Abstract
The pathogenesis of large granular lymphocytic disorders is now being unravelled. Knowledge of the various cells of origin and the (often viral) drivers of proliferation is emerging. Leukemic transformation involves acquisition of mechanisms to escape death and to continue to proliferate. In this perspective article, Drs. Zambello and Semenzato examine new etiopathogenetic clues as a rationale for innovative therapeutic approaches. See related article on page 1407.
The recently updated World Health Organization classification (WHO 2008) has made a great effort in revisiting and making order in the disorders characterized by expansions of lymphocytes with cytotoxic activities. The cytotoxic response to a specific antigen is mediated by two highly professional although extremely different players, cytotoxic T lymphocytes and natural killer (NK) cells, with the common feature of a morphological appearance of large granular lymphocytes (LGL): both are lymphocytes with coarse azurophilic granules within their cytoplasm, containing the cytotoxic weapons they are equipped with. The most striking difference is the way by which they recognize targets: T-cell or NK-cell receptors. During the last two decades, our understanding of the working mechanisms of these different types of cells has increased, leading to an impressive improvement of our knowledge of related disorders. The question is what is occurring when these systems are not working correctly? More importantly, is there a clear-cut distinction between normal and abnormal function?
Proliferations of LGL comprise a spectrum of conditions, ranging from polyclonal, usually self-limited expansions to asymptomatic clonal LGL expansions, to clearly symptomatic leukemic disease.1–3 Polyclonal expansions of LGL are usually transient and due to a viral infection, such as Epstein-Barr virus (EBV) or cytomegalovirus (CMV), neoplasm or autoimmune diseases1–3; sometimes these disorders develop after splenectomy. In contrast, clonal LGL proliferations are stably maintained for time whether or not the patients are symptomatic. In normal individuals, only a minority of circulating blood lymphocytes display the morphological characteristics of LGL. Most of these lymphocytes have a cytotoxic T-cell phenotype (CD3+ CD8+ CD4−), while only a few are NK cells (CD3− CD16+). Clonal expansions of LGL may comprise both T- and NK-cell derived diseases but the WHO considers the latter as a separate group referred to as chronic lymphoid disorders of NK cells.4,5 This separation strengthens the biological difference between the two cell types, which are clearly different although clinical manifestations of T- and NK-cell proliferations might be included within the same family. T-cell LGL (T-LGL) leukemia represents the clonal expansion of a terminally differentiated cytotoxic T-cell bearing a fully functional α /β + T-cell receptor (TCR) with the correlated CD8+ CD4− or, rarely CD4+ CD8−/+dim phenotypic pattern;6–7 expansions of γ/δ + TCR cells account for only rare cases.8 Less information is available for NK cells in chronic lymphoproliferative disorders (CLPD), although it seems that these cells are activated in vivo by persistent/active infection/stimulation. In this perspective article, we will focus on the current etiopathogenetic views of LGL disorders that suggest alternative therapeutic strategies for the treatment of these disorders, as proposed by Mohan et al. in this issue of the journal.9
The etiology of LGL leukemia is largely unknown in most cases. This is likely due to the fact that no single, specific agent can finally trigger the LGL proliferation, this latter perhaps being the expression of erroneous management of a foreign agent. In other words, different events induce the disease through a common pathogenic mechanism. Some crucial cornerstones for disease development have been identified. A number of reports strongly support the role of a chronic/persistent antigenic stimulation by an auto-antigen or a foreign infective antigen as the initial step. This would lead to the expansion of a fully differentiated effector cytotoxic LGL which is not eliminated as a consequence of an impairment of apoptotic pathways.10 This is particularly true for T-LGL leukemia. Early data suggesting the pathogenic role of EBV or human T-cell leukemia viruses (HTVL) has been reported in some but not all cases.11–13 Although no prototypic HTLV infection was demonstrated in these patients, the evidence that sera from a series of patients from Europe and USA react with the recombinant HTLV env protein p21E supports the notion that exposure to a protein containing homology to BA21 may be important in the pathogenesis of this lymphoproliferative disorder.14 It has been proposed that bone marrow, which is frequently involved in patients with LGL proliferation, represents the setting in which the putative antigen incitation takes place; furthermore, dendritic cells have been suggested to represent the target of infection in these patients.15 Compelling evidence has been provided that chronic stimulation of T cells by CMV leads to a persistent clonal expansion of CD4+/CD8−/+dim LGL, with a predominance of TCR Vβ 13.1 usage in individuals with an HLADRB1*0701 haplotype.16 A prevalent expression of this TCR Vβ region has also been reported by our group.17 All these data strongly support the view that a persistent CMV stimulation can trigger and maintain the activated/memory LGL clone in patients with a genetic predisposition. Since dysregulated autoimmune responses are frequently demonstrated in patients with LGL,17,18 such as the presence of rheumatoid factors and anti-nuclear antibodies, and since rheumatic diseases are commonly associated with LGL leukemia (particularly rheumatoid arthritis, Sjogren’s syndrome, Hashimoto’s thyroiditis and lupus erythematosus) a common immunogenetic basis for these conditions has been considered. Interestingly, a history of rheumatoid arthritis is documented in about 25% of these patients, and it has been shown that LGL leukemia patients express the HLADR4 haplotype with a higher frequency than the normal population, as occurs in patients with rheumatoid arthritis.
In recent years progress has been made in understanding the mechanisms sustaining T-LGL leukemia and CLPD-NK. It is now becoming evident that the phenotype of T-cell LGL leukemia is consistent with that of fully differentiated cytotoxic T lymphocytes. The hallmark of T-LGL leukemia lymphocytes is the failure to undergo activation-induced cell death (AICD), this event being consequent to a critical impairment of apoptotic pathways. T-LGL leukemia is characterized by an abnormal clonal expansion of antigen-primed mature cytotoxic T-lymphocytes (CTL) which successfully escape AICD and remain competent in the long-term.10 Clearance of potentially harmful antigen-stimulated effector cytotoxic T-cells after a successful immune response occurs physiologically by triggering Fas-mediated apoptosis through induction of a death-inducing signaling complex (DISC). This process, which is crucial to T-cell homeostasis, requires fine tuning between proliferation, survival, and apoptosis. Like normal activated CTL, leukemic T-LGL cells exhibit activation of multiple survival signaling pathways.6 However, unlike normal activated CTL, leukemic T-LGL cells are not sensitive to Fas-induced apoptosis,20 a process that is essential for AICD.21 Fas resistance can occur in cells with over-expression of the DISC inhibitory protein c-FLIP. This latter is constitutively expressed in leukemic LGL and prevents DISC formation and Fas-mediated apoptosis.22 Several genes are likely to be involved in the above mentioned processes and gene expression profiling has revealed that leukemic LGL are characterized by the expression of genes affecting apoptosis, regulation of TCR signaling and immune response.23 This supports the view that an uncoupling of activation and apoptotic pathways is responsible for the failure of AICD. In fact, genes that are up-regulated in leukemic LGL have anti-apoptotic functions whereas those that are down-regulated are proapoptotic. Pathway-based microarray analysis also indicated that the balance of pro-apoptotic (ceramide and its analogs) and anti-apoptotic (sphingosine-1-phosphate, S1P) sphingolipid-mediated signaling is deregulated in leukemic LGL in favor of S1P.23
Of great interest is the model suggesting that the persistence of interleukin-15 and platelet-derived growth factor is sufficient to reproduce all known deregulations in leukemic T-LGL.10 Interleukin-15 alters expression of Bcl-2 family members, i.e. Bcl-2, Bcl-XL, Bim, Noxa, and Mcl-1. Genes belonging to the BCL-2 family, such as the BCL-2 related X gene (BAX) are down-regulated, while the myeloid cell factor (MCL-1) is up-regulated. In primary leukemic LGL, Bid (which is down-regulated by interleukin-15) levels are low but are reversed following bortezomib treatment, with subsequent increases in LGL apoptosis. These data provide a novel molecular mechanism for interleukin-15 control of Bid which potentially links this cytokine to leukemogenesis through targeted proteasome degradation of Bid.24
While the hallmark of T-LGL leukemia is the proliferation of fully differentiated clonal CTL which fail to undergo AICD, the mechanism sustaining the proliferation of NK cells in CLPD-NK is less clear. Rather than being the result of a failure of apoptotic pathways, several data suggest that a persistence of antigenic stimulation represents a central mechanism in the pathogenesis of this disease. This feature is intrinsically nested in a genetic background, which determines a biased response of NK cells sustained by cells equipped with activating NK receptors. In fact, a typical feature of patients with CLPD-NK is preferential expression of the activating isoforms of killer immunoglobin-like receptors (KIR)25–27 and this pattern correlates with reduced expression of other activating receptors,25 such as natural cytotoxicity receptors. Together with a bias towards activating KIR expression, marked silencing of inhibitory KIR through increased gene methylation has been reported (Gattazzo et al., unpublished data) and a similar epigenetic mechanism has been proposed for TSC-22 gene expression silencing.28 Biochemical studies on the mechanisms sustaining the growth of NK cells in these patients demonstrated a role of RAS farnesyltrans-ferase,29 with clinical implications (see below).
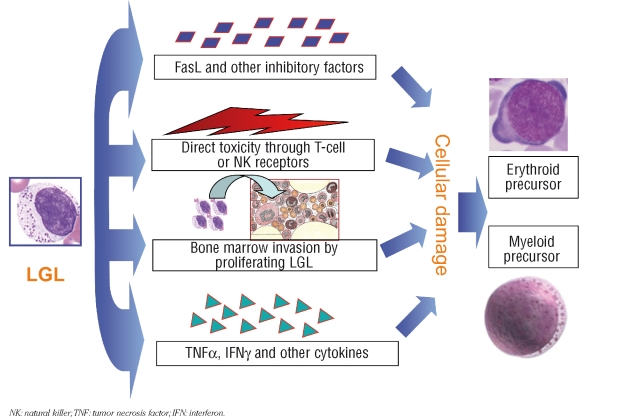
Some information is being gained on the underlying mechanisms sustaining complications of the disease (Figure 1). It should be noted that the size of the clonal population, as detected by flow cytometry, does not correlate with the severity of cytopenia. Accumulation of LGL in the bone marrow is usually not enough to account for the degree of bone marrow invasion. In addition, cytopenias are usually restricted to a single cell lineage. Alternative explanations for cytopenias in T-LGL leukemia have been proposed. There is high surface expression of Fas/Fas-L in LGL and large amounts of soluble Fas-L have been detected in sera of T-LGL patients.30 Fas-L and other inhibitory cytokines produced by LGL may lead to hematopoietic suppression through apoptosis of neutrophil precursors in the bone marrow or result in direct killing of neutrophils that express a high concentration of Fas on their surface. The correction of cytopenias in T-LGL leukemia has been associated with a disappearance or reduction in serum Fas-L levels.30 Isolated, single-lineage cytopenias may result from TCR-mediated targeting of myeloid precursors (neutropenia) or erythroid precursors (reticulocytopenic anemia). Identical or highly similar clonotypes between individual LGL patients or strikingly homologous clonotypes within the TCR repertoire of an individual patient suggest recognition of identical target antigens.31 Abnormal expression of KIR by T-LGL has been demonstrated to be associated with pure red cell aplasia32 and other NK receptors, in particular the NKG2D receptor, have been correlated with neutropenia. Abnormal production of interferon-γ and tumor necrosis factor has been claimed to play a role in the development of pure red cell aplasia. Immune-mediated phenomena, which include direct antiglobulin test positive hemolytic anemia, and (rarely) immune thrombocytopenia, have been described. As a consequence of the increased levels of circulating immune complexes in these patients, the frequent positivity of serum neutrophil antibodies is of marginal significance. Overlapping syndromes of LGL disorders, myelodysplasia, and paroxysmal nocturnal hemoglobinuria33,34 also favor the notion that an immune denominator accounts for this disease.
Figure 1.
Different mechanisms accounting for cytopenias in patients with T-LGL leukemia or CLPD-NK.
NK: natural killer;TNF: tumor necrosis factor; IFN: interferon.
The therapy of LGL disorders has long been empirical and based on anecdotal case reports or, at most, on limited series of cases. The disease may run asymptomatic for many years in the majority of patients, whereas in other cases treatment is needed, usually as a consequence of cytopenia-related manifestations. The percentage of patients who require therapy at some time during their disease ranges from 30 to 70%, according to different series.2,18 In the largest published multicenter study including 151 cases, co-ordinated by our institution, mortality after 4 years of prospective follow-up was 20% and the median survival exceeded 10 years.18 Most deaths are due to sepsis and rarely occur due to disease progression; transformation to aggressive lymphoma is rare,35 as is spontaneous disappearance of LGL lymphocytosis, also in clonal cases.36 Indications for treatment include severe and symptomatic neutropenia, transfusion-dependent anemia or thrombocytopenia as well as progressive disease (i.e., organomegaly, B symptoms and rapidly rising LGL counts). The cytopenias are usually corrected without the clone being eradicated, since this is often resistant to treatment. Immunomodulatory drugs, such as methotrexate (10 mg/m2/week), cyclosporine A (5–10 mg/kg/day) or low dose cyclophosphamide (50 to 100 mg/day)37–40 are commonly used. Corticosteroids may be useful as a part of the initial treatment to accelerate response and growth factors are often used. Adverse events are not severe and are more common with cyclosporine A. Splenectomy may be considered an option in patients with marked splenomegaly and refractory cytopenias.41
Chemotherapeutic agents currently being tested in cutaneous T-cell lymphoma and peripheral T-cell lymphoma, such as gemcitabine, liposomal doxorubicin and purine analogs (fludarabine, cladribine and nelarabine), are possible new agents that could be considered for treatment protocols for symptomatic LGL disorders. These agents should be offered to young patients, particularly when relevant bone marrow infiltration is demonstrated. Successful responses to purine analogs have been reported in the majority of patients treated with pentostatin, fludarabine and 2-chlorodeoxyadenosine,42–44 but the numbers of patients treated in this way are still very low. In selected cases, mainly refractory/relapsed patients with NK disorders, more aggressive strategies might be tried, with the use of L–asparaginase-containing regimens.45 Taking advantage of the promising results, new trials are being designed for patients to be treated upfront.46
Newer agents such as the anti-CD52 monoclonal antibody (Campath-1H), anti-CD122 and anti-CD2 are being incorporated in the therapeutic scenario. The use of anti-CD52 antibody in refractory disease is still controversial,47 with conflicting results perhaps being due to the variable expression of CD52 on the surface of LGL cells, as suggested Mohan et al. in this issue of the journal.9 Humanized MiK-β-1 monoclonal antibody is undergoing testing in a phase I open-label study in patients with T-LGL leukemia (Clinicaltrials.gov identifier: NCT 00079196). This antibody is directed toward CD122, a common subunit of interleukin-2 and inter-leukin-15 receptors. Siplizumab, a humanized anti-CD2 monoclonal antibody (MEDI-507, MedImmune, Gaithersburg, MD, USA), is undergoing testing in two phase I dose escalation studies, sponsored by the NCI and MedImmune, in patients with relapsed/ refractory CD2+ T-cell lymphoma/leukemia including T-LGL leukemia (Clinicaltrials.gov identifier: NCT 00075361, NCT 00105313). The results of these trials will help to understand the role of these molecules in the therapy of LGL disorders.
A better understanding of the signaling pathways activated in LGL neoplasms could identify other biologically targeted agents as potential candidates for inclusion in NK-cell treatment protocols. Good responses to a RAS farnesyltransferase inhibitor, tipifarnib have been obtained in patients with symptomatic T-LGL leukemia and CLPD-NK, in accordance with the findings of constitutively active signaling of the Ras/MAPK/ERK pathway.29 In addition, the proteasome inhibitor bortezomib has been reported to display anticancer activity against aggressive NK leukemia and extranodal NK/T cell lymphoma48 opening new therapeutic perspectives for patients with these diseases. Recent evidence that dasatinib, a broad-spectrum tyrosine kinase inhibitor currently used for treatment of Philadelphia chromosome-positive leukemias, induces a significant expansion of LGL cells highlights the relevance of signaling pathways in the pathogenesis of LGL disorders.49,50
In order to gain insight into the characteristics of LGL proliferations, further knowledge of the development pathways of normal LGL cell is essential. Investigation of disease pathogenesis will lead to the identification of molecular targets and the discovery of more efficacious and less toxic treatments. Finally, to extend the understanding of the pathophysiology of LGL disorders and the development of novel therapeutics, physicians should be encouraged to use National Registries and stimulate enrollment of patients.
Footnotes
Dr. Zambello is an Assistant Professor at the Hematology Section of the Department of Clinical & Experimental Medicine; Dr. Semenzato is Professor of Hematology and Head of the Department at the Padua University School of Medicine, Padua, Italy.
The authors’ research work reported here was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan), by Fondazione Berlucchi per la Ricerca sul Cancro and by Fondazione CARIPARO e CARIVERONA. The authors reported that they have no potential conflicts of interest.
References
- 1.Semenzato G, Pandolfi F, Chisesi T, De Rossi G, Pizzolo G, Zambello R, et al. The lymphoproliferative disease of granular lymphocytes. A heterogeneous disorder ranging from indolent to aggressive conditions. Cancer. 1987;60:2971–8. doi: 10.1002/1097-0142(19871215)60:12<2971::aid-cncr2820601220>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Loughran TP., Jr Clonal diseases of large granular lymphocytes. Blood. 1993;82:1–14. [PubMed] [Google Scholar]
- 3.Oshimi K, Yamada O, Kaneko T, Nishinarita S, Iizuka Y, Urabe A, et al. Laboratory findings and clinical courses of 33 patients with granular lymphocyte-proliferative disorders. Leukemia. 1993;7:782–8. [PubMed] [Google Scholar]
- 4.Chan WC, Foucar KM, Morice WG, Catovsky D. T-cell large granular lymphocytic leukaemia. In: Swerdlow SH, Campo E, Harris NL, Jaffe EJ, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 272–3. [Google Scholar]
- 5.Villamor N, Morice WG, Chan WC, Foucar K. Chronic lymphoproliferative disorders of NK cells. In: Swerdlow SH, Campo E, Harris NL, Jaffe EJ, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 274–5. [Google Scholar]
- 6.Epling-Burnette PK, Loughran TP., Jr Survival signals in leukemic large granular lymphocytes. Semin Hematol. 2003;40:213–20. doi: 10.1016/s0037-1963(03)00135-5. [DOI] [PubMed] [Google Scholar]
- 7.Baesso I, Pavan L, Boscaro E, Miorin M, Facco M, Trentin L, et al. T-cell type lymphoproliferative disease of granular lymphocytes (LDGL) is equipped with a phenotypic pattern typical of effector cytotoxic cells. Leuk Res. 2007;31:371–7. doi: 10.1016/j.leukres.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Bourgault-Rouxel AS, Loughran TP, Jr, Zambello R, Epling-Burnette PK, Semenzato G, Donadieu J, et al. Clinical spectrum of gammadelta+ T cell LGL leukemia: analysis of 20 cases. Leuk Res. 2008;32:45–8. doi: 10.1016/j.leukres.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Mohan SR, Clemente MJ, Afable M, Cazzolli HN, Bejanyan N, Wlodarski MW, et al. Therapeutic implications of variable expression of CD52 on clonal cytotoxic T cells in CD8+ large granular lymphocyte leukemia. Haematologica. 2009;94:1407–14. doi: 10.3324/haematol.2009.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang R, Shah MV, Yang J, Nyland SB, Liu X, Yun JK, et al. Network model of survival signaling in large granular lymphocyte leukemia. Proc Natl Acad Sci USA. 2008;105:16308–13. doi: 10.1073/pnas.0806447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawa-Ha K, Ishihara S, Ninomiya T, Yumura-Yagi K, Hara J, Murayama F, et al. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral DNA. J Clin Invest. 1989;84:51–5. doi: 10.1172/JCI114168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart DN, Baker BW, Inglis MJ, Nimmo JC, Starling GC, Deacon E, et al. Epstein-Barr viral DNA in acute large granular lymphocyte (natural killer) leukemic cells. Blood. 1992;79:2116–23. [PubMed] [Google Scholar]
- 13.Loughran TP, Jr, Zambello R, Ashley R, Guderian J, Pellenz M, Semenzato G, Starkebaum G. Failure to detect Epstein-Barr virus DNA in peripheral blood mononuclear cells in most patients with large granular lymphocyte leukemia. Blood. 1993;81:2723–7. [PubMed] [Google Scholar]
- 14.Loughran TP, Jr, Hadlock KG, Yang Q, Perzova R, Zambello R, Semenzato G, et al. Seroreactivity to an envelope protein of human T-cell leukemia/lymphoma virus in patients with CD3- (natural killer) lymphoproliferative disease of granular lymphocytes. Blood. 1997;90:1977–81. [PubMed] [Google Scholar]
- 15.Zambello R, Berno T, Cannas G, Baesso I, Binotto G, Bonoldi E, et al. Phenotypic and functional analyses of dendritic cells in patients with lymphoproliferative disease of granular lymphocytes (LDGL) Blood. 2005;106:3926–31. doi: 10.1182/blood-2005-05-1972. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Caballero A, Garcia-Montero AC, Barcena P, Almeida J, Ruiz-Cabello F, Tabernero MD, et al. Expanded cells in monoclonal TCRα β ‚+/CD4+/Nkα +/CD8−/+dim T-LGL lymphocytes recognize hCMV antigens. Blood. 2008;112:4609–16. doi: 10.1182/blood-2008-03-146241. [DOI] [PubMed] [Google Scholar]
- 17.Zambello R, Trentin L, Facco M, Cerutti A, Sancetta R, Milani A, et al. Analysis of the T cell receptor in the lymphoproliferative disease of granular lymphocytes: super-antigen activation of clonal CD3+ granular lymphocytes. Cancer Res. 1995;55:6140–5. [PubMed] [Google Scholar]
- 18.Pandolfi F, Loughran TP, Jr, Starkebaum G, Chisesi T, Barbui T, Chan WC, et al. Clinical course and prognosis of the lymphoproliferative disease of granular lymphocytes. A multicenter study. Cancer. 1990;65:341–8. doi: 10.1002/1097-0142(19900115)65:2<341::aid-cncr2820650227>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Semenzato G, Zambello R, Starkebaum G, Oshimi K, Loughran TP., Jr The lymphoproliferative disease of granular lymphocytes: updated criteria for diagnosis. Blood. 1997;89:256–60. [PubMed] [Google Scholar]
- 20.Lamy T, Liu JH, Landowski TH, Dalton WS, Loughran TP., Jr Dysregulation of CD95/CD95 ligand-apoptotic pathway in CD3(+) large granular lymphocyte leukemia. Blood. 1998;92:4771–7. [PubMed] [Google Scholar]
- 21.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Epling-Burnette PK, Painter JS, Zou J, Bai F, Wei S, Loughran TP., Jr Antigen activation and impaired Fas-induced death-inducing signalling complex formation in T-large granular lymphocyte leukemia. Blood. 2008;111:1610–6. doi: 10.1182/blood-2007-06-093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah MV, Zhang R, Irby R, Kothapalli R, Liu X, Arrington T, et al. Molecular profiling of LGL leukemia reveals role of sphingolipid signalling in survival of cytotoxic lymphocytes. Blood. 2008;112:770–81. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodge DL, Yang J, Buschman MD, Schaughency PM, Dang H, Bere W, et al. Interleukin-15 enhances proteasomal degradation of Bid in normal lymphocytes: implications for large granular lymphocyte leukemias. Cancer Res. 2009;69:3986–94. doi: 10.1158/0008-5472.CAN-08-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zambello R, Falco M, Della Chiesa M, Trentin L, Carollo D, Castriconi R, et al. Expression and function of KIR and natural cytotoxicity receptors in NK-type lymphoproliferative disease of granular lymphocytes. Blood. 2003;102:1797–805. doi: 10.1182/blood-2002-12-3898. [DOI] [PubMed] [Google Scholar]
- 26.Epling-Burnette PK, Painter JS, Chaurasia P, Bai F, Wei S, Djeu JY, Loughran TP., Jr Dysregulation of NK receptor expression in patients with lymphoproliferative disease of granular lymphocytes. Blood. 2004;103:3431–9. doi: 10.1182/blood-2003-02-0400. [DOI] [PubMed] [Google Scholar]
- 27.Scquizzato E, Teramo A, Miorin M, Facco M, Piazza F, Noventa F, et al. Genotypic evaluation of killer immunoglobulin-like receptors in NK-type lymphoproliferative disease of granular lymphocytes. Leukemia. 2007;21:1060–9. doi: 10.1038/sj.leu.2404634. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Ershler M, Yu L, Wei M, Hackanson B, Yokohama A, et al. TSC-22 contributes to hematopietic precursor cell proliferation and repopulation and is epigenetically silenced in large granular lymphocyte leukemia. Blood. 2009;113:5558–67. doi: 10.1182/blood-2009-02-205732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epling-Burnette PK, Sokol L, Chen X, Bai F, Zhou J, Blaskovich MA, et al. Clinical improvement by farnesyl-transferase inhibition in NK large granular lymphocyte leukemia associated with imbalanced NK receptor signalling. Blood. 2008;112:4694–8. doi: 10.1182/blood-2008-02-136382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JH, Wei S, Lamy T, Epling-Burnette PK, Starkebaum G, Djeu JY, Loughran TP. Chronic neutropenia mediated by fas ligand. Blood. 2000;95:3219–22. [PubMed] [Google Scholar]
- 31.Wlodarski MW, O'Keefe C, Howe EC, Risitano AM, Rodriguez A, Warshawsky I, et al. Pathologic clonal cytotoxic T-cell responses: nonrandom nature of the T-cell-receptor restriction in large granular lymphocyte leukemia. Blood. 2005;106:2769–80. doi: 10.1182/blood-2004-10-4045. [DOI] [PubMed] [Google Scholar]
- 32.Handgretinger R, Geiselhart A, Moris A, Grau R, Teuffel O, Bethge W, et al. Pure red-cell aplasia associated with clonal expansion of granular lymphocytes expressing killer-cell inhibitory receptors. N Engl J Med. 1999;340:278–84. doi: 10.1056/NEJM199901283400405. [DOI] [PubMed] [Google Scholar]
- 33.Huh YO, Medeiros LJ, Ravandi F, Konoplev S, Jorgensen JL, Miranda RN. T-cell large granular lymphocyte leukemia associated with myelodysplastic syndrome: a clinicopathologic study of nine cases. Am J Clin Pathol. 2009;131:347–56. doi: 10.1309/AJCP6YHI1JEXAWAP. [DOI] [PubMed] [Google Scholar]
- 34.Risitano AM, Maciejewski JP, Muranski P, Wlodarski M, O'Keefe C, Sloand EM, Young NS. Large granular lymphocyte (LGL)-like clonal expansions in paroxysmal nocturnal hemoglobinuria (PNH) patients. Leukemia. 2005;19:217–22. doi: 10.1038/sj.leu.2403617. [DOI] [PubMed] [Google Scholar]
- 35.Matutes E, Wotherspoon AC, Parker NE, Osuji N, Isaacson PG, Catovsky D. Transformation of T-cell large granular lymphocyte leukemia into a high-grade large T-cell lymphoma. Br J Haematol. 2001;115:801–6. doi: 10.1046/j.1365-2141.2001.03220.x. [DOI] [PubMed] [Google Scholar]
- 36.Winton EF, Chan WC, Check I, Colendab KW, Bongiovanni KF, Waldman TA. Spontaneous regression of a monoclonal proliferation of large granular lymphocytes associated with reversal of anemia and neutropenia. Blood. 1986;67:1427–32. [PubMed] [Google Scholar]
- 37.Loughran TP, Kidd PG, Starkebaum G. Treatment of large granular lymphocyte leukemia with oral low-dose methotrexate. Blood. 1994;84:2164–70. [PubMed] [Google Scholar]
- 38.Gabor EP, Mishalani S, Lee S. Rapid response to cyclosporine therapy and sustained remission in large granular lymphocyte leukemia. Blood. 1996;87:1199–200. [PubMed] [Google Scholar]
- 39.Osuji N, Matutes E, Tjonnfjord G, Grech H, Del Giudice I, Wotherspoon A, et al. T-cell large granular lymphocyte leukemia: a report on the treatment of 29 patients and a review of the literature. Cancer. 2006;107:570–8. doi: 10.1002/cncr.22032. [DOI] [PubMed] [Google Scholar]
- 40.Fujishima N, Sawada K, Hirokawa M, Oshimi K, Sugimoto K, Matsuda A, et al. for the PRCA Collaborative Study Group. Long-term responses and outcomes following immunosuppressive therapy in large granular lymphocyte leukemia-associated pure red cell aplasia: a nationwide cohort study in Japan for the PRCA Collaborative Study Group. Haematologica. 2008;93:1555–9. doi: 10.3324/haematol.12871. [DOI] [PubMed] [Google Scholar]
- 41.Subbiah V, Viny AD, Rosenblatt S, Pohlman B, Lichtin A, Maciejewski JP. Outcomes of splenectomy in T-cell large granular lymphocyte leukemia with splenomegaly and cytopenias. Exp Hematol. 2008;36:1078–83. doi: 10.1016/j.exphem.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sternberg A, Eagleton H, Pillai N, Leyden K, Turner S, Pearson D, et al. Neutropenia and anaemia associated with T-cell large granular lymphocyte leukemia responds to fludarabine with minimal toxicity. Br J Haematol. 2003;120:699–701. doi: 10.1046/j.1365-2141.2003.04148.x. [DOI] [PubMed] [Google Scholar]
- 43.Tsirigotis P, Venetis E, Kapsimali V, Rontogianni D, Varvitsioti E, Pappa V, et al. 2-deoxycoformycin in the treatment of T-large granular lymphocyte leukemia. Leuk Res. 2003;27:865–7. doi: 10.1016/s0145-2126(03)00019-5. [DOI] [PubMed] [Google Scholar]
- 44.Edelman MJ, O'Donnell RT, Meadows I. Treatment of refractory large granular lymphocytic leukemia with 2-chlorodeoxyadenosine. Am J Hematol. 1997;54:329–31. doi: 10.1002/(sici)1096-8652(199704)54:4<329::aid-ajh13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi M, Suzuki R, Kwong YL, Kim WS, Hasegawa Y, Izutsu K, et al. Phase I study with dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stages, relapsed or refractory extranodal NK/T-cell lymphoma and leukemia. Cancer Sci. 2008;99:1016–20. doi: 10.1111/j.1349-7006.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaccard A, Gachard N, Coppo P, Morschhauser F, Galicier L, Ysebaert L, et al. A prospective phase II trial of an L-asparaginase containing regimen in patients with refractory or relapsing extranodal NK/T-cell lymphoma. Blood. 2008;112:579A. [Google Scholar]
- 47.Ru X, Liebman HA. Successful treatment of refractory pure red cell aplasia associated with lymphoproliferative disorders with the anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) Br J Haematol. 2003;123:278–81. doi: 10.1046/j.1365-2141.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 48.Shen L, Au WY, Guo T, Wong KY, Wong ML, Tsuchiyama J, et al. Proteasome inhibitor bortezomib-induced apoptosis in natural killer(NK)-cell leukemia and lymphoma: an in vitro and in vivo preclinical evaluation. Blood. 2007;110:469–70. doi: 10.1182/blood-2007-02-072900. [DOI] [PubMed] [Google Scholar]
- 49.Kim DH, Kamel-Reid S, Chang H, Sutherland R, Jung CW, Kim HJ, et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica. 2009;94:135–9. doi: 10.3324/haematol.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23:1398–405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]