Abstract
This study was conducted to investigate the possible effect of rack type on the blastocyst yield of mouse embryo donors. The first phase of the study consisted of housing some mice (group A) in a ventilated rack and others (group B) in a static rack in the same room for 3 d, followed by euthanasia for blastocyst collection and corticosterone assay. Parametric tests were used to compare groups. The number of blastocysts per donor was lower in group A (5.0 ± 1.4 blastocysts) than group B (13.1 ± 3.7 blastocysts). Mean noise was higher in the ventilated rack (80.4 dBC) than in the static rack (69.2 dBC). Serum corticosterone concentrations did not differ between groups. For the second phase of the study, a third group of mice (group C) was housed in a static rack without a ventilated rack in the same room. The noise level for group C was even lower (45.18 ± 2.91 dBC), and the blastocyst count per donor (16.4 ± 2.4) was higher than that of group B. The mean noise levels of empty ventilated and static racks differed significantly between groups for 10 different sound frequencies. Plotting mean blastocyst production against mean rack noise revealed a negative linear relationship with good strength of correlation. These results support the earlier observation that decreased blastocyst count occurs following housing of bred C57BL/6 donor mice in ventilated cages.
Success in the creation of transgenic mice depends to a large extent on the provision of an ideal breeding environment, which includes adequate ventilation, appropriate lighting, and protection from noise. Ventilated racks may offer the advantages of keeping the air quality of the microenvironment at optimal levels and solving space issues. However, questions began to arise regarding the continued use of ventilated racks at our facility because of the observed reduction in the number of blastocysts collected from the female embryo donors. In this facility, blastocysts from C56BL/6 mice are microinjected with embryonic stem cells, usually from agouti 129/SJ mice that are provided by investigators, to create mice with gene-targeted mutations. With the increasing number of requests from numerous research groups to create transgenic mice to model human diseases, the number of blastocysts harvested from the embryo donors becomes critical. We therefore conducted the present study to compare the effects of ventilated and static racks on blastocyst production.
Materials and Methods
All experiments in this study were conducted according to regulatory guidelines under the UCLA Transgenic Facility animal use protocol with approval from the Chancellor's Animal Research Committee, which is equivalent to an institutional animal care and use committee.
Test animals.
For the first phase of the study, a total of 96 female C57BL/6J mice were used as blastocyst donors, with 48 in group A, which was housed in a ventilated rack, and 48 for group B, which was housed in a static rack in the same room. The second phase consisted of 60 female C57BL/6J mice comprising the third group (group C), which was housed in a static rack in the same room but without a ventilated rack in the room.
All blastocyst donors were obtained from the Department of Radiation Oncology (University of California–Los Angeles; principal investigator, Dr Collin McLean), which used both ventilated and static racks. The colony's C57BL/6 foundation stock originally came from The Jackson Laboratory (Bar Harbor, ME). The strain was maintained in the department's mouse breeding colony through a strict brother–sister mating scheme. To test for genetic purity, representative samples from the F24 generation were sent to Charles River Laboratories (Wilmington, MA) prior to this study. The female mice were selected randomly and transferred from the Mouse Breeding Colony to the Transgenic Core Facility Vivarium after weaning at 21 d. They were group-housed (4 or 5 female mice per cage) in their designated racks (ventilated rack for group A and static rack for groups B and C) for at least 3 d before receiving injections of synthetic hormones to synchronize estrus and cause superovulation. Male mice (C57BL/6NTac; Taconic Farms, Hudson, NY) were shipped by land from the local facility (Oxnard, CA), acclimated for 3 to 4 d in the new environment, trained for breeding with experienced female mice for 2 wk, and individually housed for a few days before superovulated female mice were placed in their cages. Both male and female mice were assigned randomly into groups. The same technician performed all procedures for all groups and followed the same time schedule—from hormone injection for superovulation to blastocyst collection—for the different experiments.
Animal husbandry.
All test animals were housed in the UCLA Transgenic Core Vivarium, an SPF barrier facility with controlled conditions of light (12:12-h light:dark cycle) and temperature (20.0 to 22.2 °C) and free access to food and water.7,8 All cages, bedding, food, and water were sterilized by autoclaving. The mice tested free serologically of the following disease agents: Sendai virus, pneumonia virus of mice, mouse hepatitis virus, mouse parvovirus, Theiler murine encephalitis virus, reovirus, rotavirus, lymphocytic choriomeningitis, Ectromelia virus, parvovirus NS1, polyomavirus, adenovirus types 1 and 2, cytomegalovirus, mouse thymic virus, K virus, cilia-associated respiratory bacillus, Mycoplasma pulmonis, and Encephalitozoon cuniculi. In addition, the colony was Helicobacter-negative by fecal PCR and free of internal and external parasites.
Superovulation and breeding technique.
This procedure was performed according to standard methods.14 Female mice were injected with 5 IU pregnant mare serum gonadotropin (NIH National Hormone and Peptide Program, UCLA, Los Angeles, CA) per 12 to 15 g of body weight, followed 47 h later with 5 IU human chorionic gonadotropin (Calbiochem, San Diego, CA). Immediately after receiving human chorionic gonadotropin, the female mice were placed into cages containing male mice for breeding, checked for vaginal plugs the following day, and transferred to new cages of the assigned racks, where they were group-housed (4 or 5 mice per cage) for 72 h until euthanasia and blastocyst collection.
Experimental design.
Housing and grouping for blastocyst collection.
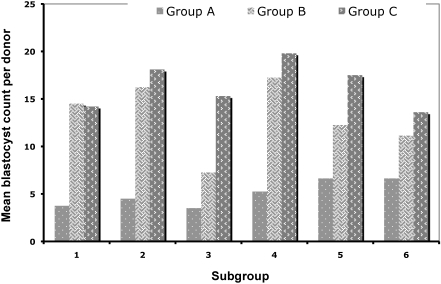
Phase 1. The first phase of the study consisted of 2 groups: group A (n = 48) was housed in a ventilated rack (Model Magnehelic, Thoren, Hazleton, PA), and Group B (n = 48) was kept in a static rack (750 SuperMouse cage, LabProducts, Seaford, DE) in the same room. Depending on the age at superovulation and blastocyst collection, each group was divided further into 6 subgroups of 8 mice. Details on the number and age of donors per subgroup are presented in Table 1. Three days after breeding, the female mice were euthanized by isoflurane overdose, the uterus was exteriorated, and the embryos flushed out with M2 media. The total number of blastocysts pooled from each subgroup was recorded and used to calculate the mean blastocyst count per subgroup (µ), mean blastocyst count per donor (x), and their standard deviations (σ and s, respectively; Table 1, Figure 1).
Table 1.
Animal groups and characteristics
| Blastocyst count |
||||
| Subgroup | Age (d) of mice at injection of PMSG | Age (d) at blastocyst collection | Total for subgroup | Mean per donor |
| Group A | ||||
| 1 | 24 | 30 | 30 | 3.8 |
| 2 | 25 | 31 | 36 | 4.5 |
| 3 | 26 | 32 | 28 | 3.5 |
| 4 | 24 | 30 | 42 | 5.3 |
| 5 | 25 | 31 | 53 | 6.6 |
| 6 | 26 | 32 | 53 | 6.6 |
| Overall mean ± 1 SD | 40.3 ± 11.0 | 5.0 ± 1.4 | ||
| Group B | ||||
| 1 | 24 | 30 | 116 | 14.5 |
| 2 | 25 | 31 | 130 | 16.3 |
| 3 | 26 | 32 | 58 | 7.3 |
| 4 | 24 | 30 | 138 | 17.3 |
| 5 | 25 | 31 | 98 | 12.3 |
| 6 | 26 | 32 | 89 | 11.1 |
| Overall mean ± 1 SD | 105.0 ± 29.5 | 13.1 ± 3.7 | ||
| Group C | ||||
| 1 | 25 | 31 | 142 | 14.2 |
| 2 | 26 | 32 | 181 | 18.1 |
| 3 | 27 | 33 | 153 | 15.3 |
| 4 | 25 | 31 | 198 | 19.8 |
| 5 | 26 | 32 | 175 | 17.5 |
| 6 | 27 | 33 | 136 | 13.6 |
| Overall mean ± 1 SD | 164.0 ± 24.0 | 16.4 ± 2.4 | ||
PMSG, pregnant mare serum gonadotropin
For groups A and B, each subgroup contained 8 donor mice; subgroups for group C contained 10 donor mice each.
Figure 1.
Group comparison of the mean blastocyst counts collected from each donor kept in ventilated (group A) and static (groups B and C) racks. The racks holding groups A and B were held concurrently in the same room; group C was the only rack in the room. The housing period was from acclimation to 96 h after breeding. Each group of 48 mice was allocated into 6 subgroups of 8 mice each; data from each subgroup were pooled and used to generate group means. Overall group mean blastocyst count per donor: group A, 5.0 ± 1.4 blastocysts; group B, 13.1 ± 3.7 blastocysts; group C, 16.4 ± 2.4 blastocysts,Paired Student t tests (P < 0.05; df = 5) revealed significant differences between groups A and B (P = 0.002) and groups B and C (P = 0.002).
Phase 2. The ventilated rack was removed from the room, and a similar experiment was conducted by using only the static rack to house group C, which consisted of 6 subgroups of 10 female mice. The same data set for this group was collected and presented in Table 1 and Figure 1.
Rack Noise Level Measurement.
Experiment A. The sound pressure levels (dBC) in the 2 types of racks were measured while housing mice from the 3 treatment groups during the 3 d prior to blastocyst collection. Four sites were tested on each rack (upper left, upper right, lower left, and lower right corners) by using a sound-level meter (model 2900, Quest Technologies, Oconomowoc, WI) set at the C-frequency weighting mode. No other husbandry activity was taking place during the time of testing. All results were reported by using dBC as the unit of measurement to denote the sound-pressure level or amplitude as well as the frequency-weighting mode applied for taking the measurements. The instrument was calibrated prior to testing, and all measurements were performed with the help of personnel from the UCLA Environmental Health and Safety Department.
Experiment B. The mean noise levels of the empty ventilated and static racks were measured at 10 different frequencies from 31.5 to 16,000 Hz of the C-frequency weighting mode of a sound-level meter. This measurement was conducted immediately after the completion of phase 2 of the study.
Corticosterone assay.
Blood samples collected through cardiocentesis at the time of euthanasia from groups A (n = 12) and B (n = 12) were assayed individually in triplicate for corticosterone by using a commercially available ELISA test kit (ACE, Cayman Chemical Company, MI), a competitive enzyme immunoassay based on the competition between corticosterone and corticosterone–acetylcholinesterase conjugate or tracer for a limited number of corticosterone-specific rabbit antiserum binding sites. The product of the enzymatic reaction has a distinct yellow color, which is measured spectrophotometrically and expressed as absorbance units (AU). The intensity of this color is proportional to the amount of acetylcholinesterase bound to the well and inversely proportional to the amount of free corticosterone present in the well.2 The sample reactions were evaluated relative to the standard curve from 8 control sera with known corticosterone concentrations, and expressed as the percentage of the absorbance of the particular sample or standard well to that of the maximal amount of tracer that the antiserum bound in the absence of free corticosterone. The assay was performed and the serum corticosterone content in picograms per milliliter calculated according to the manufacturer's recommendations.2
Data analysis.
Blastocyst counts.
The paired Student t test (significance level, P < 0.05; df = 5; Excel 5.0, Microsoft, Redmond, WA, and SPSS for Windows Version 12.0.1, Chicago, IL) was used to compare the subgroup and individual blastocyst counts of groups A and B. Because of the different number of animals used per subgroup in group C, only the mean blastocyst count per donor was used to compare group C with groups A and B. This parameter was derived by dividing the subgroup's total number of pooled blastocysts by the number of animals in the subgroup (Table1).
Rack noise levels.
The results of experiment A were analyzed by using 1-factor ANOVA. For Experiment B, the data were compared by using 2-factor ANOVA. Excel 5.0, Microsoft, was used to run these analyses.
Test for correlation.
Correlation between blastocyst production and rack noise level was assessed by plotting each group's mean for rack noise levels as the independent variable on the x axis against the corresponding group's mean for blastocyst count produced per donor as the dependent variable on the y axis. Computation was done for 2 coefficients, namely: a) the Pearson product moment coefficient of correlation (r) to measure linear correlation and indicate the direction of correlation and b) the correlation of determination (R2) to show the strength of correlation.9,11
Corticosterone assay.
The mean percentages of absorbance for groups A and B were compared by using paired Student t tests (Excel 5.0, Microsoft).
Results
Blastocyst counts.
Phase 1.
Group B showed significantly (P < 0.05) higher mean blastocyst counts (subgroup, 105.0 ± 29.5 blastocysts; individual donor, 13.1 ± 3.7 blastocysts) than did group A (subgroup, 40.3 blastocysts ± 10.97; individual donor, 5.0 ± 1.37; Table 1, Figure 1).
Phase 2.
Removal of the ventilated rack from the room and using only the static rack to house the blastocyst donors further increased blastocyst production. Group C showed a significantly (P < 0.05) higher mean blastocyst count per donor (16.4 ± 2.4 blastocysts) than did group B (13.1 ± 3.7 blastocysts per donor; Table 1, Figure 1).
Rack noise levels.
Experiment A.
The ventilated rack had a significantly (P < 0.05) higher mean noise level (80.40 ± 1.25 dBC) than did the static rack (69.22 ± 0.88 dBC). In the absence of the ventilated rack, the mean noise level for group C's static rack (45.18 ± 2.91 dBC) was significantly (P < 0.05) lower than the static rack used for Group B (Figure 2 A).
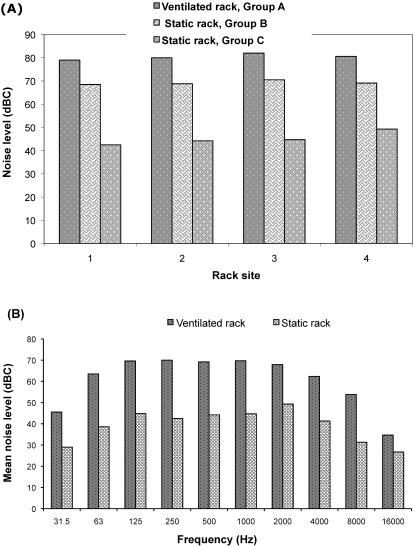
Figure 2.
(A) Noise levels (in dBC) measured by a sound-level meter set on C-frequency weighting scale from 4 sites (1, upper left corner; 2, upper right corner; 3, lower left corner; 4, lower right corner) on the racks used to house groups A, B, and C from the acclimation period to 72 h after breeding. Overall group mean noise level: group A, 80.4 ± 1.25 dBC; group B, 69.22 ± 0.88 dBC; group C, 45.18 ± 2.91 dBC. One-way ANOVA revealed significant differences between groups A and B (P = 0.00007) and groups B and C (P = 0.00004). (B) Mean noise levels of empty ventilated and static racks at various frequencies. Noise levels of the ventilated rack (mean, 60.62 ± 12.18 dBC) were significantly (2-way ANOVA, P < 0.05) higher than those of the static rack (mean, 39.24 ± 7.65 dBC).
Experiment B.
At specific sound frequencies, the emptied ventilated rack had a significantly (P < 0.05) higher noise level (60.62 ± 12.18 dBC) than did the emptied static rack (x = 39.24 ± 7.65 dBC; Figure 2 B).
Test for correlation.
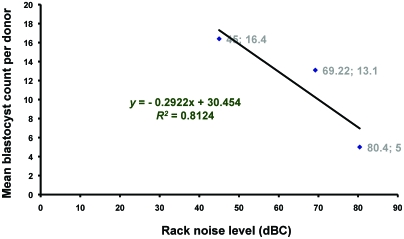
Simple linear regression analysis revealed a negative linear relationship between the mean blastocyst production per donor and mean rack noise level (r = –0.9013) and a good correlation of determination (R2 = 0.8123, P = 0.285; Figure 3).
Figure 3.
Simple linear regression analysis showing negative linear relationship between rack noise level and blastocyst production (r = –0.9013; P = 0.285).
Corticosterone assay.
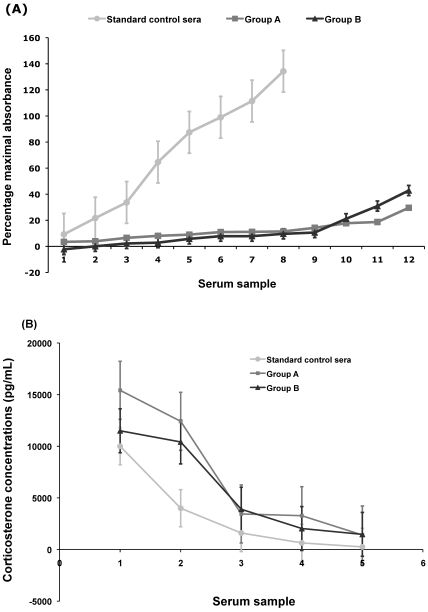
The mean absorbance values of the assayed sera from groups A (percentage of maximal absorbance, 12.08%) and B (11.62%) were greater than or equal to those of the 3 vendor-supplied control sera with the highest corticosterone concentrations. Values for groups A and B did not differ significantly (P = 0.819, Figure 4 A). The corticosterone concentrations (pg/mL) of most of the samples exceeded the standard curve's linearity; the 5 lowest values from each group are shown in Figure 4 A.
Figure 4.
Corticosterone assays. (A) Percentage of maximal absorbance. Note that most of the absorbance values of groups A (ventilated rack; squares) and B (static rack; triangles) are outside the linearity of the standard curve (circles); only 3 or 4 samples lie within the standard curve's first 3 control sera with the highest corticosterone concentrations. Mean maximal serum corticosterone for groups A (12.07%) and B (11.62%) did not differ significantly (n = 12; df = 11; paired Student T-test). (B) Corticosterone concentrations (pg/mL) of 5 samples each from groups A (mean, 7192.63 pg/mL) and B (mean, 5864.11 pg/mL) whose values were within the linearity of the standard curve. The corticosterone levels of the remaining 7 samples from each group were beyond the upper limit of the standard curve.
Discussion
The negative effects of noise on the behavior, physiology, and various phases of growth and development in both humans and animals have been described in the literature.3,4,12,13,18,21 The present study was conducted to investigate an earlier observation at our institution, in which bred superovulated embryo donors tended to produce fewer blastocysts when kept in ventilated racks. In our current results, blastocyst production decreased as the holding rack's noise level increased, as indicated by the negative linear correlation coefficient (r = –0.9013) and good coefficient of determination (R2 = 0.8123), with rack noise level as the independent variable and blastocyst count as the dependent variable. The correlation coefficient, r, shows the direction of the correlation (whether positive or negative), whereas the coefficient of determination, R2, demonstrates the strength of the correlation.9,11 Given the design and conditions of this study and based on the definition of R2,9,11 81.23% of the reduction in blastocyst production (y) was presumed to be associated with the increased rack noise level (x). However, the correlation was not significant (P = 0.285), probably because only 3 data points were available.
Four corners of the racks used to house groups A, B, and C were tested for noise levels to take into account the collective sound coming from various sources within and around the racks, including the rack ventilation system, room air conditioner, air handler, and laminar flow hood. The measurements were done using a sound level meter set at C-frequency weighting mode, which can detect audible sounds for fit, healthy humans from 20 Hz to 20 kHz.6 This setting was used in the current study for the following reasons. First, the sounds produced by ventilating systems were reported to be in the low-frequency range.4 In addition, chronic exposure to low-frequency noise (less than 500 Hz) and vibration can cause genotoxic effects in exposed mice and humans.20 Furthermore, this setting covers most of the sound frequencies applied in acoustic startle reflex tests, which are used in mouse phenotyping protocols (4, 12, and 20 kHz).24,26 Depending on the amplitude, the hearing-frequency range of a mouse can vary from a minimum of 1 to 16.4 kHz to a maximum of 94 kHz,4,6,25 all of which are detectable by the frequency-weighting mode we used. Finally, although rats and mice are known to be sensitive to ultrasonic frequencies (greater than 20 kHz),6 we did not measure the sound pressure level under this frequency spectrum because the animals themselves emit ultrasonic vocalizations,16 which can serve as confounders that increase the noise levels in both ventilated and static racks; the disadvantage of this aspect of the experimental design is the exclusion of ultrasonic sounds from other sources that may have been present in the room at the time of testing.
Regardless of the frequency range, the numerical magnitude of a sound is expressed as the sound pressure level in logarithmic decibel units, with 0 as the reference minimal threshold and 120 dB as the approximate threshold of pain.6,17 In rodents, the recommended sound pressure level maximum is 85 dB, above which they are predisposed to auditory and nonauditory effects, including eosinopenia, increased adrenal weight, and decreased fertility.8 For the current study's experiment A, group A's ventilated rack had a mean noise level of 80.40 dBC, which was below this recommended limit but still significantly louder by 10.78 dBC than was the noise detected from group B's static rack (69.22 dBC; Figure 2). An increase of 10 dB within the hearing range is perceived as a doubling in loudness,10 and this change may have contributed to Group A's significantly decreased blastocyst counts relative to those of group B (Table 1, Figure 1). The noise level detected in the static rack appeared to have originated mainly from the ventilated rack, because with removal of the ventilated rack from the room during phase 2 of the study, the sound pressure level in the static rack for group C (45.18 dB) declined by at least another 20 dB whereas the mean blastocyst count increased by 3.3 blastocysts compared with those of group B (Figures 1 and 2).
The significantly higher noise level in the ventilated rack used in this study can be attributed to the blower–filter unit, which was mounted on top of the rack. The ventilated rack's observed mean noise level (80.4 dB) was comparable to another study's macroenvironmental noise of 74 to 80 dB produced by 3 commercially available individually ventilated caging systems15 and close to the values applied in 1 study in which preimplantation mortality occurred when mice were exposed intermittently to a noise range of 83 to 95 dB during gestation.27 The noise produced by these racks may have served as 1 of the stress factors leading to reproductive problems such as suppression of spermatogenesis, spontaneous abortion, reduced fertility, and failure of embryo implantation.1,8,27 The complex mechanism through which stress interferes with reproductive processes is described adequately in the literature.1,12,19 Briefly, stress-induced reproductive failures start at the highest level of the hypothalamus–pituitary–gonadal and hypothalamus–pituitary–adrenocortical pathways: decreased secretion of hypothalamic gonadotropin-releasing hormone eventually leads to decreased plasma levels of follicle-stimulating and luteinizing hormones, whereas activation of the hypothalamus–pituitary–adrenocortical axis stimulates secretion of corticotrophin-releasing hormone, resulting in increased synthesis and release of corticosteroids. Luteinizing hormone is important in the maintenance of pregnancy, and during stress, reduction of plasma levels of this hormone also occur from the inhibitory effects of cytokines, specifically interleukin 1β.1 Moreover, failure of embryo implantation may be related to reduced uterine blood flow due to a stress-induced increase in catecholamine (epinephrine and norepinephrine) levels5,12,19.
Depending on the species, stress-induced corticosteroids may be in the form of cortisol or corticosterone. Corticosterone, the corticosteroid usually found in mice, was assayed in the present study by using serum as test specimen. During phase 1, blood was collected from the ventilated and nonventilated groups as a terminal procedure shortly before blastocyst collection. High levels of corticosterone were detected, most of which exceeded those of the standard curve's 3 control sera containing the highest corticosterone concentrations (Figure 4 A, B). Groups A and B did not differ significantly in this regard, and the high levels seen can probably be attributed primarily to the stress of transporting the animals from the vivarium to the laboratory and handling prior to euthanasia. To document stress-induced reproductive failures in future studies, fecal corticosterone can be assayed in place of serum corticosterone during the 3-d housing period before blastocyst collection, because fecal corticosterone assays are neither invasive nor influenced by handling and restraint.22 Although our findings seem to support earlier observations at our institution in which lower blastocyst counts were associated with the use of ventilated racks, more thorough assessment of the stress levels in groups placed in ventilated and static racks is warranted. In addition to the application of a less-invasive sample collection method for corticosterone assay, we also recommend testing catecholamine and luteinizing hormone levels at multiple time points from breeding to blastocyst collection.
The current study has limitations. Because only 1 ventilated rack was used and considering variations in product features and specifications, the observations and conclusion drawn from the current study should not be generalized to the possible effects of all commercially available ventilated racks on mouse blastocyst production. Moreover, susceptibility to the negative effects of noise varies according to mouse strain,23 and the conclusion drawn from this study can be applied only to the strain we used, C57BL/6J.
Acknowledgments
The authors wish to thank Jimmy Shaw and Kevin Chu (Environmental Health and Safety, University of California—Los Angeles) for sharing their technical expertise in measuring the rack noise levels.
References
- 1.Balm PHM. 1999. Stress physiology in animals, p 133. Sheffield (UK): Sheffield Academic Press [Google Scholar]
- 2.Cayman Chemical Company 2003. Instruction manual—corticosterone EIA kit. Ann Arbor (MI): Cayman Chemical Company [Google Scholar]
- 3.Clough G. 1982. Environmental effects on animals used in biomedical research. Biol Rev Camb Philos Soc 57:487–523 [DOI] [PubMed] [Google Scholar]
- 4.Faith R, Miller SJ. 2007. The need for sound and vibration standards in US research animal rooms. [Cited 28 Mar 2009]. Available at http://www.alnmag.com/articles.asp?pid=265
- 5.Geber WF. 1966. Developmental effects of chronic maternal audiovisual stress on the rat fetus. J Embryol Exp Morphol 16:1–16 [PubMed] [Google Scholar]
- 6.Heffner HE, Heffner RS. 2007. Hearing ranges of laboratory animals. J Am Assoc Lab Anim Sci 46:20–22 [PubMed] [Google Scholar]
- 7.Hessler JR, Leary SL. 2002. Design and management of laboratory animal facilities, p 931–948. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed.New York (NY): Academic Press [Google Scholar]
- 8.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals, p 36. Washington (DC): National Academies Press [Google Scholar]
- 9.Lowry R. Concepts and applications of inferential statistics. VassarStats: website for statistical computation. [cited 28 March 2009]. Available at http://faculty.vassar.edu/lowry/webtext.html
- 10.Med-Associates 2008. Acoustic startle reflex test components. [cited 2008 December 15]. Available at http://www.med-associates.com/startle/startle.htm
- 11.Mendenhall W. 1987. Linear regression and correlation, p 481–548. In: Introduction to probability and statistics, 7th ed.Boston (MA): Duxbury Press [Google Scholar]
- 12.Meyer RE, Aldrich TE, Easterly CE. 1989. Effects of noised and electromagnetic fields on reproductive outcomes. Environ Health Perspect 81:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milligan SR, Sales GD, Khirnykh K. 1993. Sound levels in rooms housing laboratory animals: an uncontrolled daily variable. Physiol Behav 53:1067–1076 [DOI] [PubMed] [Google Scholar]
- 14.Monastersky GM, Geistfeld JG. 2002. Transgenic and knockout mice, p 1130–1135. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed.New York (NY): Academic Press [Google Scholar]
- 15.Perkins SE, Lipman NS. 1996. Evaluation of microenvironmental conditions and noise generation in three individually ventilated rodent caging systems and static isolator cages. Contemp Top Lab Anim Sci 35:61–65 [PubMed] [Google Scholar]
- 16.Portfors CV. 2007. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci 46:28–34 [PubMed] [Google Scholar]
- 17.Product Technology Partners 2008. Noise measurement briefing. [cited 15 December 2008]. Available at http://www.ptpart.co.uk/show.php?contentid=70
- 18.Sales GD, Wilson KJ, Spencer KEV, Milligan SR. 1988. Environmental ultrasound in laboratories and animal houses: a possible cause for concern in the welfare and use of laboratory animals. Lab Anim 22:369–375 [DOI] [PubMed] [Google Scholar]
- 19.Shnider SM, Wright RG, Levinson G, Roizen MF, Wallis KL, Rolbin SH, Craft JB. 1979. Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology 50:524–527 [DOI] [PubMed] [Google Scholar]
- 20.Silva MJ, Dias A, Barreta A, Nogueira PJ, Castelo-Branco NAA, Boavida MG. 2002. Low frequency noise and whole-body vibration cause increased levels of sister chromatid exchange in splenocytes of exposed mice. Teratog Carcinog Mutagen 22:195–203 [DOI] [PubMed] [Google Scholar]
- 21.Sobrian SK, Vaughn VT, Ashe WK, Markovic B, Djuric V, Jankovic BD. 1997. Gestational exposure to loud noise alters the development and postnatal responsiveness of humoral and cellular compoents of the immune system in offspring. Environ Res 73:227–241 [DOI] [PubMed] [Google Scholar]
- 22.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 23.Turner JG, Parrish JL, Hughes LF, Toth LA, Carpary DM. 2005. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 55:12–23 [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Su H, Copenhagen LD, Vaishnav S, Pieri F, Shope CD, Brownell WE, De Biasi M, Paylor R, Bradley A. 2002. Urocortin-deficient mice display normal stress-induced anxiety behavior and autonomic control butan impaired acoustic startle response. Mol Cell Biol 22:6605–6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willott JF. 2007. Factors affecting hearing in mice, rats, and other laboratory animals. J Am Assoc Lab Anim Sci 46:23–27 [PubMed] [Google Scholar]
- 26.Willott JF, Tanner L, O'Steen J, Johnson KR, Bogue MA, Gagnon L. 2003. Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav Neurosci 117:716–727 [DOI] [PubMed] [Google Scholar]
- 27.Zakem HB, Alliston CW. 1974. The effects of noise levels and elevated ambient temperatures upon selected reproductive trains of female Swiss–Webster mice. Lab Anim Sci 24:469–475 [PubMed] [Google Scholar]