Hodgkin’s lymphoma (HL) is a lymphoproliferative malignancy of B-cell origin, with an age-adjusted incidence of 2.3–3.1 per 100,000 in the Western world.1 HL is subdivided into classical HL and the rare entity nodular lymphocyte predominant HL (~5% of all HL cases). Based on characteristics of reactive infiltrates and morphology of Reed-Sternberg cells, classical HL is further divided into the following four subtypes: nodular sclerosis (NS), mixed cellularity (MC), lymphocyte-depleted (LD), and lymphocyte-rich (LR).1 Studies have shown that these subtypes have different age-incidence curves, gender ratio, and racial discrepancy.1
The etiology of HL is largely unknown; however, clues about causes have been suggested by the bimodal distribution of age at diagnosis observed in developed countries and by higher risks in males, in persons with higher socioeconomic status, and in smaller families.2 Young adult–onset HL is thought to arise as a consequence of delayed primary infection with Epstein–Barr virus.3 Inherited genetic factors play a significant role in the etiology.4,5
Personal history of autoimmune diseases is consistently associated with increased risk of non-Hodgkin’s lymphoma.6 Recent data also indicate that the risk of HL is increased following autoimmune diseases.7–9 We recently analyzed the association of a personal history of autoimmune conditions in 7,476 HL patients compared to 18,573 controls, and found several autoimmune conditions to be strongly associated with HL, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), sarcoidosis, and immune thrombocytopenic purpura (ITP).10 We found an overall 2.7-fold increased risk for all systemic autoimmune diseases combined. Furthermore, a family history of sarcoidosis and ulcerative colitis were also associated with HL risk, suggesting a role for shared susceptibility factors. To our knowledge, no prior study has evaluated risk for HL following autoimmune disease by HL subtype.
To improve our understanding in this area, we have extended our previous study to evaluate the association of a personal history of autoimmune disease (from the Inpatient Registry) and subsequent risk of HL subtypes. Using high-quality population-based data from Sweden, we identified 9,314 HL patients (median age 49.5 years, 42% females) and 37,069 matched controls. A total of 1,601 (17%) of the patients had information regarding HL subtype using ICD10 diagnoses. We analyzed the three most common subtypes identified (NS, n=1,072; MC, n=364, and LR, n=122), and only those conditions previously identified as being significant in a multivariate analysis. Using logistic regression models adjusted for age, sex, calendar period, and region, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) as measures of relative risks for each condition using logistic regression. To limit the influence of detection bias, we excluded autoimmune disease diagnosed less than one year prior to HL diagnosis.
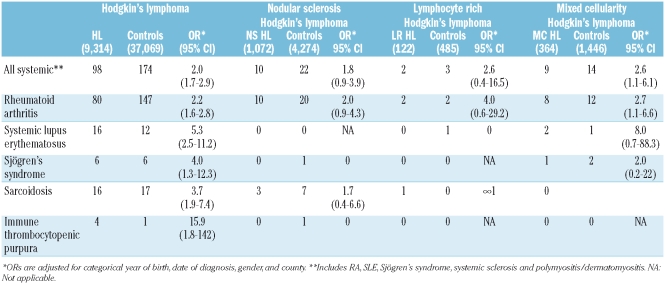
First, in analyses including all HL cases, we found an increased risk for HL among persons with a personal history of any systemic autoimmune disease (OR=2.0; 95% CI 1.7–2.9), and specifically for RA (2.2; 1.6–2.8), SLE (5.3; 2.5–11.2), Sjögren’s syndrome (4.0; 1.3–12.3), sarcoidosis (3.7; 1.9–7.4) and ITP (15.9; 1.8–142) (Table 1). Second, in analyses stratified by HL subtype, a personal history of any systemic autoimmune disease (2.6; 1.1–6.1) and RA (2.7; 1.1–6.6) were associated with and increased risk for MC HL. We did not find any significant associations between a personal history of an autoimmune disease and a subsequent risk of developing HL NS (Table 1).
Table 1.
Relative risk of Hodgkin’s lymphoma and subtypes in relation to autoimmune disease.
Our findings are important for several reasons. We show for the first time that the risk for HL following a personal history of autoimmune disease may vary by HL subtype. Autoimmunity was associated with a significantly elevated risk for MC HL, but there were no significant associations with NS HL and the risk estimates were generally lower. Interestingly, we found a long latency from the date of autoimmune diagnosis to the date of MC HL (mean time =15.4 years) suggesting that long-term chronic immune stimulation may play a role in the causation of MC HL. Taken together, our observations support the hypothesis that the etiology of HL might differ by HL subtype. Also, we confirmed that Sjögren’s syndrome is associated with a highly increased risk of HL.
We used high-quality data,11 however, the nature of this study is hypothesis-generating and one has to interpret our findings with caution due to the low numbers of cases by subtype, hampering further stratified analyses.
In conclusion, our findings support a role for chronic immune stimulation in the etiology of HL. Our novel finding of a different risk pattern by HL subtypes suggests the operation of separate pathogenetic mechanisms and needs to be supported by other study groups.
Footnotes
Funding: this research was supported by the Intramural Research Program of the NIH and the National Cancer Institute; and by grants from the Swedish Cancer Society, Stockholm County Council, and the Karolinska Institutet Foundations. The authors have no conflict of interest to declare.
References
- 1.Swerdlov SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. [Google Scholar]
- 2.MacMahon B. Epidemiology of Hodgkin’s disease. Cancer Res. 1966;26:1189–201. [PubMed] [Google Scholar]
- 3.Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med. 2003;349:1324–32. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 4.Goldin LR, Pfeiffer RM, Gridley G, Gail MH, Li X, Mellemkjaer L, et al. Familial aggregation of Hodgkin lymphoma and related tumors. Cancer. 2004;100:1902–8. doi: 10.1002/cncr.20189. [DOI] [PubMed] [Google Scholar]
- 5.Mack TM, Cozen W, Shibata DK, Weiss LM, Nathwani BN, Hernandez AM, et al. Concordance for Hodgkin’s disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N Engl J Med. 1995;332:413–8. doi: 10.1056/NEJM199502163320701. [DOI] [PubMed] [Google Scholar]
- 6.Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 7.Landgren O, Björkholm M, Montgomery SM, Hjalgrim H, Sjöberg J, Goldin LR, Askling J. Personal and family history of autoimmune diabetes mellitus and susceptibility to young-adult-onset Hodgkin lymphoma. Int J Cancer. 2006;118:449–52. doi: 10.1002/ijc.21347. [DOI] [PubMed] [Google Scholar]
- 8.Ekström K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A, Askling J. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 2003;48:963–70. doi: 10.1002/art.10939. [DOI] [PubMed] [Google Scholar]
- 9.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 10.Landgren O, Engels EA, Pfeiffer RM, Gridley G, Mellemkjaer L, Olsen JH, et al. Autoimmunity and susceptibility to Hodgkin lymphoma: a population-based case-control study in Scandinavia. J Natl Cancer Inst. 2006;98:1321–30. doi: 10.1093/jnci/djj361. [DOI] [PubMed] [Google Scholar]
- 11.Turesson I, Linet MS, Björkholm M, Kristinsson SY, Goldin LR, Caporaso NE, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003. Int J Cancer. 2007;121:2260–6. doi: 10.1002/ijc.22912. [DOI] [PubMed] [Google Scholar]