Abstract
To evaluate the role of root-synthesized ABA in regulating growth and stomatal behaviour under well-watered conditions, isogenic wild-type (WT) and ABA-deficient flacca (flc) tomato (Solanum lycopersicum) were reciprocally and self-grafted just below the cotyledonary node. Since flc scions had lower leaf water potentials due to higher transpiration rates, a subset of all graft combinations was grown under a shoot misting treatment to minimize differences in shoot water status. Misting did not alter the relative effects of the different graft combinations on leaf area. WT scions had the greatest leaf area and lowest whole plant transpiration rate irrespective of the rootstock, implying that shoot ABA biosynthesis was sufficient to account for a WT shoot phenotype. In WT scions, the rootstock had no effect on detached leaf ethylene evolution or xylem concentrations of ABA or the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC). In flc scions, although the WT rootstock suppressed stomatal conductance of individual leaves, there was no detectable effect on whole plant transpiration rate. However, leaf area of flc/WT (scion/rootstock) plants increased 1.6-fold compared to flc self-grafts. WT rootstocks increased xylem ABA concentration in flc scions (relative to flc self-grafts) up to 3-fold, and resulted in xylem ACC concentrations and detached leaf ethylene evolution similar to WT scions. Since the WT rootstock normalized shoot ethylene relations but only partially restored the leaf area of flc scions (relative to that of WT scions), shoot ABA biosynthesis can directly promote leaf area via an unknown, ethylene-independent, mechanism.
Keywords: ABA, ACC, ethylene, flacca, grafting, leaf area, rootstock
Introduction
The plant hormone abscisic acid (ABA) has many physiological roles in the plant including control of stomatal behaviour, enhancement of root hydraulic conductance, and regulation of gene expression (Davies and Jones, 1991). One approach to elucidate the role of ABA in planta has been the use of ABA-deficient mutants, which show higher stomatal conductance and transpiration rates (Tal, 1966; Nagel et al., 1994; Fambrini et al., 1995), lower leaf water potential (Jones et al., 1987; Nagel et al., 1994; Fambrini et al., 1995) and relative water content (Nagel et al., 1994; Fambrini et al., 1995; Dodd, 2003), decreased root (Tal and Nevo, 1973; Nagel et al., 1994) and whole plant (Nagel et al., 1994) hydraulic conductance, and decreased leaf area (Jones et al., 1987; Sharp et al., 2000; Dodd, 2003) compared with wild-type (WT) plants. These phenotypes can be partially restored with exogenous ABA treatment (Imber and Tal, 1970; Nagel et al., 1994; Sharp et al., 2000). More recently, constitutive overexpression of genes for ABA biosynthesis has shown that increased ABA levels decreases stomatal conductance and transpiration rates and increases leaf water potential and root hydraulic conductance (Thompson et al., 2007; Parent et al., 2009).
Reciprocal grafting of ABA-deficient mutants and WT plants has determined the influence of root ABA biosynthesis on the shoot phenotype (Jones et al., 1987; Cornish and Zeevaart, 1988; Fambrini et al., 1995; Chen et al., 2002, 2003; Holbrook et al., 2002; Christmann et al., 2007). When maintained under well-watered conditions, shoots of WT/mutant (scion/rootstock) plants were usually indistinguishable from WT self-grafts in terms of biomass (Chen et al., 2002), leaf area (Chen et al., 2002; Holbrook et al., 2002), stomatal behaviour or transpiration rate (Jones et al., 1987; Fambrini et al., 1995; Holbrook et al., 2002), leaf water potential (Fambrini et al., 1995), and shoot or leaf ABA concentration (Fambrini et al., 1995; Chen et al., 2002), in spite of lower leaf xylem sap (Holbrook et al., 2002) and root exudate (Chen et al., 2002) ABA concentration. Such data imply that shoot ABA biosynthesis by WT scions is sufficient to maintain a WT shoot phenotype, independently of root ABA biosynthesis.
By contrast, the shoot phenotype of mutant/WT plants can differ from mutant self-grafts under well-watered conditions, with partial phenotypic reversions of leaf area (Cornish and Zeevaart, 1988; Holbrook et al., 2002), stomatal conductance (Jones et al., 1987), transpiration rate (Chen et al., 2002), leaf water potential (Fambrini et al., 1995), and leaf ABA concentration (Fambrini et al., 1995; Chen et al., 2002), emphasizing the importance of the rootstock. However, this behaviour is not always consistent between studies, in that mutant/WT plants can be indistinguishable from mutant self-grafts in terms of stomatal conductance (Holbrook et al., 2002) and leaf area or biomass (Chen et al., 2002). Within a single study, the effectiveness of a WT rootstock in phenotypically reverting a mutant scion can vary according to the physiological process studied. For example, the leaf area of the ABA-deficient tomato sitiens scions grafted onto WT rootstocks exceeded that of sitiens self-grafts, even though transpiration rates were as high as sitiens self-grafts (Holbrook et al., 2002), implying that different processes are differentially sensitive to root-synthesized ABA.
However, it can be difficult to evaluate the specific contribution of the root for several reasons. Firstly, in some experiments the site of the graft union is not explicitly reported and a considerable length of stem (sometimes with leaves attached) can comprise the ‘rootstock’ (Cornish and Zeevaart, 1988). Secondly, the re-circulation of ABA between xylem and phloem (reviewed in Dodd, 2005) means that xylem sap collected from transpiring leaves of reciprocal grafts apparently had ABA concentrations intermediate between mutant and WT self-grafts (Holbrook et al., 2002), indicating that xylem ABA concentration ([X-ABA]) of chimeric grafts depends on both root and shoot genotypes. Thirdly, [X-ABA] varies with distance along the transport pathway in non-grafted plants (Jokhan et al., 1999), indicating ABA exchange between xylem parencyhma cells and the xylem lumen. Such exchange suggests that the site of the graft union may influence scion ABA relations, thus our experiments evaluating the influence of the root system established the graft union just below the cotyledonary node.
Another difficulty of interpreting the role of ABA in such grafting experiments is a confounding of ABA status and leaf water status, in that different graft combinations differ in shoot water status (Fambrini et al., 1995; Chen et al., 2002). Despite this caveat, in most examples where plants have been manipulated to grow at two different shoot water statuses but subjected to similar edaphic conditions, no effect of shoot water status was observed (reviewed in Dodd, 2005). Methods of increasing shoot water status of ABA-deficient scions (to that of WT scions) include growing in different humidity regimes (Sharp et al., 2000; LeNoble et al., 2004), applying pressure to the root systems (Termaat et al., 1985; Dodd et al., 2002) or misting the shoot (Stirzaker et al., 1997). For practical reasons, the latter was adopted to allow a large number of grafted tomato plants to be grown in a single experiment.
A further difficulty with interpreting the role of ABA in such grafting experiments is that ABA-deficient mutants can show multiple changes in phytohormone status. Shoot tissues of the ABA-deficient tomato mutants notabilis and flacca (flc) produce 1.6–2.3-fold more ethylene than WT plants (Tal et al., 1979; Hussain et al., 2000) even when grown at an equivalent or higher shoot water status than WT plants (Sharp et al., 2000), as did the ABA-deficient aba2-1 Arabidopsis mutant (LeNoble et al., 2004). Foliar ABA sprays decreased leaf ethylene evolution and increased leaf area of flc (Sharp et al., 2000) and aba2-1 (LeNoble et al., 2004) plants. Moreover, blocking ethylene perception in ABA-deficient mutants chemically (foliar sprays of an inhibitor of ethylene action: Sharp et al., 2000) or genetically (the aba2-1 etr1-1 double mutant shows both ABA deficiency and ethylene insensitivity: LeNoble et al., 2004) increased leaf area, suggesting that an important role of endogenous ABA is to limit the growth inhibitory action of ethylene. Root export of the immediate precursor of ethylene, 1-aminocyclopropane-1-carboxylic acid (ACC) can quantitatively account for shoot ethylene evolution of well-drained and flooded tomato plants (Else and Jackson, 1998), thus xylem ACC concentration of reciprocally grafted flc and WT tomatoes was measured.
Our objective was to determine the influence of the rootstock on the shoot phenotype (shoot water status, transpiration rate and leaf area, xylem sap ABA and ACC concentrations, detached leaf ethylene evolution) of well-watered reciprocally grafted flc and WT tomatoes. Plants were grown in the presence and absence of shoot misting, allowing phenotypic comparisons of different graft combinations at a similar shoot water status. To allow xylem sap collection from the same plant on several occasions during an experiment, single leaves were detached and pressurized in a Scholander-type pressure chamber, a technique that provides a reasonable estimate of [X-ABA] in the transpiration stream (Borel and Simmoneau, 2002). It was hypothesized that rootstock influences on shoot physiology were primarily mediated by differences in chemical, not hydraulic, signalling.
Materials and methods
Plant material and culture
Isogenic wild-type and flacca (flc) genotypes of tomato (Solanum lycopersicum Mill. cv. Ailsa Craig) were used. The flc genotype is impaired in the oxidation of ABA-aldehyde to ABA (Taylor et al., 1988), giving leaf ABA concentrations only 26–33% of the wild type (Neill and Horgan, 1985; Sharp et al., 2000). During germination and subsequent growth (except as detailed below), plants were maintained in a single walk-in controlled environment room (3×4 m) at the Lancaster Environment Centre under a 12 h photoperiod (09.00 h to 21.00 h) and 16 °C to 26 °C temperatures. Atmospheric evaporative demand varied between 0.2 kPa and 1.2 kPa. Metal halide lamps (HQI-T 400N, Osram, St Helens, UK) were 1.2 m above bench height and provided 180 μmol m−2 s−1 photosynthetic photon flux density (PPFD) at bench height and 800 μmol m−2 s−1 at the maximum height of WT/WT plants.
Seeds were sown in a well-watered peat-based substrate (Levingtons M3, Levington Horticulture Ltd., Ipswich, UK) in seedling trays, with a single seed in each separate compartment (3 cm deep×2 cm×2 cm). After 14 d, when the first true leaf had emerged, seedlings were transferred to 0.37 l pots filled with a loam-based substrate (John Innes No 2, J Arthur Bowers, Lincoln, UK). Seedlings were watered daily for a further 2 weeks prior to grafting.
Graft unions were established just below the cotyledonary node as previously described (Chen et al., 2002). A wedge was cut in the rootstock, the shoot tip inserted, and the union bound with parafilm (‘M’ laboratory film, Pechiney, Chicago, USA). Plants were immediately covered with transparent plastic bags, secured with elastic bands around the pots and left to establish for 2–3 weeks. Towards the end of this period, plastic bags were removed for short intervals (1–2 h) each day, which gradually lengthened (4–6 h) to harden the plants to the growth environment.
Three to four weeks after grafting, plants were transplanted to 3.0 l pots containing the same substrate (John Innes No 2). Volumetric water content of this substrate at field capacity was 0.43 cm3 cm−3, and the bulk density when dry was 0.55 g cm−3. Plants were watered immediately following transplanting and daily thereafter, and allowed to establish for 7–10 d. Before measurements were initiated, one plant of each graft combination was randomly assigned to a block, and blocks of four randomly distributed plants were arranged in the walk-in controlled environment room. The position of each block within the room, and the position of each plant within a block was re-randomized daily. Side-shoots were removed daily and floral trusses were removed at the first sign of petal colour in the first flower.
Shoot misting experiment
Since flc scions have higher transpiration rates than WT scions in reciprocally grafted plants (Chen et al., 2002; Holbrook et al., 2002), it was expected that flc scions would have a lower leaf water potential (Ψleaf). To investigate the effect of Ψleaf on growth of the graft combinations, some plants from all graft combinations were misted. Misted and unmisted plants were paired (to ensure similar leaf area between treatments before misting commenced) 1 week after transplanting to the 3.0 l pots. This experiment was conducted in a greenhouse compartment at the Lancaster Environment Centre during January–February 2005. Plants received natural daylight supplemented with artificial lighting (250 μmol m−2 s−1 at bench height) supplied by SON-T Agro 400W sodium lamps (Philips, Guildford, UK) on an 11 h photoperiod (09.00 h to 20.00 h). Average minimum and maximum temperatures during the experiment were 17 °C and 26 °C, respectively. Plants of all graft combinations were misted intermittently with tap water using a mist propagation unit that was automatically triggered using a leaf wetness sensor. The misting unit occupied one side of a bench (3×2 m) which was separated from the other side by a clear plastic sheet strung across the bench 1 m above the surface (to prevent spray drift from the nozzles, located 0.8 m above the surface, reaching unmisted plants). Plants were grouped in blocks of four (comprising one plant of each graft combination) and each block was moved daily to a different position along the long axis of the bench, to minimize effects of a heterogeneous spray distribution. The position of each plant within a block with respect to the short axis of the bench was also re-randomized daily, for similar reasons. Since an unknown mist volume fell on the soil at the top of each pot, it was not possible to determine the transpiration of misted plants gravimetrically. The amount of water supplied to these plants was decreased (relative to unmisted plants in the same experiment) trying to ensure a similar soil water content (θ) between misted and unmisted plants. Although plants grown under the misting treatment showed a different diurnal variation in θ than unmisted plants, similar mean θs were achieved.
Physiological measurements
Whole plant transpiration was estimated gravimetrically, and corrected for evaporation from ‘blank’ pots (without a plant). Watering occurred daily (at the beginning of the photoperiod) or twice-daily according to the transpirational needs of the plant. The maximum soil water deficit in a 24 h period was determined prior to the morning watering by measuring the apparent dielectric constant of the upper 6 cm of substrate using a theta probe (ML2X, Delta-T, Cambridge, UK). Readings were converted from microvolts to volumetric soil moisture content (θ), based on a two point calibration (field capacity and oven-dried soil) with the same substrate. Soil water potential at known volumetric soil water contents (made by adding known volumes of water to known volumes of oven-dry soil in a cup, which was sealed with parafilm and allowed to equilibrate overnight) were measured with a soil psychrometer (Model WP4, Decagon Devices Inc., Pullman, Washington, USA). This technique showed that soil water potential was indistinguishable from 0 MPa at all water contents exceeding 0.18 cm3 cm−3.
Leaf water potential (Ψleaf) was determined using a Scholander-type pressure chamber (Plant Moisture Systems, Santa Barbara, CA, USA). The chamber was lined with moistened filter paper, and measurements were made between 11.30 h and 17.30 h on fully expanded leaves. To check whether Ψleaf of these leaves were representative of those of expanding leaves, a fully expanded leaf (mean area, across all four graft combinations, of 214 cm2) and an expanding leaf (mean area of 53 cm2) were sampled on two consecutive days in one experiment. Following measurement of Ψleaf, an overpressure of 0.4 MPa was applied to leaves for 60–120 s, to ensure collection of sufficient xylem sap for analysis. To check whether the overpressure applied to leaves affected ABA concentration of the sap sample, on one occasion leaves were sampled at 0.1 MPa (one or two leaves per plant) and 0.4 MPa (one leaf per plant). Samples were frozen in liquid nitrogen and stored at –20 oC prior to determination of ABA and ACC concentration.
Leaf area of all excised leaves was recorded with a Li-3100 Area Meter (Li-Cor Inc., Lincoln, Nebraska, USA) following measurement of Ψleaf and sap collection. At the end of each experiment, the area of all remaining leaves was measured, allowing whole plant transpiration rate (per unit leaf area) to be calculated for the 24 h period preceding harvest. Total leaf area developed during the experiment (including those leaves harvested for Ψleaf determination) was calculated and the number of leaves (>1 cm2) counted. Abscission of older leaves (predominantly in flc scions) was recorded by counting the nodal scars, to obtain the total number of leaves developed during the experiment.
Single leaf gas exchange was determined between 11.00 h to 13.30 h using infrared gas analysis (CIRAS-2, PP Systems, Hitchin, UK). Leaves were allowed to equilibrate at ambient temperature, constant CO2 concentration (37.5 Pa) and PPFD (350 μmol m−2 s−1) for 3 min before data were recorded.
Sap analysis
Sap ABA concentration was measured by radioimmunoassay (Quarrie et al., 1988), using the monoclonal antibody AFRC MAC 252 (kindly provided by Dr G Butcher, Babraham Bioscience Technologies, Cambridge, UK). Sap volumes used in the assay were typically 25 μl (WT scions) and 50 μl (flc scions). It was sometimes necessary to pool sap from several plants of the same graft combination to ensure sufficient sap for analysis.
Sap ACC concentration was determined by GC-MS (Smets et al., 2003). Xylem sap (100–200 μl) was made up to 80% (v/v) with methanol, to which 15 ng of a [2H4] ACC internal standard (Olchemim, Olomouc, Czech Republic) was added and the sample left overnight at –20 °C. Following the procedure of Persson and Näsholm (2001), the sample was dried to the water phase, acidified to pH <3.0 with 3–4 drops of 0.01 M HCl using one drop of 1% Bromophenol Blue as an indicator. Solid-phase extraction (SPE) was used to purify the sample over a strong cation-exchange resin (Extract Clean SCX 200 mg/4 ml, Grace Davison Discovery Sciences, Lokeren, Belgium), pre-conditioned with 6 ml of deionized water. Following sample loading, the column was washed with 2 ml of water:methanol (1:8 v/v) to remove interfering compounds, and then ACC bound to the column was eluted by washing twice with 750 μl of 4 M ammonium hydroxide. The eluent was dried under a stream of nitrogen and stored at –20 °C prior to further analysis.
For derivatization, samples were transferred to 2 ml amber vials, using 200 μl of methanol, then dried under a stream of nitrogen and redissolved in 50 μl of acetone. To this, 2 μl of 1-ethylpiperidine and 10 μl of bromopentafluorotoluene were added, and capped vials incubated for 45 min at 60 °C on a heating block. Derivatized samples were then dried under a stream of nitrogen. Samples were further purified within the same vial by liquid–liquid extraction, by adding 400 μl of ethyl acetate and 400 μl of water, capping vials, vortexing, standing for 30 min, and then discarding the lower aqueous phase. The ethyl acetate phase was then washed with 400 μl of water for a second time, and after this had carefully been discarded, the sample was dried under a stream of nitrogen, resuspended and dissolved in 30 μl methanol, and transferred to a vial insert.
The ACC-bis-pentafluorobenzyl samples were analysed by auto-injection onto a gas chromatograph connected to a mass spectrometer (6890N GC and 5975 Inert MSD, Agilent Technologies, UK). A 0.5 μl sample was injected into a cool on-column (COC) inlet with a temperature gradient of 150–325 °C in 2 min, and focused onto a JandW 50 m×0.25 mm×0.25 μm film DB-5MS UI column (HiChrom Ltd, Reading, UK), using helium (99.995%, BOC Gases, Guildford, UK) as a carrier gas at a flow rate of 1.5 ml min−1. The GC oven was set to 150 °C for 11 min, ramped at 20 °C min−1 to 300 °C, and then held for 10 min. The retention times for [2H4] ACC-bis-PFB and ACC-bis-PFB were 17.14 and 17.16 min, respectively. Analytes were ionized by negative chemical ionization, using methane (99.999%, Argo International Ltd, Basildon, UK) at a pressure of 2.2×10−24 mbar, a source temperature of 150 °C and a voltage of 150 eV. For maximum sensitivity, the deprotonated molecules at m/z 280 for ACC-bis-PFB and m/z 284 for [2H4] ACC-bis-PFB were followed in selective ion monitoring (SIM) mode, using a dwell time of 100 ms for each. Resultant ion chromatograms were integrated using Enhanced MSD Chemstation software (Agilent Technologies, UK), and sample ACC concentrations calculated with reference to a calibration curve.
Detached leaf ethylene evolution
The rate of ethylene evolution was determined by placing detached leaves (1–2 g FW) in 27 cm3 glass boiling tubes containing 5 cm2 of filter paper saturated with water. Each tube was flushed with air for 1 min prior to closure with a Suba-Seal (SLS, Nottingham, UK), and then incubated under 100 μmol PPFD m−2 s−1 for 1 h. Using a disposable plastic syringe, a 1 ml headspace sample was withdrawn and manually injected into a gas chromatograph (6890N, Agilent Technologies, Wokingham, UK) fitted with a JandW HP-AL/S (50 m×0.537 mm×15.0 μm) column (HiChrom Ltd, Reading, UK). The inlet temperature was 250 °C, and the oven was set at 100 °C for the first 5 min to resolve ethylene, then ramped at 15 °C min−1 to 150 °C and held for 1.5 min to drive off any water vapour introduced onto the column. Helium carrier gas flowed at 5.7 ml min−1, and detection was by flame ionization at 250 °C. The rate of ethylene evolution was calculated with reference to peak areas of known ethylene standards (99.995%, BOC Special Gases, Manchester, UK) and corrected for tissue fresh weight and the proportion of total headspace taken for analysis. Preliminary experiments showed stable ethylene evolution rates within 1 h of leaf detachment, suggesting a limited wound-induced ethylene response.
Statistics
With the exception of shoot misting (which was done only once), several experiments were performed as described above. Each physiological analysis was repeated at least twice, and data from representative experiments are illustrated. When measurements were made several times during an experiment (eg leaf water potential, xylem ABA concentration), ANOVA was used to determine any effects of measurement occasion. Within a single experiment, analysis of variance (ANOVA) was used to detect significant effects of root and shoot genotypes, the misting treatment and any interactions, with means discriminated (P <0.05) using Tukey's HSD test. When two specific treatments were compared, Student's unpaired t tests (P <0.05) were used.
Results
Shoot misting experiment
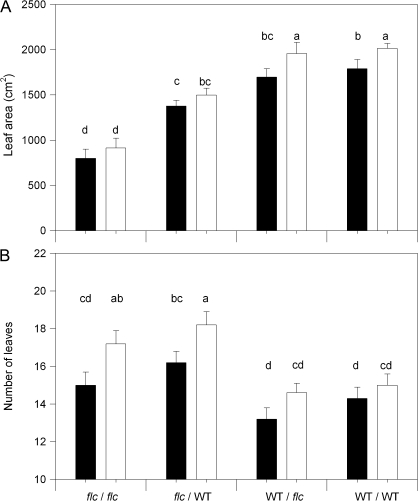
ANOVA showed highly significant (P <0.01) effects of misting, both root and shoot genotypes, and the scion×rootstock interaction on leaf area (Table 1). Although misting decreased leaf area (averaged across all graft combinations) by 11%, leaf area differences between the graft combinations were similar, independent of whether misting was applied (Fig. 1A). Transient overwatering of misted plants may have decreased leaf area since misted plants had a higher soil water content (θ) than unmisted plants on some days (Table 2). Leaf area of WT/WT plants was statistically similar to that of WT/flc plants, while leaf area of flc/WT and flc/flc plants was decreased by 24% and 55%, respectively (Fig. 1A). These data indicate that the effect of the rootstock on leaf area was dependent on the scion.
Table 1.
Analysis of variance of physiological variables in the shoot misting experiment
| Effect or Interaction | Leaf area | Leaf number | Ψleaf | [X-ABA]leaf |
| (Fig. 1A) | (Fig. 1B) | (Fig. 3A) | (Fig. 3B) | |
| Scion | <0.001 | <0.001 | <0.001 | <0.001 |
| Rootstock | <0.001 | 0.049 | 0.008 | 0.69 |
| Misting | 0.005 | 0.001 | <0.001 | 0.73 |
| Scion×Rootstock | 0.002 | 0.76 | 0.27 | 0.52 |
| Scion×Misting | 0.31 | 0.26 | 0.026 | 0.87 |
| Rootstock×Misting | 0.90 | 0.63 | 0.44 | 0.91 |
| Scion×Rootstock×Misting | 0.86 | 0.76 | 0.11 | 0.72 |
P values are presented for each main effect or interaction.
Fig. 1.
Whole plant leaf area (A) and number of leaves >1 cm long (B) of WT/WT, WT/flc, flc/WT, and flc/flc plants exposed to shoot misting (black bars) and unmisted (white bars). Data are means±SE of 5–6 plants, with significant (P <0.05) differences between treatments according to Tukey's HSD test denoted by different letters above the bars. P values for three-way ANOVA are reported in Table 1.
Table 2.
Minimum soil volumetric water content (cm3 cm−3) of reciprocally grafted wild-type (WT) and flacca (flc) plants in the shoot misting experiment
| Graft | Unmisted (UM) | Misted (M) | Number of days | ||
| UM <M | UM=M | UM >M | |||
| WT/WT | 0.30±0.01 c | 0.31±0.00 b | 4 | 10 | 1 |
| WT/flc | 0.32±0.02 b | 0.32±0.01 b | 2 | 11 | 2 |
| flc/WT | 0.31±0.01 bc | 0.30±0.01 b | 1 | 14 | 0 |
| flc/flc | 0.33±0.01 a | 0.33±0.02 a | 2 | 13 | 0 |
Field capacity was 0.43 cm3 cm−3. Mean soil water content for each graft combination was calculated daily, and data comprise means ±SE of 15 d of measurements. Values followed by different letters within a column are significantly different at the 0.05 level according to Tukey's HSD test. The number of days for which the water content of pots grown under shoot misting exceeded (UM <M), were equivalent to (UM=M), or were less than (UM >M) unmisted pots, for each graft combination, is also given.
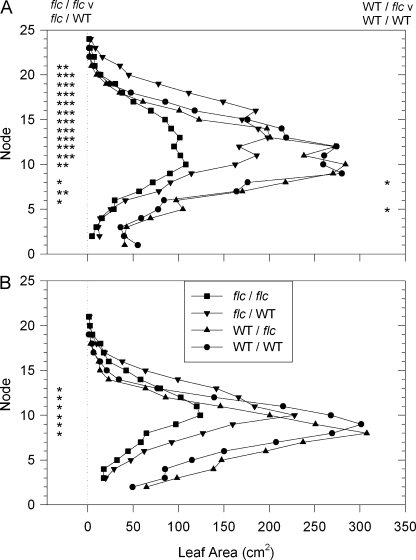
Decreased leaf area of flc scions could not be attributed to decreased leaf initiation, as they had more leaves than WT scions, independent of rootstock (Fig. 1B). Instead, differences in the area of fully expanded leaves (Fig. 2) caused differences in total leaf area.
Fig. 2.
Leaf area at different nodes of unmisted WT/WT (black circles),WT/flc (black triangles), flc/WT (black inverted triangles), and flc/flc (black squares) plants in two separate experiments (A) and (B). Data are means of at least five plants, SEs have been omitted for clarity. Significant effects of the rootstock for the WT/WT versus WT/flc comparison, and for the flc/WT versus flc/flc comparison are given on the right and left sides of the panels respectively, where significant differences as determined by Student's unpaired t test are indicated thus: * P <0.05, ** P <0.01, *** P <0.001.
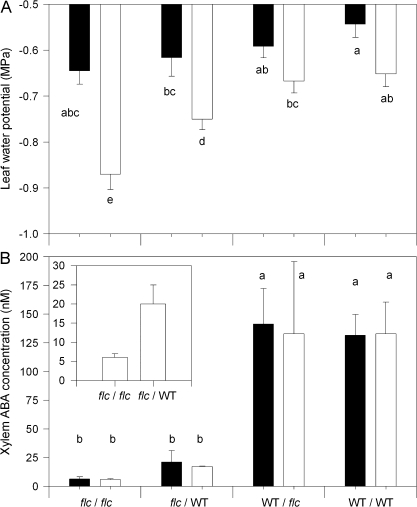
ANOVA also showed highly significant (P <0.01) effects of misting and both root and shoot genotypes on leaf water potential (Ψleaf) (Table 1). In unmisted plants, WT/WT and WT/flc plants had the same Ψleaf, with that of flc/WT and flc/flc plants decreased by 0.10 MPa and 0.22 MPa, respectively (Fig. 3A). The decreased Ψleaf of flc scions could not be attributed to a decrease in θ as watering of flc/flc plants maintained a slightly higher θ than all other graft combinations while flc/WT plants had a similar θ to WT/flc and WT/WT plants (Table 2). Although leaf area (Fig. 1A) and Ψleaf (Fig. 3A) were positively correlated in unmisted plants, artificially increasing Ψleaf of flc scions by shoot misting did not restore leaf area to that WT/WT plants (Fig. 1A), even though mean Ψleaf of the different graft combinations were within 0.1 MPa of each other (Fig. 3A).
Fig. 3.
Leaf water potential (A) and leaf xylem ABA concentration (B) of WT/WT, WT/flc, flc/WT, and flc/flc plants exposed to shoot misting (black bars) and unmisted (white bars). Data are means ±SE of 5–6 (A) and 2–5 (B) plants, with significant (P <0.05) differences between treatments according to Tukey's HSD test denoted by different letters above the bars. P values for three-way ANOVA are reported in Table 1. The inset in (B) indicates leaf xylem ABA concentration of flc/WT and flc/flc plants pooled from both misted and unmisted plants.
When xylem sap was collected by pressurizing detached leaves, xylem ABA concentration ([X-ABA]) was dependent only on the scion genotype, and independent of rootstock and misting (Table 1; Fig. 3B). Xylem ABA concentration of WT scions was 10-fold higher than flc scions and rootstock-independent. However, pooling data from the misted and unmisted plants (since misting had no significant effect on [X-ABA]) showed that the mean (±SE) [X-ABA] of flc/WT plants (20±5 nM, n=5) was 3.2-fold higher than flc/flc plants (6±1 nM, n=5: see Fig. 3B, inset).
Experiments with unmisted plants
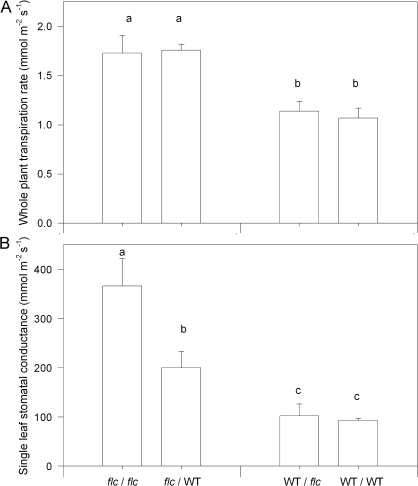
Whole plant transpiration rates (Eplant) of unmisted plants were measured gravimetrically at the end of each experiment. ANOVA showed highly significant (P <0.001) effects of scion genotype on Eplant, but no significant effect of rootstock or the scion×rootstock interaction (Table 3). Expressed on a leaf area basis, Eplant of flc/WT and flc/flc plants were c. 1.6-fold greater that WT/flc and WT/WT plants (Fig. 4A). However, single leaf measurements of stomatal conductance (gs) showed significant (P <0.05) effects of scion, rootstock and their interaction (Table 3; Fig. 4B). Although rootstock had no impact on gs of WT scions, gs of flc/flc leaves was significantly (P <0.05) higher than flc/WT leaves.
Table 3.
Analysis of variance of physiological variables in experiments with no misting treatment
| Effect or Interaction | Eplant (Fig. 4A) | gs (Fig. 4B) | C2H4 (Fig. 6A) | [X-ACC]leaf (Fig. 6B) |
| Scion | <0.001 | <0.001 | 0.064 | 0.14 |
| Rootstock | 0.90 | 0.011 | 0.025 | 0.036 |
| Scion×Rootstock | 0.6 | 0.021 | 0.14 | 0.041 |
P values are presented for each main effect or interaction.
Fig. 4.
Whole plant transpiration rate (A) and single leaf stomatal conductance (B) of unmisted WT/WT, WT/flc, flc/WT, and flc/flc plants. Data are means ±SE of 5–6 plants, with significant (P <0.05) differences between treatments according to Tukey's HSD test denoted by different letters above the bars. P values for two-way ANOVA are reported in Table 3.
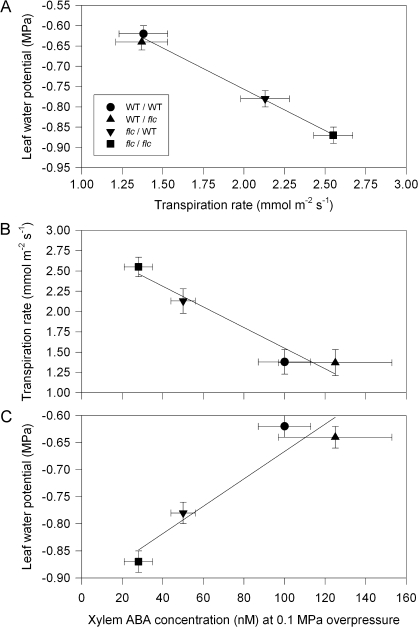
Measuring transpiration rate (Eleaf), [X-ABA] and Ψleaf of each leaf allowed these variables to be correlated. Leaf water potential decreased as single leaf transpiration rate increased (Fig. 5A). Transpiration rate decreased as [X-ABA] increased (Fig. 5B), thus leaf water potential decreased as [X-ABA] decreased (Fig. 5C).
Fig. 5.
Correlation of leaf water potential and single leaf transpiration rate (A), single leaf transpiration rate and xylem ABA concentration (B) and leaf water potential and xylem ABA concentration (C) for unmisted WT/WT (black circles),WT/flc (black triangles), flc/WT (black inverted triangles), and flc/flc (black squares) plants. Data are means ±SE of 5–6 plants.
Differences in [X-ABA] between flc/WT and flc/flc plants were confirmed in two additional experiments with unmisted plants. When data from three measurement occasions in one experiment were pooled (since measurement occasion had no significant effect on [X-ABA]), the mean (±SE) [X-ABA] of flc/WT plants (17±2 nM, n=10) was 2.7-fold higher than flc/flc plants (6±1 nM, n=9). When data from xylem saps collected at two different overpressures (0.1 versus 0.4 MPa) were pooled (since overpressure had no significant effect on [X-ABA]), the mean (±SE) [X-ABA] of flc/WT plants (42±5 nM, n=12) was 1.7-fold higher than flc/flc plants (25±3 nM, n=7).
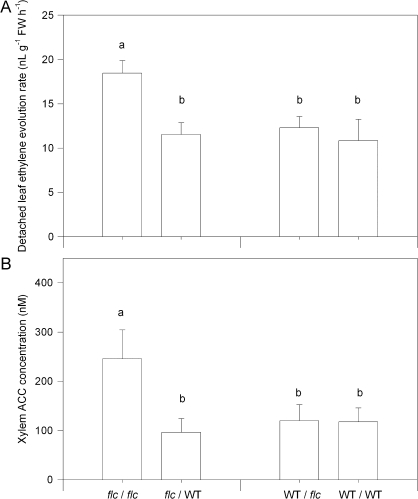
The rootstock had no effect on detached leaf ethylene evolution (Fig. 6A) or xylem ACC concentration (Fig. 6B) of WT scions. However, a WT rootstock decreased detached leaf ethylene evolution (Fig. 6A) and xylem ACC concentration (Fig. 6B) of flc scions to levels equivalent to WT scions. For both variables, there were significant (P <0.05) rootstock effects although for xylem ACC concentration, the effect of the rootstock was dependent on the scion (Table 3).
Fig. 6.
Detached leaf ethylene evolution (A) and leaf xylem ACC concentration (B) of unmisted WT/WT, WT/flc, flc/WT, and flc/flc plants. Data are means ±SE of 6–12 plants, with significant (P <0.05) differences between treatments according to Tukey's HSD test denoted by different letters above the bars. P values for two-way ANOVA are reported in Table 3.
Discussion
Much work has tried to elucidate the role of root-synthesized ABA in regulating plant responses to soil drying, by correlating root (and more usually xylem) ABA concentrations with shoot physiology (reviewed in Dodd, 2005), and by investigating reciprocal grafts of wild-type plants and ABA-deficient mutants (Fambrini et al., 1995; Chen et al., 2002, 2003; Holbrook et al., 2002; Christmann et al., 2007). The latter studies have generally concluded that stomatal closure is independent of root-synthesized ABA. Interestingly, [X-ABA]leaf of ‘two root, one shoot’ grafted WT plants exposed to heterogeneous soil moisture was best explained by accounting for the ABA contributions from each root system (Dodd et al., 2008). Furthermore, scion vigour of tomatoes grafted on different rootstocks and grown under moderate salinity was negatively correlated with the [X-ACC]/[X-ABA] ratio (Albacete et al., 2009), suggesting that the role of rootstock-mediated changes in ABA status will continue to be investigated in different contexts. Under well-watered conditions here, for several shoot variables (leaf area, gs and [X-ACC]leaf), rootstock effects were dependent on the scion (ie scion×rootstock interaction was significant: Tables 1, 3) thus each scion genotype is discussed separately.
An ABA-deficient rootstock has minimal effects on WT scions
In WT scions, decreased export of root-synthesized ABA had no significant effect on whole plant leaf area or number (Fig. 1), leaf water potential (Fig. 3A), whole plant transpiration rate (Fig. 4A) and single leaf stomatal conductance (Fig. 4B). Only in one experiment at specific nodes did the leaf area of WT/flc and WT/WT plants differ (Fig. 2A). Although reciprocal grafting allows the influence of the rootstock on shoot phenotype to be assessed, such influence did not extend to differences in xylem hormone concentration beyond the graft union in WT scions. When sap was collected from attached leaves by pressurizing the root system of well-watered plants (balancing pressure <600 kPa), [X-ABA] of WT scions was independent of whether the rootstock was WT or sitiens (Holbrook et al., 2002). Similarly, when sap was collected by pressurizing detached leaves, [X-ABA] and [X-ACC] of WT/flc plants was indistinguishable from WT self-grafts (Figs 3B, 6B), although the [X-ABA] of sap exuded from the root system of WT/flc plants was half that of WT self-grafts (Chen et al., 2002). Since this discrepancy implies that the transport pathway modifies [X-ABA] in transit in WT/flc plants, it is important to determine whether the explanation is technical (owing to the method of sap collection) or physiological.
The latter conclusion is supported by the correlation between measured [X-ABA] and whole plant (cf. Figs 3B, 4A) or single leaf transpiration rate (Fig. 5B). Nevertheless, application of pneumatic pressure to detached leaves (as in this study) may force symplastic sap into the xylem lumen (Jachetta et al., 1986; Borel and Simmoneau, 2002) thus contributing to the measured [X-ABA]. However, since the overpressure applied (0.1 MPa versus 0.4 MPa) did not affect [X-ABA]leaf, it seems that this technique provides a robust estimate of in vivo [X-ABA]. There are also concerns that the collection of xylem sap from roots via exudation (as in Chen et al., 2002) overestimates xylem hormone concentration, because solute concentration increases as xylem flow rate declines (reviewed in Dodd, 2005). Since root exudation rate increases with root ABA concentration (Nagel et al., 1994), differences in root exudate [X-ABA] between WT/WT and WT/flc plants will be minimized as sap from WT roots should flow faster (thus decreasing [X-ABA]) than sap from flc roots. Therefore the higher root exudate [X-ABA] of WT/WT plants compared to WT/flc plants (Chen et al., 2002) probably underestimates in vivo differences in [X-ABA] as it exits the root system. A higher [X-ABA] above than below the graft union of WT/flc plants implies enrichment of the transpiration stream by scion-sourced ABA, and such shoot mediation of [X-ABA] explains the failure of ABA-deficient rootstocks to influence shoot physiology of WT scions (Holbrook et al., 2002; Christmann et al., 2007). Such shoot enrichment of the root-supplied ABA message implies that the influence of root ABA biosynthesis will be most apparent in ABA-deficient scions.
A WT rootstock partially phenotypically reverts flc scions
In flc scions, increased export of root-synthesized ABA significantly increased leaf area (Figs 1A, 2) and leaf water potential (Fig. 3A) and decreased single leaf stomatal conductance (Fig. 4B) but not whole plant transpiration rate (Fig. 4A). Furthermore, a WT rootstock increased [X-ABA] of flc/WT plants relative to flc self-grafts, in both sap exuded from the root system of hydroponically grown plants (Chen et al., 2002) and collected from detached leaves of well-watered soil-grown plants (Fig. 3B, inset). However, the failure of the WT rootstock to increase [X-ABA] of flacca scions to the levels of WT scions indicates that the contribution of the root system to [X-ABA] of these well-watered plants was relatively small. Rather, basipetal transport of ABA in the phloem to the root system and its subsequent recirculation in the xylem (reviewed in Dodd, 2005) seems to be the dominant influence on [X-ABA] of flc/WT plants. Further ‘hormone flow modelling’ experiments (sensu Jiang and Hartung, 2008) in such grafted plants will be important to define more precisely the contributions of root versus shoot to xylem hormone concentrations.
In flc scions, a WT rootstock also decreased leaf xylem ACC concentration (Fig. 6B), in similar proportions to the decrease in detached leaf ethylene evolution (Fig. 6A). Proportional changes in xylem ACC and leaf ethylene evolution suggests that root ACC export quantitatively accounts for shoot ethylene evolution of well-drained and flooded tomato plants (Else and Jackson, 1998). Decreased ethylene evolution of flc scions (Fig. 6A) in response to an additional supply of ABA from the roots (Fig. 3B, inset) is consistent with observations that foliar ABA sprays normalized leaf ethylene evolution of ABA-deficient flc tomato (Sharp et al., 2000) and aba2-1 Arabidopsis (LeNoble et al., 2004). Furthermore, that leaf area of the aba2-1 etr1-1 double mutant (with both ABA deficiency and ethylene insensitivity) was intermediate between aba2-1 and WT plants suggests a direct (ethylene-independent) promotion of leaf area by ABA (LeNoble et al., 2004). Similarly, that a WT rootstock normalized shoot ethylene relations, but only partially restored leaf area of flc scions (leaf area of flc/WT plants was 75% of WT/WT plants), suggests that additional shoot-synthesized ABA directly promotes normal leaf area. However, whether root-synthesized ABA directly promotes leaf area independently of ethylene awaits further experiments where ethylene perception of flc/WT plants is blocked.
Our understanding of the mechanism(s) by which ABA or ethylene regulate growth has advanced on several fronts: determining whether effects are chemical or hydraulic in nature by measuring and/or manipulating leaf water relations (the approach adopted here), and measuring cell wall properties and the activities of selected cell wall loosening enzymes of growing tissues in response to hormone application (Wu et al., 1994, 1996). With the latter approach, it can be difficult to be certain whether enzyme activity is actually regulating, or simply responding to, changes in growth that may be mediated by additional (not measured) factors. For this reason, several laboratories have taken a comprehensive proteomic (Zhu et al., 2007) and genomic (Spollen et al., 2009) approach to gain further insights into complex growth regulatory mechanisms in specific tissues differing in growth rates. Combining such approaches with high throughput, multi-analyte physicochemical quantification of plant hormones (Albacete et al., 2009) in growing tissues of available plant hormone mutants represents a powerful approach to understanding the chemical regulation of growth.
However, the tight coupling of leaf growth rate with hydraulic properties of maize genotypes differing in ABA status (Parent et al., 2009) emphasizes the importance of determining whether the growth promotion of flc/WT plants is via chemical or hydraulic factors, especially since partial ABA-induced stomatal closure (Fig. 4B) of flc/WT plants increased Ψleaf (Fig. 3A). Differences in Ψleaf between the graft combinations (Figs 3A, 5A) were generally consistent with those reported for reciprocally grafted wilty and WT sunflower (Helianthus annuus) plants (Fambrini et al., 1995). A WT rootstock increased Ψleaf of mutant scions, but not to the level of WT scions. The simplest interpretation of these differences in Ψleaf between graft combinations is that Eleaf controls Ψleaf (Fig. 5A) and not vice versa. Since [X-ABA] controls Eleaf (Fig. 5B), scions with the lowest [X-ABA] also have the lowest Ψleaf (Fig. 5C). However, differences in stomatal behaviour cannot always explain Ψleaf in reciprocal grafts involving ABA-deficient mutants. In well-watered sunflower plants, gs (and presumably Eleaf) of mutant scions grafted on WT rootstocks was equivalent to WT self-grafts (Fambrini et al., 1995), implying that the decreased Ψleaf of wilty/WT grafts must be due to differences in the resistance to water flow elsewhere in the scion or rootstock. In such cases, direct measurements of stem and root hydraulic conductance are needed to account for the differences in Ψleaf.
Decreased gs (Fig. 4B) and Eleaf (Fig. 5) of flc/WT plants relative to flc self-grafts did not decrease whole plant transpiration rate (Eplant) (Fig. 4A). Fully expanded leaves (selected for ease of xylem sap collection and measurement of gas exchange) were exposed to lower light intensities lower in the plant canopy, and thus may not have a dominant influence on Eplant. Furthermore, stomatal measurements made at only one time point during the day may not always reflect stomatal behaviour over the course of the whole day. Despite these issues, Eplant of the different graft combinations was generally consistent with [X-ABA] measured in single detached leaves (cf. Figs 3B, 4A). The 2–3-fold increase in [X-ABA] (and possibly bulk leaf ABA concentration: Cornish and Zeevaart, 1988; Chen et al., 2002) of flc/WT plants when compared to flc/flc plants, was apparently insufficient to significantly impact on Eplant, implying that a threshold ABA concentration is required to prevent excessive transpiration.
Increased leaf area of flc/WT plants relative to flc/flc self-grafts (Fig. 1A) was independent of shoot water status, as relative effects of the different graft combinations were similar in the presence and absence of a shoot misting treatment. This is consistent with laboratory experiments showing that, although shoot misting transiently stimulated tomato leaf growth, a subsequent growth depression following each misting event resulted in misting having no net effect on whole plant leaf area when integrated over periods of days (Stirzaker et al., 1997). Thus chemical, and not hydraulic, influences were apparently responsible for the phenotypes of the different graft combinations observed under well-watered conditions.
Conclusion
Increased leaf area of flc scions on a WT rootstock (compared to flc self-grafts) could not be attributed to improved leaf water relations (cf. Figs 1A, 3A), but rather the normalization of shoot ethylene relations (Fig. 6) and perhaps a direct promotion of growth by root-synthesized ABA by an, as yet, unidentified mechanism. By contrast, the failure of the flc rootstock to influence either physiology of, or [X-ABA] and [X-ACC] in, WT scions indicate a limited role for root-synthesized ABA and that shoot ABA biosynthesis is sufficient to confer a WT shoot phenotype.
Acknowledgments
We thank the Tomato Genetic Resources Centre for supply of mutant seed and Maureen Harrison (Lancaster) for plant care during the pre-grafting stage. BBSRC (Grant ref: BB/DO12821/1) supported this work.
Glossary
Abbreviations
- Eleaf, Eplant
leaf and whole plant transpiration rate
- flc
flacca
- gs
stomatal conductance
- Ψleaf
leaf water potential
- θ
(volumetric) soil water content
- WT
wild-type
- [X-ABA], [X-ACC]
xylem ABA and ACC concentrations
- [X-ABA]leaf
leaf xylem ABA concentration
- [X-ACC]leaf
leaf xylem ACC concentration
References
- Albacete A, Martínez-Andújar C, Ghanem ME, Acosta M, Sánchez-Bravo J, Asins MJ, Cuartero J, Lutts S, Dodd IC, Pérez-Alfocea F. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence and increased leaf area and crop productivity in salinised tomato. Plant, Cell and Environment. 2009;32:928–938. doi: 10.1111/j.1365-3040.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- Borel C, Simonneau T. Is the ABA concentration in the sap collected by pressurizing leaves relevant for analysing drought effects on stomata? Evidence from ABA-fed leaves of transgenic plants with modified capacities to synthesize ABA. Journal of Experimental Botany. 2002;53:287–296. doi: 10.1093/jexbot/53.367.287. [DOI] [PubMed] [Google Scholar]
- Chen G, Fu XP, Lips SH, Sagi M. Control of plant growth resides in the shoot, and not in the root, in reciprocal grafts of flacca and wild-type tomato (Lycopersicon esculentum) in the presence and absence of salinity stress. Plant and Soil. 2003;256:205–215. [Google Scholar]
- Chen G, Lips SH, Sagi M. Biomass production, transpiration rate and endogenous abscisic acid levels in grafts of flacca and wild-type tomato (Lycopersicon esculentum) Functional Plant Biology. 2002;29:1329–1335. doi: 10.1071/PP01263. [DOI] [PubMed] [Google Scholar]
- Christmann A, Weiler EW, Steudle E, Grill E. A hydraulic signal in root-to-shoot signalling of water shortage. The Plant Journal. 2007;52:167–174. doi: 10.1111/j.1365-313X.2007.03234.x. [DOI] [PubMed] [Google Scholar]
- Cornish K, Zeevaart JAD. Phenotypic expression of wild-type tomato and three wilty mutants in relation to abscisic acid accumulation in roots and leaflets of reciprocal grafts. Plant Physiology. 1988;87:190–194. doi: 10.1104/pp.87.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Jones HG. Abscisic acid: physiology and biochemistry. Oxford, UK: Bios Scientific Publishers; 1991. [Google Scholar]
- Dodd IC. Leaf area development of ABA-deficient and wild-type peas at two levels of nitrogen supply. Functional Plant Biology. 2003;30:777–783. doi: 10.1071/FP02227. [DOI] [PubMed] [Google Scholar]
- Dodd IC. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant and Soil. 2005;274:251–270. [Google Scholar]
- Dodd IC, Egea G, Davies WJ. ABA signalling when soil moisture is heterogeneous: decreased photoperiod sap flow from drying roots limits ABA export to the shoots. Plant, Cell and Environment. 2008;31:1263–1274. doi: 10.1111/j.1365-3040.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- Dodd IC, Munns R, Passioura JB. Does shoot water status limit leaf area of nitrogen-deprived barley? Journal of Experimental Botany. 2002;53:1765–1770. doi: 10.1093/jxb/erf030. [DOI] [PubMed] [Google Scholar]
- Else MA, Jackson MB. Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycopersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Australian Journal of Plant Physiology. 1998;25:453–458. [Google Scholar]
- Fambrini M, Vernieri P, Toncelli ML, Rossi VD, Pugliesi C. Characterization of a wilty sunflower (Helianthus annuus L) mutant. III. Phenotypic interaction in reciprocal grafts from wilty mutant and wild-type plants. Journal of Experimental Botany. 1995;46:525–530. [Google Scholar]
- Holbrook NM, Shashidhar VR, James RA, Munns R. Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. Journal of Experimental Botany. 2002;53:1503–1514. [PubMed] [Google Scholar]
- Hussain A, Black CR, Taylor IB, Roberts JA. Does an antagonistic relationship between ABA and ethylene mediate shoot growth when tomato (Lycopersicon esculentum Mill.) plants encounter compacted soil? Plant, Cell and Environment. 2000;23:1217–1226. [Google Scholar]
- Imber D, Tal M. Phenotypic reversion of flacca, a wilty mutant of tomato, by abscisic acid. Science. 1970;169:592–593. doi: 10.1126/science.169.3945.592. [DOI] [PubMed] [Google Scholar]
- Jachetta JJ, Appleby AP, Boersma L. Use of the pressure-vessel to measure concentrations of solutes in apoplastic and membrane-filtered symplastic sap in sunflower leaves. Plant Physiology. 1986;82:995–999. doi: 10.1104/pp.82.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Hartung W. Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. Journal of Experimental Botany. 2008;59:37–43. doi: 10.1093/jxb/erm127. [DOI] [PubMed] [Google Scholar]
- Jokhan AD, Harink RJ, Jackson MB. Concentration and delivery of abscisic acid in xylem sap are greater at the shoot base than at a target leaf nearer to the shoot apex. Plant Biology. 1999;1:253–260. [Google Scholar]
- Jones HG, Sharp CS, Higgs KH. Growth and water relations of wilty mutants of tomato (Lycopersicon esculentum Mill.) Journal of Experimental Botany. 1987;38:1848–1856. [Google Scholar]
- LeNoble ME, Spollen WG, Sharp RE. Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. Journal of Experimental Botany. 2004;55:237–245. doi: 10.1093/jxb/erh031. [DOI] [PubMed] [Google Scholar]
- Nagel OW, Konings H, Lambers H. Growth rate, plant development and water relations of the ABA-deficient mutant sitiens. Physiologia Plantarum. 1994;92:102–108. [Google Scholar]
- Neill SJ, Horgan R. Abscisic acid production and water relations in wilty tomato mutants subjected to water deficiency. Journal of Experimental Botany. 1985;36:1222–1231. [Google Scholar]
- Parent B, Hachez C, Redondo E, Simonneau T, Chaumont C, Tardieu F. Drought and ABA effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiology. 2009;149:2000–2012. doi: 10.1104/pp.108.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Näsholm T. A GC-MS method for determination of amino acid uptake by plants. Physiologia Plantarum. 2001;113:352–358. doi: 10.1034/j.1399-3054.2001.1130308.x. [DOI] [PubMed] [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. A monoclonal antibody to (S)-abscisic acid: its characterization and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. Journal of Experimental Botany. 2000;51:1575–1584. doi: 10.1093/jexbot/51.350.1575. [DOI] [PubMed] [Google Scholar]
- Smets R, Claes V, Van Onckelen H, Prinsen E. Extraction and quantitative analysis of 1-aminocyclopropane-1-carboxylic acid in plant tissue by gas chromatography coupled to mass spectrometry. Journal of Chromatography A. 2003;993:79–87. doi: 10.1016/s0021-9673(02)01817-4. [DOI] [PubMed] [Google Scholar]
- Spollen WG, Tao W, Valloyidan B, et al. Spatial distribution of transcript changes in the maize primary root elongation zone at low water potential. BMC Plant Biology. 2009;8:32. doi: 10.1186/1471-2229-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirzaker RJ, Hayman PT, Sutton BG. Misting of tomato plants improves leaf water status but not leaf growth. Australian Journal of Plant Physiology. 1997;24:9–16. [Google Scholar]
- Tal M. Abnormal stomatal behavior in wilty mutants of tomato. Plant Physiology. 1966;41:1387–1391. doi: 10.1104/pp.41.8.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Imber D, Erez A, Epstein E. Abnormal stomatal behaviour and hormone imbalance in flacca, a wilty mutant of tomato. V. Effect of abscisic acid on indoleacetic acid metabolism and ethylene evolution. Plant Physiology. 1979;63:1044–1048. doi: 10.1104/pp.63.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M, Nevo Y. Abnormal stomatal behavior and root resistance, and hormonal imbalance in three wilty mutants of tomato. Biochemical Genetics. 1973;8:291–300. doi: 10.1007/BF00486182. [DOI] [PubMed] [Google Scholar]
- Taylor IB, Linforth RST, Al Naieb RJ, Bowman WR, Marples BA. The wilty tomato mutants flacca and sitiens are impaired in the oxidation of ABA-aldehyde to ABA. Plant, Cell and Environment. 1988;11:739–745. [Google Scholar]
- Termaat A, Passioura JB, Munns R. Shoot turgor does not limit shoot growth of NaCl-affected wheat and barley. Plant Physiology. 1985;77:869–872. doi: 10.1104/pp.77.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf area. Plant Physiology. 2007;143:1905–1917. doi: 10.1104/pp.106.093559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, Sharp RE, Durachko DM, Cosgrove DJ. Growth maintenance of the maize primary root at low water potentials involves increases in cell-wall extension properties, expansin activity, and wall susceptibility to expansins. Plant Physiology. 1996;111:765–772. doi: 10.1104/pp.111.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, Spollen WG, Sharp RE, Hetherington PR, Fry SC. Root growth maintenance at low water potentials: increased activity of xyloglucan endotransglycosylase and its possible regulation by abscisic acid. Plant Physiology. 1994;106:607–615. doi: 10.1104/pp.106.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JM, Alvarez S, Marsh EL, et al. Cell wall proteome in the maize primary root elongation zone. II. Region-specific changes in water soluble and lightly ionically bound proteins under water deficit. Plant Physiology. 2007;145:1533–1548. doi: 10.1104/pp.107.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]