NKG2D is an activating and co-stimulatory receptor expressed on natural killer cells and T cells that confers multiple effects in the immune control of microbial infections and malignant disease. As a result, donor and recipient polymorphisms in the NKG2D gene may have an effect on the outcome of allogeneic hematopoietic stem cell transplantations (HSCT). In this study, Dr. Espinoza and colleagues found that the donor NKG2D-HNK1 haplotype was linked to improved overall survival and impaired transplant-related mortality after unrelated, myeloablative conditioned HSCT of patients suffering from hematologic malignancies and reported to the Japan Marrow Donor Program.
Keywords: NKG2D, HNK1, LNK1, unrelated donor, bone marrow transplantation, single nucleotide polymorphism
Abstract
Background
NKG2D, an activating and co-stimulatory receptor expressed on natural killer cells and T cells, plays pivotal roles in immunity to microbial infections as well as in cancer immunosurveillance. This study examined the impact of donor and recipient polymorphisms in the NKG2D gene on the clinical outcomes of patients undergoing allogeneic T-cell-replete myeloablative bone marrow transplantation using an HLA-matched unrelated donor.
Design and Methods
The NKG2D polymorphism was retrospectively analyzed in a total 145 recipients with hematologic malignancies and their unrelated donors. The patients underwent transplantation following myeloablative conditioning; the recipients and donors were matched through the Japan Marrow Donor Program.
Results
In patients with standard-risk disease, the donor NKG2D-HNK1 haplotype, a haplotype expected to induce greater natural killer cell activity, was associated with significantly improved overall survival (adjusted hazard ratio, 0.44; 95% confidence interval, 0.23 to 0.85; p=0.01) as well as transplant related mortality (adjusted hazard ratio, 0.42; 95% confidence interval, 0.21 to 0.86; p=0.02), but had no impact on disease relapse or the development of grade II–IV acute graft-versus-host disease or chronic graft-versus-host disease. The NKG2D polymorphism did not significantly influence the transplant outcomes in patients with high-risk disease.
Conclusions
These data suggest an association between the donor HNK1 haplotype and better clinical outcome among recipients, with standard-risk disease, of bone marrow transplants from HLA-matched unrelated donors.
Introduction
Hematopoietic stem cell transplantation (SCT) is a potentially curative treatment for a range of hematologic malignancies. Although the use of an HLA-matched unrelated donor is well accepted when an HLA-identical sibling donor is unavailable, the risk of transplantation- related complications may be increased.1 Despite improvements in clinical and supportive care, transplant- related life-threatening complications, including graft-versus-host disease (GVHD), infections and disease relapse, remain an enormous obstacle to overcome.2 Although HLA matching is the major genetic determinant of clinical outcome after allogeneic SCT, recent evidence suggests that non-HLA immune-associated genes are also implicated.3 Previous investigations have revealed that several single nucleotide polymorphisms (SNP) which affect individual immune response to infections and inflammatory reactions are associated with the risk of GVHD and transplant outcomes.4–15
NKG2D is an activating and co-stimulatory receptor belonging to the C-type lectin-like family of transmembrane proteins and is expressed as a homodimer on natural killer (NK) cells, CD8+ αβ+ T cells, γδ+ T cells and activated macrophages.16–18 The ligands for NKG2D, such as MHC class I-chain related proteins (MICA and MICB), UL16 binding proteins are usually absent or expressed at very low levels in normal cells but are up-regulated by cellular stress including heat shock and microbial infections and are frequently expressed in epithelial tumor cells.19 Ligand engagement of NKG2D triggers cell-mediated cytotoxicity and co-stimulates cytokine production through a DAP10-phosphoinositol 3-kinase dependent pathway and plays an important role in the elimination of tumors and infected cells.16–18,20
Recently, SNP were identified between LNK1 and HNK1 haplotypes of the NKG2D gene.21 In Japanese individuals, the HNK1 haplotype is associated with greater activity of NK cells in the peripheral blood21,22 and a lower prevalence of cancers originating from epithelial cells.21,23,24 The present study investigates the impact of donor and recipient polymorphisms in the NKG2D gene on the clinical outcomes of patients undergoing allogeneic myeloablative bone marrow transplantation using an HLA allele-matched unrelated donor.
Design and Methods
Patients
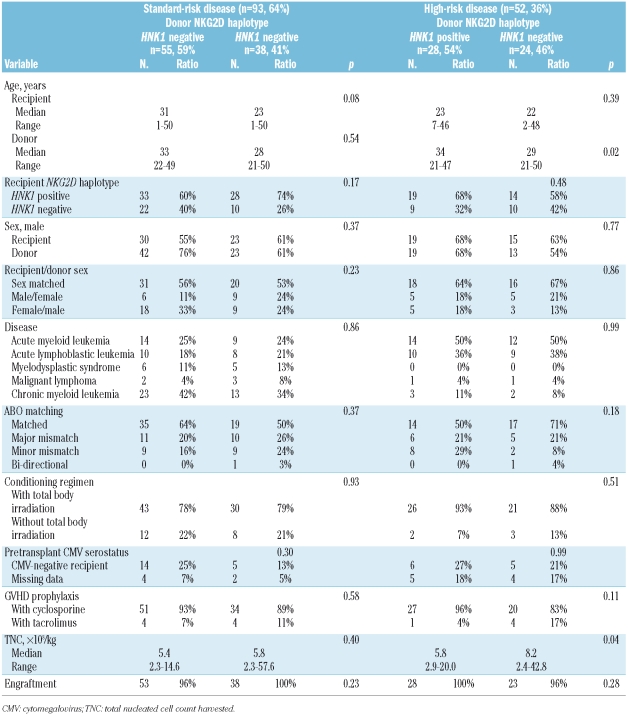
NKG2D genotyping was performed on a total 145 recipients with hematologic malignancies and their unrelated donors who were part of the Japan Marrow Donor Program (JMDP). The recipients underwent transplantation, following myeloablative conditioning, with T-cell-replete marrow from an HLA-A, -B, -C, -DRB1 allele-matched donor between November 1995 and March 2000. HLA genotypes of the HLA-A, -B, -C, and -DRB1 alleles of the patients and donors were determined by the Luminex microbead method described previously. (Luminex 100 System; Luminex, Austin, TX, USA).25,26 No patient had a history of prior transplantation. The final clinical survey of these patients was completed by November 1, 2007. Diagnoses were acute myeloid leukemia (n=49; 34%), acute lymphoblastic leukemia (n=37; 26%), chronic myeloid leukemia (n=41; 28%), myelodysplastic syndrome (n=11; 8%) and malignant lymphoma (n=7; 5%), (Table 1). The recipients were defined as having standard risk disease if they had acute myeloid or lymphoblastic leukemia in first complete remission, malignant lymphoma in complete remission, chronic myeloid leukemia in any chronic phase or myelodysplastic syndrome. All other patients were designated as having high-risk disease. Myeloid malignancies included acute myeloid leukemia, chronic myeloid leukemia and myelodysplastic syndrome, whereas lymphoid malignancies included acute lymphoblastic leukemia and malignant lymphomas. Cyclosporine or tacrolimus- based regimens were used in all patients for GVHD prophylaxis whereas anti-T-cell therapy, such as anti-thymocyte globulin and ex vivo T-cell depletion, was not. All patients and donors gave their written informed consent to molecular studies, according to the declaration of Helsinki, at the time of transplantation. The project was approved by the Institutional Review Board of Kanazawa University Graduate School of Medicine and the JMDP.
Table 1.
Characteristics of the donors and recipients.
NKG2D genotyping
NKG2D was genotyped using the TaqMan-Allelic discrimination method27 with a 9700-HT real time polymerase chain reaction (PCR) system (Applied Biosystems, Foster City, CA, USA) and results were analyzed using allelic discrimination software (Applied Biosystems). The genotyping assay was conducted in 96-well PCR plates. The amplification reaction contained template DNA, TaqMan universal master mix and a specific probe (product No. C_9345347_10; Applied Biosystems) for rs1049174, a single locus featuring a G-C substitution to distinguish between the HNK1 (G) and LNK1 (C) haplotypes of the NKG2D gene.21,23,24
Data management and statistical analysis
Data were collected by the JMDP using a standardized report form. Follow-up reports were submitted at 100 days, 1 year and annually after transplantation. Pre-transplant cytomegalovirus serostatus was routinely tested only in patients but not in their donors. Engraftment was confirmed by an absolute neutrophil count of more than 0.5×109/L for at least 3 consecutive days. Acute and chronic GVHD were diagnosed and graded using established criteria.28,29 Overall survival was defined as the number of days from transplantation to death from any cause. Disease relapse was defined as the number of days from transplantation to disease relapse. Transplant-related mortality was defined as death without relapse. Any patients who were alive at the last-follow-up date were censored. When collecting data, only the main cause of death was recorded if two or more causes were combined. Data on etiological agents of infections, postmortem changes and supportive care (including prophylaxis of infections and therapy of GVHD, which were given on an institutional basis), were not available for this cohort of patients. The analysis was performed using Excel 2007 (Microsoft Corp, Redmond, WA, USA), OriginPro version 8.0J (Lightstone Inc, Tokyo, Japan), and R (The R Foundation for Statistical Computing, Perugia, Italy).30 The probability of overall survival was calculated using the Kaplan-Meier method and compared using the log-rank test. The probabilities of transplant-related mortality, disease relapse, acute GVHD, chronic GVHD, and each cause of death were compared using the Grey test31 and analyzed using cumulative incidence analysis,30 considering relapse, death without disease relapse, death without acute GVHD, death without chronic GVHD, and death without each cause as respective competing risks. The analysis was stratified for patients with standard-risk disease and high-risk disease to take into account the already recognized prognostic differences. The variables considered were recipient age at time of transplantation, sex, recipient cytomegalovirus serostatus before transplantation, disease characteristics (disease type and disease lineage), donor characteristics (age, sex, sex compatibility, and ABO compatibility), transplant characteristics (total body irradiation-containing regimen, tacrolimus versus cyclosporine, and total nucleated cell count harvested per recipient weight). The median was used as the cut-off point for continuous variables. The χ2 test and Mann-Whitney test were used to compare results of two groups. The Hardy-Weinberg equilibrium for the NKG2D gene polymorphism was tested using the Haploview program.32 Multivariate Cox models were used to evaluate the hazard ratio associated with the NKG2D polymorphism. Covariates found to be statistically significant in univariate analyses (p≤0.10) were included in the models. For both the univariate and multivariate analyses, p values were two-sided and outcomes were considered to be statistically significant with p≤0.05.
Results
Frequencies of NKG2D haplotype
The NKG2D gene polymorphism was analyzed in 145 pairs of unrelated donors-recipients of bone marrow following myeloablative conditioning (Table 1). The haplotype frequencies of LNK1/LNK1, HNK1/LNK1 and HNK1/HNK1 were 43%, 42% and 15%, respectively in donors and 35%, 45% and 20%, respectively in recipients. These frequencies were similar to those reported in previous studies in Japanese populations21,24 and were in accordance with the Hardy-Weinberg equilibrium (p=0.80).
Transplant outcomes according to NKG2D haplotype
With a median follow-up of 115 months among survivors (range, 74 to 140 months), 30 recipients (21%) had relapsed or progressed and 62 (47%) had died. Three patients (2%) died before engraftment. The analysis of the influence of the NKG2D genotype on clinical outcomes after transplantation was stratified according to whether the recipients had standard-risk disease or high-risk disease to account for the already recognized prognostic difference. The overall survival at 5 years in patients with standard-risk disease was 63% while that of patients with high-risk disease was 44% (p=0.06). The 5-year cumulative incidences of trasplant-related mortality were 32% and 27%, respectively (p=0.33) and those of disease relapse were 10% and 31%, respectively (p=0.0006).
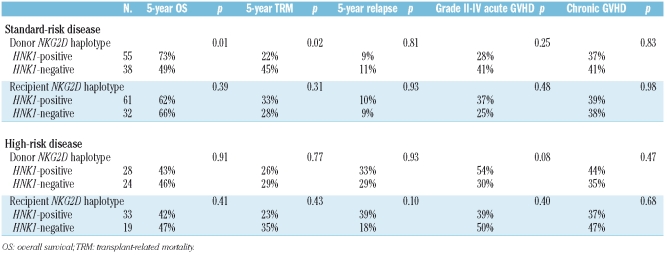
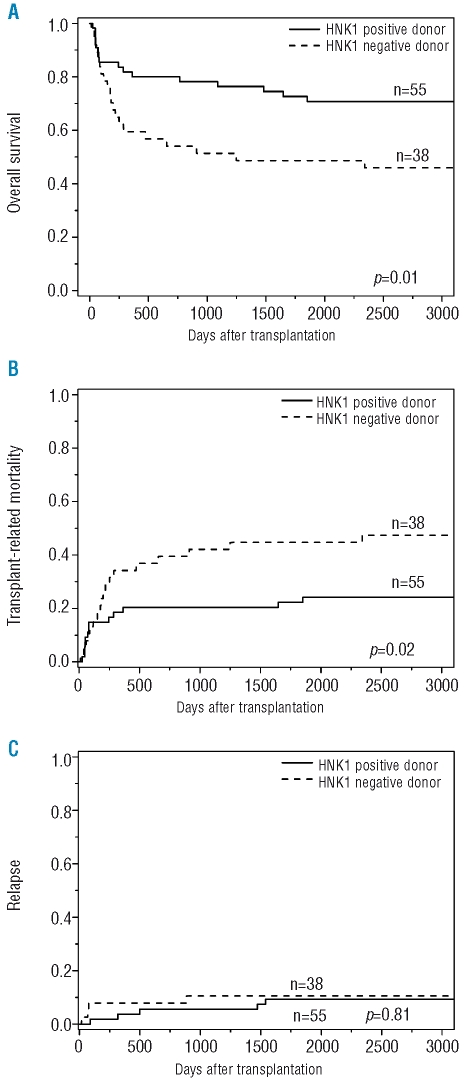
The transplant outcomes according to NKG2D genotype are summarized in Table 2. Patients with standard-risk disease receiving transplants from donors with the HNK1 haplotype had a significantly better 5-year overall survival (73% vs. 49%, p=0.01; Figure 1A) and lower transplant-related mortality rate (22% vs. 45%, p=0.02; Figure 1B) than those receiving transplants from donors without the HNK1 haplotype. No difference was noted in disease relapse in relation to the donors’ polymorphism (9% vs. 11%, p=0.81; Figure 1C) or in the development of grades II to IV acute GVHD (28% vs. 41%, p=0.25) or chronic GVHD (37% vs. 41%, p=0.83). When patients with acute myeloid leukemia or myelodysplastic syndrome were separately analyzed, there was still no difference in disease relapse in relation to NKG2D polymorphisms (data not shown). In patients with high-risk disease, the donor HNK1 haplotype had no significant effects on transplant outcomes (Table 2).
Table 2.
Univariate analysis of the association of NKG2D polymorphisms with clinical outcomes after transplantation.
Figure 1.
Kaplan-Meier analysis of (A) overall survival, (B) cumulative incidence of transplant-related mortality and (C) disease relapse after transplantation according to the donor NKG2D polymorphism in patients with standard-risk disease. Patients with donors with the HNK1 haplotype had better overall survival and lower transplant-related mortality. Donor haplotype had no significant impact on disease relapse.
Multivariate analysis
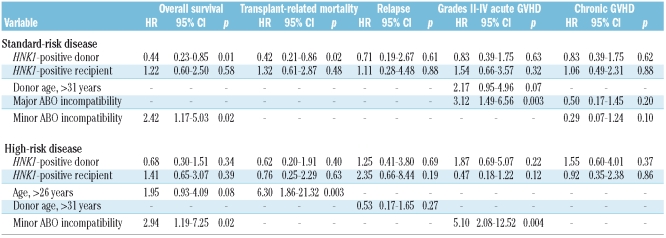
Any factors found to be significant in univariate analyses were included in the multivariate analysis. When patients with standard-risk disease were analyzed, the HNK1 haplotype in donors remained statistically significant in multivariate analyses for both overall survival and transplant-related mortality (Table 3). The presence of the HNK1 haplotype in the donor resulted in better overall survival (hazard ratio, 0.44; 95% confidence interval, 0.23 to 0.85; p=0.01) and transplant-related mortality (hazard ratio, 0.42; 95% confidence interval, 0.21 to 0.86; p=0.02). The donor and recipient HNK1 haplotype did not significantly influence the transplant outcomes in patients with high-risk disease.
Table 3.
Multivariate analysis of the association of NKG2D polymorphisms with clinical outcomes after transplantation.
Main causes of death
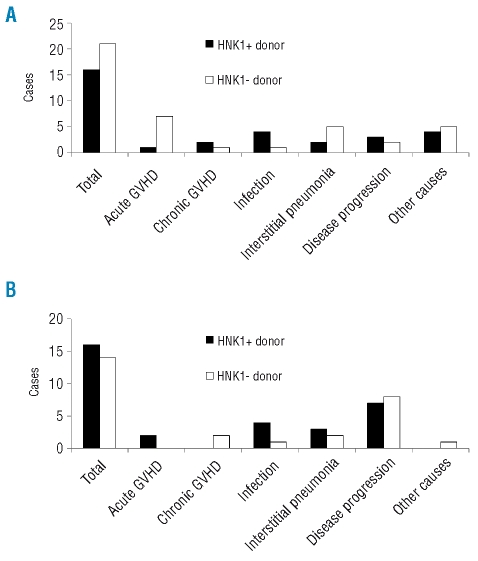
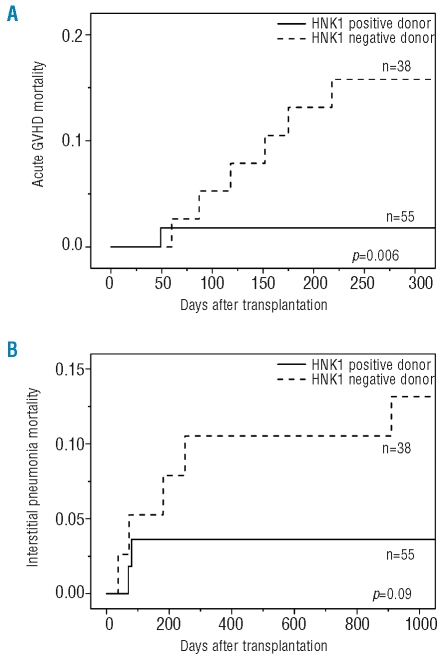
The main causes of death according to the HNK1 haplotype of the donors and recipients are illustrated in Figure 2A for patients with standard-risk disease, and in Figure 2B for those with high-risk disease. In patients with standard-risk disease receiving transplants from HNK1-negative donors, the most frequent cause of death was acute GVHD, followed by interstitial pneumonia. Transplants from HNK1-positive donors resulted in a statistically significantly reduced incidence of death attributed to acute GVHD (Figure 3A; p=0.006) as well as a trend toward a lower incidence of death attributed to interstitial pneumonia (Figure 3B; p=0.09). Other causes of death did not differ according to the HNK1 haplotype.
Figure 2.
Main causes of death after transplantation according to the NKG2D polymorphism in patients with (A) standard-risk disease (B) high-risk disease.
Figure 3.
Cumulative incidence of deaths due to (A) acute GVHD and (B) interstitial pneumonia after transplantation in patients with standard-risk disease. The HNK1 haplotype in donors was associated with a significantly lower incidence of deaths due to acute GVHD (p=0.006) as well as a trend toward a lower incidence of deaths due to interstitial pneumonia (p=0.09).
Discussion
The current study showed an association between the NKG2D-HNK1 haplotype in unrelated donors of HLA-matched myeloablative bone marrow transplants (haplotype frequency, 61%) and a significantly reduced transplant-related mortality and better overall survival for their recipients with standard-risk disease. The polymorphism of the donor NKG2D gene did not influence disease relapse or the development of grades II to IV acute GVHD or chronic GVHD in the patients. One possible explanation for the absence of the beneficial effects of the HNK1 haplotype in patients with high-risk disease may be that the number of cases in the study was insufficient for a meaningful assessment of the effect. Alternatively, disease progression may precede the emergence of the potential advantageous effects of the HNK1 donor haplotype that could protect the recipient from severe transplant-related complications. There was a larger difference in disease relapse between patients with standard-risk disease and those with high-risk disease: 10% and 31% at 3 years after transplantation, respectively.
NKG2D plays important roles in immunity to microbial infections and is especially prominent in controlling viral and bacterial infections.16 Therefore, the reduced transplant-related mortality in patients with standard-risk disease receiving grafts from donors with the HNK1 haplotype in this study might be a consequence of increased resistance to infections in the recipients. However, the hypothesis is too speculative because of the unavailability of data on causes of infections in this cohort. Further studies will be needed to clarify whether the HNK1 haplotype in donors can effectively protect patients against infections.
Several studies have shown that NK cell activity has an important role in the outcomes of patients undergoing allogeneic transplantation.33,34 Alloreactive NK cells reduced the risk of relapse of acute myeloid leukemia without increasing the incidence of GVHD, resulting in a marked improvement of event-free survival in a series of haploidentical transplant recipients.35,36 In HLA-identical sibling transplants, the absence of HLA-C and HLA-B ligand for donor-inhibitory killer immunoglobulin-like receptors (KIR) provided benefits in terms of survival and relapse of patients with acute myeloid leukemia and myelodysplastic syndrome in recipients of T-cell-depleted SCT.37 On the other hand, the JMDP found that KIR ligand mismatch was unfavorably correlated with relapse of leukemia and survival in patients undergoing T-cell-replete unrelated bone marrow transplants.38 All patients in the present study received grafts from an HLA-A, -B, and -C allele-matched donor, implying KIR ligand match between each patient and donor. It is an open question whether the NKG2D polymorphism could affect the outcomes of patients undergoing transplantation with KIR-mismatched grafts.
In this study, major and minor ABO incompatibilities between the donor and recipient tended to be associated with poorer transplant outcomes, regardless of the risk category of the disease. These findings are compatible with those of a previous study by the JMDP,39 although the impact of ABO incompatibilities on SCT outcomes is controversial.
This study also identified age as a significant predictive factor for transplant-related mortality in the patients with standard-risk disease. This is consistent with the results of a previous study40 showing that age over 35 years increased the risk of transplant-related mortality after allogeneic myeloablative SCT in high-risk patients.
A possible limitation of this study is the fact that no direct evidence is yet available regarding the ability of NKG2D polymorphisms to protect against microbial infections. The association observed between the NKG2D haplotype and transplant outcome might be due to another genetic polymorphism in linkage disequilibrium responsible for a better transplant outcome. One candidate gene is NKG2F (KLRC4), which is located in the NK complex region adjacent to the NKG2D gene, because an intrinsic SNP (rs2617171) in the gene has been reported to be in complete linkage with the NKG2D genotype.24 Alternatively, polymorphisms may not be directly associated with controlling infection, but rather may be associated with other factors, such as sensitivity to treatment against GVHD or protection against organ toxicities related to transplants, which also influence the transplant outcome. These hypotheses have yet to be verified give the insufficient evidence.
Polymorphisms in genes encoding for nucleotide-binding oligomerization domain 2 (NOD2)/caspase recruitment domain 15 (CARD15),9 heme oxygenase-1 (HO-1) promoter,6 the Toll-like receptor 4,4 CC chemokine ligand (CCL) 5 promoter,32 transforming growth factor (TGF) β1,11 interleukin (IL) 12, tumor necrosis factor (TNF) α,15 IL-23,5 mannose-binding lectin (MBL),10 Fcγ receptor IIa (FcγRIIa), myeloperoxidase (MPO), FcγRIIIb, IL-1Ra, IL-10,12 Fc receptor-like 3 (FCRL3), peptidylarginine deiminase citullinating enzymes 4 (PADI4)13 and methylenetetrahydrofolate reductase (MTHFR)14 have been shown to influence the outcome after allogeneic SCT. Most of them are associated with the development of GVHD. Only the NOD2/CARD15 and HO-1 promoter polymorphisms have a significant impact on overall survival after SCT. Furthermore, the impact of the HO-1 promoter polymorphisms depends on donor cells but not on recipient cells, as observed with the NKG2D polymorphism which, in the donor, was shown to be significantly associated with overall survival in the present study. This may prompt the determination of the donor NKG2D polymorphism prior to SCT in order to choose the best donor, expected to minimize transplant-related mortality after SCT, when multiple donors for a patient are available. Otherwise, prior information on the donor NKG2D polymorphism may be helpful in selecting risk-specific appropriate precautions following transplantation.
In conclusion, the present data suggest that the NKG2D polymorphism, in addition to HLA disparity between recipients and donors, affects prognosis after a bone marrow transplant from an unrelated donor. However, care should be made in drawing conclusions because the number of patients in the present study was small. The finding of a gene polymorphism may not be equivalent to differences in gene expression, which may be influenced by multiple factors because the NKG2D receptor is found on many tissues and cells.41 Experimental evidence is required to substantiate the effect of the NKG2D polymorphism on immune function. We next plan to conduct a prospective study to confirm these results and to extend this investigation to other transplantation settings, such as related donor SCT, reduced-intensity SCT, HLA-mismatched SCT and SCT for patients with non-hematologic malignancies.
Acknowledgments
we are indebted to Drs. Hiroko Oshima, Masanobu Oshima and Atsushi Hirao, Mrs. Shinichi Ohmae and Katsuya Nakata, and Ms. Kaori Matsuura at Kanazawa University, and Drs. Keitaro Matsuo and Takakazu Kawase at the Aichi Cancer Center Research Institute for their technical assistance. We thank all of the JMDP transplant teams who contributed patients and donors to this study.
Footnotes
Authorship and Disclosures
JLE and AT designed and performed the research, and contributed to the same aspects of the work; AT, JLE and SN wrote the paper; AT, YKa, and SOh performed the statistical analyses; MO, HS, HA, KM, SOk, MI, TF, YM, and YKo contributed to data collection.
The authors reported no potential conflicts of interest.
Funding: this study was supported by grants from the Ministry of Health, Labor and Welfare, and the Ministry of Education, Culture, Sports and Technology, and funds from the Mitani Research and Development Assistance Organization (Kanazawa, Japan) and by the Japan Leukemia Research Fund (Tokyo, Japan).
References
- 1.Weisdorf DJ, Anasetti C, Antin JH, Kernan NA, Kollman C, Snyder D, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–7. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005;36:757–69. doi: 10.1038/sj.bmt.1705140. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson AM, Middleton PG, Rocha V, Gluckman E, Holler E. Genetic polymorphisms predicting the outcome of bone marrow transplants. Br J Haematol. 2004;127:479–90. doi: 10.1111/j.1365-2141.2004.05216.x. [DOI] [PubMed] [Google Scholar]
- 4.Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008;359:1766–77. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmaagacli AH, Koldehoff M, Landt O, Beelen DW. Relation of an inter-leukin-23 receptor gene polymorphism to graft-versus-host disease after hematopoietic-cell transplantation. Bone Marrow Transplant. 2008;41:821–6. doi: 10.1038/sj.bmt.1705980. [DOI] [PubMed] [Google Scholar]
- 6.Gerbitz A, Hillemanns P, Schmid C, Wilke A, Jayaraman R, Kolb HJ, et al. Influence of polymorphism within the heme oxygenase-I promoter on overall survival and transplantation-related mortality after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:1180–9. doi: 10.1016/j.bbmt.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Holler E, Rogler G, Brenmoehl J, Hahn J, Herfarth H, Greinix H, et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood. 2006;107:4189–93. doi: 10.1182/blood-2005-09-3741. [DOI] [PubMed] [Google Scholar]
- 8.Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104:889–94. doi: 10.1182/blood-2003-10-3543. [DOI] [PubMed] [Google Scholar]
- 9.Mayor NP, Shaw BE, Hughes DA, Maldonado-Torres H, Madrigal JA, Keshav S, et al. Single nucleotide polymorphisms in the NOD2/CARD15 gene are associated with an increased risk of relapse and death for patients with acute leukemia after hematopoietic stem-cell transplantation with unrelated donors. J Clin Oncol. 2007;25:4262–9. doi: 10.1200/JCO.2007.12.1897. [DOI] [PubMed] [Google Scholar]
- 10.Mullighan CG, Heatley S, Doherty K, Szabo F, Grigg A, Hughes TP, et al. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 2002;99:3524–9. doi: 10.1182/blood.v99.10.3524. [DOI] [PubMed] [Google Scholar]
- 11.Noori-Daloii MR, Rashidi-Nezhad A, Izadi P, Hossein-Nezhad A, Sobhani M, Derakhshandeh-Peykar P, et al. Transforming growth factor-β1 codon 10 polymorphism is associated with acute GVHD after allogenic BMT in Iranian population. Ann Transplant. 2007;12:5–10. [PubMed] [Google Scholar]
- 12.Rocha V, Franco RF, Porcher R, Bittencourt H, Silva WA, Jr, Latouche A, et al. Host defense and inflammatory gene polymorphisms are associated with outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;100:3908–18. doi: 10.1182/blood-2002-04-1033. [DOI] [PubMed] [Google Scholar]
- 13.Shimada M, Onizuka M, Machida S, Suzuki R, Kojima M, Miyamura K, et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br J Haematol. 2007;139:458–63. doi: 10.1111/j.1365-2141.2007.06797.x. [DOI] [PubMed] [Google Scholar]
- 14.Soydan E, Topcuoglu P, Dalva K, Arat M. The impact of methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism on transplant-related variables after allogeneic hematopoietic cell transplantation in patients receiving MTX as GVHD prophylaxis. Bone Marrow Transplant. 2008;42:429–30. doi: 10.1038/bmt.2008.184. [DOI] [PubMed] [Google Scholar]
- 15.Viel DO, Tsuneto LT, Sossai CR, Lieber SR, Marques SB, Vigorito AC, et al. IL2 and TNFA gene polymorphisms and the risk of graft-versus-host disease after allogeneic haematopoietic stem cell transplantation. Scand J Immunol. 2007;66:703–10. doi: 10.1111/j.1365-3083.2007.02021.x. [DOI] [PubMed] [Google Scholar]
- 16.Burgess SJ, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan JE. The NKG2D receptor: immunobiology and clinical implications. Immunol Res. 2008;40:18–34. doi: 10.1007/s12026-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 17.Hyka-Nouspikel N, Phillips JH. Physiological roles of murine DAP10 adapter protein in tumor immunity and autoimmunity. Immunol Rev. 2006;214:106–17. doi: 10.1111/j.1600-065X.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 19.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 20.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Imai K, Morishita Y, Hayashi I, Kusunoki Y, Nakachi K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res. 2006;66:563–70. doi: 10.1158/0008-5472.CAN-05-2776. [DOI] [PubMed] [Google Scholar]
- 22.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 23.Furue H, Kumimoto H, Matsuo K, Suzuki T, Hasegawa Y, Shinoda M, et al. Opposite impact of NKG2D genotype by lifestyle exposure to risk of aerodigestive tract cancer among Japanese. Int J Cancer. 2008;123:181–6. doi: 10.1002/ijc.23456. [DOI] [PubMed] [Google Scholar]
- 24.Furue H, Matsuo K, Kumimoto H, Hiraki A, Suzuki T, Yatabe Y, et al. Decreased risk of colorectal cancer with the high natural killer cell activity NKG2D genotype in Japanese. Carcinogenesis. 2008;29:316–20. doi: 10.1093/carcin/bgm260. [DOI] [PubMed] [Google Scholar]
- 25.Kawase T, Morishima Y, Matsuo K, Kashiwase K, Inoko H, Saji H, et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood. 2007;110:2235–41. doi: 10.1182/blood-2007-02-072405. [DOI] [PubMed] [Google Scholar]
- 26.Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoko H, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998;339:1177–85. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 28.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 29.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 30.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–7. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 31.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Jung HD, Lee NY, Sohn SK. Single nucleotide polymorphism of CC chemokine ligand 5 promoter gene in recipients may predict the risk of chronic graft-versus-host disease and its severity after allogeneic transplantation. Transplantation. 2007;84:917–25. doi: 10.1097/01.tp.0000284583.15810.6e. [DOI] [PubMed] [Google Scholar]
- 33.Dulphy N, Haas P, Busson M, Belhadj S, Peffault de Latour R, Robin M, et al. An unusual CD56(bright) CD16(low) NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol. 2008;181:2227–37. doi: 10.4049/jimmunol.181.3.2227. [DOI] [PubMed] [Google Scholar]
- 34.Hamby K, Trexler A, Pearson T, Larsen C, Rigby M, Kean L. NK cells rapidly reject allogeneic bone marrow in the spleen through a perforinand Ly49D-dependent, but NKG2D-independent mechanism. Am J Transplant. 2007;7:1884–96. doi: 10.1111/j.1600-6143.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- 35.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik W, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 36.Ruggeri L, Mancusi A, Burchielli E, Capanni M, Carotti A, Aloisi T, et al. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol Dis. 2008;40:84–90. doi: 10.1016/j.bcmd.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Hsu K, Keever-Taylor C, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–84. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morishima Y, Yabe T, Matsuo K, Kashiwase K, Inoko H, Saji H, et al. Effects of HLA allele and killer immunoglobulin-like receptor ligand matching on clinical outcome in leukemia patients undergoing transplantation with T-cell-replete marrow from an unrelated donor. Biol Blood Marrow Transplant. 2007;13:315–28. doi: 10.1016/j.bbmt.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Kimura F, Sato K, Kobayashi S, Ikeda T, Sao H, Okamoto S, et al. Impact of ABO-blood group incompatibility on the outcome of recipients of bone marrow transplants from unrelated donors in the Japan Marrow Donor Program. Haematologica. 2008;93:1686–93. doi: 10.3324/haematol.12933. [DOI] [PubMed] [Google Scholar]
- 40.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–33. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 41.Collins RW. Human MHC class I chain related (MIC) genes: their biological function and relevance to disease and transplantation. Eur J Immunogenet. 2004;31:105–14. doi: 10.1111/j.1365-2370.2004.00457.x. [DOI] [PubMed] [Google Scholar]