Hereditary thrombocythemia is a rare disease characterized by increased megakaryopoiesis and overproduction of platelets. Germline mutations have been identified in the genes for thrombopoietin (THPO) and its receptor, MPL. This study suggests that the recurrent MPL (S505N) mutation found in eight Italian families with hereditary thrombocythemia is likely due to a founder effect.
Keywords: hereditary thrombocythemia, MPL, mutation, founder effect, TPO
Abstract
Background
Hereditary thrombocythemia is a rare disease characterized by increased megakaryopoiesis and overproduction of platelets. Germ line mutations have been identified in the genes for thrombopoietin (THPO) and its receptor, MPL. A clustering of familial cases with the MPL-G1073A mutation that results in a serine to asparagine substitution (S505N) has been recently reported in Italy. Here we performed haplotype analysis in nine families (eight Italian and one Japanese) with hereditary thrombocythemia carrying the MPL-S505N mutation in the MPL gene.
Design and Methods
The MPL gene was examined by genomic DNA sequencing. Haplotype analysis was performed using microsatellites and single nucleotide polymorphisms.
Results
Analysis of microsatellite markers and single nucleotide polymorphisms in the eight Italian families with hereditary thrombocythemia revealed the presence of a common haplotype compatible with a founder effect, which may have originated 23 generations ago. This haplotype was rarely observed in 132 unrelated individuals and was absent in a Japanese family with the MPL-S505N mutation.
Conclusions
The recurrent MPL-S505N mutation found in the eight Italian families with hereditary thrombocythemia is likely due to a founder effect.
Introduction
Hereditary thrombocythemia (HT) is a rare disease characterized by sustained megakaryopoiesis with overproduction of platelets, with clinical features resembling those of sporadically occurring essential thrombo-cythemia.1 Thrombopoietin (THPO) and its receptor MPL regulate platelet production by stimulating proliferation and maturation of megakaryocytes.2 Activating germ line mutations in the THPO and MPL genes have been described in HT families. The four THPO gene mutations known to date are all located in the 5’ untranslated region of the mRNA sequence,3–8 which contains upstream open reading frames that inhibit the translation of the THPO mRNA. The mutations remove the inhibitory upstream open reading frames and lead to increased translation of the THPO mRNA causing elevated serum levels of thrombopoietin and overproduction of platelets.3,6,9 A G>A transition in position 1073 of the MPL gene (G1073A), which changes a serine to an asparagine at amino acid position 505 (S505N) in the transmembrane domain of MPL protein, was first identified in a Japanese family with HT.10 This mutant MPL protein is hyperactive and stimulates megakaryopoiesis resulting in excessive platelet production.10 Interestingly, the identical activating mutation was found in mouse Mpl in a retroviral mutagenesis screening.11 THPO mutations have not been identified in patients with sporadically occurring thrombocythemia,12 but recently the MPL-S505N mutation was described also in patients with non-familial thrombocythemia.13 Furthermore, mutations altering the codon encoding tryptophan in position 515 (W515) located in the juxtamembrane domain of the MPL protein have been found in patients with sporadic myeloproliferative disorders, in particular primary myelofibrosis and essential thrombocythemia.14,15 However, no familial cases of MPL-W515 mutations have been reported to date. Recently, a recessive germ-line mutation changing a proline to leucine at amino acid 106 (P106L) in the MPL gene was reported in families with thrombocytosis.16
We screened for mutations in the MPL gene in 14 families with thrombocytosis and low or normal serum levels of thrombopoietin and found one Italian family with the MPL-S505N mutation. In contrast, in another study, the MPL-S505N mutation was detected in four of five families of Italian descent.17 The clustering of the MPL-S505N mutation in Italian HT families suggested that the mutation may have originated from a single mutation that occurred many generations ago and that these families are distantly related and inherited the MPL-S505N mutation from the same individual. A founder effect for a disease-causing germline mutation can arise when a new population is established by a very small number of individuals who include a carrier of the mutation. One example is the VHL 598C>T mutation that causes Chuvash polycythemia and has been reported to originate from a single founder event.18 Here we tested the hypothesis that the G>A transition in the Italian families with MPL-S505N is the result of a founder effect.
Design and Methods
Patients and clinical features
Blood samples were collected at the study centers in Basel (Switzerland), Rome (Italy), and Nagoya (Japan) following approval by the local ethics committees (Ethik Kommission Beider Basel, and the institutional boards of Rome and Nagoya). Written consent was obtained from all patients in accordance with the Declaration of Helsinki. Five of the families (A, B, C, D and J) have been previously described.10,17 In family E with the MPL-S505N mutation, the proposita, a 5-year-old girl, was referred to us because of asymptomatic thrombocytosis since birth and the suspicion that she had essential thrombocythemia. However, clonogenic assays were negative, and family history and blood cell counts revealed that the father and grandmother also had high platelet counts on repeated occasions. Initial screening for the MPL mutation was performed on DNA from 13 additional Caucasian families with thrombocytosis originating from the UK (5 pedigrees), Italy (2), Spain (2), Switzerland (1), Israel (1), Germany (1) and USA (1). Family F consisted of two sisters (23 and 21 years old) with asymptomatic thrombocytosis, referred to us because of the suspicion of essential thrombocythemia. Family G consisted of three female patients aged 23, 42 and 61 years at diagnosis. Family H included two patients (father and son) aged 71 and 31 years, respectively. DNA from 132 unrelated patients with sporadic myeloproliferative disorders (kindly provided by Prof. Mario Cazzola and Dr. Francesco Passamonti) was used to determine the frequency of the MPL-S505N haplotype.
MPL gene sequencing
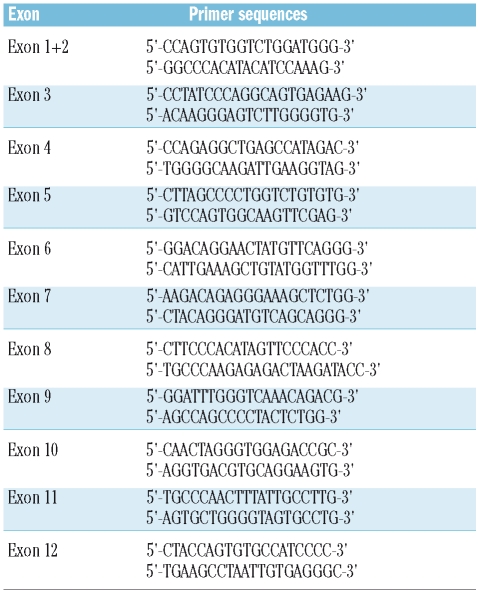
Genomic DNA was extracted from blood or buccal swabs using the DNeasy Blood & Tissue Kit from QIA-GEN (QIAGEN Spa, Milan, Italy) or DNAzol (Invitrogen, Milan, Italy), after isolation of peripheral granulocytes.15 All exons including the intron/exon boundaries of the MPL gene were sequenced from polymerase chain reaction (PCR) fragments amplified from genomic DNA. The primer sequences are shown in Table 1. The PCR conditions were 95ºC for 2 min, 94ºC for 30 s, 60ºC for 30 s and 72ºC for 1 min for 35 cycles. Sequencing was performed on an Applied Biosystems 3130 DNA sequencer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocols.
Table 1.
Sequencing primers for the MPL gene.
Haplotype analysis
Family E was genotyped using 12 microsatellite markers on chromosome 1p. One marker, TC340/341, was newly derived from the genomic sequence of chromosome 1 (FAM-CATGATGGGATAAGTGTCTTCG and GTTTCTTCCTGGTGATGGCTTTC). One marker (CA214/215) has been described previously,3 the others (D1S493, D1S2676, D1S2830, D1S463, D1S1882E, D1S1758E, D1S545, D1S447, D1S1808E, D1S2737) were derived from the UniSTS database. The PCR products were analyzed using an Applied Biosystems 3130 genetic analyzer and Genemapper software package version 3.5 (Applied Biosystems, Foster City, CA, USA). A haplotype co-segregating with thrombocytosis was derived from the segregation of markers within pedigree E. The sizes of the PCR products of the co-segregating microsatellite markers were compared between affected members of the nine families. Among the 12 microsatellite markers we tested, nine were informative in family E and allowed us to define the disease haplotype. Of these nine markers, four were informative (D1S463, D1S545, TC340/341, D1S447) and enabled us to define the smallest co-segregating haplotype shared in the eight Italian families. Genotyping with the four microsatellite markers shared by all eight Italian families was performed on DNA from 132 unrelated Italian control individuals. To identify haplotypes and their frequencies we used the Haplore program, which effectively analyzes data from tightly linked microsatellite loci.19
Single nucleotide polymorphism genotyping and association analysis
Genome-wide single nucleotide polymorphism (SNP) genotyping was undertaken for one affected family member from each family with the Affymetrix GeneChip Human Mapping 500K Nsp I according to the Affymetrix GeneChip Mapping Assay Manual (Affymetrix Inc., Santa Clara, CA, USA). The SNP calls were generated by GeneChip DNA Analysis Software. The association between SNP alleles and thrombocytosis was assessed using simple Pearson’s χ2 tests, implemented in SPSS v. 15 for Windows.
Dating the origin of the S505N mutation
The time of the origin of the mutation was calculated using the formula: number of generations =log((1−Q)/(1−Pn))/log(1-θ), as described before.18 Q is the proportion of haplotypes in diseased subjects that are not the disease haplotype. Pn is the allele frequency of the minor allele for each marker, and θ is the recombination fraction assuming that 1 Mb = 1 cM. In this analysis, Q =0 for all of the Italian families.
Results
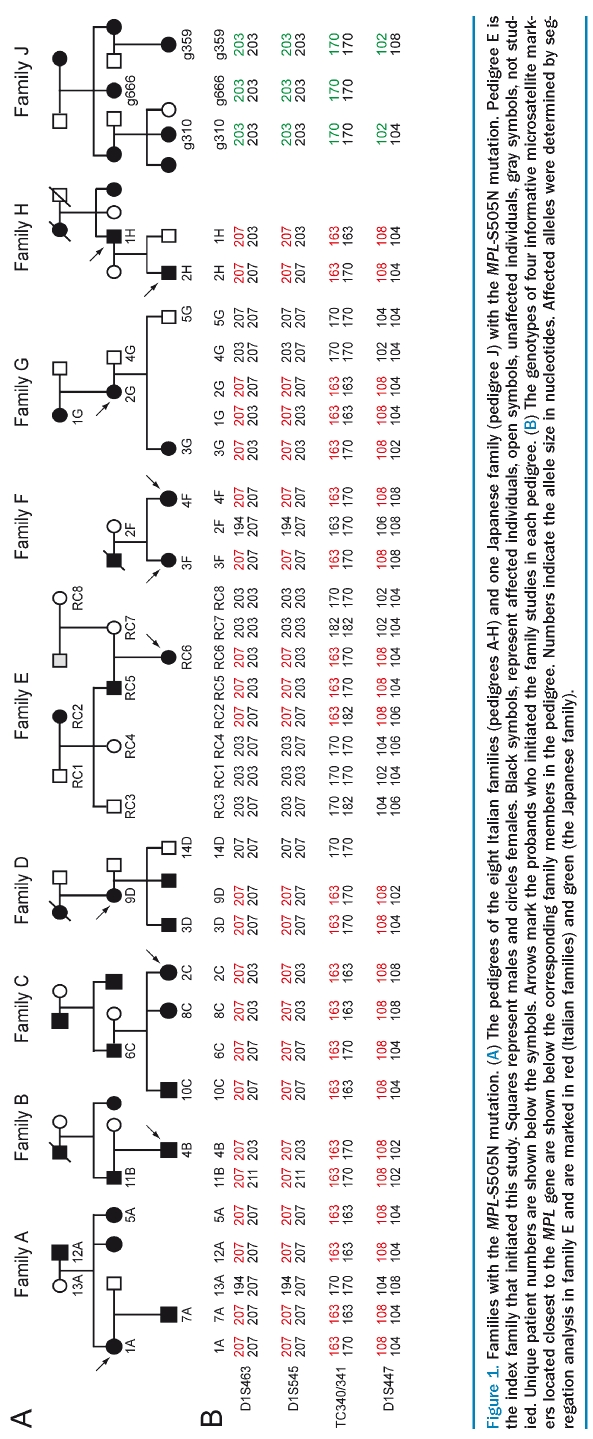
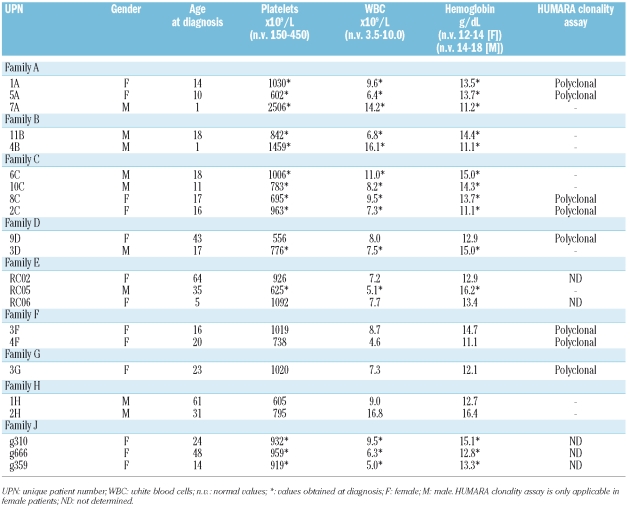
We screened for the presence of mutations in the MPL gene in 14 families with thrombocytosis and low or normal serum levels of thrombopoietin. All exons and intron/exon boundaries of the MPL gene were sequenced and the MPL-S505N mutation was detected in one Italian pedigree (Figure 1A, family E). Since the frequency of the MPL-S505N mutation in our series (1/14) contrasted with the high frequency recently reported in another series of familial cases from Italy (4/5),17 and our family as well as these four families came from the same region in the vicinity of Rome (Figure 1A, families A-D), we hypothesized that the high frequency of the MPL-S505N may represent a founder effect. We, therefore, initiated a collaborative study. In addition to these five families with MPL-S505N, we included three new families from the same region (Figure 1A, pedigrees F-H) that were identified after the report on clustering of MPL-S505N had been published.17 For comparison, we also analyzed the DNA from affected family members of a Japanese family, in which the MPL-S505N was first described (Figure 1A, pedigree J).10 The laboratory findings of affected family members with the MPL-S505N mutation are summarized in Table 2. JAK2-V617F was not detected in any of the affected family members (data not shown).
Figure 1.
Families with the MPL-S505N mutation. (A) The pedigrees of the eight Italian families (pedigrees A-H) and one Japanese family (pedigree J) with the MPL-S505N mutation. Pedigree E is the index family that initiated this study. Squares represent males and circles females. Black symbols, represent affected individuals, open symbols, unaffected individuals, gray symbols, not studied. Unique patient numbers are shown below the symbols. Arrows mark the probands who initiated the family studies in each pedigree. (B) The genotypes of four informative microsatellite markers located closest to the MPL gene are shown below the corresponding family members in the pedigree. Numbers indicate the allele size in nucleotides. Affected alleles were determined by segregation analysis in family E and are marked in red (Italian families) and green (the Japanese family).
Table 2.
Summary of laboratory data of affected family members with the MPL-S505N mutation.
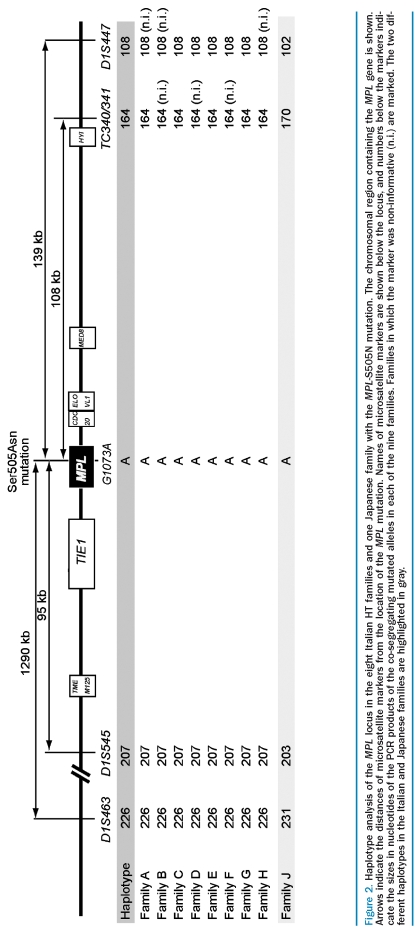
We performed haplotype analysis in affected family members using microsatellite markers located in the vicinity of the MPL mutation. A founder effect is expected to result in sharing of allelic sequence polymorphisms in the vicinity of the MPL mutation (linkage disequilibrium due to a common ancestor). We examined 12 microsatellite markers within 7–18 Mb of the MPL gene locus and found nine markers that were informative and allowed us to determine the disease haplotype that co-segregated with thrombocytosis in family E. We then tested whether the same haplotype is also present in affected family members of the other pedigrees. For the four microsatellite markers that are closest to the location of the MPL-S505N mutation (D1S463, D1S545, TC340/341 and D1S447), we found that at least one PCR product of the identical size as the disease allele in family E was present in all 19 affected family members of the other pedigrees (Figure 1B). These four microsatellite markers are located between 95 kilobases (kb) and 1,290 kb from the MPL mutation (Figure 2). In contrast, the affected family members in the Japanese pedigree displayed differently sized alleles for the same markers (Figure 1B and 2). These results are in favor of a founder effect in the eight Italian families. To determine how frequently alleles of the same size can be obtained by chance in a general population, we genotyped DNA from 132 unrelated patients with sporadic myeloproliferative disorders from Italy using primers for the same four microsatellite markers (Online Supplementary Table S1). We found allele sizes corresponding to the disease haplo-type with all four microsatellite markers in only 6 of these 132 individuals compared to in 22/22 familial cases of Italian descent (p=6×10−27). Logical inference of haplotypes using Haplore showed that no controls inherited the same haplotype as was observed in all cases. If only the two microsatellite markers located most proximal to the mutation were considered, 38/132 of unrelated individuals showed allele sizes corresponding to the disease haplotype, compared to 22/22 familial cases (p=2.3×10−10). The allele frequencies in all individuals tested are listed in Online Supplementary Table S1. To further confirm the strength of this association, we performed GeneChip Human Mapping 500K Nsp I for one affected family member from each of the nine families with HT. The genotypes of the 45 SNP within the region defined by the microsatellites showed a possible common haplotype in all eight Italian families but not the Japanese family (data not shown). However, the SNP data were less informative than the microsatellite analysis, because the SNP were less polymorphic and fewer family members were tested. Together, these results strongly suggest that a founder effect is responsible for the increased frequency of MPL-S505N mutation in familial thrombocytosis in Italy.
Figure 2.
Haplotype analysis of the MPL locus in the eight Italian HT families and one Japanese family with the MPL-S505N mutation. The chromosomal region containing the MPL gene is shown. Arrows indicate the distances of microsatellite markers from the location of the MPL mutation. Names of microsatellite markers are shown below the locus, and numbers below the markers indicate the sizes in nucleotides of the PCR products of the co-segregating mutated alleles in each of the nine families. Families in which the marker was non-informative (n.i.) are marked. The two different haplotypes in the Italian and Japanese families are highlighted in gray.
To estimate the time of origin of the MPL-S505N mutation in the Italian families, we used an approach suggested by Risch.20 For the four microsatellite markers D1S463, D1S545, TC340/341 and D1S447, the number of generations to a common ancestor are 22.16, 22.74, 23.30 and 30.74, respectively. Since the distance between the markers is small and there are no haplotypes that are not shared between all the carriers, reporting about the number of generations is primarily driven by the population allele frequency of the markers and not by the shared haplotype in the affected cases. Despite this limitation these results are surprisingly consistent and suggest that the families had a common ancestor about 23 generations ago.
Discussion
In this study we demonstrated that a clustering of the MPL-S505N mutation in eight Italian families with HT is due to a common founder ancestor approximately 23 generations ago. Since this germ-line mutation is rare in the general population, the founder effect explains the high frequency of this mutation in the Italian families. The MPL-S505N mutation in a Japanese family with HT appears to have originated independently from that of the Italian HT families. Mutations that persist following their occurence in a single founder may reflect selection favoring heterozygotes, e.g. by providing the carriers with a survival advantage. However, there is no obvious reason why thrombocytosis should be an advantage. Our previous study on two families with thrombocytosis carrying an identical mutation in the splice donor of THPO intron 3 showed no evidence for a founder effect.8 These families were from Holland and Poland and affected family members displayed a clinical phenotype very similar to that of patients with the MPL-S505N mutation. Recently, several Arabic families with HT have been found to carry a homozygous or heterozygous P106L mutation in the MPL gene,16 with clinical phenotypes indistinguishable from those of other HT families. The possibility of a founder effect in these families with a P106L mutation has not yet been examined. Other mutations in MPL, showing a less stringent association with thrombocytosis, have been described, including MPL-K39N (also called MPL Baltimore) and MPL-S204P.21–23 Thus, HT is a disease-phenotype caused by a variety of disease-causing mutations and in a large proportion of HT families the disease-causing mutation remains to be determined.
Acknowledgments
we thank Prof. Mario Cazzola and Dr. Francesco Passamonti for DNA from unrelated patients with sporadic myeloproliferative disorders.
Footnotes
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
KL performed the research, analyzed data and wrote the paper, MM performed the research and analyzed data, BR analyzed clinical data, CIA performed genetic analyses, LT, FG, JD, HK and LML analyzed clinical data, RCS designed the research, analyzed data and wrote the paper.
The authors reported no potential conflicts of interest.
Funding: this work was supported by grants from the Swiss National Science Foundation (310000-108006/1), the Swiss Cancer League (OCS-01742-08-2005) and the Krebsliga Beider Basel to RCS; by Prin 2006, Ministero Università e Ricerca Scientifica (Rome, Italy) and by Fondi d’Ateneo, Progetti D1 2006–2007, Università Cattolica (Rome, Italy) to LT and LML; and in part by EC FP6 EICOSANOX grant (LSHM-CT-2004-005033) to BR.
References
- 1.Skoda R, Prchal JT. Lessons from familial myeloproliferative disorders. Semin Hematol. 2005;42:266–73. doi: 10.1053/j.seminhematol.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Kaushansky K. Thrombopoietin. N Engl J Med. 1998;339:746–54. doi: 10.1056/NEJM199809103391107. [DOI] [PubMed] [Google Scholar]
- 3.Wiestner A, Schlemper RJ, van der Maas AP, Skoda RC. An activating splice donor mutation in the thrombopoietin gene causes hereditary thrombocythaemia. Nat Genet. 1998;18:49–52. doi: 10.1038/ng0198-49. [DOI] [PubMed] [Google Scholar]
- 4.Ghilardi N, Wiestner A, Kikuchi M, Oshaka A, Skoda RC. Hereditary thrombocythemia in a Japanese family is caused by a novel point mutation in the thrombopoietin gene. Br J Haematol. 1999;107:310–6. doi: 10.1046/j.1365-2141.1999.01710.x. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T, Okabe M, Sanada M, Kurosawa M, Suzuki S, Kobayashi M, et al. Familial essential thrombocythemia associated with one-base deletion in the 5′-untranslated region of the thrombopoietin gene. Blood. 1998;92:1091–6. [PubMed] [Google Scholar]
- 6.Ghilardi N, Skoda RC. A single-base deletion in the thrombopoietin (TPO) gene causes familial essential thrombocytosis through a mechanism of more efficient translation of TPO mRNA. Blood. 1999;94:1480–2. [PubMed] [Google Scholar]
- 7.Jorgensen MJ, Raskind WH, Wolff JF, Bachrach HR, Kaushansky K. Familial thrombocytosis associated with overproduction of thrombopoietin due to a novel splice donor site mutation. Blood. 1998;92(Suppl 1):205. [Abstract] [Google Scholar]
- 8.Liu K, Kralovics R, Rudzki Z, Grabowska B, Buser AS, Olcaydu D, et al. A de novo splice donor mutation in the thrombopoietin gene causes hereditary thrombocythemia in a Polish family. Haematologica. 2008;93:706–14. doi: 10.3324/haematol.11801. [DOI] [PubMed] [Google Scholar]
- 9.Ghilardi N, Wiestner A, Skoda RC. Thrombopoietin production is inhibited by a translational mechanism. Blood. 1998;92:4023–30. [PubMed] [Google Scholar]
- 10.Ding J, Komatsu H, Wakita A, Kato-Uranishi M, Ito M, Satoh A, et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood. 2004;103:4198–200. doi: 10.1182/blood-2003-10-3471. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura T, Onishi M, Yahata T, Kanakura Y, Asano S. Activating mutations of the transmembrane domain of MPL in vitro and in vivo: incorrect sequence of MPL-K, an alternative spliced form of MPL. Blood. 1998;92:2596–7. [PubMed] [Google Scholar]
- 12.Harrison CN, Gale RE, Wiestner AC, Skoda RC, Linch DC. The activating splice mutation in intron 3 of the thrombopoietin gene is not found in patients with non-familial essential thrombocythaemia. Br J Haematol. 1998;102:1341–3. [PubMed] [Google Scholar]
- 13.Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D, et al. MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort. Blood. 2008;112:141–9. doi: 10.1182/blood-2008-01-131664. [DOI] [PubMed] [Google Scholar]
- 14.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108:3472–6. doi: 10.1182/blood-2006-04-018879. [DOI] [PubMed] [Google Scholar]
- 16.El-Harith HA, Roesl C, Ballmaier M, Germeshausen M, Frye-Boukhriss H, von Neuhoff N, et al. Familial thrombocytosis caused by the novel germ-line mutation p.Pro106Leu in the MPL gene. Br J Haematol. 2009;144:185–94. doi: 10.1111/j.1365-2141.2008.07430.x. [DOI] [PubMed] [Google Scholar]
- 17.Teofili L, Giona F, Martini M, Cenci T, Guidi F, Torti L, et al. Markers of myeloproliferative diseases in childhood polycythemia vera and essential thrombocythemia. J Clin Oncol. 2007;25:1048–53. doi: 10.1200/JCO.2006.08.6884. [DOI] [PubMed] [Google Scholar]
- 18.Liu E, Percy MJ, Amos CI, Guan Y, Shete S, Stockton DW, et al. The worldwide distribution of the VHL 598C>T mutation indicates a single founding event. Blood. 2004;103:1937–40. doi: 10.1182/blood-2003-07-2550. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Zhao H. A comparison of several methods for haplotype frequency estimation and haplotype reconstruction for tightly linked markers from general pedigrees. Genet Epidemiol. 2006;30:423–37. doi: 10.1002/gepi.20154. [DOI] [PubMed] [Google Scholar]
- 20.Risch N, de Leon D, Ozelius L, Kramer P, Almasy L, Singer B, et al. Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat Genet. 1995;9:152–9. doi: 10.1038/ng0295-152. [DOI] [PubMed] [Google Scholar]
- 21.Moliterno AR, Williams DM, Gutierrez-Alamillo LI, Salvatori R, Ingersoll RG, Spivak JL. Mpl Baltimore: a thrombopoietin receptor polymorphism associated with thrombocytosis. Proc Natl Acad Sci USA. 2004;101:11444–7. doi: 10.1073/pnas.0404241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams DM, Kim AH, Rogers O, Spivak JL, Moliterno AR. Phenotypic variations and new mutations in JAK2 V617F-negative polycythemia vera, erythrocytosis, and idiopathic myelofibrosis. Exp Hematol. 2007;35:1641–6. doi: 10.1016/j.exphem.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Standen G, Clench T. Rapid detection of MPL Baltimore using LightCycler technology and melting curve analysis. Br J Haematol. 2008;140:714–6. doi: 10.1111/j.1365-2141.2008.06984.x. [DOI] [PubMed] [Google Scholar]