Abstract
To develop a means of euthanasia to support rapid time-course pharmacokinetic studies in mice, we compared retroorbital and intravenous lateral tail vein injection of ketamine–xylazine with regard to preparation time, utility, tissue distribution, and time to onset of euthanasia. Tissue distribution and time to onset of euthanasia did not differ between administration methods. However, retroorbital injection could be performed more rapidly than intravenous injection and was considered to be a technically simple and superior alternative for mouse euthanasia. Retroorbital ketamine–xylazine, CO2 gas, and intraperitoneal pentobarbital then were compared as euthanasia agents in a rapid time-point pharmacokinetic study. Retroorbital ketamine–xylazine was the most efficient and consistent of the 3 methods, with an average time to death of approximately 5 s after injection. In addition, euthanasia by retroorbital ketamine–xylazine enabled accurate sample collection at closely spaced time points and satisfied established criteria for acceptable euthanasia technique.
Matching the attributes of the euthanasia method to different applications and study designs is an important consideration in selecting the euthanasia method for an in vivo study. Methods of euthanasia should adhere to the AVMA Guidelines on Euthanasia, ACLAM Task Force Guidelines on Euthanasia, and other references reiterating similar principles.1,3,7,11,16, According to these guidelines, an acceptable euthanasia method is characterized by: (1) rapid loss of consciousness; (2) reliability; (3) safety of personnel; (4) irreversibility; (5) compatibility with study requirements; (6) minimal negative emotional effect on observers and personnel; and (7) compatibility with subsequent evaluation, examination, or use of tissue sample.1,7 The purpose of the current set of studies was to compare commonly accepted means of euthanasia in mice with a novel method: retroorbital ketamine–xylazine euthanasia.
Ketamine–xylazine is a commonly used combination for anesthesia and euthanasia in mice.4,14,28 In our experience, ketamine–xylazine is most often given intraperitoneally as an anesthetic combination. When used for euthanasia purposes, typically an overdose of the anesthetic is administered intraperitoneally followed by a secondary means of euthanasia, such as exsanguination, thoracotomy, or cervical dislocation.
For intravenous drug administration in mice, the retroorbital injection method is a technically simple, easily learned, reproducible, and rapid procedure, particularly as compared with intravenous tail vein dosing. Retroorbital injection has been shown to be interchangeable with the intravenous tail vein injection technique when parenteral access is desired in the mouse.9,10,12,18,20,22,29 The variability, technical demand, and other negative aspects of intravenous tail vein dosing in mice make the retroorbital method desirable.9,10,12,18,20,22,29 To minimize any potential associated pain or distress, retroorbital injections typically are given to anesthetized mice.15 This practice is feasible when mice are intended to recover after the injection; however, use of the retroorbital technique for euthanasia has not been documented. One goal of the current study was to demonstrate the adherence of retroorbital injection of ketamine–xylazine to the previously stated principles regarding euthanasia, with emphasis on the humaneness of the technique.
We developed the retroorbital ketamine–xylazine euthanasia technique to support rapid time-course mouse pulmonary pharmakokinetics studies in drug development. For these types of studies, which involve direct delivery of compounds to the lungs, intratracheal dosing is often the preferred method because of its reproducibility, reliability, and translatability to the clinic setting.19,21,23,24,26,27 One key factor that affects the efficacy and potency of these drug candidates is their residence time in the lungs. Pharmacokinetics studies focus on the distribution, clearance, and metabolism of chemical or drug entities that are introduced into the body. Pulmonary pharmacokinetics parameters are often assessed in serum, lung tissue, and bronchoalveolar lavage fluid. Because of rapid local clearance of the compounds, the quality of these data relies on the precision of sample collection, particularly from early time points that often are within minutes of each other.
When pulmonary pharmacokinetics studies are performed in mice, groups of animals are euthanized at specific time points after dosing to enable collection of tissue samples for concentration measurements over a time course. The method of euthanasia chosen for these studies must be nontraumatic and incorporate the attributes of rapid onset, ease of execution, reproducibility, and the ability to preserve tissues and samples. Currently, pulmonary pharmacokinetics studies use a variety of euthanasia techniques, including CO2 exposure, intraperitioneal barbiturate overdose, cervical dislocation, and decapitation.13,19,23,26 These methods, when used in rapid time-point pharmacokinetics studies, have attributes that can confound the results.6,8,11,17 Cervical dislocation and decapitation result in rapid death but are traumatic in nature. These techniques damage the trachea and cervical region, making it difficult or impossible to lavage the lungs after the procedure. These methods also confound the results by causing hemorrhage into various tissues and contaminating the lung tissue. Other euthanasia methods, such as CO2 exposure and intraperitoneal pentobarbital overdose are less traumatic, but the time to death is delayed and variable, thus preventing precise timing for tissue harvest.2,5,8,11,17 Therefore, the goals of this study were to examine the utility of a novel method of euthanasia, retroorbital administration of ketamine–xylazine for euthanasia of mice. We here demonstrate its positive effect on the quality of in vivo data, and show that retroorbital administration of ketamine–xylazine meets the criteria for acceptable euthanasia. Retroorbital administration of ketamine–xylazine for euthanasia of mice is ideal for pulmonary pharmacokinetics studies and for any study needing a rapid, nontraumatic means of euthanasia.
Materials and Methods
The Pfizer Institutional Animal Care and Use Committee reviewed and approved the animal use in these studies. The animal care and use program is fully AAALAC-accredited.
Male BALB/cAnNCrl mice (approximate weight, 25 g; Charles River Laboratories, Wilmington, MA) were housed and fed in an environment in accordance with established principles under the Guide for the Care and Use of Laboratory Animals.16 They were free of pathogens, parasites, and opportunists. The mice were housed 5 animals per cage in standard polycarbonate cages on corncob bedding (Bed-o'cobs, The Andersons, Maumee, OH) and maintained on a 12:12-h light:dark cycle. They were fed ad libitum (Lab Diet 5001, Purina Mills International, St Louis, MO) and had unrestricted access to water. The mice were allowed to acclimate in our facility for 4 d prior to the study.
Production of fluticasone nanosuspension formulation for pharmakokinetics study dosing.
Fluticasone (Sequoia Research Products, Oxford, UK) is a well-known, FDA-approved compound that could be used in a standard pulmonary pharmacokinetics study. An unique nanosuspension created at Pfizer was used. To produce the nanosuspension formulation of fluticasone, a bench-scale wet milling (micronization) device was used. Fluticasone, an appropriate amount of multisized glass beads (0.5 to 0.75 mm), and Tween 80 [0.5% (w/w) in PBS (pH 7.4); used as a surfactant and wetting agent] were combined in a scintillation vial. The mixture was stirred at 1200 revolutions per minute for 24 h with occasional shaking. The stock formulation then was harvested, and potency was checked by HPLC–diode array detection, and its solid state was checked by powder X-ray diffraction. Thermal gravimetric analysis with simultaneous differential thermal analysis (TGA/SDTA851e, Mettler,) was performed. Particle size distribution was determined on a Beckman Coulter LS 230 particle size analyzer using the small volume accessory (Beckman Coulter, Miami, FL). A polarization intensity differential scattering obscuration water optical model was employed. Particle size distribution was computed by software using Mie scattering theory (Beckman Coulter, Fullerton, CA). Formulation concentration, homogeneity, chemical stability, and solid-state stability were performed according to the same procedures described.
Comparison of retroorbital and intravenous techniques for ketamine–xylazine euthanasia.
Two groups of 7 naïve mice were euthanized with 100 µL of a 10:1 mixture of ketamine (100 mg/mL; Ketaset, Fort Dodge, Fort Dodge, IA) and xylazine (100 mg/mL; Anased, Lloyd Laboratories, Shenandoah, IA) delivered either retroorbitally or by injection into the lateral tail vein.
The retroorbital injection was given after a scruff restraint of the mouse, followed by injection of ketamine–xylazine into the retroorbital sinus through a 26-gauge needle inserted into the lateral canthus of either orbit. For intravenous tail vein injection, mice were placed under a heat lamp to promote vascular dilation and facilitate dosing. The mice then were restrained to allow access to the tail while keeping the body still. The time to death after treatment was recorded for each technique. The time spent under the heat lamp was not included in the total procedural time for intravenous injections. The average time to death was determined from the end of the injection to the point of clinical death (lack of respiration and heart beat). After clinical death, a terminal blood sample was collected by cardiocentesis. Brain, heart, and lung tissue were harvested for determination of ketamine–xylazine concentration.
Brain, lung, and heart tissue was weighed and homogenized individually in 2 mL 70% acetonitrile in 15-mL plastic centrifuge tubes (Corning, Corning, NY). Blood samples were placed into plasma separator tubes (Microcontainer Plasma Separator Tubes, Becton–Dickinson, Franklin Lakes, NJ) and centrifuged at 7826 × g for 6 min. The tissue and plasma samples were frozen at –80 °C until analysis.
Determination of tissue ketamine–xylazine concentration.
Tissue ketamine–xylazine quantification was performed by using HPLC and mass spectrometry (Pharmacokinetics Dynamics and Metabolism Department, Pfizer, St Louis, MO). The analysis system comprised a triple-quadrupole mass spectrometer (API4000, Applied Biosystems, Foster City, CA) with an atmospheric pressure electrospray ionization source (MDS SCIEX, Concord, Ontario, Canada) and 2 pumps with a controller (LC-10ADvp, Shimadzu, Columbia, MD). A 10-μL sample of homogenized tissue or plasma was injected onto an Altima-C18 column (2.1 × 50 mm; 3.0 μm; Alltech, Deerfield, IL) and eluted by a mobile phase with initial conditions of 2% solvent B for 1 min followed by a gradient of 2% solvent B to 50% solvent B (100% in the case of xylazine) over 2 min (solvent A: 95% H2O–5% acetonitrile with 0.1% formic acid; solvent B: 100% acetonitrile with 0.1% formic acid); 50% solvent B (100% in the case of xylazine) then was held for 1 min, followed by an immediate return to initial conditions and maintained for 1 min, with a flow rate of 0.4 mL/min. By using the positive-ion mode, protonated molecules were formed by using an ion-spray voltage of 5000 V (2500 V in the case of xylazine), declustering potential of 56 eV (61 eV in the case of xylazine), entrance potential of 10 eV, and source temperature of 300 °C for ketamine (250° for xylazine). The product ion was generated at a collision energy of 29 eV (18eV for xylazine). For measuring ketamine, mass transition of m/z 238.1 (221 xylazine) to m/z 124.8 (90 xylazine) was used. The peak areas of all the analytes, standards blanks, and internal standard were quantified by using Analyst 1.4.1 (MDS SCIEX, Ontario, Canada).
Pulmonary pharmacokinetics study.
For this portion of the study, 72 naïve male BALB/C mice were dosed intratracheally with a 0.8-mg/mL nanosuspension of fluticasone at 1 mg/kg. After the animals were sedated with inhaled isoflurane (Aerrane, Baxter, Norfolk, UK), each was restrained through a scruff hold. The mouth was held open with a thin metal wire placed below the upper incisors. The tongue was retracted forward to expose the back of the oral cavity. The fluticasone then was pipetted into the opening of the larynx. After dosing, the animals were returned to their cages.
All dosed animals were divided randomly into 3 arms; 6 mice from each group were euthanized at each of 4 designated time points after dosing (5, 15, 30, and 60 min) by use of 1 of 3 euthanasia methods: (1) inhalation of 100% CO2 in a nonprecharged 4-L chamber at a moderate fill rate; (2) intraperitioneal pentobarbital (100 mg/kg; Sleepaway, Fort Dodge), and (3) retroorbital injection of ketamine–xylazine (100 µL of a 10:1 ketamine–xylazine solution; Ketaset, Fort Dodge; Anased, Lloyd Laboratories). At the point of clinical death (lack of heart beat and respiration), a terminal blood sample, bronchoalveolar lavage fluid, and lung tissue was harvested for pharmakokinetics analysis.
Lung tissue was weighed and homogenized in 3 mL 70% acetonitrile in 15-mL plastic centrifuge tubes (Corning). Blood samples were placed into plasma separator tubes (BD Micro container Plasma Separator Tubes, Becton–Dickinson) and centrifuged at 7826 × g for 6 min. The tissue and plasma samples were frozen at –80 °C until analysis.
Determination of tissue concentration of fluticasone.
Analysis of plasma and lung samples for fluticasone concentration was performed by using HPLC–mass spectroscopy according to the same methods described for ketamine, with the following exceptions: the initial 10-µL injected sample was eluted by a mobile phase with initial conditions of 10% solvent B for 1 min, followed by a gradient of 10% solvent B to 100% solvent B over 2 min (solvent A: 95% H2O–5% acetonitrile with 0.1% formic acid; solvent B: 100% acetonitrile with 0.1% formic acid), 100% solvent B held for 1 min, followed by an immediate return to initial conditions maintained for 1 min, with a flow rate of 0.4 mL/min. The declustering potential was 80eV with a source temperature of 400 °C. The product ion was generated at collision energy of 19 eV for measuring the fluticasone mass transition of m/z 502.1 to m/z 313.7.
The halflifes of fluticasone in the lungs and plasma of the study mice were calculated based on the theoretical concentration of fluticasone after intratracheal dosing (for lung tissue) or first measurement (in the case of plasma). The starting plasma concentration is different for each euthanasia technique due to the different time to onset of death for each method.
Statistical methods.
A 2-tailed t test (LabStats, Computer Lab Solutions, Farmington, UT) was used to compare the means of the tissue concentrations of the retroorbital versus the lateral tail vein injection. A confidence interval of 95% or a P value of less than or equal to 0.05 was considered statistically significant.
In addition, 1-way ANVOA (LabStats, Computer Lab Solutions) was used to compare the means of lung fluticasone concentration at each time point in the pharmacokinetics study among the 3 euthanasia techniques and to compare the means of plasma fluticasone concentration at each time point in the pharmacokinetics study among the 3 methods. A confidence interval of 95% or a P value of less than or equal to 0.05 was considered statistically significant.
Results
Both retroorbital and intravenous administration of ketamine–xylazine to mice rapidly resulted in clinical death (lack of heart beat and respiration). Average time to clinical death for the retroorbital method was 5 s, and the average time to death for the intravenous method was 3 s (the time from end of injection to clinical death). The primary difference between the 2 techniques was the preparation time required for the injection. The retroorbital method requires approximately 10 s to execute. With intravenous tail vein injection, the tail must be prewarmed to dilate the vessel, with the mouse restrained in a way that allowed access to the tail for the injection. The total time from cage removal to death by tail vein injection was approximately 60 s, not including the time spent under the heat lamp for vessel dilation. Therefore the difference in time between the 2 methods was approximately 50 s for each animal, with the retroorbital method less time-consuming overall.
The average concentrations of ketamine and xylazine in the brain, lung, heart, and plasma after retroorbital euthanasia and intravenous euthanasia (Table 1) were compared with 2-tailed t tests. The tests revealed no significant differences in mean tissue concentrations between the 2 methods.
Table 1.
Concentrations of ketamine and xylazine in various tissues after clinical death
| Route of administration | Average concentration (µg/mL) |
||
| Tissue | Ketamine | Xylazine | |
| Brain | Retroorbital | 85.67 | 5.69 |
| Intravenous | 88.49 | 5.92 | |
| Lung | Retroorbital | 653.71 | 56.71 |
| Intravenous | 521.53 | 47.33 | |
| Heart | Retroorbital | 335.57 | 15.83 |
| Intravenous | 374.29 | 16.42 | |
| Plasma | Retroorbital | 5091.43 | 543.57 |
| Intravenous | 4759.57 | 390.35 | |
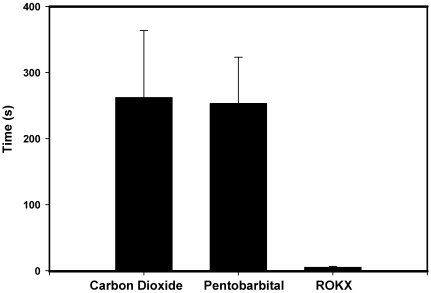
In the fluticasone pharmacokinetics study, the average time to clinical death for CO2 inhalation was 262 s, for intraperitoneal pentobarbital was 253 s, and for retroorbital ketamine–xylazine was 5.3 s (Figure 1). Statistical testing was not done to compare average times to clinical death in light of the pronounced differences in time between retroorbital ketamine–xylazine and the other 2 methods.
Figure 1.
Time to clinical death. ROKX, retroorbital injection of ketamine–xylazine.
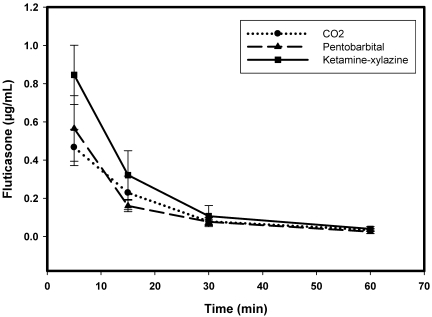
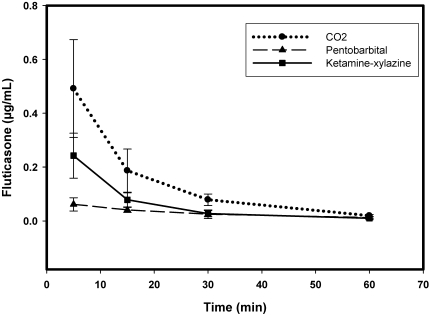
The concentration of fluticasone in plasma and homogenated lung tissue was determined at various time points (Figures 2 and 3). At 5 min after dosing, the lung concentration of fluticasone was different in the 3 groups euthanized with different techniques. The fluticasone level was the highest in the ketamine–xylazine euthanized group. The corresponding plasma level is the lowest when compared with other methods at this time point. Plasma fluticasone levels were higher in the CO2 euthanasia group as compared with the other 2 methods of euthanasia. By 30 min, the plasma and lung levels were almost identical from all 3 groups of animals. The variability in the data for each group decreased at later time points as the clearance rate is slower at these time points.
Figure 2.
Pharmakokinetics profile of fluticasone in the lungs as determined by using the 3 different euthanasia techniques.
Figure 3.
Plasma pharmakokinetics profile of fluticasone as determined by using the 3 different euthanasia techniques.
For all 3 euthanasia methods, mean lung concentrations of fluticasone were significantly different (P < 0.05) by 1-way ANOVA at the 5- and 15-min time points; mean plasma fluticasone at these time points were different between these 3 methods as well. At the 30-min time point, drug concentrations in lung did not differ significantly between the euthanasia methods, but differences in plasma concentration between methods remained at the 30-min point. All significant differences in plasma and lung concentrations between the 3 different methods were lost at 60 min.
Table 2 shows the calculated half life of fluticasone in lung and plasma. The pulmonary half life was calculated based on the theoretical starting concentration of fluticasone. The plasma half life was calculated based on the first concentration since the plasma concentration is 0 at time 0. Depending on the euthanasia technique, the half life of fluticasone in lung and plasma varied by as much as 5 min.
Table 2.
Halflife of intratracheal fluticasone in plasma and lung
| Euthanasia method | Calculated halflife (min) | |
| Lung | Carbon dioxide | 8 |
| Pentobarbital | 9 | |
| Ketamine–xylazine | 13 | |
| Plasma | Carbon dioxide | 12 |
| Pentobarbital | 17 | |
| Ketamine–xylazine | 13 |
Discussion
The combination of ketamine and xylazine is a common anesthetic regimen in mice.4,14,28 The use of this combination by retroorbital injection for euthanasia has not been reported previously. Comparison of retroorbital and intravenous injection of ketamine–xylazine revealed that the concentrations of ketamine–xylazine do not differ between these methods. This finding suggests that the target organs and mechanism of action for both methods are likely identical. This information, combined with the time required to complete each procedure, suggests that the retroorbital method is a superior option when a fast, technically simple, and nontraumatic means of euthanasia is desired.9,10,12,18,20,22,29
An important factor demonstrated in these studies was that retroorbital injection of ketamine–xylazine met all established guidelines for euthanasia. This method was rapid, easily reproducible, irreversible, required minimal restraint, and could be learned quickly. It is also safe for personnel, is humane and nontraumatic, and can accommodate study requirements. These attributes are in line with published accepted principles of euthanasia.1,7,11,16,3 Several sources, including the 2nd edition of The Mouse in Biomedical Research advocate the use of anesthesia during retroorbital injections.15 This guideline is due, in part, to the theorized pain and distress involved with retroorbital injections in recovering mice. The reference does not mention use of retroorbital injections for euthanasia purposes. As we present here, clinical death after retroorbital injection of ketamine–xylazine was rapid—within seconds of injection—thus minimizing concerns of the technique causing anything more than momentary pain or distress. As demonstrated in the fluticasone pharmacokinetics study, this method allowed collection of tissue samples at precise time points.
Cervical dislocation and decapitation are both rapid means of euthanasia, but they can cause complications that are not amenable to certain studies. Some of these complications include hemorrhage into the thorax or lungs, bruising and damage to cervical structures and trachea, and even adverse changes to abdominal organs.8,11,17 Commonly used nontraumatic means of euthanasia, such as CO2 or barbiturate overdose, resulted in variable time to death. Slow onset of death does not support sample collection at precise time points. In contrast, retroorbital injection of ketamine–xylazine enabled us to adhere to tight, rapid time lines and allowed parallel processing of several animals. The slow and variable onset of death after CO2 exposure and pentobarbital overdose hindered precision in sample collection and directly affected the pharmacokinetics profile, especially at early time points. Other concerns with the use of CO2 in pharmacokinetics studies involve its potential negative effect on the metabolism of compounds due to pH changes as well as potential adverse effects to various tissues.2,6
Because the clearance of drug candidates from the lungs is most rapid upon delivery, these rapid changes in lung concentration must be captured during the early time points for an accurate calculation of compound half life. Fluticasone was present and measurable in plasma as early as 5 min after administration. The lung levels of fluticasone were the highest in the retroorbitally euthanized animals at the early time points and second highest in plasma at the same time point (because of more drug remaining in lung tissue). Because of the rapidity of the retroorbital euthanasia method, more compound was captured in the lungs, before clearance into plasma. This finding demonstrates the necessity of a rapid onset and efficient euthanasia method to capture these dynamic events. The data also clearly indicate that the method of euthanasia influenced the perceived pharmacokinetics profile.
The lung and plasma concentrations of fluticasone differed significantly between the 3 euthanasia methods at the 5-min time point. At the 15-min time point, this variability was less prominent but still significant; these differences became insignificant at later time points. This finding demonstrated that the method of euthanasia can directly affect the data at the closely spaced early time points, where precision in sampling time is most crucial. The variability between methods could be due to additional factors, such as euthanasia metabolism effects, prolonged and variable time to death, and procedure-related stress. These factors should be addressed in future studies.
For the pentobarbital-injected mice, the plasma level of fluticasone at 5 min was low in comparison with the other methods, but the lung levels of fluticasone were relatively high. One potential reason for these findings is that pentobarbital may affect the metabolism of fluticasone and its clearance from the lungs into the circulation, thus decreasing systemic availability. This theory is supported in part by a study showing similar phenomena with oxacillin levels in rats.25 In that study, oxacillin was administered to rats that were either anesthetized with pentobarbital or remained conscious. Samples of portal and arterial blood were obtained to measure plasma concentrations of oxacillin in both groups. The metabolism of oxacillin was significantly different in the 2 groups. Further, the hepatic clearance of oxacillin was lower and mean absorption time and absolute bioavailability both higher in anesthetized rats. Pentobarbital could have a similar effect on the drug clearance from lung in our study.
The calculated pulmonary half life of fluticasone varied with the euthanasia technique, most likely due to a direct effect of the method of euthanasia. The calculation of halflife requires accurate measurement of the starting concentration in the tissue. For a euthanasia method with a long onset time, accurately capturing these time sensitive tissue concentrations would be impossible.
The retroorbital ketamine–xylazine injection technique offers an option of mouse euthanasia that might be used in various areas of research where rapid, nontraumatic, technically simple, and consistent means of euthanasia are desired. This technique satisfies the requirements for sound euthanasia as described in guidelines from the AVMA, ACLAM Task Force, and others. Future studies should explore the utility of euthanasia through retroorbital ketamine–xylazine in various study designs and the possible application of this technique in other rodent species.
References
- 1.American Veterinary Medical Association. 2007. [Internet] AVMA guidelines on euthanasia, 2007 update, p 4–11. [Cited Jan 8 2009]. Available at http://www.avma.org/issues/animal_welfare/euthanasia.pdf.
- 2.Angus DW, Baker JA, Mason R, Martin IJ. 2008. The potential influence of CO2 as an agent for euthanasia on the pharmacokinetics of basic compounds in rodents. Drug Metab Dispos 36:375–379 [DOI] [PubMed] [Google Scholar]
- 3.Artwohl J, Brown P, Corning B, Stein S; ACLAM Task Force Report of the ACLAM Task Force on rodent euthanasia. 2006. J Am Assoc Lab Anim Sci 45:98–105 [PubMed] [Google Scholar]
- 4.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17 [PMC free article] [PubMed] [Google Scholar]
- 5.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161 [DOI] [PubMed] [Google Scholar]
- 6.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia and euthanasia with rats. Lab Anim Sci 47:376–385 [PubMed] [Google Scholar]
- 7.Donovan J, Brown P. 2006. Euthanasia, unit 1.8 In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM. Current protocols in immunology. Hoboken (NJ): John Wiley and Sons [Google Scholar]
- 8.Feldman DB, Gupta BN. 1976. Histopathological changes in laboratory animals resulting from various methods of euthanasia. Lab Anim Sci 26:218–221 [PubMed] [Google Scholar]
- 9.Folberg R, Leach L, Valyi-Nagy K, Lin AY, Apushkin MA, Ai Z, Barak V, Majumdar D, Pe'er J, Maniotis AJ. 2007. Modeling the behavior of uveal melanoma in the liver. Invest Ophthalmol Vis Sci 48:2967–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes NG, Boni M, Primo CC. 1997. The invasive behavior of Cryptococcus neoformans: a possibility of direct access to the central nervous system? Mycopathologia 140:1–11 [DOI] [PubMed] [Google Scholar]
- 11.Green CJ. 1987. Euthanasia. In: Tuffery A. Laboratory animals: an introduction for new experimenters, p 171–177. Chichester (UK): Wiley Interscience [Google Scholar]
- 12.Hall SL, Lau KH, Chen S, Felt J, Gridley D, Yee J, Baylink D. 2007. An improved mouse Sca1+ cell-based bone marrow transplantation model for use in gene- and cell-based therapeutic studies. Acta Haematol 117:24–33 [DOI] [PubMed] [Google Scholar]
- 13.Hanninen M, Vliagoftis H. 2005. The role of mast cells in a mouse model of stress-induced airway inflammation. Univ Alberta Health Sci J 2:11– 13 [Google Scholar]
- 14.Hawk T, Leary S, Morris T. 2005. Formulary for laboratory animals, 3rd ed, p 56, 62 Ames (IA): Iowa State University Press [Google Scholar]
- 15.Hayward AM, Lemke LB, Bridgeford EC, Theve EJ, Jackson CN, Cunliffe-Beamer TL, Marini RP. 2007. Biomethodology and surgical techniques. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research: normative biology, husbandry, and models, p 452–454. Burlington (MA): Academic Press [Google Scholar]
- 16.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [DOI] [PubMed] [Google Scholar]
- 17.Iwarsson K, Rehbinder C. 1993. A study of different euthanasia techniques in guinea pigs, rats, and mice. Animal response and post mortem findings. Scan J Lab Animl Sci. 20:191–205 [Google Scholar]
- 18.Nanni C, Pettinato C, Ambrosini V, Spinelli A, Trespidi S, Rubello D, Al-Nahhas A, Franchi R, Alavi A, Fanti S. 2007. Retroorbital injection is an effective route for radiopharmaceutical administration in mice during small-animal PET studies. Nucl Med Commun 28:547–553 [DOI] [PubMed] [Google Scholar]
- 19.Nurkiewicz T, Porter D, Barger M, Castronova V, Boegehold M. 2004. Particulate matter exposure impairs systemic microvascular endothelium dependant dilation. Environ Health Perspect 112: 1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price JE, Barth RF, Johnson CW, Straubus AE. 1984. Injection of cells and monoclonal antibodies into mice: comparison of tail vein and retroorbital routes. Proc Soc Exp Biol Med 177:347–353 [DOI] [PubMed] [Google Scholar]
- 21.Serkova NJ, Van Rheen Z, Tobias M, Pitzer JE, Wilkinson JE, Stringer KA. 2008. Utility of magnetic resonance imaging and nuclear magnetic resonance- based metabolomics for quantification of inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol 295:L152–L161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP. 2008. Comparison of the lateral tail vein and the retroorbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY) 37:26–32 [DOI] [PubMed] [Google Scholar]
- 23.Stringer KA, Tobias M, Dunn JS, Campos J, Vaan Rheen Z, Masharaf M, Nayar R. 2008. Accelerated dosing frequency of a pulmonary formulation of tissue plasminogen activator is well tolerated in mice. Clin Exp Pharmacol Physiol 35:1454–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tung D.2008. Personal communication.
- 25.Ueda S, Yamaoka K, Nakagawa T. 1999. Effect of pentobarbital anesthesia on intestinal absorption and hepatic first pass metabolism of oxacillin in rats, evaluated by portal–systemic concentration difference. J Pharm Pharmacol 51:585–589 [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Hussain S, He Y, Pasula R, Smith P, Martin W. 2001. Genetically engineered macrophages expressing INFγ restore alveolar immune function in SCID mice. Proc Natl Acad Sci USA 98:14589–14594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q, Martin R, Rino J, Breed R, Torres R, Chu H. 2007. IL23-dependent IL17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect 9:78–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q, Ming Z, Dart AM, Du XJ. 2007. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin Exp Pharmacol Physiol 34: 499–507 [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Naraharisetti SD, Wang H, Unadkat SD, Hebert MF, Mao Q. 2008. The breast cancer resistance protein (Bcrp1, Abcg2) limits fetal distribution of glyburide in the pregnant mouse: an Obstetric–Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research study. Mol Pharmacol 73:949–959 [DOI] [PubMed] [Google Scholar]