Abstract
Clitoromegaly in the neonatal period is an important morphologic sign that can be useful for sexual determination in aberrant cases. In rhesus monkeys, differentiation of the external genitalia occurs early during gestation (at 55 to 60 d) and is complete by approximately 80 d. Most of the critical steps in genital differentiation in primates occur prenatally. We sought to determine clitoral size in normal rhesus monkeys (Macaca mulatta) and possible effects of age and inheritance. Clitoral length was highly variable and had no relationship to fertility. Statistical evaluation revealed no association in the distribution of daughters with and without clitoris between mothers with and without clitoris. However, even when mated with several female monkeys, some male macaques produced primarily daughters without clitoris.
Normal pregnancy is associated with high circulating levels of total testosterone due to synthesis of testosterone–estradiol-binding globulin as well as free testosterone and androstenedione in plasma. Protective mechanisms against maternal and fetal virilization counterbalance this biologic hyperandrogenism. An important concern is to evaluate the risk of virilization of a female fetus: the earlier the hyperandrogenism occurs during pregnancy, the higher the risk of fetal virilization.33 During normal pregnancy, high circulating levels of androgens do not lead to fetal virilization. This phenomenon has been attributed to the high levels of estrogen, progesterone, and sex-hormone–binding globulin during pregnancy, which interfere with the biologic activity of androgens,6,10,18 and to placental aromatase, which rapidly converts androstenedione to estrone and 16-hydroxytestosterone to estriol.31 Unless compromised (for example, due to aromatase deficiency), the primate placenta is highly robust at inactivating and conjugating androgens, much more so that are nonprimate placentae.1,30 Therefore, virilization of female offspring in primates due to excess maternal androgen is rare.
Sexual differentiation is a sequential and regulated process. Sex chromosomes, established at the moment of fertilization, determines the gonadal sex, which in turn, leads to development of the phenotypic sex, when the masculine or feminine urogenital system is formed. Alterations in any phase of this developmental process during embryogenesis could result in uncontrolled sexual differentiation. Known causes of abnormality in sexual development are environmental (such as the ingestion of virilizing medication during gestation), abnormal sex chromosomes, development of neonatal defects of multifactorial etiology (such as in most cases of hypospadias), and isolated mutation of genes. In general, a specific diagnosis can be established after genetic, endocrine, clinical, and chromosomal evaluations.17
Clitoral hypertrophy has many causes, and androgen is closely associated with clitoral formation. Androgen-induced clitoral hypertrophy is divided into fetal and maternal sources. In regard to fetal sources, congenital adrenal hyperplasia (a well-known cause of clitoral hypertrophy) and deficiencies in cytochromes P450 C21 and C11 are predominantly virilizing disorders. Maternal sources include iatrogenic causes (testosterone and its related steroids, oral progestogens, and diethylstilbestrol), virilizing ovarian or adrenal tumor, luteoma of pregnancy, and congenital hyperplasia.16 An observation that the clitoris appears overly prominent may lead unnecessary diagnostic investigation. In contrast, misdiagnosis can result when clitoromegaly is interpreted as normal for age and species. The current study was undertaken to establish normal standards for the clitoral length of female rhesus monkeys and factors that might influence this parameter.
Materials and Methods
A case of putative clitoral enlargement of unknown origin in a juvenile female rhesus monkey (Macaca mulatta) at the Center for Laboratory Animal Breeding (Oswaldo Cruz Foundation / FIOCRUZ, Rio de Janeiro, Brazil) was presented for evaluation. After several diagnostic evaluations, we confirmed that this animal lacked chromosomal abnormalities (42XX) and phenotypic alterations, had normal levels of testosterone (0.14 ng/mL) and cortisol (56 µg/dL), and became pregnant. From this finding, we decided to measure the clitoris of all 285 female rhesus macaques (age, 11 mo to 21 y) in this particular breeding colony during their annual medical evaluations.2 For all monkeys, the same investigator measured clitoral length (from base to tip) by using a steel caliper graduated in millimeters. Animals were anesthetized with ketamine hydrochloride (10 mg/kg IM) and placed in lateral decubitus position.
The rhesus macaques in this colony were housed in single-male breeding groups of 20 to 25 animals in cages of 48 m2; received filtered, treated water ad libitum through automatic stainless-steel water bottles; and were fed a commercial primate chow (Nuvilab Primates 6030, Nuvitab, Colombo, Brazil) in the morning and supplemented with fruits and vegetables in the afternoon, which first were immersed in a 2% sodium hypochlorite solution for parasitologic and bacteriologic control. The enclosures were swept clean daily and washed with pressurized water 3 times each week; no chemical products are used. The breeding colony was maintained in compliance with Brazilian law and approved by the Ethics Commission on Animal Experimentation of the Oswaldo Cruz Foundation under protocol number P.0042/00.
Statistical analysis.
Data underwent to Shapiro–Wilk, Kolmogorov–Smirnov, and Anderson–Darling analyses to determine whether clitoral length was normally distributed; trials were performed in which the sample both included and excluded female rhesus that lacked clitoris (coded with the value 0). In both cases, these distributions diverged significantly from normality. Therefore, we compared clitoral length across age groups [infant (age, 0 to 12 mo; n = 4), juvenile (13 to 48 mo; n = 137), young adult (49 to 72 mo; n = 59), and adult (73 mo and older; n = 85)] by using the nonparametric Kruskal–Wallis test. In addition, we calculated the Spearman correlation coefficient between clitoral length (in cm) and age (in months). Finally, we compared the distribution of daughters with and without clitoris between mothers with and without clitoris by using the χ2 test. A significance level of 0.05 was used for all tests.
Results
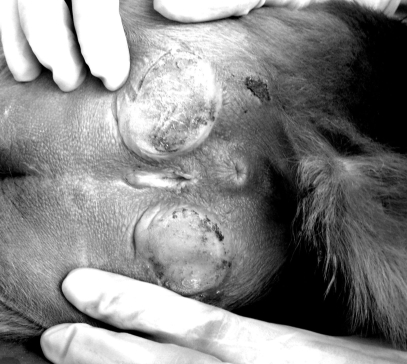
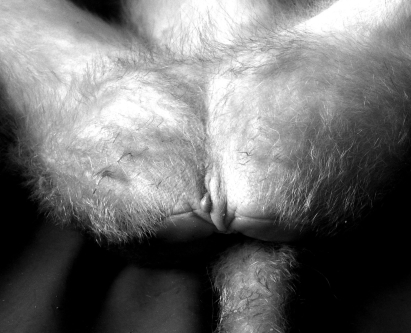
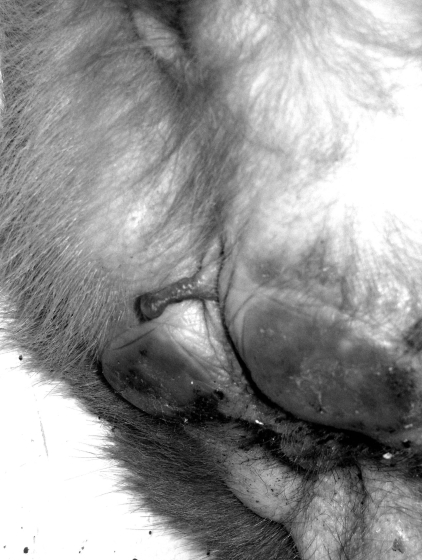
Clitoral length among our study population of 285 female rhesus monkeys varied widely, ranging from complete absence (Figure 1), through various lengths (Figure 2), to a maximum of 1.51 cm (Figure 3). Clitoral length was greatly heterogeneous when compared across age groups (H = 84.92; P = 0.000; Table 1). Spearman correlation between age and clitoral length was highly significant also (r = 0.631; P = 0.000).
Figure 1.
Female rhesus lacking a clitoris.
Figure 2.
Female rhesus with clitoris of intermediate length.
Figure 3.
Female rhesus with long clitoris.
Table 1.
Analysis of clitoral length (cm) in a colony of rhesus macaques
| Age group | n | Average | Standard deviation | Minimum | Maximum |
| Infant | 2 | 0.435 | 0.050 | 0.40 | 0.47 |
| Juvenile | 79 | 0.513 | 0.167 | 0.15 | 0.94 |
| Young adult | 47 | 0.752 | 0.248 | 0.37 | 1.51 |
| Adult | 76 | 0.863 | 0.201 | 0.42 | 1.37 |
| Total | 204 | 0.698 | 0.254 | 0.15 | 1.51 |
Results of Kruskal–Wallis test: H = 84.92; df = 3; P = 0.000
Based on observations of behavior and reproductive failure, clitoral size was not associated with either fertility or reproductive behavior in this colony. Among the 285 animals analyzed, 80 (28%) presented with either rudimentary clitoris (with mild salience) or totally lacked the structure. All monkeys that lacked clitoris when first evaluated, regardless of age, showed no difference in this feature 1 y later. Further, no association was detected when we compared the distribution of daughters with and without clitoris with mothers with and without clitoris (χ2 = 1.75; P = 0.1854; odds ratio, 1.84; Table 2). In addition, some males when mated with several females showed an increased incidence of daughters and granddaughters without clitoris while others showed an increased incidence of daughters and granddaughters with clitoris. From the 30 reproductive males in the colony, 6 generated the majority of daughters and granddaughters with clitoris (15% without clitoris of 52), while 5 presented predominance of descendents without clitoris (40% of 41). Between the examined females, 78 were not descendents of these 30 males.
Table 2.
Distribution of presence or absence of clitoris
| Mother |
|||
| Daughter | Present | Absent | Total |
| Clitoris present | 164 | 12 | 176 |
| Clitoris absent | 67 | 9 | 76 |
| Total | 231 | 21 | 252 |
Odds ratio = 1.84; χ2 = 1.754; P = 0.1854
Discussion
Genital anatomy is the main determinant of sex assignment in newborn humans.9 Human females exposed to excess prenatal androgens due to congenital adrenal hyperplasia36 or exogenous androgenic compounds37 are born with various degrees of genital virilization, including clitoral hypertrophy, labial fusion, displacement of the urethral opening, and development of a penis. About 25% of normal women have an enlarged clitoris.32
The circulating levels of some androgens increase during normal pregnancy.6,22,23,28 Testosterone concentration increases during the first trimester of pregnancy, with further increases in androstenodione levels during the second half of gestation.4,23,28 This mechanism is explained by the induction of the synthesis of testosterone–estradiol-binding globulin and increases in plasma levels of free testosterone and androstenedione.33 In contrast, dehydroepiandrosterone sulfate in the maternal circulation decreases with advancing gestation.8,11,28
During normal human fetal development, clitoral length reaches its maximum during the 27th week of gestation, with little change thereafter during gestation and infancy.24,27 Thereafter, clitoral size may increase gradually, as reflected by the clitoral index in normal females, from a mean of about 4 mm in childhood to 20 mm in the fourth decade of life to approximately 30 mm in the postmenopausal years.20 Significant changes or progressive growth in clitoral length in women of reproductive age and differences in clitoral size attributable to race or ethnic group have not been found.35 Normal ranges of genital measurements in women demonstrated that far greater diversity in clitoral length than previously documented.21 Isolated congenital absence of the clitoris with otherwise normal or near-normal external genitalia is rare in humans.5 Usually, hypoplasia of the clitoris occurs in the setting of multiple congenital anomalies associated with genetic aberrations.25 Agenesis of the clitoris is much rarer and has only been reported twice as an isolated finding.5,34
In rhesus monkeys, differentiation of the external genitalia occurs in the beginning of gestation period, at about day 55 to 60,26 and is complete by approximately by day 80.2 Alterations in the prenatal androgen environment of both male and female rhesus monkeys affect genital differentiation, endocrine function, and growth of rhesus monkey infants.18 Therefore, most of the critical steps in genital differentiation in primates occur prenatally. However, postnatal manipulation of testosterone circulation levels altered clitoral development in infant rhesus macaques, and rates of growth were greatest during the first 2 mo of life.7 Therefore, the early postnatal period represents a period during which clitoral growth normally occurs in rhesus monkeys and which is influenced by circulating testosterone levels. Exposure of female rhesus fetuses to testosterone through injection of pregnant females with testosterone propionate masculinized the development of the genitalia.13,15 The degree of masculinization varied with the timing and duration of administration of testosterone propionate.14 Beginning at approximately 3 mo of age, the clitoral growth rate of primates decreases, despite the continued presence of high testosterone circulation levels.7
Despite the lack of normative data regarding clitoral size in rhesus monkeys (Macaca mulatta), the presence of clitoromegaly or clitoral agenesis could be important physical findings that direct veterinarians to further diagnostic evaluation. Studies conducted to elucidate the importance of androgen-mediated induction of the extreme masculinization of the external genitalia in female spotted hyenas revealed placental production of testosterone and its transfer to female and male fetuses through the umbilical vein.38 Such placental androgens may well modulate the development of clitoral morphology. The immature primate reproductive tract and external genitalia express androgen receptors during the first trimester of gestation, but during the second trimester, the female reproductive tract and external genitalia (except for the clitoris) lose this expression.12,29 Therefore clitoral hypertrophy alone at any time after the first trimester could indicate androgen excess.
The large variation in clitoral length among our rhesus females may reflect normal variation in testosterone levels during gestation and in the ability to convert it into estradiol, given that no drugs or hormones had been administered in this breeding colony. The present study was conducted based on the pedigree of the colony, and we found no relationship between mother and daughter with respect to presence or absence of the clitoris. However the male breeder might transmit absence of the clitoris. We noted that the daughters of some male monkeys that had been mated with several females all lacked clitoris and that, when mated with other males, these aclitoral females birthed daughters with clitoris; however there is a 10% probability error with regard to paternity.3 To clarify the genetic basis of this trait, further studies will require use of the sons of aclitoral females as breeders and analyze their offspring.
The odds ratio obtained (1.84) indicates a significant relation between mothers with daughters without clitoris for mothers and daughters with clitoris. However, the sample size is small and should be increased. Although the OR calculated of the Table 2 was not significant, it shows that mother/daughter with same phenotype are almost twice as frequent as mother/daughter with different phenotypes.
Clitoromegaly during the neonatal period is an important morphologic sign that should direct clinicians to further diagnostic evaluation. Here we provide previously unavailable data regarding objective assessment of normal clitoral size in rhesus monkeys. This information may help in interpretations concerning aberrant sexual determination in this species.
Acknowledgment
This work was supported by Fundação de Amparo a Pesquisa no Estado do Rio de Janeiro.
References
- 1.Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. 2008. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod 79:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbot DH, Dumesic DA, Eisner JR, Kemnitz JW, Goy RW. 1997. The prenatally androgenized female rhesus monkey as a model for PCOS, p 369–382. In: Azziz R, Nestler JE, Dewailly D. Androgen-excess disorders in women. Philadelphia (PA): Lippincott Williams and Wilkins [Google Scholar]
- 3.Andrade MCR, Ribeiro CT, Silva VF, Molinaro EM, Gonçalves MAB, Cabello PH, Leite JPG. 2004b. Biologic data of Macaca mulatta, Macaca fascicularis, and Saimiri sciureus used for research at the Fiocruz Primate Center. Mem Inst Oswaldo Cruz 99:581–589 [PubMed] [Google Scholar]
- 4.Bammann BL, Coulam CB, Yang NS. 1980. Total and free testosterone during pregnancy. Am J Obstet Gynecol 137:293–298 [DOI] [PubMed] [Google Scholar]
- 5.Bellemare S, Dibden L. 2005. Absence of the clitoris in a 13-year-old adolescent: medical implications for child and adolescent health. J Pediatr Adolesc Gynecol 18:415–418 [DOI] [PubMed] [Google Scholar]
- 6.Berger NG, Repke JT, Woodruff JD. 1984. Markedly elevated serum testosterone in pregnancy without fetal virilization. Obstet Gynecol 63:260–262 [PubMed] [Google Scholar]
- 7.Brown GR, Nevison CM, Fraser HM, Dixson AF. 1999. Manipulation of postnatal testosterone levels affects phallic and clitoral development in infant rhesus monkeys. Int J Androl 22:119–128 [DOI] [PubMed] [Google Scholar]
- 8.Buster JE, Chany RJ, Preston DL, Elashoff RM, Cousins LM, Abraham GE, Hobel CJ, Marshall JR. 1979. Interrelationship of circulating maternal steroid concentrations in third trimester pregnancy. II. C18- and C19-steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosteronesulfate, androstenedione, testosterone, dehydrotestosterone. J Clin Endocrinol Metab 48:139–142 [DOI] [PubMed] [Google Scholar]
- 9.Diamond M, Sigmundson HK. 1997. Sex reassignment at birth: long-term review and clinical implications. Arch Pediatr Adolesc Med 151:298–304 [DOI] [PubMed] [Google Scholar]
- 10.Forest MG, Ances IG, Tapper AJ, Migeon CJ. 1971. Percentage binding of testosterone, androstenedione, and dehydroisoandrosterone in plasma at the time of delivery. J Clin Endocrinol Metab 32:417–425 [DOI] [PubMed] [Google Scholar]
- 11.Gant NF, Hutchinson HT, Siiteri PK, MacDonald PC. 1971. Study of the metabolic clearance rate of dehydroisoandrosterone sulfate in pregnancy. Am J Obstet Gynecol 111:555–563 [DOI] [PubMed] [Google Scholar]
- 12.Goto M, Hanley KP, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA. 2006. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest 116:953–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goy RW. 1968. Organizing effects of androgen on the behaviour of rhesus monkey, p 12–31. In: Michael RP. Endocrinology of human behaviour. New York (NY): Oxford University Press [Google Scholar]
- 14.Goy RW, Bercovitch FB, McBrair MC. 1988. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Horm Behav 22:552–571 [DOI] [PubMed] [Google Scholar]
- 15.Goy RW, Phoenix CH. 1972. The effects of testosterone propionate administered before birth on the development of behavior in genetic female rhesus monkeys. UCLA Forum Med Sci 15:193–201 [PubMed] [Google Scholar]
- 16.Grumbach MM. 1992. Disorders of sex differentiation, p 906. In: Williams RH. Textbook of endocrinology, 6th ed.Philadelphia (PA): WB Saunders [Google Scholar]
- 17.Grumbach MM, Hughes IA, Conte FA. 2002Disorders of sex differentiation. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. Williams textbook of endocrinology, 10th ed.Philadelphia (PA): WB Saunders [Google Scholar]
- 18.Hensleigh PA, Carter RP, Grotjan HE. 1975. Fetal protection against masculinization with hyperreactio luteinalis and virilization. J Clin Endocrinol Metab 40:816–823 [DOI] [PubMed] [Google Scholar]
- 19.Herman RA, Jones B, Mann DR, Wallen K. 2000. Timing of prenatal andrgen exposure: anatomical and endocrine effects on juvenile male and female rhesus monkeys. Horm Behav 38:52–66 [DOI] [PubMed] [Google Scholar]
- 20.Huffman JW, Dewhurst CJ, Capraro VJ. 1981. The gynecology of childhood and adolescence, 2nd ed, p 27–28. Philadelphia (PA): WB Saunders [Google Scholar]
- 21.Lloyd J, Crouch NS, Minto CL, Liao LM, Creighton SM. 2005. Female genital appearance: ‘normality’ unfolds. BJOG 112:643–646 [DOI] [PubMed] [Google Scholar]
- 22.McClamrock HD, Adashi EY. 1992. Gestational hyperandrogenism. Fertil Steril 57:257–274 [DOI] [PubMed] [Google Scholar]
- 23.Mizuno M, Lobotsky J, Lloyd CW, Kobayashi T, Murasawa Y. 1968. Plasma androstenedione and testosterone during pregnancy and in the newborn. J Clin Endocrinol Metab 28:1133–1142 [DOI] [PubMed] [Google Scholar]
- 24.Oberfield SE, Mondok A, Shahrivar F, Klein JF, Levine LS. 1989. Clitoral size in full-term infants. Am J Perinatol 6:453–454 [DOI] [PubMed] [Google Scholar]
- 25.Omar HA, Hummel MD, Jones EA, Perkins KC. 1999. Hypoplastic external genitalia in association with X;autosome chromosome translocation. J Pediatr Adolesc Gynecol 12:161–164 [DOI] [PubMed] [Google Scholar]
- 26.Prahalada S, Tarantal AF, Harris GS, Ellsworth KP, Clarke AP, Skiles GL, MacKenzie KI, Kruk LF, Ablin DS, Cukierski MA, Peter CP, VanZwieten MJ, Hendrichx AG. 1997. Effects of finasteride, a type 2 5-XXX reductase inhibitor, on fetal development in the rhesus monkey (Macaca mulatta). Teratology 55:119–131 [DOI] [PubMed] [Google Scholar]
- 27.Riley WJ, Rosenbloom AL. 1980. Clitoral size in infancy. J Pediatr 96:918–919 [DOI] [PubMed] [Google Scholar]
- 28.Rivarola MA, Forest MG, Migeon CJ. 1968. Testosterone, androstenedione and dehydroepiandrosterone in plasma during pregnancy and at delivery: concentration and protein binding. J Clin Endocrinol Metab 28:34–40 [DOI] [PubMed] [Google Scholar]
- 29.Shapiro E, Huang HY, Wu XR. 2000. Uroplakin and androgen receptor expression in the human fetal genital tract: insights into the development of the vagina. J Urol 164:1048–1051 [DOI] [PubMed] [Google Scholar]
- 30.Sir-Petermann T, Maliqueo M, Ange B, Lara HE, Pe'rez-Bravo F, Recabarren SE. 2002. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 17:2573–2579 [DOI] [PubMed] [Google Scholar]
- 31.Smith SW, Axelrod LR. 1969. Studies on the metabolism of steroid hormones and their precursors by the human placenta at various stages of gestation. II. In vitro metabolism of 3 β-hydroxyandrost-5-en-17-one. J Clin Endocrinol Metab 29:1182–1190 [DOI] [PubMed] [Google Scholar]
- 32.Tagatz GE, Kopher RA, Nagel TC, Okagaki T. 1979. The clitoral index: a bioassay of androgenic stimulation. Obstet Gynecol 54:562–564 [PubMed] [Google Scholar]
- 33.Thorin-Savouré A, Kuhn JM. 2002. Hyperandrogenism and pregnancy. Ann Endocrinol (Paris) 63:443–451[Article in French] [PubMed] [Google Scholar]
- 34.Vanelli M, Bernasconi S, Balestrazzi P, Gionannelli G. 1978. Congenital and isolated absence of the clitoris. Arch Fr Pediatr 35:165–167[Article in French] [PubMed] [Google Scholar]
- 35.Verkauf BS, Von Thron J, O'Brien WF. 1992. Clitoral size in normal women. Obstet Gynecol 80:41–44 [PubMed] [Google Scholar]
- 36.White PC, New MI, Dupont B. 1987. Congenital adrenal hyperplasia (first of two parts). N Engl J Med 316:1519–1524 [DOI] [PubMed] [Google Scholar]
- 37.Wilkins L. 1960. Masculinization of female fetus due to use of orally given progestins. J Am Med Assoc 172:1028–1032 [DOI] [PubMed] [Google Scholar]
- 38.Yalcinkaya TM, Siiteri PK, Vigne JL, Licht P, Pavgi S, Frank LG, Glickman SE. 1993. A mechanism for virilization of female spotted hyenas in utero. Science 260:1929–1931 [DOI] [PubMed] [Google Scholar]