Individual variations in response and/or toxicity to anti-cancer agents is common. The antifolate agent methotrexate is frequently used in maintenace therapy of acute lymphoblastic leukemia. The findings of this study suggest that genotyping of folate polymorphisms might be useful in adult acute lymphoblastic leukemia to optimize methotrexate therapy, reducing the associated toxicity with possible effects on survival.
Keywords: gene polymorphisms, adult acute lymphoblastic leukemia, methotrexate-related toxicity, folate
Abstract
Background
The antifolate agent methotrexate is an important component of maintenance therapy in acute lymphoblastic leukemia, although methotrexate-related toxicity is often a reason for interruption of chemotherapy. Prediction of toxicity is difficult because of inter-individual variability susceptibility to antileukemic agents. Methotrexate interferes with folate metabolism leading to depletion of reduced folates.
Design and Methods
The aim of this study was to investigate the influence of polymorphisms for folate metabolizing enzymes with respect to toxicity and survival in adult patients with acute lymphoblastic leukemia treated with methotrexate maintenance therapy. To this purpose, we evaluated possible associations between genotype and hematologic and non-hematologic toxicity and effects on survival at 2 years of follow-up in patients with acute lymphoblastic leukemia.
Results
Polymorphisms in the genes encoding for methylenetetrahydrofolate reductase (MTHFR 677C>T) and in dihydrofolate reductase (DHFR 19 bp deletion) significantly increased the risk of hepatotoxicity in single (odds ratio 5.23, 95% confidence interval 1.13–21.95 and odds ratio 4.57, 95% confidence interval 1.01–20.77, respectively) and in combined analysis (odds ratio 6.82, 95% confidence interval 1.38–33.59). MTHFR 677C>T also increased the risk of leukopenia and gastrointestinal toxicity, whilst thymidylate synthase 28 bp repeat polymorphism increased the risk of anemia (odds ratio 8.48, 95% confidence interval 2.00–36.09). Finally, patients with MTHFR 677TT had a decreased overall survival rate (hazard ratio 2.37, 95% confidence interval 1.46–8.45).
Conclusions
Genotyping of folate polymorphisms might be useful in adult acute lymphoblastic leukemia to optimize methotrexate therapy, reducing the associated toxicity with possible effects on survival.
Introduction
Chemotherapy protocols currently used in adults for the treatment of acute lymphoblastic leukemia (ALL), have increased the complete remission rate,1 although therapy-related toxicity remains one of the reasons for treatment interruption or discontinuation, which may increase relapse risk.2 Prediction of toxicity is difficult because of wide interpatient variation in pharmacokinetics and pharmacodynamics of antileukemic agents and because the same toxicity can be attributable to different drugs.
An important component of ALL maintenance therapy is the antifolate agent methotrexate. This drug enters cells transported by the reduced folate carrier (RFC) and its mechanism of action consists mainly in competitive inhibition of the dihydrofolate reductase (DHFR) enzyme.3 DHFR is responsible for the reduction of dihydrofolate into tetrahydrofolate. Dihydrofolate is also generated during deoxythymidylate synthesis,4 catalyzed by the thymidylate synthase (TS) enzyme which uses as substrates 5,10-methylene-tetrahydrofolate and deoxyuridylate. 5,10-methylene-tetrahydrofolate is, in turn, the substrate of methylenetetrahydrofolate reductase (MTHFR), a key folate enzyme that generates 5-methyl-tetrahydrofolate, necessary for the remethylation of homocysteine into methionine. Thus, methotrexate-induced inhibition of DHFR results in the depletion of reduced forms of tetrahydrofolate (including 5,10-methylene-tetrahydrofolate), contributing to the inhibition of nucleic acid synthesis and favoring cell death.5
Despite its clinical success, methotrexate can be associated with serious toxicities in a considerable number of patients. There is evidence that toxicity from anticancer drugs can be affected by inherited polymorphisms in genes encoding for drug-metabolizing enzymes. Polymorphisms of the methotrexate transporter (i.e. RFC 80G>A),6 methotrexate targets (i.e. DHFR 19-bp deletion7 and TS enhancer 28-bp tandem repeat),8 and folate-metabolizing enzyme (i.e. MTHFR 677 C>T9 and 1298 A>C)10 can influence the effectiveness and the toxic effects of methotrexate. The relationship between polymorphisms affecting folate metabolism and methotrexate-related toxicity has been studied mainly in childhood ALL,2,11–15 while only a few studies have investigated this relationship in adult patients with hematologic diseases.16,17 In particular, in adult ALL only MTHFR polymorphisms have been investigated and associated with increased methotrexate-related toxicity.18,19
The aim of our study was to investigate the influence of polymorphisms directly involved in the methotrexate pharmacological pathway in relation to the outcome of adult ALL patients treated with methotrexate maintenance therapy. To this purpose we analyzed the effects of the association of polymorphisms in MTHFR, DHFR, RFC and TS genes on therapy-related toxicity and survival.
Design and Methods
Patients
Cases were 122 Italian individuals (all Caucasians) with newly diagnosed ALL according to the World Health Organization (WHO) classification.20 Patients were recruited from the Units of Hematology at the Universities of Ferrara (n=45), Catania (n=17) and Pavia (n=60) in the period between January 2000 and July 2005. ALL cases were persons aged 18–80 years old with a mean age at diagnosis of 43.5±18.2 years; 53% of them were male. Of all patients, 83% had B-ALL and 17% had TALL. Inclusion criteria were age at least 18 years, absence of other active malignancy, and lack of infection by human immunodeficiency virus-1. Patients were classified into two subgroups on the basis of the cytogenetic abnormalities present: patients with chromosome translocations with known adverse prognostic significance such as t(9;22), t(4;11) were classified as having a high cytogenetic risk, while patients with the other karyotypes formed the standard-risk group. Peripheral blood samples were collected from all the patients at the date of diagnosis by venipuncture, before any pharmacological treatment. At the time of blood collection, patients gave written informed consent to their participation in the study. The study was approved by the local Ethics Committee.
Therapy and toxicity evaluation
Patients recruited from Ferrara and Catania were treated according to the GIMEMA ALL 0496 protocol 21,22 while those recruited in Pavia received the Hyper-CVAD regimen.23 Both protocols included maintenance therapy with methotrexate administered weekly at a dosage of 15 or 20 mg/m2 (for the GIMEMA ALL 0496 and Hyper-CVAD regimes, respectively) for 2-3 years. Hematologic (leukopenia, anemia, thrombocytopenia), and non-hematologic (hepatic and gastrointestinal) toxicity was graded according to WHO criteria (grades 0–4).24 Toxicity was evaluated by independent clinicians before any genotyping was carried out and before they knew the hypothesis of the study. Therefore, toxicity was assessed in a blinded fashion with respect to the patients’ genotypes. The highest grade of WHO toxicity (hematologic or non-hematologic) observed in each patient during the maintenance therapy period was recorded.
Genotype analyses
DNA was isolated from peripheral whole blood using the QIAmp DNA kit (QIAGEN GmbH, Hilden, Germany). Genotyping for MTHFR and RFC polymorphisms was performed using polymerase chain reaction (PCR) followed by restriction-fragment length polymorphism analysis. The genotyping protocols for the MTHFR 677C>T and MTHFR 1298A>C polymorphisms were performed according to Gemmati et al.25 The genotyping protocol used for RFC 80G>A was adapted from Chango et al.6 as follows: initial denaturation step of 3 min at 94°C, 36 cycles of 94°C for 30 s, 59°C for 30 s, 72°C for 55 s, and a final extension step of 72°C for 7 min. The PCR product was digested by CfoI (Sigma-Aldrich, Milan, Italy). The genotyping protocol for the DHFR 19-bp deletion was adapted from Johnson et al.7 as follows: initial denaturation step of 4 min at 94°C, 36 cycles of 94°C for 55 s, 62°C for 55 s, 72°C for 55 s, and a final extension step of 72°C for 12 min. The genotyping protocol for the TS polymorphism was performed as described by Horie et al.8 PCR products were analyzed by electrophoresis on a 10% polyacrylamide gel and visualized with ethidium bromide. All primers were supplied by Sigma-Genosys (Milan, Italy). All PCR cycles were performed in a Peltier Thermal Cycler (PTC-200; M. J. Research, Inc., Watertown, MA, USA). DNA digestion, if required, was performed according to the suppliers’ instructions. Genotypes were confirmed by re-genotyping a random selection of samples for each polymorphism investigated. There were no discrepancies between genotypes determined in duplicate. The possibility of inaccurate genotyping performed on leukemic blast DNA, due to loss of heterozygosity, cannot be excluded, although we did not find any discordance between the number of combined MTHFR genotypes observed (i.e 677C>T and 1298A>C) and that expected by linkage disequilibrium (data not shown).
Statistical analysis
In this study, the primary outcome of interest was the development of toxicity in patients receiving homogeneous maintenance therapy with methotrexate. By univariate analysis, odds ratios (OR) and 95% confidence intervals (95% CI) were used to estimate the risk of developing different grades of toxicity after therapy in patients with each specific genotype. By multivariate logistic regression analysis, adjusted OR were calculated, with the dependent variable being toxicity grades according to the WHO criteria. The multivariate model included sex, age, ALL lineage, cytogenetic risk (considered as high or standard) and the polymorphisms as covariates and they were checked for possible interaction or confounding effects. If a covariate had an effect of 10% or more, then it was considered a confounding factor and the model was adjusted for it. To evaluate the impact of the polymorphisms on different toxicity grades, we performed two series of analyses: the first compared the presence at any toxicity grade (grades 1–4) versus the absence (grade 0) and the second compared intermediate/high toxicity grades (2–4) versus no toxicity/low grades (0–1), to further evaluate whether polymorphisms were associated with more severe toxicities. In this way, the patients were subdivided in two different toxicity grade groups and all patients were simultaneously included in the analyses. To analyze whether the two treatment protocols were associated with different toxicities, we used the χ2 test to compare the number of patients who developed toxicity in each treatment protocol, accounting for the different genotypes.
The secondary outcome of the study was the association between survival or relapse rates, and the genotypes. Overall survival was calculated as the time from the date of diagnosis to the occurrence of death or the end of follow-up. Relapse free-survival was calculated as the time from the date of diagnosis to the occurrence of the first relapse. Overall and relapse-free survival rates were estimated at 2 years of follow-up by Kaplan-Meier analysis and differences were assessed by the log-rank test. The associated hazard risk (HR) and 95% CI were used to estimate the probability of developing an event in the period of follow-up. Hazard risks were calculated by means of Cox’s proportionate hazards modeling. A p value less than or equal to 0.05 was considered statistically significant. All analyses were performed by Systat V.5.0 (Systat Inc., Evanston, IL, USA) and the SPSS Statistical Package (SPSS Inc., Chicago, IL, USA).
Results
Patients’ baseline characteristics
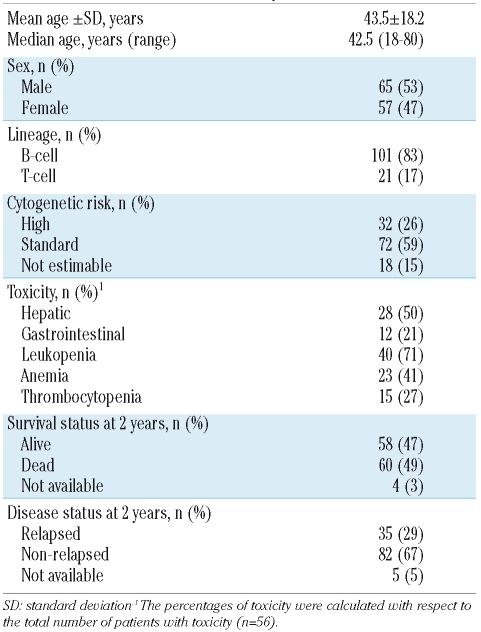
The patients’ characteristics are reported in Table 1. Clinical data about therapy-related toxicity were available for 94 patients. There was no difference in the number of patients developing each kind of toxicity according to the treatment protocol used; thus all patients were analyzed together. Among all patients who developed toxicity (n=56), the prevalences of hematologic and non-hematologic toxicities were as follows: hepatic toxicity (n=28; 50%), gastrointestinal toxicity (n=12; 21%), leukopenia (n=40; 71%), anemia (n=23; 41%), and thrombocytopenia (n=15; 27%). Furthermore, when we considered other measures indicating that patients tolerated the chemotherapy poorly, such as discontinuation of treatment (planned when the white blood cell count was lower than 2×109/L), a reduction of methotrexate dose and a delay in administering chemotherapy, we did not find significant associations with genotypes for any of the polymorphisms (data not shown).
Table 1.
Clinical characteristics of the patients.
Clinical data about survival were available for 118 patients in relation to overall survival and for 117 patients in relation to relapse-free survival (Table 1). After 2 years of follow-up, 47% of all patients were alive. Globally, 29% of the patients relapsed. The median time to death or relapse was 22 and 13 months, respectively. The genotype frequencies were in Hardy-Weinberg equilibrium. The frequency of the polymorphisms affecting folate metabolism in T-cell and B-cell ALL patients, analyzed by the χ2 test, did not differ significantly between the two groups (data not shown).
Analysis of toxicity in association with the polymorphisms affecting folate metabolism: grade 0 versus grades 1–4
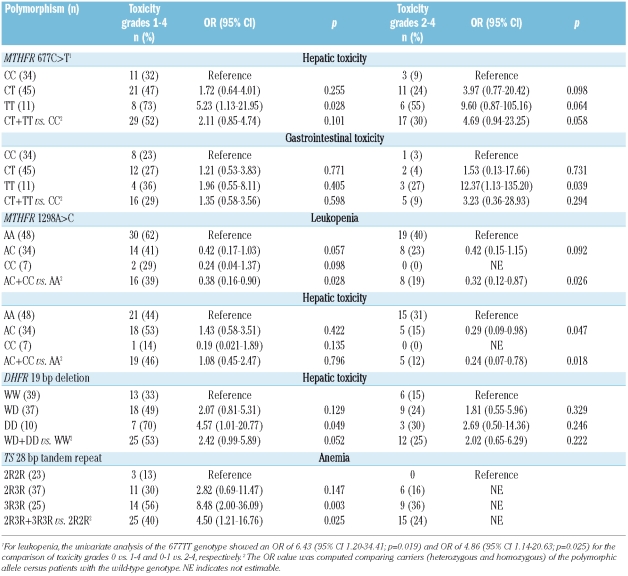
For the polymorphisms investigated, Table 2 shows the risk evaluation of developing several kinds of toxicity when we compared the presence (WHO grades 1–4) with the absence (WHO grade 0) of toxicity, calculated by multivariate logistic regression models.
Table 2.
Toxicities (grades 0 vs. 1–4 and grades 0–1 vs. 2–4) associated with folate pathway polymorphisms in multivariate analysis.
MTHFR 677C>T
Hepatic toxicity increased proportionally to the number of 677T alleles present in the genotype of patients (32% CC, 47% CT and 73% TT). Patients with the TT genotype had a 5.23-fold increased risk of developing hepatic toxicity (p=0.028) when compared to patients with the wild-type genotype (CC). Furthermore, TT patients also had a 6.43-fold increased risk of leukopenia (p=0.019), although this was found only in univariate analysis.
MTHFR 1298A>C
Leukopenia was underrepresented among patients carrying the 1298C allele (AC+CC) compared to among patients with the wild-type genotype (AA), with a significant risk reduction of 2.63-fold (OR= 0.38; p=0.028).
DHFR 19 bp deletion
Hepatic toxicity was 2.07- and 4.57-fold increased in patients with the heterozygous (WD) and homozygous (DD) genotype, respectively, compared to in patients with the wild-type genotype (WW), indicating a possible additive gene-dosage effect. Furthermore, when we considered all carriers of the deletion allele (WD+DD), a 2.42-fold increased risk was confirmed (p=0.052).
TS 28 bp tandem repeat
Anemia was significantly more frequent in patients with the 3R3R genotype (p=0.003) than in those with the wild-type genotype (2R2R), with the former patients showing an 8.48-fold increased risk. Furthermore comparing all carriers of the 3R allele (2R3R+3R3R) with patients with the 2R2R genotype, the increased risk remained statistically significant (OR 4.50; p=0.025).
RFC 80G>A
No significant association was found between this polymorphism and the toxicities investigated (data not shown).
Combined analysis between MTHFR 677C>T and DHFR 19 bp deletion polymorphisms
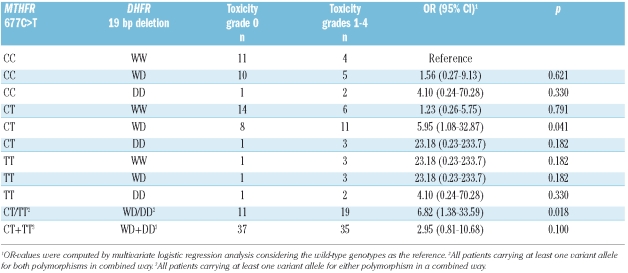
As both MTHFR 677C>T and DHFR 19 bp deletion polymorphisms resulted in associations with an increase in hepatic toxicity, we evaluated them in a combined analysis (Table 3). All combinations yielded OR greater than the unit-value, although increases reached statistical significance only for those combinations for which there were appreciable numbers of cases. In fact, when we considered the presence of at least one polymorphic allele for both polymorphisms (i.e. CT/TT-WD/DD subgroup) with respect to the double wild-type (CC-WW), the risk of developing hepatic toxicity exceeded the risks found for each single polymorphism (OR 6.82; p=0.018).
Table 3.
Combined analysis for MTHFR 677C>T and DHFR 19 bp deletion polymorphisms and risk of hepatic toxicity (WHO grade 0 vs. grades 1–4).
Analysis of toxicity in association with the polymorphisms affecting folate metabolism: grades 0–1 versus grades 2–4
We performed a further analysis of toxicity by comparing patients with no or low grade toxicity (WHO grades 0–1) with those with intermediate-high grades (WHO grades 2–4) (Table 2).
MTHFR 677C>T
Patients with the TT genotype had a an increased risk of gastrointestinal toxicity (OR 12.37; p=0.039), not found in the previous analysis (grade 0 vs. 1–4). The risk of hepatic toxicity was confirmed and slightly increased, but not statistically significantly so, in patients with both the CT (OR 3.97; p= 0.098) and TT (OR 9.60; p=0.064) genotypes by multivariate analysis (Table 2); in univariate analysis the results were statistically significant (CT: OR 3.33, p=0.084; TT: OR 11.99, p=0.003; CT+TT: OR 4.47, p=0.025). Similarly, the risk of developing leukopenia was significantly increased only in univariate analysis for TT patients (OR 4.86, p=0.025) with respect to those with the wild-type genotype.
MTHFR 1298A>C
Hepatic toxicity was greatly underrepresented among patients carrying the 1298C allele. In fact, no patients with the homozygous genotype (CC) were affected by hepatic toxicity. Thus, the 1298 C-carriers had a 4.17-fold risk reduction (OR 0.24, p=0.018) of developing hepatic adverse effects with respect to patients with 1298 AA. Similarly, leukopenia was underrepresented among patients carrying the 1298C allele, with a 3.12-fold risk reduction for 1298 C-carriers (OR 0.32, p=0.026) with respect to those with the wild-type genotype.
No significant association was found for the other investigated polymorphisms affecting folate metabolism.
Survival analysis and polymorphisms
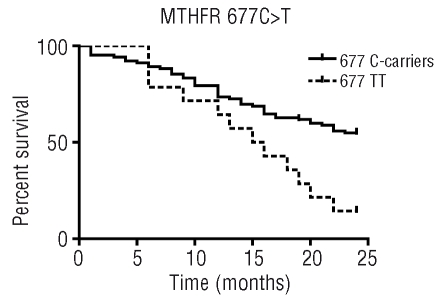
Kaplan-Meier analysis of overall survival curves showed significant results for MTHFR 677C>T polymorphism. At 2 years of follow-up, the different overall survival was evident for TT cases compared to 677C-carriers (Log-rank p=0.005) (Figure 1). In fact, at 2 years a similar percentage of 677CC and 677CT patients (55%) were alive, whereas only 14% of patients with 677TT were alive, with an associated HR of 2.37 (95% CI 1.46–8.45) for the 677TT genotype.
Figure 1.
Kaplan-Meier analysis evaluating the association between overall survival and MTHFR 677C>T polymorphism in 118 adult patients with ALL.
No association was found between overall survival and the other polymorphisms investigated. We also calculated the relationship between relapse-free survival and polymorphisms. In this series of analyses we found no significant associations, although for the RFC 80G>A polymorphism we observed a reduction of relapse-free survival at 2 years of follow-up among 80A-carriers with respect to that of patients with the wild-type genotype (log-rank p=0.235; HR 1.60, 95% CI 0.72–3.55; p=0.251).
Discussion
In this study we evaluated the influence of folate polymorphisms, directly involved in the methotrexate pharmacological pathway, on toxicity and survival in adult ALL patients. We found that the MTHFR 677C>T polymorphism was associated with hepatic toxicity, leukopenia and gastrointestinal toxicity. Specifically, patients harboring the 677T allele exhibited a substantial 5–6-fold increase in incidence of hepatic toxicity and leukopenia (Table 2). In addition, considering a comparison between lower and intermediate-high grade gastrointestinal toxicity (Table 2), 50% of patients had the TT genotype, attributing a 12-fold increased risk to this genotype. As far as concerns MTHFR 1298A>C, we found an association between this polymorphism and leukopenia and hepatic toxicity (Table 2) but in the opposite direction with respect to that reported for MTHFR 677C>T. This might be due to the known linkage disequilibrium existing between the 677T and 1298C alleles.
In recent years, several studies have investigated the relationship between MTHFR polymorphisms and toxicity during methotrexate therapy in childhood ALL.2,11,12,14,15,26,27 Most of these studies did not find significant associations between the 677T allele and toxicity,2,12,14,26,27 although one study reported a lower rate of episodes of toxicity among patients carrying the 677T allele15 and another study showed that individuals with the 677T allele more frequently had to interrupt methotrexate treatment, suggesting that the MTHFR 677C>T serves as a predictor of toxicity during maintenance chemotherapy.11 These different results are probably attributable to several factors including the methotrexate-dose, treatment protocol, ethnic background, and number of patients analyzed. In line with our findings, decreased hematologic toxicity associated with the 1298 AC/CC genotypes was previously described in children with ALL treated with high-dose methotrexate; nevertheless these studies did not find greater toxicity associated with the 677T allele.12,27
In contrast to what has been observed in childhood ALL, in adult ALL patients treated with methotrexate previous studies have shown a correlation between the MTHFR 677C>T and increases of mucositis and, hepatic and hematologic toxicity,18,19 in line with our results. It should be noted that the doses of methotrexate administered to our patients during ALL maintenance therapy were lower than the doses used in childhood ALL. The influence of the polymorphism might be more clearly expressed during the low-dose methotrexate regimen explaining, in part, the different results reported in studies of ALL in adults and children.
This study provides new evidence regarding the influence of the DHFR 19 bp deletion polymorphism on methotrexate toxicity in adult ALL. Homozygosity for DHFR 19 bp deleted allele was significantly associated with increased hepatic toxicity. As DHFR and MTHFR are two key functionally linked enzymes involved in folate metabolism (DHFR starts a folate reduction process preceding and favoring the formation of 5,10-methylene-tetrahydrofolate, the substrate for MTHFR) and the MTHFR 677C>T and DHFR 19 bp deletion were both associated with an increase in hepatic toxicity, we investigated the two polymorphisms in a combined analysis, to evaluate a possible additive effect. We found that the risk was even higher when the two polymorphisms were present in combination (Table 3) with respect to the risk obtained for each polymorphism. However, in combined analysis the small number of patients carrying certain haplotypes limited the possibility of estimating the role of each specific haplotype. To our knowledge, no study has been reported on a relationship between the DHFR 19 bp deletion polymorphism and toxicity in adult ALL, although DHFR gene variants, including the DHFR 19 bp deletion, were investigated in a previous study performed in childhood ALL, in which a correspondence between worse ALL outcome and specific DHFR genotypes was found.13
A further considerable result of our study concerns the TS enhancer repeat polymorphism and a higher risk of developing anemia in patients with the 3R3R genotype. Recently, this polymorphism was studied in childhood ALL; no association with toxicity was found,2,12,27 although the 3R3R genotype was associated with a higher risk of hematologic relapse,28 poorer outcome13 and shorter event-free survival.29,30 Finally, no association was identified between the RFC 80G>A polymorphism and the toxicities investigated.
Folate metabolism, crucial for important cellular events such as DNA synthesis and DNA methylation, may be affected by polymorphisms in genes codifying key folate enzymes. Indeed, it has been reported that MTHFR 677C>T and 1298A>C polymorphisms reduce the activity of the MTHFR enzyme9,10 leading to an increase of homocysteine levels. Furthermore, the DHFR 19-bp deletion polymorphism7 has been associated with an increase of gene expression and homocysteine levels.31
Globally, considering the role of the polymorphisms investigated and the results obtained in this study, we speculate that the increased toxicity associated with specific genotypes might be explained by an imbalance in folate homeostasis. In particular, with regards to the increased hepatic toxicity in patients with MTHFR 677TT and DHFR 19 bp DD genotypes, we hypothesize an additive impact of methotrexate therapy and MTHFR-DHFR polymorphisms on the unbalancing of the folate cycle,32 via homocysteine. This consideration is based on the following evidence: first, an acute elevation in homocysteine levels has been observed after methotrexate administration;33–35 secondly, the MTHFR 677C>T and DHFR 19 bp deletion polymorphisms have been reported to cause an increase homocysteine;9,31 thirdly, homocysteine seems to have a role in hepatotoxicity by elevating liver enzymes.36,37 Nevertheless, the cellular mechanisms explaining effects of the different genotypes on the occurrence of toxicity remain to be fully clarified.
A second goal of this study was to investigate the impact of the polymorphisms on survival. The overall survival rate of MTHFR 677TT carriers was lower than that of patients carrying MTHFR C alleles (Figure 1). A limited amount of evidence has been reported on the influence of MTHFR polymorphisms on survival in adults. Our results are in line with those previously reported by Chiusolo et al., showing an association between the 677TT variant and a reduced survival among adult ALL patients treated with methotrexate-based maintenance therapy.19 Similarly, the 677T variant was found to be associated with lower disease-free survival or relapse-free survival in two childhood ALL studies.26,38 Our result could be partly explained by the fact that 677TT patients conserve more 5,10-methylene-tethahydrofolate in their cells than do carriers of the other genotypes. This condition may counteract the folate-depleting effect of methotrexate and affect methotrexate-efficacy and survival. The analysis of relapse-free survival performed in our study showed no significant associations with polymorphisms, although patients carrying the RFC 80A allele had a lower probability of relapse-free survival. Though not statistically significant, our results were in line with those of Laverdiere et al.,39 who showed that children with ALL carrying the 80A allele had a higher risk of relapse or death during induction and maintenance methotrexate therapy. These data seem to suggest a role for the RFC 80G>A polymorphism in relapse risk and it would be interesting to confirm and extend the findings in a larger population.
Previous studies have found a different clinical responses to treatment protocols for specific ALL subtypes, with a relatively unfavorable prognosis for T-lineage ALL.40 In particular, Kager et al.41 found a lower ability of T-ALL cells to accumulate methotrexate polyglutamate (an important determinant of the antileukemic effects of methotrexate) associated with an ALL subtype–specific expression patterns for folate pathway genes. In our study, in order to identify potential differences in relation to histology, we compared, for each polymorphism, the genotype distribution in T-ALL vs. B-ALL patients. We did not find significant differences for any polymorphism, probably, in part, because of the low number of T-ALL patients. In this regard, further investigations in larger ALL subtype groups might be interesting to identify a possible role for folate pathway gene polymorphisms.
In conclusion, this is the first study to have identified a significant role of folate pathway polymorphisms, such as DHFR 19 bp deletion and TS 28 bp repeat, on methotrexate-related toxicity and survival in adult ALL patients. Furthermore, the study confirmed the influence of MTHFR polymorphisms on ALL outcome. Our findings suggest that pharmacogenetics influence methotrexate therapy-related toxicity in adult ALL, enhancing the risk of hematologic and non-hematologic toxicity, and have an impact on survival. Identification of new genotype/phenotype associations could enable tailored therapy taking into account the genetic background of the patient. We are, however, aware of the limitations of our study due to the small sample size, thus definitive conclusions should be drawn with extreme caution, and further larger studies are needed to confirm the present findings.
Footnotes
The authors reported no potential conflicts of interest.
Funding: this work was supported by the Fondazione CARICENTO (Italy) and by the Italian Association Against Leukemia and Lymphoma (AIL, section of Ferrara).
Authorship and Disclosures
AO was the principal investigator, designed the study, analyzed and interpreted data, performed the statistical analysis, and wrote the article. MDM and DG co-ordinated the research, interpreted data, participated in the statistical analysis and gave interesting suggestions for the paper. MGDP, MR, CA and FDR recruited the patients and collected clinical data. AP, FFM, LC and AO performed the experimental and molecular biology work. AC obtained funding support and interpreted data.
References
- 1.Hoelzer D, Gokbuget N, Ottmann O, Pui CH, Relling MV, Appelbaum FR, et al. Acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2002:162–92. doi: 10.1182/asheducation-2002.1.162. [DOI] [PubMed] [Google Scholar]
- 2.Kishi S, Cheng C, French D, Pei D, Das S, Cook EH, et al. Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood. 2007;109:4151–7. doi: 10.1182/blood-2006-10-054528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo-Sorbello GS, Bertino JR. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica. 2001;86:121–7. [PubMed] [Google Scholar]
- 4.Chen MJ, Shimada T, Moulton AD, Cline A, Humphries RK, Maizel J, et al. The functional human dihydrofolate reductase gene. J Biol Chem. 1984;259:3933–43. [PubMed] [Google Scholar]
- 5.Krajinovic M, Moghrabi A. Pharmacogenetics of methotrexate. Pharmacogenomics. 2004;5:819–34. doi: 10.1517/14622416.5.7.819. [DOI] [PubMed] [Google Scholar]
- 6.Chango A, Emery-Fillon N, de Courcy GP, Lambert D, Pfister M, Rosenblatt DS, et al. A polymorphism (80G->A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Mol Genet Metabol. 2000;70:310–15. doi: 10.1006/mgme.2000.3034. [DOI] [PubMed] [Google Scholar]
- 7.Johnson WG, Stenroos ES, Spychala JR, Chatkupt S, Ming SX, Buyske S. New 19 bp deletion polymorphism in intron-1 of dihydrofolate reductase (DHFR): a risk factor for spina bifida acting in mothers during pregnancy? Am J Med Gen. 2004;124:339–45. doi: 10.1002/ajmg.a.20505. [DOI] [PubMed] [Google Scholar]
- 8.Horie N, Aiba H, Oguro K, Hojo H, Takeishi K. Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Fun. 1995;20:191–7. doi: 10.1247/csf.20.191. [DOI] [PubMed] [Google Scholar]
- 9.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nature Gen. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Gen Metabol. 1998;64:169–72. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 11.Shimasaki N, Mori T, Torii C, Sato R, Shimada H, Tanigawara Y, et al. Influence of MTHFR and RFC1 polymorphisms on toxicities during maintenance chemotherapy for childhood acute lymphoblastic leukemia or lymphoma. J Pediatr Hematol Oncol. 2008;30:347–52. doi: 10.1097/MPH.0b013e318165b25d. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Tissing WJ, de Jonge R, van Zelst BD, Pieters R. Polymorphisms in folate-related genes: association with side effects of high-dose methotrexate in childhood acute lymphoblastic leukemia. Leukemia. 2008;22:1798–800. doi: 10.1038/leu.2008.66. [DOI] [PubMed] [Google Scholar]
- 13.Dulucq S, St-Onge G, Gagne V, Ansari M, Sinnett D, Labuda D, et al. DNA variants in dihydrofolate reductase gene and outcome in childhood ALL. Blood. 2008;111:3692–700. doi: 10.1182/blood-2007-09-110593. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi H, Okamura N, Yagi M, Noro Y, Moriya Y, Nakamura T, et al. Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet. 2007;52:166–71. doi: 10.1007/s10038-006-0096-z. [DOI] [PubMed] [Google Scholar]
- 15.Costea I, Moghrabi A, Laverdiere C, Graziani A, Krajinovic M. Folate cycle gene variants and chemotherapy toxicity in pediatric patients with acute lymphoblastic leukemia. Haematologica. 2006;91:1113–6. [PubMed] [Google Scholar]
- 16.Gemmati D, Ongaro A, Tognazzo S, Catozzi L, Federici F, Mauro E, et al. Methylenetetrahydrofolate reductase C677T and A1298C gene variants in adult non-Hodgkin’s lymphoma patients: association with toxicity and survival. Haematologica. 2007;92:478–85. doi: 10.3324/haematol.10587. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich CM, Yasui Y, Storb R, Schubert MM, Wagner JL, Bigler J, et al. Pharmacogenetics of methotrexate: toxicity among marrow transplantation patients varies with the methylenetetrahydrofolate reductase C677T polymorphism. Blood. 2001;98:231–4. doi: 10.1182/blood.v98.1.231. [DOI] [PubMed] [Google Scholar]
- 18.Chiusolo P, Reddiconto G, Casorelli I, Laurenti L, Sora F, Mele L, et al. Preponderance of methylenetetrahydrofolate reductase C677T homozygosity among leukemia patients intolerant to methotrexate. Ann Oncol. 2002;13:1915–8. doi: 10.1093/annonc/mdf322. [DOI] [PubMed] [Google Scholar]
- 19.Chiusolo P, Reddiconto G, Farina G, Mannocci A, Fiorini A, Palladino M, et al. MTHFR polymorphisms’ influence on outcome and toxicity in acute lymphoblastic leukemia patients. Leuk Res. 2007;31:1669–74. doi: 10.1016/j.leukres.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 20.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 21.Mancini M, Vegna ML, Castoldi GL, Mecucci C, Spirito F, Elia L, et al. Partial deletions of long arm of chromosome 6: biologic and clinical implications in adult acute lymphoblastic leukemia. Leukemia. 2002;16:2055–61. doi: 10.1038/sj.leu.2402640. [DOI] [PubMed] [Google Scholar]
- 22.Vitale A, Guarini A, Ariola C, Meloni G, Perbellini O, Pizzuti M, et al. Absence of prognostic impact of CD13 and/or CD33 antigen expression in adult acute lymphoblastic leukemia. Results of the GIMEMA ALL 0496 trial. Haematologica. 2007;92:342–8. doi: 10.3324/haematol.10385. [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–61. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. WHO Handbook For Reporting Results of Cancer Treatment. World Health Organization; Geneva: 1979. WHO Offset Pubblication no. 48. [Google Scholar]
- 25.Gemmati D, Ongaro A, Scapoli GL, Della Porta M, Tognazzo S, Serino ML, et al. Common gene polymorphisms in the metabolic folate and methylation pathway and the risk of acute lymphoblastic leukemia and non-Hodgkin’s lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2004;13:787–94. [PubMed] [Google Scholar]
- 26.Aplenc R, Thompson J, Han P, La M, Zhao H, Lange B, et al. Methylenetetrahydrofolate reductase polymorphisms and therapy response in pediatric acute lymphoblastic leukemia. Cancer Res. 2005;65:2482–7. doi: 10.1158/0008-5472.CAN-04-2606. [DOI] [PubMed] [Google Scholar]
- 27.Pakakasama S, Kanchanakamhaeng K, Kajanachumpol S, Udomsubpayakul U, Sirachainan N, Thithapandha A, et al. Genetic polymorphisms of folate metabolic enzymes and toxicities of high dose methotrexate in children with acute lymphoblastic leukemia. Ann Hematol. 2007;86:609–11. doi: 10.1007/s00277-007-0274-x. [DOI] [PubMed] [Google Scholar]
- 28.Rocha JC, Cheng C, Liu W, Kishi S, Das S, Cook EH, et al. Pharmacogenetics of outcome in children with acute lymphoblastic leukemia. Blood. 2005;105:4752–8. doi: 10.1182/blood-2004-11-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krajinovic M, Costea I, Chiasson S. Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet. 2002;359:1033–4. doi: 10.1016/S0140-6736(02)08065-0. [DOI] [PubMed] [Google Scholar]
- 30.Krajinovic M, Costea I, Primeau M, Dulucq S, Moghrabi A. Combining several polymorphisms of thymidylate synthase gene for pharmacogenetic analysis. Pharmacogen J. 2005;5:374–80. doi: 10.1038/sj.tpj.6500332. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Gammon MD, Wetmur JG, Rao M, Gaudet MM, Teitelbaum SL, et al. A functional 19-base pair deletion polymorphism of dihydrofolate reductase (DHFR) and risk of breast cancer in multivitamin users. Am J Clin Nutr. 2007;85:1098–102. doi: 10.1093/ajcn/85.4.1098. [DOI] [PubMed] [Google Scholar]
- 32.Gemmati D, De Mattei M, Catozzi L, Della Porta M, Serino ML, Ambrosio C, et al. DHFR 19-bp insertion/deletion polymorphism and MTHFR C677T in adult acute lymphoblastic leukaemia: is the risk reduction due to intracellular folate unbalancing? Am J Hematol. 2009;84:526–9. doi: 10.1002/ajh.21451. [DOI] [PubMed] [Google Scholar]
- 33.Refsum H, Wesenberg F, Ueland PM. Plasma homocysteine in children with acute lymphoblastic leukemia: changes during a chemotherapeutic regimen including methotrexate. Cancer Res. 1991;51:828–35. [PubMed] [Google Scholar]
- 34.Broxson EH, Jr, Stork LC, Allen RH, Stabler SP, Kolhouse JF. Changes in plasma methionine and total homocysteine levels in patients receiving methotrexate infusions. Cancer Res. 1989;49:5879–83. [PubMed] [Google Scholar]
- 35.van Ede AE, Laan RF, Blom HJ, Boers GH, Haagsma CJ, Thomas CM, et al. Homocysteine and folate status in methotrexate-treated patients with rheumatoid arthritis. Rheumatology. 2002;41:658–65. doi: 10.1093/rheumatology/41.6.658. [DOI] [PubMed] [Google Scholar]
- 36.van Ede AE, Laan RF, Blom HJ, Huizinga TW, Haagsma CJ, Giesendorf BA, et al. The C677T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis Rheum. 2001;44:2525–30. doi: 10.1002/1529-0131(200111)44:11<2525::aid-art432>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.Celtikci B, Leclerc D, Lawrance AK, Deng L, Friedman HC, Krupenko NI, et al. Altered expression of methylenetetrahydrofolate reductase modifies response to methotrexate in mice. Pharmacogen Genom. 2008;18:577–89. doi: 10.1097/FPC.0b013e32830058aa. [DOI] [PubMed] [Google Scholar]
- 38.Krajinovic M, Lemieux-Blanchard E, Chiasson S, Primeau M, Costea I, Moghrabi A. Role of polymorphisms in MTHFR and MTHFD1 genes in the outcome of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2004;4:66–72. doi: 10.1038/sj.tpj.6500224. [DOI] [PubMed] [Google Scholar]
- 39.Laverdiere C, Chiasson S, Costea I, Moghrabi A, Krajinovic M. Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood. 2002;100:3832–4. doi: 10.1182/blood.V100.10.3832. [DOI] [PubMed] [Google Scholar]
- 40.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–48. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 41.Kager L, Cheok M, Yang W, Zaza G, Cheng Q, Panetta JC, et al. Folate pathway gene expression differs in subtypes of acute lymphoblastic leukemia and influences methotrexate pharmacodynamics. J Clin Invest. 2005;115:110–7. doi: 10.1172/JCI22477. [DOI] [PMC free article] [PubMed] [Google Scholar]