This study suggest that non-melanoma skin cancer may be a novel clinical predictor of worse chronic lymphocytic leukemia outcome.
Keywords: chronic lymphocytic leukemia, non-melanoma skin cancer, squamous cell carcinoma, susceptibility, pathogenesis, immune disruption, immunosuppression, survival, prognosis
Abstract
We investigated whether a previous diagnosis of non-melanoma skin cancer among chronic lymphocytic leukemia patients is a predictor of poor outcome. Using the Swedish Cancer Registry, we conducted a population-based study to evaluate the survival patterns among chronic lymphocytic leukemia patients with and without non-melanoma skin cancer. Cox proportional hazards regression models were used and Kaplan-Meier curves were constructed. Of a total of 12,041 chronic lymphocytic leukemia cases identified, 236 cases, including 111 squamous cell cancer, had a prior history of non-melanoma skin cancer. Chronic lymphocytic leukemia patients with a prior history of non-melanoma skin cancer had a 1.29-fold (95% CI 1.10–1.52; p=0.0024) increased risk of dying; and those with a history of squamous cell cancer had a further elevated 1.86-fold (95% CI 1.46–2.36; p<0.0001) risk of dying. Kaplan-Meier plots showed that patients with a history of non-melanoma skin cancer, particularly those with squamous cell cancer, had significantly poorer survival than chronic lymphocytic leukemia patients without non-melanoma skin cancer (p<0.0001; log-rank test). Non-melanoma skin cancer may be a novel clinical predictor of worse chronic lymphocytic leukemia outcome.
Introduction
Chronic lymphocytic leukemia (CLL) is a neoplastic disease characterized by the accumulation of small, mature-appearing lymphocytes in the bone marrow, blood, and lymphoid tissues. CLL accounts for 30% of all leukemias and is the most common form of leukemia among older adults in Western countries.1 Data from the United States Surveillance, Epidemiology, and End Results (SEER) Registry estimate the incidence rate of CLL to be 3.9/100,000 person-years with a median age at diagnosis of 72 years.2 During 2008 it is estimated that 15,110 new cases of CLL will be diagnosed and 4,390 people will die of CLL in the US.3 In Sweden, the incidence of CLL is 6.5/100,000 and 3.1/100.000 person-years for males and females, respectively. Although advanced age, Caucasian race, and family history of certain hematologic malignancies are recognized risk factors, the etiology of CLL is unknown.1,2,4,5 The clinical course of CLL varies enormously among patients, reflected in the overall survival ranging from months to decades.6 The identification of novel clinical predictors of outcome in CLL is of intense interest.7 Non-melanoma skin cancers (NMSC) are the most common malignancies in Caucasian populations.8 Indeed, squamous cell cancer (SCC) was the fourth most common cancer in men and women in Sweden in 1998, with an annual incidence of 30.2/100,000 and 14.1/100, 000, respectively. Currently, SCC is the most rapidly increasing cancer in Sweden.9,10 Multiple case reports described subclinical cases of CLL incidentally identified during re-excisions of SCC and basal cell cancer.11, 12 Prior studies found individuals with a history of SCC to have a 2-fold increased risk of developing CLL,13 non-Hodgkin’s lymphoma (NHL) and Hodgkin’s disease.14
In Finland,15 Denmark16 and Switzerland17 increased risks for both CLL and non-Hodgkin's lymphoma have also been reported. Furthermore, two recent investigations found increased mortality among lymphoma patients with a prior history of skin cancer.18,19 However, in both studies, CLL patients were combined with other NHL subtypes. We conducted a large population-based study including 12,041 CLL patients diagnosed in Sweden between 1958 and 2003 to assess survival patterns among CLL patients with and without a diagnosis of NMSC.
Design and Methods
Data resources and patients
The nationwide Swedish Cancer Registry has been in operation since 1958 with very high diagnostic accuracy.20 We identified 11,805 cases in whom CLL was the primary tumor and who did not develop NMSC before or after the diagnosis of CLL during the study period. We also identified 236 NMSC cases (including 111 SCC cases) in whom CLL occurred after a diagnosis of NMSC registered on the Swedish Cancer Registry between January 1, 1958 and December 31, 2003 (International Classification of Diseases [ICD] 7th Revision codes: CLL 72041 and NMSC 191). We selected SCC patients with the ICD-O-1 codes 80513 (verrucous carcinoma), 80703 (SCC), 80702 (SCC in situ), 80812 (Bowen’s disease), 80743 (SCC, spindle cell type) and 80763 (microinvasive SCC). For each person, information on date of birth, date of diagnosis of CLL, and gender; and for CLL patients with NMSC, date of skin cancer diagnosis and histological subtype were collected. Through record linkage with the Cause of Death Register, we collected information on vital status. Approval for the study was obtained from the Swedish National Review Board and the IRB of NIH Office of Human Subjects Research.
Statistical analysis
The assumption of proportional hazards was first examined by assessing the effect of NMSCs on death. The relative risk (RR) for dying was estimated by using Cox proportional hazards regression analysis21 with years since diagnosis of CLL as the time scale. Models were adjusted for age at first CLL diagnosis (quartiles), sex, and calendar period of first CLL diagnosis (quartiles) to investigate the association between the survival time and NMSC. Five year survival analysis was conducted. Kaplan-Meier plots were constructed stratified by NMSC status to compare the survival curves for CLL patients with a previous history of NMSC and patients in whom CLL was the primary tumor but who did not develop NMSC before or after the CLL diagnosis. Differences were tested formally by the log-rank test. Further, we assessed the relationship between CLL mortality and latency of NMSC by the time between diagnosis of NMSC and CLL diagnosis (<1 year, 1–5 years, or >5 years) and compared the difference in the RR among three latency categories to those without NMSC. The difference in the RR were examined by the number of registrations of NMSC, gender, and age at CLL (<70 vs. >70 years) to those without an NMSC. All statistical analyses were performed using the SAS statistical package (SAS Institute, Inc., Cary, North Carolina, USA).
Results and Discussion
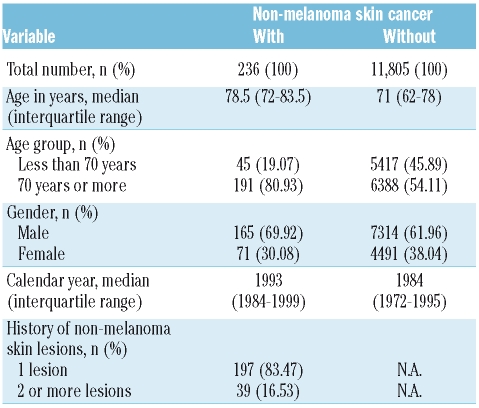
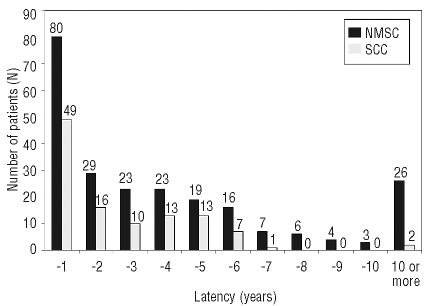
This is the largest population-based study and included 12,041 CLL cases. Between 1958–2003, 11,805 cases in whom CLL was the primary tumor and 236 NMSC cases in whom CLL occurred after a diagnosis of NMSC were registered on the Swedish Cancer Registry (Table 1A). The median age at CLL diagnosis for individuals with and without a history of NMSC was 78.5 and 71 years, respectively. While 70% of the NMSC patients were men, 30% were women. Of the 236 patients with NMSC, 83.5% of cases had one registered skin NMSC lesion and 16.5% had two or more registered NMSC lesions (Table 1A). Of 236 CLL cases who had a prior history of NMSC, 111 had at least one SCC, prior to their CLL diagnosis (Table 1B). There were no cases with an ICD code specific for basal cell carcinoma. Thirty-three percent (80/236) of NMSC and 44% (49/111) of SCC cases preceded the diagnosis of CLL by less than one year (Figure 1).
Table 1A.
Characteristics of chronic lymphocytic leukemia patients.
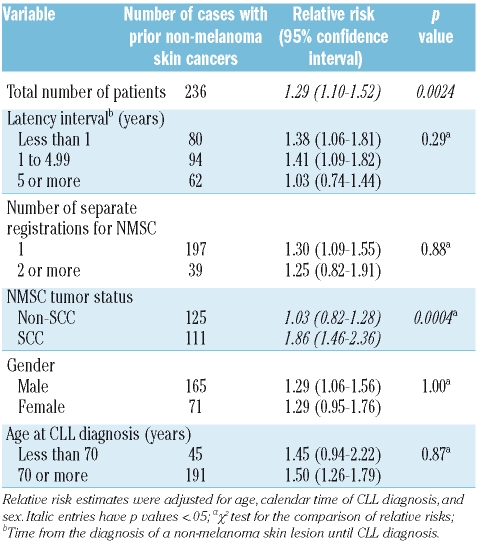
Table 1B.
Relative risk for death (all-cause mortality) after diagnosis of chronic lymphocytic leukemia in patients with a previous history of non-melanoma skin cancer.
Figure 1.
Time between first diagnosis of non-melanoma skin cancers, including squamous cell cancer, and chronic lymphocytic leukemia diagnosis.
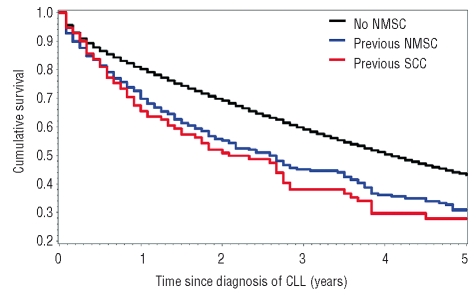
We showed for the first time that CLL patients with a history of NMSC had a significantly increased mortality compared to CLL patients without NMSC (RR=1.29, 95% CI 1.10–1.52) (Table 1B). Mortality was slightly higher among individuals diagnosed with NMSC within one year (RR=1.38, 95% CI 1.06–1.81) and 1–5 years (RR=1.41; 95% CI 1.09–1.82) prior to CLL diagnosis compared to those diagnosed with NMSC more than five years (RR= 1.03; 95% CI 0.74–1.44) prior to CLL diagnosis. However, the difference among groups was not statistically significant (p=0.29). Similarly, mortality did not differ by number of registrations for NMSC (1 or ≥2) (p=0.88), gender (p=1.00), or age at CLL diagnosis (<70 years vs. >70 years) (p=0.87) (Table 2). CLL patients with a prior history of SCC had a further elevated 1.86-fold (95% CI 1.46–2.36; p<0.0001) risk of dying. Kaplan-Meier plots showed that CLL patients with a prior history of NMSC (31%) or SCC (28%) had significantly poorer overall 5-year survival rates than those patients with CLL without NMSC (43%) (p<0.0001, log-rank test) (Figure 2). Among the 236 NMSC cases, there were 149 deaths of which 64 (43%) were attributed to CLL within the 5-year follow-up period. Among 111 SCC cases, there were 69 deaths of which 31 (45%) were attributed to CLL within the 5-year follow-up period. Individually, causes other than CLL accounted for a small proportion of deaths and accounted for similar proportions in the NMSC and the SCC group.
Figure 2.
Kaplan-Meier plots showing 5-year cumulative survival after diagnosis of chronic lymphocytic leukemia among patients with and without non-melanoma skin cancer and squamous cell carcinoma, respectively.
We showed for the first time that CLL patients with a previous diagnosis of NMSC have a significantly decreased survival compared with CLL patients without NMSC before and after the CLL diagnosis. Our investigation has unique new findings and expands the findings of two previous studies that found increased mortality among lymphoma patients with a prior history of skin cancer.22,23 However, in both studies, CLL patients were combined with other non-Hodgkin’s lymphoma (NHL) subtypes. The association of NMSC, especially SCC, and CLL is of increased interest since it has been observed in numerous CLL patients across the United States and worldwide. However, this association has been very little studied and is under-reported in the literature. The relationship between NMSC and CLL, as well as the increased mortality in CLL patients with a prior history of NMSC is complex. The underlying pathogenic mechanisms for both of these associations remain unknown but they may be related. Clinical and epidemiological studies suggest that diminished immune function is a risk factor for CLL. In CLL, there is profound T-cell dysfunction, suggesting that these lymphocytes are unable to maintain immune surveillance and cytotoxic functions,18 allowing mutagenic events to persist and progress. This mechanism is similar to immunosuppression-related skin cancer in solid organ transplant recipients. Both UV radiation and CLL are thought to cause immunosuppression, possibly accounting for the increased incidence of NMSC in patients with CLL.
The association of NMSC and immunosuppression is well-documented.23 Unfortunately, we were unable to investigate immune status in our cohort since we did not have laboratory or other clinical data. Human papilloma virus infection has been reported to play a role in the oncogenesis of SCC, especially in organ transplant recipients.24 It is also possible that the predisposition for CLL and NMSC is due to shared genetic susceptibility. In conclusion, immunological factors, in combination with viral, genetic and/or environmental factors may all contribute to the association between NMSC and CLL as well as the increased mortality in CLL patients with a prior history of NMSC.
Strengths of this study include its large size. We used a population-based cohort design, which minimized recall-bias and allowed us to evaluate risk by age and gender. We restricted the study to NMSC cases prior to CLL diagnosis to avoid CLL treatment-related effects and to eliminate survival bias. Limitations include lack of clinical and therapeutic data, and centralized pathology review. Reliance on central cancer data is a limitation because it can lead to under-ascertainment of CLL.20 Furthermore, a fraction of the CLL patients in our study coded without history of NMSC likely had a history of NMSC; thus any bias should have been toward a null association. In a Finnish25 and a US study,26 no excess mortality was associated with a diagnosis of NMSC compared to the general population. In Denmark superior survival was associated with BCC, but inferior survival with SCC.27 Therefore, it is possible that part of the excess mortality in CLL patients with SCC may be explained by an increased mortality observed in patients with SCC compared to the general population. We could not assess whether SCC itself contributed to excess mortality in our study because we did not have access to data regarding the general population. NMSC might be a surrogate marker for an underlying systemic immunosuppressed state preceding CLL diagnosis as well as a novel clinical marker of outcome in a group of CLL patients.
Our findings may have clinical impact in that CLL patients with a prior history of NMSC might have more aggressive disease. In addition, physicians should have a high index of suspicion of CLL in patients with NMSC, especially SCC who have new onset of symptoms such as lymphadenopathy, fever, weight loss, and/or night sweats. Improving our understanding of CLL and its related precursor states will ultimately lead to early CLL diagnosis and treatment, and identification of novel molecular targets.2,28,29
Additional studies should evaluate individual risk factors for CLL prognosis by incorporating more clinical data regarding treatment and immune parameters. The possibility that subclinical CLL may be present at the time of NMSC diagnosis should be addressed, as we observed a trend for decreased survival with shorter latency between NMSC diagnosis and CLL diagnosis. Future epidemiological and molecular studies are needed to explore the underlying mechanisms of our findings.
Acknowledgments
the authors thank Ms. Fereshte Ebrahim, The National Board of Health and Welfare, Stockholm, Sweden, for important efforts in setting up this database.
Footnotes
Authorship and Disclosures
JRT and OL designed, conducted studies and wrote the manuscript; MB, SYK and OL obtained institutional review board approval and data; ZW performed statistical analyses; PB prepared a graph. All authors were involved in the analyses and the interpretation of the results. All authors read, gave comments and approved the final version of the manuscript. JRT, OL and ZW have full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors reported no potential conflict of interest.
Funding: this research was supported in parts by the Intramural Program of the National Cancer Institute, National Institutes of Health, Bethesda, Maryland; and grants from the Swedish Cancer Society, Stockholm County Council, and the Karolinska Institutet Foundations.
References
- 1.Linet MS, Devesa SS, Morgan GJ. The Leukemias. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. ed Third. New York City: Oxford University Press; 2006. pp. 841–71. [Google Scholar]
- 2.Ries LAG, Harkins D, Krapcho M. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 3.Anonymous: Cancer facts & figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 4.Goldin LR, Pfeiffer RM, Li X, Hemminki K. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish Family-Cancer Database. Blood. 2004;104:1850–4. doi: 10.1182/blood-2004-01-0341. [DOI] [PubMed] [Google Scholar]
- 5.Goldin LR, Landgren O, McMaster ML, Gridley G, Hemminki K, Li X, et al. Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev. 2005;14:2402–6. doi: 10.1158/1055-9965.EPI-05-0346. [DOI] [PubMed] [Google Scholar]
- 6.Kay NE, O’Brien SM, Pettitt AR, Stilgenbauer S. The role of prognostic factors in assessing ‘high-risk’ subgroups of patients with chronic lymphocytic leukemia. Leukemia. 2007;21:1885–91. doi: 10.1038/sj.leu.2404802. [DOI] [PubMed] [Google Scholar]
- 7.Center for Epidemiology. Cancer Incidence in Sweden 1998. Stockholm; Sweden: 2000. [Google Scholar]
- 8.Wassberg C, Thorn M, Johansson AM, Bergstrom R, Berne B, Ringborg U. Increasing incidence rates of squamous cell carcinoma of the skin in Sweden. Acta Derm Venereol. 2001;81:268–72. doi: 10.1080/00015550152572903. [DOI] [PubMed] [Google Scholar]
- 9.Moreno C, Montserrat E. New prognostic markers in chronic lymphocytic leukemia. Blood Rev. 2008;22:211–9. doi: 10.1016/j.blre.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Trakatelli M, Ulrich C, del Marmol V, Euvrard S, Stockfleth E, Abeni D. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol. 2007;156 (Suppl 3):1–7. doi: 10.1111/j.1365-2133.2007.07861.x. [DOI] [PubMed] [Google Scholar]
- 11.Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256–9. doi: 10.1111/j.0303-6987.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 12.Padgett JK, Parlette HL, 3rd, English JC., 3rd A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in Mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769–71. doi: 10.1046/j.1524-4725.2003.29194.x. [DOI] [PubMed] [Google Scholar]
- 13.Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an association between non-Hodgkin’s lymphoma and skin cancer. BMJ. 1995;310:1491–5. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wassberg C, Thörn M, Yuen J, Ringborg U, Hakulinen T. Second primary cancers in patients with squamous cell carcinoma of the skin: a population-based study in Sweden. Int J Cancer. 1999;80:511–5. doi: 10.1002/(sici)1097-0215(19990209)80:4<511::aid-ijc5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 15.Teppo L, Pukkala E, Saxén E. Multiple cancer - an epidemiologic exercise in Finland. J NatL Cancer Inst. 1985;75:207–17. [PubMed] [Google Scholar]
- 16.Frisch M, Melbye M. New primary cancers after squamous cell skin cancer. Am J Epidemiol. 1995;141:916–22. doi: 10.1093/oxfordjournals.aje.a117358. [DOI] [PubMed] [Google Scholar]
- 17.Levi F, Randimbison L, La Vecchia CL, Erler G, Te VC. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol. 1997;146:734–9. doi: 10.1093/oxfordjournals.aje.a009349. [DOI] [PubMed] [Google Scholar]
- 18.Hjalgrim H, Frisch M, Storm HH, Glimelius B, Pedersen JB, Melbye M. Non-melanoma skin cancer may be a marker of poor prognosis in patients with non-Hodgkin’s lymphoma. Int J Cancer. 2000;85:639–42. doi: 10.1002/(sici)1097-0215(20000301)85:5<639::aid-ijc7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Askling J, Sørensen P, Ekbom A, Frisch M, Melbye M, Glimelius B, et al. Is history of squamouscell skin cancer a marker of poor prognosis in patients with cancer? Ann Intern Med. 1999;131:655–9. doi: 10.7326/0003-4819-131-9-199911020-00004. [DOI] [PubMed] [Google Scholar]
- 20.Turesson I, Linet MS, Björkholm M, Kristinsson SY, Goldin LR, Caporaso NE, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003. Int J Cancer. 2007;121:2260–6. doi: 10.1002/ijc.22912. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables (with discussion) J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 22.Scrivener S, Goddard RV, Kaminski ER, Prentice AG. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk Lymphoma. 2003;44:383–9. doi: 10.1080/1042819021000029993. [DOI] [PubMed] [Google Scholar]
- 23.Thomson MA, Suggett NR, Nightingale PG, Milford DV, Baumann U, Kelly DA, et al. Skin surveillance of a U.K. paediatric transplant population. Br J Dermatol. 2007;156:45–50. doi: 10.1111/j.1365-2133.2006.07546.x. [DOI] [PubMed] [Google Scholar]
- 24.Stockfleth E, Nindl I, Sterry W, Ulrich C, Shmook T, Meyer T. Human Papillomaviruses in transplant-associated skin cancers. Dermatol Surg. 2004;30:604–9. doi: 10.1111/j.1524-4725.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 25.Marti GE, Rawstron AC, Ghia P, Hillmen P, Houlston RS, Kay N, et al. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130:325–32. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 26.Marti GE, Carter P, Abbasi F, Washington GC, Jain N, Zenger V, et al. B-cell monoclonal lymphocytosis and B-cell abnormalities in the setting of familial B-cell chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2003;52:1–12. doi: 10.1002/cyto.b.10013. [DOI] [PubMed] [Google Scholar]
- 27.Storm HH, Engholm G. Relative survival of Danish cancer patients diagnosed 1981 to 1997 and followed to 2001. A status report. Ugeskr Laeger. 2002;164:2855–64. [PubMed] [Google Scholar]
- 28.Karjalainen S, Salo H, Teppo L. Basal cell and squamous cell carcinoma of the skin in Finland. Site distribution and patient survival. Int J Dermatol. 1989;28:445–50. doi: 10.1111/j.1365-4362.1989.tb02503.x. [DOI] [PubMed] [Google Scholar]
- 29.Jensen AØ, Bautz A, Olesen AB, Karagas MR, Sørensen HT, Friis S. Mortality in Danish patients with nonmelanoma skin cancer, 1978–2001. Br J Dermatol. 2008;159:419–25. doi: 10.1111/j.1365-2133.2008.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]