The degree of the globin chain imbalance is the pathogenetic clue to the clinical phenotype of thalassemia syndromes. This paper reports a duplication of the α globin gene locus in a group of hetereozygous β-thalassemia patients with the unexplained phenotype of thalassemia intermedia.
Keywords: thalassemia intermedia, α-globin gene quadruplication, silent β thalassemia, MLPA
Abstract
Ten patients with thalassemia intermedia with variable severity and apparent simple heterozygosis for β0 39 C>T nonsense mutation were submitted to clinical, hematologic and molecular studies. The presence of an unknown molecular defect (silent β-thalassemia) unlinked to the β cluster interacting with the heterozygous β thalassemia, was previously postulated in these families. Analysis of the α globin gene cluster with PCR-based methods (MLPA, GAP-PCR, digestion with restriction enzymes) detected complex rearrangements in the α cluster. A duplication of the α globin gene locus, including the upstream regulatory region, was present in all the patients, associated in some of them with deletion or non-deletion α thalassemia. The variability of the clinical phenotype correlates with the degree of the globin chain imbalance. The presence of α globin cluster duplication should be considered in patients heterozygote for β-thalassemia with thalassemia intermedia phenotype and in the carriers of suspected silent β thalassemia.
Introduction
Variable clinical manifestations are associated with β-thalassemia spanning from the transfusion-dependent condition of thalassemia major to the asymptomatic thalassemia carrier state. Thalassemia intermedia lies between the two extreme phenotypes and comprises a broad spectrum of clinical conditions of different severity having in common the non-transfusion dependence. Part of the clinical heterogeneity of thalassemia intermedia depends on different and only partially defined molecular mechanisms, the most common being homozygosity for mild β-thalassemia mutations, compound heterozygosity for mild/silent and severe β-thalassemia mutations, co-inheritance with homozygous β thalassemia of α thalassemia or of genetic determinants able to increase the production of fetal hemoglobin (HPFH).1–3 Less commonly, the phenotype of thalassemia intermedia has been reported in subjects carrying only one β globin gene defect. Among them 3 groups have been defined: (i) the dominantly inherited β thalassemia mutations (also reported as inclusion body thalassemias);4,5 (ii) the co-existence of somatic deletions of a region of chromosome 11 p15;6,7 (iii) co-inheritance of triplicated α globin genes with excessive α globin production.8–10 Recently cases of simple β thalassemia heterozygosity presenting with an intermediate to severe phenotype due to duplications of the complete α globin gene cluster, including the upstream regulatory element HS-40, have been reported;11 (iv) interaction between severe and silent β thalassemia mutations.12,13
Herein, we report the molecular characterization of 10 individuals belonging to four families, with the clinical phenotype of thalassemia intermedia of variable severity due to the association of α globin gene quadruplication with heterozygous β thalassemia. These families have been reported before and to explain the thalassemia intermedia phenotype, we postulated the presence of an unknown molecular defect (silent β thalassemia), unlinked to the β globin gene cluster, interacting with the heterozygous β globin gene mutation.12,13
Design and Methods
We studied the clinical and hematologic characteristics and the molecular bases of 10 patients (5 males and 5 females) with β thalassemia intermedia belonging to four Sardinian families (two of them are related). Peripheral blood samples were obtained as part of the clinical study after informed consent.
Hematologic analysis
Red blood cell indices were determined with Coulter Max –M ( I.L. Milan, Italy ). Hb A2 and Hb F quantitation was performed by high performance liquid chromatography, HPLC (Variant, BIO-RAD, Milan Italy). Globin chain synthesis analysis was performed on the peripheral blood reticulocytes following the method of Kan et al.14 Appropriate methods have been used to exclude the presence of associated red blood cell membrane defects or enzymatic deficiencies.
DNA analysis
DNA extraction was performed according to standard methods. Mutation analysis of the β globin gene including the promoter up to 600bp was performed by direct DNA sequencing. The HS2 and HS3 cores have also been sequenced. α-globin genotype was defined by GAP PCR for the deletional defects and the triple α gene arrangement, and by digestion of amplification products with NcoI and HphI restriction enzymes for non-deletional defects.15 To detect the less common anti-α 4.2 triplication, the Southern blot analysis was carried out.
To investigate for the presence of extended deletions or duplications on the α cluster, MLPA method (multiplex ligation-dependent probe amplification) was performed using a set of 25 probes, covering a region of 170 Kb in the α cluster on the short arm of the chromosome 16 (MRC-Holland, Amsterdam, Nederland, P140B HBA probemix).16
Results and Discussion
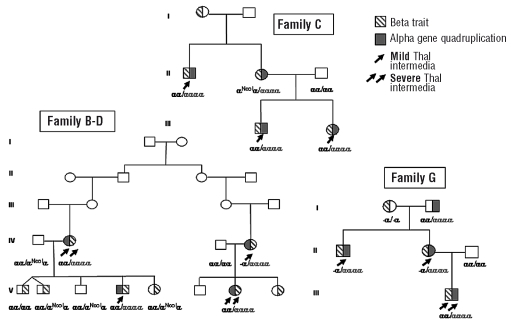
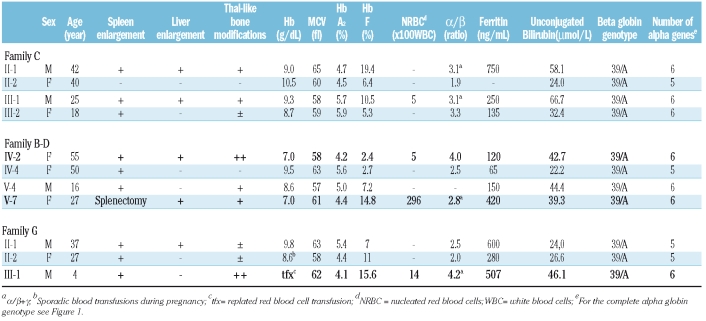
Pedigrees of the families, clinical and hematologic data are reported in Figure 1 and Table 1.
Figure 1.
Pedigrees of the families.
Table 1.
Clinical and hematologic data of the patients (in bold the patients with the most severe phenotype).
All patients with thalassemia intermedia (indicated by the arrows) have moderate to severe microcytic anemia (Hb 7.0–9.8 g/dL, MCV 57-65 fL), increased HbA2 (>4.1%) and HbF (2.7–19.4%) and spleen enlargement. Nine have moderate to marked thalassemia-like bone modifications. Four patients have nucleated red blood cells in their peripheral blood. Serum ferritin and unconjugated bilirubin are normal or moderately increased. Gallstones are present in 4 patients. All patients have normal cardiac function (left ventricle ejection fraction >60%) and pulmonary hypertension was evident. Among the patients, two different severity degrees can be identified. Patients IV-2 and V-7 in family B-D and patient III-1 in family G have a more severe phenotype (Table 1 in bold) [lower hemoglobin levels, marked splenomegaly, severe thalassemia-like bone modifications, need for splenectomy for worsening of anemia in V-7, and needs for repeated red blood cell transfusions from age 4 in III-1 and markedly unbalanced α/β globin chain synthesis ratio (α/β 4.0 – 4.2)]. Patients IV-4 and V-4 from family B-D, patients II-1, III-1 and III-2 from family C and patients II-1 and II-2 from family G have a milder phenotype and less unbalanced α/β globin chain synthesis ratio (Table 1). Subject II-2 from family C, despite the presence of α cluster duplication, has the clinical phenotype of the β tha-lassemia carrier state: no clinical symptoms, hemoglobin level of 10.5 g/dL, lowest α/β globin synthesis ratio (1.9).
DNA analysis
Despite the extended analysis of the β globin gene cluster [β globin gene sequence including the polymorphic (AT)n (T) sequence at position -530, which has been associated in some studies17,18 with silent β thalassemia, and sequencing of the HS2 and HS3 core19,20 of the Locus Control Region], as previously reported,12,13 all patients have as only abnormality the codon 39 C→T nonsense mutation in one β globin gene and a completely normal sequence in the other. With family studies we easily excluded that the determinant responsible for the severe phenotype resided within the β globin gene cluster because other members of the family heterozygotes for the β 39 mutation (I-1 in family C, V-1, V-2, V-5 and V-8 in family B-D, and I-1 in family G) had the typical carrier state phenotype. Moreover, haplotype analysis according to Orkin et al.21 showed the β 39 mutation associated with haplotype 1 in B-D family and 2 in C and G families.
α globin gene analysis excluded in all patients with thalassemia intermedia the triple α globin gene arrangement, while revealed in 2 of them (IV-4 in family B-D and II-1, II-2 in family G) the presence of the -α 3.7 deletion and in a patient of family C (II-2) the presence of the α2initiation codon ATG>ACG mutation (indicated as aNcoI in Figure 1 since this mutation is recognized by the NcoI restriction enzyme). MLPA analysis of the α-globin cluster revealed in all patients with thalassemia intermedia a complete duplication of the α-globin gene cluster spanning in one family 170 Kb region from the telomere while in the other families the duplication was larger than 170kb. The duplications include the regulatory elements MCS-R1, MCS-R2(HS40), MCS-R3 and, MCS-R4. This genotype was also present in subject I-2 in G family with normal hematologic parameters (MCV 80 fL, HbA2 2.9%, Hb 15.2 g/dL and HbF 0.9%) but globin chain synthesis imbalance (α/β 1.9)
This study describes a group of patients belonging to four Sardinian families, simple heterozygotes for β thalassemia with a variable thalassemia intermedia phenotype. We have previously reported these families, postulating the presence of an unknown silent molecular defect unlinked to β cluster interacting with the β globin gene mutation.12,13 Similar cases have been described by Faà et al.22 Using the high resolution MLPA method we have been able to identify the reason for the worsening of the symptoms in the simple β thalassemia carriers of our families. In fact, beside the heterozygosity for β thalassemia, they have a duplication of the α globin locus and some of them a coexistent deletional or non-deletional α thalassemia. The duplications spanning at least 170 Kb include all the regulatory elements of the α globin gene cluster. The final result of the α globin cluster rearrangements is the presence of one or two additional α globin genes, which cause a more severe imbalance of the α/β globin chain synthesis ratio, as compared to the simple β thalassemia carrier state. The increased α/β imbalance enhances the ineffective erythropoiesis, resulting in evident clinical symptoms. It is interesting to note that the severity of the clinical phenotype seems to be partly related to the number of the α globin genes and to the type of interacting α thalassemia. In fact subjects with six α globin genes have the highest α/β ratio (3.1–4.2) and the most severe phenotype, while subjects with duplication and interacting α thalassemia, and hence with five functional α genes, have a milder phenotype and a milder α/β ratio (2.0–2.5). However, among the subjects with six α globin genes 3 have a more severe and 4 a milder clinical phenotype. The reasons of this interfamiliar (family C versus family B-D and G) and intrafamiliar (V-4 versus IV-2 and V-7 in family B-D) variability have not been defined. However, the individual variability could be related to the ubiquitine proteolytic pathway, the process which eliminates the excess of unbounded globin chains in the cell, previously postulated as being variable from person to person in the some family.23 In this respect subject II-2 from family C, who has a non-deletional α thalassemia defect, known to be more severe than the deletional α thalassemia and therefore able to further reduce the α/β imbalance, presents the lowest α/β ratio (1.9) and the mildest phenotype, not very different from a simple β thalassemia carrier state, except for a more pronounced anemia. From these data it seems that the critical threshold of globin chain imbalance above which clinical symptoms appear is an α/β ratio of 2.0. It is interesting to note that the subject with only α globin gene duplication (I-2 in G family) shows a silent hematologic phenotype and normal HbA2 but imbalanced α/β ratio, further proving that the extra α globin genes are functional.
It is well known that the presence of increased α globin chain production in heterozygotes for β-thalassemia, as a result of co-inherited triple α globin arrangement, converts the typical clinically asymptomatic β thalassemia carrier state into that of thalassemia intermedia.8–10,24 Other α globin gene rearrangements associated with β thalassemia carrier state and resulting in thalassemia intermedia, have been reported.25 However, the phenotype of the patients with these genetic combinations is variable and depends both on the severity of the β thalassemia allele and on the number of excess α globin genes. Recently Harteveld et al.16 described segmental duplications of the α globin gene cluster causing thalassemia intermedia in 2 subjects hetorozygotes for β thalassemia. One patient with the typical thalassemia intermedia phenotype, had six active α globin genes, as a result of duplication, the other with a transfusion-dependent phenotype had a duplication of the α locus in one chromosome and the common anti-α 3.7 triplication in trans resulting in a total of seven active α genes. Globin chain synthesis analysis was not reported in this study. In our families, 3 of the subjects with six α globin genes have a more severe phenotype the reasons for which have not been clarified and the presence of another not yet identified genetic determinant cannot be excluded. Since families are originating from distant areas and the extension of the duplication seem to be different, at least in one family, α globin gene quadruplication is expected to be a not uncommon defect.
In conclusion, we have demonstrated that α globin cluster duplications should be considered in cases of thalassemia intermedia phenotypes presenting with simple heterozygosis and in carriers of suspected silent β thalassemia. The reported duplication and other similar defects could possibly not be uncommon in Sardinia and elsewhere.
Acknowledgments
the authors would like to thank Maria Franca Desogus for editorial assistance.
Footnotes
Authorship and Disclosures
RG wrote the paper and takes primary responsibility for it. MCS and MEP performed the laboratory work and contributed to writing the paper. LP and DL performed the laboratory work and participated in the analysis of the data. NG recruited the patients and collected the clinical data.
The authors reported no potential conflicts of interest.
Funding: this work was supported by a grant from the Ministero dell’Istruzione, dell’Università e della Ricerca and by a grant from Regione Sardegna (L.R. 11, 1990, PRIN 2006).
References
- 1.Galanello R, Cao A. Relationship between genotype and phenotype. Thalassemia intermedia. Ann N Y Acad Sci. 1998;850:325–33. doi: 10.1111/j.1749-6632.1998.tb10489.x. [DOI] [PubMed] [Google Scholar]
- 2.Thein SL. Genetic modifiers of b-thalassemia. Haematologica. 2005;90:649–60. [PubMed] [Google Scholar]
- 3.Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalssemia. Proc Natl Acad Sci USA. 2008;105:1620–5. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thein SL. Structural Variants with a β-thalassemia phenotype. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge, UK: Cambridge University Press, Cambridge, UK; 2001. pp. 342–55. [Google Scholar]
- 5.Fei YJ, Stoming TA, Kutlar A, Huisman THJ, Stamatoyannopoulos G. One form of inclusion body b-thalassemia is due to a GAA→TAA mutation at codon 121 of the β chain. Blood. 1989;73:1075–7. [PubMed] [Google Scholar]
- 6.Badens C, Mattei MG, Imbert AM, Lapoumérouliee C, Martini N, Michel G, et al. A novel mechanism for thalassaemia intermedia. Lancet. 2002;359:132–3. doi: 10.1016/s0140-6736(02)07338-5. [DOI] [PubMed] [Google Scholar]
- 7.Galanello R, Perseu L, Perra C, Maccioni L, Barella S, Longinotti M, et al. Somatic deletion of the normal β-globin gene leading to thalassemia intermedia in heterozygous β-thalassaemic patients. Br J Haematol. 2004;127:604–6. doi: 10.1111/j.1365-2141.2004.05237.x. [DOI] [PubMed] [Google Scholar]
- 8.Galanello R, Ruggeri R, Paglietti E, Addis M, Melis MA, Cao A. A family with segregating triplicated α globin loci and β thalassaemia. Blood. 1983;62:1035–40. [PubMed] [Google Scholar]
- 9.Camaschella C, Kattamis AC, Petroni D, Roetto A, Sivera P, Sbaiz L, et al. Different hematological phenotypes caused by the interaction of triplicated α-globin genes and heterozygous β-thalassaemia. Am J Haematol. 1997;55:83–8. doi: 10.1002/(sici)1096-8652(199706)55:2<83::aid-ajh6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Traeger-Synodinos J, Kanavakis E, Vrettou C, Maragoudaki E, Michael T, Metaxotou-Mavromati A. The triplicated α-globin gene locus in β-thalassaemia heterozygotes: clinical, haematological, biosynthetic and molecular studies. Br J Haematol. 1996;95:467–71. doi: 10.1046/j.1365-2141.1996.d01-1939.x. [DOI] [PubMed] [Google Scholar]
- 11.Harteveld CL, Refaldi C, Cassinerio E, Cappellini MD, Giordano PC. Segmental duplications involving the α-globin gene cluster are causing β-thalassemia intermedia phenotypes in β-thalassemia heterozygous patients. Blood Cells Mol Dis. 2008;40:312–6. doi: 10.1016/j.bcmd.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Galanello R, Maccioni L, Rosatelli C, Ibba P, Nurchi AM, Cao A. A genetic combination of silent β-thalassaemia, high Hb A2 β-thalassaemia, and single α globin gene deletion causing mild thalassaemia intermedia. J Med Genet. 1984;21:153–6. doi: 10.1136/jmg.21.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasperini D, Perseu L, Melis MA, Maccioni L, Sollaino MC, Paglietti E, et al. Heterozygous β-thalassemia with thalassemia intermedia phenotype. Am J Haematol. 1998;57:43–7. doi: 10.1002/(sici)1096-8652(199801)57:1<43::aid-ajh7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Kan YW, Schwartz E, Nathan DG. Globin chain synthesis in the α thalassemia syndrome. J Clin Invest. 1968;47:2515–22. doi: 10.1172/JCI105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanello R, Sollaino C, Paglietti E, Barella S, Perra C, Doneddu I, et al. a-Thalassemia carrier identification by DNA analysis in the screening for thalassemia. Am J Haematol. 1998;59:273–8. doi: 10.1002/(sici)1096-8652(199812)59:4<273::aid-ajh2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Harteveld CL, Voskamp A, Phylipsen M, Akkermans N, den Dunnen JT, White SJ, et al. Nine unknown rearrangements in 16p13.3 and 11p15.4 causing α- and β-thalassaemia characterised by high resolution multiplex ligation-dependent probe amplification. J Med Genet. 2005:42922–31. doi: 10.1136/jmg.2005.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg PE, Williams DM, Qian RL, Cohen RB, Cao SX, Mittelman M, et al. A common protein binds to two silencers 5' to the human beta-globin gene. Nucleic Acids Res. 1989;17:8833–52. doi: 10.1093/nar/17.21.8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murru S, Loudianos G, Porcu S, Sciarratta GV, Agosti S, Parodi MI, et al. A β-thalassaemia phenotype not linked to the β-globin cluster in an Italian family. Br J Haematol. 1992;81:283–7. doi: 10.1111/j.1365-2141.1992.tb08221.x. [DOI] [PubMed] [Google Scholar]
- 19.Oner C, Dimovski AJ, Altay C, Gurgey A, Gu YC, Huisman TH, et al. Sequence variations in the 5' hypersensitive site-2 of the locus control region of β S chromosomes are associated with different levels of fetal globin in hemoglobin S homozygotes. Blood. 1992;79:813–9. [PubMed] [Google Scholar]
- 20.Talbot D, Philipsen S, Fraser P, Grosveld F. Detailed analysis of the site 3 region of the human β-globin dominant control region. EMBO J. 1990;9:2169–77. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orkin SH, Kazazian HH, Jr, Antonarakis SE, Goff SC, Boehm CD, Sexton JP, et al. Linkage of β-thalassemia mutations and α-globin gene polymorphisms with DNA polymorphisms in human α-globin gene cluster. Nature. 1982;296:627–31. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- 22.Faà V, Meloni A, Moi L, Ibba G, Travi M, Vitucci A, et al. Thalassaemia-like carriers not linked to the β-globin gene cluster. Br J Haematol. 2006;132:640–50. doi: 10.1111/j.1365-2141.2005.05915.x. [DOI] [PubMed] [Google Scholar]
- 23.Giordano PC, Harteveld CL, Michiels JJ, Terpstra W, Schelfhout LJDM, Appel IM, et al. Phenotype variability of the dominant β-thalassemia induced in four Dutch families by the rare cd121 (G→T) mutation. Ann Hematol. 1998;77:249–255. doi: 10.1007/s002770050453. [DOI] [PubMed] [Google Scholar]
- 24.Bianco I, Lerone M, Foglietta E, Deidda G, Cappabianca MP, Morlupi L, et al. Phenotypes of individuals with a β thal classical allele associated either with a β thal silent allele or with a globin gene triplication. Haematologica. 1997;82:513–25. [PubMed] [Google Scholar]
- 25.Beris P, Darbellay R, Hochmann A, Pradervand E, Pugin P. Interaction of heterozygous β (0)-thalassemia and triplicated α globin loci in a Swiss-Spanish family. Klin Wochenschr. 1991;69:710–4. doi: 10.1007/BF01649440. [DOI] [PubMed] [Google Scholar]