 4B). At higher external Ni2+ concentrations, the leak rate of Ni2+ across the IMV membrane is expected to be higher. Thus, the IMV lumen may not have been completely devoid of Ni2+ at the higher external Ni2+ concentrations. Leakage of Ni2+ across the IMV membrane would result in an underestimate of the transport efficiency as determined by the fluorescence-based assay. However, significant fluorescence dequenching was still observed after preincubating the IMVs with 20 μM NiCl2 overnight (not shown), indicating that the IMVs are highly impermeable to Ni2+. Because the gel-based assay was the most direct measure of the amount of transported protein, the accuracy of the gel-based approach for determining transport efficiency was likely higher. Most of our subsequent experiments were performed at 5 μM Ni2+, where the difference between the transport efficiencies determined by the two transport assays was within the error of the measurements.
A Single Turnover Assay
Kinetic information obtained from a single turnover assay can provide a more detailed picture of enzyme turnover than that from a multiple turnover assay because it is easier to determine fundamental rate constants from a single reaction cycle. Because the above fluorescence-based transport assay can be performed with nanomolar concentrations of precursor protein, we investigated the possibility that single SecYEG translocation cycles were reported by the observed kinetics. Under conditions where the functional SecYEG concentration is lower than the precursor concentration, reaction completion is expected to take longer at higher precursor concentrations because multiple turnover cycles would be required to transport the excess precursor. In contrast, if the functional SecYEG concentration is in excess over the precursor concentration, the reaction time is expected to be invariant over a range of precursor concentrations because under these conditions each SecYEG translocon would be expected to transport at most one precursor protein. Quantitative Western blotting revealed that the concentration of SecY in a typical transport assay ([IMV] (A280) = 1) was at least ∼400 nM (not shown), though not all molecules may have been active. A binding assay revealed that the number of functional proOmpA-binding sites on the IMVs under the same conditions ([IMV] (A280) = 1) was >40 nM (Figure 4C). A Scatchard analysis, though inaccurate for the precursor concentration range used, nonetheless suggests that the functional SecYEG concentration was likely >100 nM (Figure 4D). This contrasts with the precursor concentration used for typical fluorescence-based transport measurements (≤10 nM). The transport time and transport efficiency measured over a range of precursor concentrations (2–10 nM) were essentially independent of precursor concentration (Figure 4, E and F). Because the precursor concentrations in these assays were well below the concentration of SecA-dependent proOmpA binding sites on the membrane and well below the precursor saturation level of ≥300 nM (Supplemental Figure S4B), these data support the hypothesis that more SecYEG translocons translocated precursors at higher precursor concentrations. We conclude that the precursor concentration was significantly lower than the functional SecYEG concentration and therefore that it was unlikely that a single translocon transported multiple precursor proteins. Thus, the above described fluorescence-based transport assay reports no more than a single turnover cycle of the SecYEG translocon.
Transport Time Dependence on Precursor Length
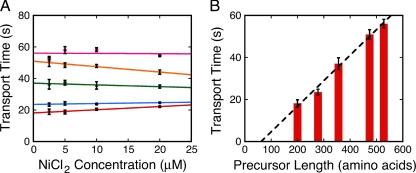
To test the hypothesis that the translocation of the unfolded polypeptide through the SecYEG pore is the rate-limiting step of precursor transport, we investigated the dependence of transport time on precursor length. To correct for any differential effect of Ni2+ on the translocation rates of the series of precursor proteins described in Figure 1A, we measured the transport time dependence on Ni2+ concentration for each precursor protein (Figure 5A). We then extrapolated to 0 μM Ni2+ to estimate the transport time for the various precursor proteins in the absence of Ni2+. We found that longer precursor proteins transported more slowly than shorter precursor proteins (Figure 5B), in agreement with previous results (Tomkiewicz et al., 2006). This finding is consistent with the hypothesis that longer precursor proteins take longer to thread their way through the SecYEG pore. Note that a precursor length versus translocation rate plot for each Ni2+ concentration tested in Figure 5A yields the same conclusion. Such a plot is shown for 5 μM NiCl2 in Supplemental Figure S9.
Figure 5.
Effects of different precursor lengths on transport time. (A) Transport time dependence on NiCl2 concentration for different precursor lengths. All precursors concentrations were 10 nM (n = 2 for each): red, 0.53XproOmpA-HisC; blue, 0.77XproOmpA-HisC; green, proOmpA-HisC; orange, 1.34XproOmpA-HisC; and pink, 1.51XproOmpA-HisC. [IMV] (A280) = 1. (B) Transport time dependence on precursor length. Total precursor length was plotted against the 0 μM NiCl2 transport time (τ), which was estimated by linear extrapolation from the data shown in A. The dashed line is a linear fit to the data, with an x-intercept of 61 amino acids.
Effect of Low ATP Concentration on Transport Time
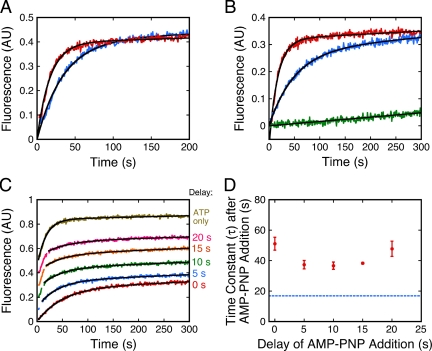
We next investigated the effect of low ATP concentration on the transport kinetics. The goal was to reduce the ATP concentration sufficiently so that the ATP (i.e., SecA)-dependent step(s) of translocation clearly limited the overall translocation rate. The Km of SecA for ATP is ∼0.05 mM (de Keyzer et al., 2002). When the ATP concentration was decreased from 0.25 to 0.02 mM, i.e., to a concentration near the Km, the transport time was slower by about twofold, and the transport kinetics were still well fit by a single-exponential (Figure 6A). These data indicate that even under ATP limiting conditions, a single rate-limiting step is sufficient to explain the observed kinetics.
Figure 6.
Effect of low ATP concentration and AMP-PNP on transport rate. (A) ATP rate-limiting conditions. The transport rate of proOmpA-HisC-Atto565 at a low ATP concentration (0.02 mM ATP, blue, τ = 34 ± 1 s, n = 3) was about twofold slower than the rate at a high ATP concentration (0.25 mM ATP, red, τ = 17 ± 1 s, n = 3). Because of the necessity of maintaining a low constant ATP concentration, the transport reactions in this figure contained an ATP-regenerating system (5 mM creatine phosphate and 0.2 mg/ml creatine phosphokinase). The IMV preparation used in this figure exhibited a linear baseline drift (see B). Thus, the data are fit by a single-exponential with a linear slope. (B) Effect of AMP-PNP. AMP-PNP alone (0.25 mM, green) does not promote proOmpA-HisC-Atto565 transport. The rate observed for ATP + AMP-PNP (0.25 mM each, blue, τ = 51 ± 7 s, n = 3) was about threefold slower than that observed for ATP alone (0.25 mM, red, τ = 17 ± 1 s, n = 3). The AMP-PNP reaction was fit to a line that represents the baseline drift. Transport efficiencies were similar in the absence (45 ± 2%) and presence (42 ± 2%) of AMP-PNP. (C) Delayed addition of AMP-PNP. Under the conditions of B, AMP-PNP was added 0, 5, 10, 15, 20 s, or never after ATP addition, as indicated. Curves are displaced for clarity. (D) Transport rate observed after AMP-PNP addition. The τ's from the fits in C are plotted against the AMP-PNP addition time (n = 3). The transport rate observed in the presence of ATP alone is identified by the dashed line. For A–D: [IMV] (A280) = 1; [proOmpA-HisC-Atto565] = 10 nM.
Effect of AMP-PNP on Transport Time
AMP-PNP, a nonhydrolyzable analog of ATP, does not promote full translocation of precursor proteins. One model is that AMP-PNP promotes the SecA “inserted state” because the “deinsertion” part of the SecA ATPase cycle requires ATP hydrolysis (Economou and Wickner, 1994; Economou et al., 1995; Nishiyama et al., 1999). Consistent with these earlier results, we observed that AMP-PNP was unable to promote precursor transport (Figure 6B). However, when ATP and AMP-PNP were present in an equimolar ratio, precursor transport occurred at a rate about threefold slower than that observed when ATP alone was present (Figure 6B). One possible explanation of these data are that the PMF was reduced in the presence of AMP-PNP. This explanation was ruled out, however, by using nigericin to adjust the ATP-generated PMF to PMF levels observed in the presence of ATP + AMP-PNP (Supplemental Figure S5). An alternate explanation is that AMP-PNP dissociated after binding to SecA and that this dissociation occurred on a time scale similar to ADP dissociation after ATP hydrolysis. This possibility is supported by the similar transport rates observed when reactions were preincubated with AMP-PNP and when ATP and AMP-PNP were added simultaneously (Supplemental Figure S6), i.e., AMP-PNP does not generate a trapped intermediate by high-affinity binding. According to this picture, the transport time was dictated by a direct competition between binding of AMP-PNP and ATP.
The PMF is essential for rapid and efficient precursor transport (Supplemental Figure S7). One model is that ATP is required at the beginning of transport and that the PMF is sufficient to drive the later stages of precursor translocation (Schiebel et al., 1991; Driessen and van der Does, 2002). We tested this picture as follows. If ATP hydrolysis is only required at the beginning of transport, addition of AMP-PNP after these initial stages are completed should have no (or little) effect on the observed transport rate. This was not observed. On AMP-PNP addition 0, 5, 10, 15, or 20 s after ATP addition, the reaction kinetics immediately converted from a fast ATP-driven transport rate to a slower ATP+AMP-PNP-driven transport rate (Figures 6, C and D). These data indicate that AMP-PNP can inhibit the translocation rate after the initial stages of transport.
Simulated Kinetics for a Series of First-Order Reactions
If the repetitive ATP-dependent conformational cycling of SecA rate-limits the translocation of a precursor protein through the SecYEG translocon by directly coupling SecA conformational changes to precursor movement through the pore, the transport kinetics should follow a model that assumes a series of first-order reactions (Equation 1). Simulated kinetics for a series of n first-order reactions indicate that as n increases, an initial lag phase lengthens and the product formation rate after this lag phase becomes progressively faster. These features are readily apparent when fluorescence-based transport assay data are fit according to Equation 1 with n = 1–4 (Figure 7). The best fit is a single exponential (n = 1). For all of the single turnover experiments described above, the precursor translocation kinetics were best fit by n ≤ 2, even for longer precursors for which a lag phase according to Equation 1 should have been more obvious (Supplemental Figure S8, A–E). This conclusion is not affected by the fact that the first 3 s of data were typically discarded because of mixing artifacts (Supplemental Figure S8F). Thus, the data indicate that proOmpA translocation is rate-limited by 1–2 slow kinetic step(s).
Figure 7.
Simulated transport kinetics. The transport kinetics of proOmpA-HisC were fit by numerical simulation. The simulations (Berkeley Madonna) assume n identical rate constants for a series of 1 (red), 2 (green), 3 (orange), and 4 (blue) first-order reactions (see Equation 1). The fluorescence data (black) were obtained under the conditions of Figure 2D (n = 5). The data are normalized to the n = 1 fit (τ = 27 ± 3 s). For n ≠ 1, the baseline was allowed to float. Residuals and the root-mean-square deviations (RMSDs) for each fit are shown in the top four panels.
DISCUSSION
This study reports the development of a fluorescence-based kinetic assay that reveals information about the series of reaction steps within a single Sec transport cycle. The data support the following major conclusions. First, the reaction sequence reported by the assay is rate-limited by at most 1–2 kinetic step(s) under fully energized conditions. Second, under ATP-limiting conditions, the detected transport steps are also rate-limited by 1–2 kinetic step(s). Third, the time necessary to complete the detected reaction steps is approximately linearly dependent on the MW of the precursor protein. And fourth, ATP is utilized at a late stage of the observable kinetics. The implications of these findings are now discussed.
For the reported kinetics, the initial reaction mixture was heterogeneous: ∼20% of proOmpA was bound to the IMVs before ATP addition (Figure 4C). How long did it take those precursor proteins that eventually transported to become bound to a SecYEG complex after ATP addition? If the transport time were largely dictated by the time required for the SecB/precursor complex to bind to the SecYEG complex, it should be correlated with the diffusion constant of the SecB/precursor complex. Diffusion constants are approximately dependent on the cubed-root of MW (Berg, 1993). Hence, the data in Figure 5B strongly argue against the hypothesis that translocation time is dominated by the membrane binding rate. If the precursor binding rate does not dominate the translocation time, could it at least contribute significantly to the translocation kinetics? Such a possibility would require at least two slow kinetic steps: the precursor binding step and some other step. Considering all our data, a single exponential fit (n = 1) was usually the best fit, although an n = 2 fit was also reasonable on some occasions (Supplemental Figure S8). Thus, the kinetic fits are consistent with this two slow step possibility. However, the strong transport time dependence on MW, rather then the cubed-root of the MW, argues that any diffusion component must be small. We therefore conclude that the time required for the precursor to bind to the SecYEG complex likely contributes minimally (<∼20%) to the total fluorescence-based transport kinetics.
As discussed in the last paragraph, the initial population of states that existed at the beginning of the transport kinetics was heterogeneous, but all precursor proteins were kinetically at an early stage in transport. What was the ending point of the observed kinetics? One possibility is that Ni2+ was released from the 6xHis-tag of the precursor proteins after full translocation through the SecYEG translocation channel. This appears unlikely based on the slow spontaneous off-rate of the bound Ni2+ (Supplemental Equation S1a; Supplemental Figure S3C). More likely is the possibility that the Ni2+ was released from the precursor proteins before full translocation (Supplemental Equation S1b), e.g., because the Ni2+ chelation structure(s) could not be accommodated by the translocation channel. The somewhat faster kinetics observed for the fluorescence-based transport assay than for the gel-based transport assay (Figure 3B) is consistent with this hypothesis. One interpretation is that the fluorescence-based transport assay reported translocation of the first ∼65% of proOmpA-HisC, and the gel-based transport assay reported translocation of the entire precursor protein (Figure 3B). Alternatively, considering that the time constant of the observed kinetics is approximately linearly correlated with precursor length (Figure 5B and Supplemental Figure S9), the x-intercept could report the point at which the Ni2+ was forced to dissociated from the precursor protein. According to this argument, the Ni2+ dissociated when ∼60 amino acids, or ∼17% of the proOmpA-HisC protein, remained to be translocated through the SecYEG channel.
Larger time constants were obtained for longer precursors (Figure 5B and Supplemental Figure S9), suggesting that such precursors took more time to transport across the membrane. As discussed earlier, precursor binding to the membrane was likely not the dominant rate-limiting step. Thus, the precursor length most likely exerted its effect on the translocation kinetics after the precursors bound to the IMVs. One possibility is that longer precursor proteins are more difficult to unfold and/or release from SecB, steps that likely occur concomitant with translocation through the SecYEG pore. The kinetics of SecB dissociation during translocation are unknown. We consider it unlikely that the release rate of SecB from precursors of different length would be both linearly dependent on precursor length and always occur in a single rate-limiting step. Another possible step that could contribute to limiting transport rate is the oligomerization of SecYEG complexes (Mori et al., 2003; Scheuring et al., 2005; Osborne and Rapoport, 2007). However, recent structural work suggests that monomeric SecYEG is sufficient for precursor transport (Ménétret et al., 2007; Erlandson et al., 2008a; Zimmer et al., 2008). Moreover, it is unclear how SecYEG oligomerization rate could be linearly dependent on precursor length. A third possibility is that longer precursor proteins require longer times to thread through the SecYEG translocation pore and that the translocation kinetics are dominated by this translocation step. This explanation is simple and intuitive, and, at this juncture, is considered most likely to be correct. As discussed in the previous paragraph, the time constants obtained from the fluorescence-based transport assay do not appear to reflect the time required to transport the entire precursor protein through the SecYEG channel. Nonetheless, it is reasonable that the data reflect the time required for the majority of the length of most of the precursors tested to migrate through this channel. With this in mind, a model in which SecA mechanically drives precursor translocation in ∼5 kDa units per ATPase cycle predicts at least ∼4–5 sequential first-order reaction steps to translocate the first ∼230 amino acids of proOmpA (∼65% of the protein). This model is inconsistent with the single exponential kinetics observed (Figure 7). Interestingly, Tomkiewicz et al. (2006) found a length-dependent lag in the transport kinetics of various proOmpA-derived precursor proteins and therefore interpreted their results as supporting a SecA-driven stepwise translocation model. However, their data were collected in the absence of a PMF, which does not reflect the normal in vivo situation. Further, we tried to reproduce the lag phase with proOmpA-HisC under similar conditions, but were unsuccessful (Supplemental Figure S7).
The conclusion that precursor translocation through the SecYEG pore occurs in a single rate-limiting step contradicts the dominant model that has guided the Sec translocation field for over a decade (Wickner and Leonard, 1996), namely, that repetitive cycles of SecA conformational changes drive precursor translocation. On the one hand, it could be argued that this implies that the translocation of the precursor through the pore simply cannot be the rate-limiting step of transport. As discussed in the last paragraph, we cannot at this stage completely rule out this possibility. However, it is unclear what other transport step could depend on precursor length. Is there an alternative to the dominant model? If, as our data suggest, the SecA ATPase does not mechanically drive precursor movement through the SecYEG pore in a series of discrete steps, what could the ATP be used for and how might the precursor migrate through the translocation pore? The speed of particle diffusion on the nanoscale is generally underappreciated. Using direct imaging techniques, proteins have been observed transiting through the ≥50-nm-long central channel of nuclear pores in <2 ms (Yang and Musser, 2006). Though the shape of precursor proteins transiting through the SecYEG complex and the characteristics of the SecYEG channel itself are very different from those of the nuclear transport system (including that the SecYEG channel is about an order of magnitude shorter in length), it is physically reasonable that an unfolded polypeptide could diffuse through an open SecYEG pore in milliseconds (Simon et al., 1992). The actual translocation rate could be much slower than that predicted by simple diffusion models because of strong interactions between the precursor protein and the transport machinery (Chauwin et al., 1998). We postulate here that the gating mechanisms necessary to prevent free access to the translocation channel requires energy. ATP could be required for SecA to promote opening of the channel in the presence of precursor protein. In this way, ATP hydrolysis is not directly coupled to precursor movement. Direct coupling implies that for every ATP molecule hydrolyzed, the transport machinery moves the precursor protein a specific distance. Instead, ATP hydrolysis could be indirectly coupled to precursor translocation. For example, ATP hydrolysis could be required to open or to keep the channel open, and when open, the precursor randomly diffuses through the translocation channel. One precursor molecule could transport entirely upon hydrolysis of a single ATP molecule. Other identical precursor molecules could require many ATP molecules. If translocation through the SecYEG channel is fast, a histogram of individual translocation times would follow an exponential distribution, where the average translocation time would be related to the translocation efficiency of a single attempt. This model predicts that ATP hydrolysis is required during the entire period that the precursor is transiting (or attempting to transit) through the SecYEG channel, consistent with the data in Figure 6D. A lower ATP concentration or the presence of AMP-PNP would reduce the open probability of the channel, leading to a slower transport rate (Figure 6, A and B). The presence of a PMF could enhance translocation speed either due to an electrophoretic effect or due to the effect of the PMF on the conformations or binding affinities of the precursor or the elements of the translocation system (Andersson and von Heijne, 1994; Cao et al., 1995; Delgado-Partin and Dalbey, 1998; Nishiyama et al., 1999).
In conclusion, a newly developed fluorescence-based assay allows high time-resolution (1 s) kinetic analysis of Sec precursor transport. This approach provides a powerful new method to probe the details of the mechanism of Sec protein transport. The observable reaction sequence is rate-limited by 1–2 reaction step(s). Further work is required to definitively identify the rate-limiting step of transport. At this juncture, the simplest and most intuitive explanation for the length dependence of the transport time is that the movement of the precursor protein through the SecYEG pore is the slow step of transport. If this conclusion is correct, our data are inconsistent with the commonly invoked mechanism wherein precursor movement through the SecYEG pore is mechanically driven by a series of rate-limiting, repetitive conformation cycling steps of the SecA ATPase. Instead, we suggest a model in which ATP hydrolysis is indirectly coupled to precursor translocation. Single molecule experiments would be extremely useful for further examination of the precursor transport mechanism.
Supplementary Material
Acknowledgments
We thank A. Driessen for the SecYEG and SecB overexpression plasmids; D. Oliver for the SecA overexpression plasmid; and T. Yahr and W. Wickner for the proOmpA overexpression plasmid. This research was supported by the National Institutes of Health Grant GM065534 and the Welch Foundation Grant BE-1541.
Glossary
Abbreviations used:
- β-ME
β-mercaptoethanol
- BSA
bovine serum albumin
- IMV
inverted membrane vesicle
- OmpA
outer membrane protein A
- PMF
proton motive force
- RT
room temperature
- TCA
trichloroacetic acid.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-01-0075) on August 5, 2009.
References
- Andersson H., von Heijne G. Membrane protein topology: effects of delta mu H+ on the translocation of charged residues explain the ‘positive inside’ rule. EMBO J. 1994;13:2267–2272. doi: 10.1002/j.1460-2075.1994.tb06508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bageshwar U. K., Musser S. M. Two electrical potential dependent steps are required for transport by the Escherichia coli Tat machinery. J. Cell Biol. 2007;179:87–99. doi: 10.1083/jcb.200702082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. Random Walks in Biology. Princeton, NJ: Princeton University Press; 1993. [Google Scholar]
- Cao G., Kuhn A., Dalbey R. E. The translocation of negatively charged residues across the membrane is driven by the electrochemical potential: evidence for an electrophoresis-like membrane transfer mechanism. EMBO J. 1995;14:866–875. doi: 10.1002/j.1460-2075.1995.tb07068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauwin J. F., Oster G., Glick B. S. Strong precursor-pore interactions constrain models for mitochondrial protein import. Biophys. 1998;J. 74:1732–1743. doi: 10.1016/S0006-3495(98)77884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke E., Guthrie B., Lecker S., Lill R., Wickner W. ProOmpA is stabilized for membrane translocation by either purified E. coli trigger factor or canine signal recognition particle. Cell. 1988;54:1003–1011. doi: 10.1016/0092-8674(88)90115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbey R. E., Chen M. Sec-translocase mediated membrane protein biogenesis. Biochim. Biophys. Acta. 2004;1694:37–53. doi: 10.1016/j.bbamcr.2004.03.009. [DOI] [PubMed] [Google Scholar]
- de Keyzer J., van der Does C., Driessen A. The bacterial translocase: a dynamic protein channel complex. Cell. Mol. Life Sci. 2003;60:2034–2052. doi: 10.1007/s00018-003-3006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Keyzer J., van der Does C., Driessen A.J.M. Kinetic analysis of the translocation of fluorescent precursor proteins into Escherichia coli membrane vesicles. J. Biol. Chem. 2002;277:46059–46065. doi: 10.1074/jbc.M208449200. [DOI] [PubMed] [Google Scholar]
- Delgado-Partin V. M., Dalbey R. E. The proton motive force, acting on acidic residues, promotes translocation of amino-terminal domains of membrane proteins when the hydrophobicity of the translocation signal is low. J. Biol. Chem. 1998;273:9927–9934. doi: 10.1074/jbc.273.16.9927. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., van der Does C. Protein export in bacteria. In: Dalbey R., von Heijne G., editors. Protein Targeting, Transport & Translocation. San Diego: Academic Press; 2002. pp. 47–73. [Google Scholar]
- Economou A., Pogliano J. A., Beckwith J., Oliver D. B., Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83:1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- Economou A., Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Eichler J., Wickner W. Both an N-terminal 65-kDa domain and a C-terminal 30-kDa domain of SecA cycle into the membrane at SecYEG during translocation. Proc. Natl. Acad. Sci. USA. 1997;94:5574–5581. doi: 10.1073/pnas.94.11.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson K. J., Miller S.B.M., Nam Y., Osborne A. R., Zimmer J., Rapoport T. A role for the two-helix finger of the SecA ATPase in protein translocation. Nature. 2008a;455:984–988. doi: 10.1038/nature07439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandson K. J., Or E., Osborne A. R., Rapoport T. Analysis of polypeptide movement in the SecY channel during SecA-mediated protein translocation. J. Biol. Chem. 2008b;283:15700–15715. doi: 10.1074/jbc.M710356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes P., de Wit J. G., van der Wolk J.P.W., Kimsey H. H., Kumamoto C. A., Driessen A.J.M. Preprotein transfer to the Escherichia coli translocase requires the co-operative binding of SecB and the signal sequence to SecA. Mol. Microbiol. 1998;29:1179–1190. doi: 10.1046/j.1365-2958.1998.00997.x. [DOI] [PubMed] [Google Scholar]
- Gold V.A.M., Duong F., Collinson I. Structure and function of the bacterial Sec translocon. Mol. Membr. Biol. 2007;24:387–394. doi: 10.1080/09687680701416570. [DOI] [PubMed] [Google Scholar]
- Jilaveanu L. B., Oliver D. B. In vivo membrane topology of Escherichia coli SecA ATPase reveals extensive periplasmic exposure of multiple functionally important domains clustering on one face of SecA. J. Biol. Chem. 2007;282:4661–4668. doi: 10.1074/jbc.M610828200. [DOI] [PubMed] [Google Scholar]
- Kapanidis A., Ebright Y. W., Ebright R. H. Site-specific incorporation of fluorescent probes into protein: hexahistidine-tag-mediated fluorescent labeling with (Ni2+):nitrilotriacetic acid)n-fluorochrome conjugates. J. Am. Chem. Soc. 2001;123:12123–12125. doi: 10.1021/ja017074a. [DOI] [PubMed] [Google Scholar]
- Kaufmann A., Manting E. H., Veenendaal A.K.J., Driessen A.J.M., van der Does C. Cysteine-directed cross-linking demonstrates that helix 3 of SecE is close to helix 2 of SecY and helix 3 of a neighboring SecE. Biochemistry. 1999;38:9115–9125. doi: 10.1021/bi990539d. [DOI] [PubMed] [Google Scholar]
- Kawasaki S., Mizushima S., Tokuda H. Membrane vesicles containing overproduced SecY and SecE exhibit high translocation ATPase activity and countermovement of protons in a SecA- and presecretory protein-dependent manner. J. Biol. Chem. 1993;268:8193–8198. [PubMed] [Google Scholar]
- Kim Y. J., Rajapandi T., Oliver D. SecA protein is exposed to the periplasmic surface of the E.coli inner membrane in its active state. Cell. 1994;78:845–853. doi: 10.1016/s0092-8674(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Ménétret J.-F., et al. Ribosome binding of a single copy of the SecY complex: implications for protein translocation. Mol. Cell. 2007;28:1083–1092. doi: 10.1016/j.molcel.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Mitchell C., Oliver D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Microbiol. 1993;10:483–497. doi: 10.1111/j.1365-2958.1993.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Mori H., Tsukazaki T., Masui R., Kuramitsu S., Yokoyama S., Johnson A. E., Kimura Y., Akiyama Y., Ito K. Fluorescence resonance energy transfer analysis of protein translocase. SecYE from Thermus thermophilus HB8 forms a constitutive oligomer in membranes. J. Biol. Chem. 2003;278:14257–14264. doi: 10.1074/jbc.M300230200. [DOI] [PubMed] [Google Scholar]
- Nishiyama K., Fukuda A., Morita K., Tokuda H. Membrane deinsertion of SecA underlying proton motive force-dependent stimulation of protein translocation. EMBO J. 1999;18:1049–1058. doi: 10.1093/emboj/18.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne A. R., Rapoport T. Protein translocation is mediated by oligomers of the SecY complex with one SecY copy forming the channel. Cell. 2007;129:97–110. doi: 10.1016/j.cell.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Ramamurthy V., Oliver D. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmic exposed regions and modulation by ATP binding. J. Biol. Chem. 1997;272:23239–23246. doi: 10.1074/jbc.272.37.23239. [DOI] [PubMed] [Google Scholar]
- Richmond T. A., Takahashi T. T., Shimkhada R., Bernsdorf J. Engineered metal binding sites on green fluorescence protein. Biochem. Biophys. Res. Commun. 2000;268:462–465. doi: 10.1006/bbrc.1999.1244. [DOI] [PubMed] [Google Scholar]
- Sato K., Mori H., Yoshida M., Tagaya M., Mizushima S. Short hydrophobic segments in the mature domain of proOmpA determine its stepwise movement during translocation across the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 1997;272:5880–5886. doi: 10.1074/jbc.272.9.5880. [DOI] [PubMed] [Google Scholar]
- Scheuring J., Braun N., Nothdurft L., Stumpf M., Veenendaal A. K., Kol S., van der Does C., Driessen A. J., Weinkauf S. The oligomeric distribution of SecYEG is altered by SecA and translocation ligands. J. Mol. Biol. 2005;354:258–271. doi: 10.1016/j.jmb.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Driessen A.J.M., Hartl F.-U., Wickner W. ΔμH+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991;64:927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Simon S., Peskin C., Oster G. What drives the translocation of proteins? Proc. Natl. Acad. Sci. USA. 1992;89:3770–3774. doi: 10.1073/pnas.89.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkiewicz D., Nouwen N., van Leeuwen R., Tans S., Driessen A. J. SecA supports a constant rate of preprotein translocation. J. Biol. Chem. 2006;281:15709–15713. doi: 10.1074/jbc.M600205200. [DOI] [PubMed] [Google Scholar]
- Uchida K., Mori H., Mizushima S. Stepwise movement of preproteins in the process of translocation across the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 1995;270:30862–30868. doi: 10.1074/jbc.270.52.30862. [DOI] [PubMed] [Google Scholar]
- van den Berg B., Clemons W.M.J., Collinson I., Modil Y., Hartmann E., Harrison S. C., Rapoport T. A. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- van der Wolk J.P.W., de Wit J. G., Driessen A.J.M. The catalytic cycle of the Escherichia coli SecA ATPase comprises two distinct preprotein translocation events. EMBO J. 1997;16:7297–7304. doi: 10.1093/emboj/16.24.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrontou E., Economou A. Structure and function of SecA, the preprotein translocase motor. Biochim. Biophys. Acta. 2004;1694:67–80. doi: 10.1016/j.bbamcr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Wickner W., Leonard M. R. Escherichia coli preprotein translocase. J. Biol. Chem. 1996;271:29514–29516. doi: 10.1074/jbc.271.47.29514. [DOI] [PubMed] [Google Scholar]
- Xie K., Dalbey R. E. Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nat. Rev. Microbiol. 2008;6:234–244. doi: 10.1038/nrmicro3595. [DOI] [PubMed] [Google Scholar]
- Yang W., Musser S. M. Nuclear import time and transport efficiency depend on importin β concentration. J. Cell Biol. 2006;174:951–961. doi: 10.1083/jcb.200605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J., Nam Y., Rapoport T. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature. 2008;455:936–945. doi: 10.1038/nature07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.