Abstract
It has previously been shown that hydraulic conductance in bur oak leaves (Quercus macrocarpa Michx.), measured with the high pressure flow meter technique (HPFM), can significantly increase within 30 min following exposure to high irradiance. The present study investigated whether this increase could be explained by an increase in the cell-to-cell pathway and whether the response is linked to changes in the transcript level corresponding to aquaporin genes. Four cDNA sequences showing high similarity to members of the aquaporin gene family from other plant species were characterized from bur oak leaves and the expression levels of these cDNA sequences were examined in leaves by quantitative real-time PCR (QRT-PCR). No change was found in the relative transcript abundance corresponding to these four putative aquaporin genes in leaves with light-induced high hydraulic conductance (exposed to high irradiance) compared to leaves with low hydraulic conductance (exposed to low irradiance). However, in sun leaves that were exposed to different light levels prior to leaf collection (full sunlight, shade, and covered with aluminium foil for 16 h), the relative transcript levels of two of the putative aquaporin genes increased several-fold in shaded leaves compared to the sun-exposed or covered leaves. When the leaves were pressure-infiltrated with the apoplastic tracer dye trisodium 3-hydroxy-5,8,10-pyrenetrisulphonate (PTS3, 0.02%), there was no change in the PTS3 concentration of leaf exudates collected in ambient light or in high irradiance, but there was a small apoplastic acidification. There was also no change in PTS3 concentration between the leaves infiltrated under high irradiance with 0.02% PTS3 or with 0.1 mM HgCl2 in 0.02% PTS3. The results suggest that the putative aquaporin genes that were identified in the present study probably do not play a role in the light responses of hydraulic conductance at the transcript level, but they may function in regulating water homeostasis in leaves adapted to different light conditions. In addition, it is shown that high irradiance induced changes in the pH of the apoplast and that there does not appear to be a significant shift to the cell-to-cell mediated water transport in bur oak leaves exposed to high irradiance as measured by the apoplastic tracer dye.
Keywords: Aquaporins; leaf hydraulic conductance; high-pressure flow meter; irradiance; transcript level; trisodium 3-hydroxy-5,8,10-pyrenetrisulphonate
Introduction
The ability of leaves to transport water, as measured by the hydraulic conductance of leaves, is a dynamic characteristic (Sack and Holbrook, 2006). While water flow across leaves follows the apoplastic, symplastic, and transmembrane pathways (Steudle, 2000; Maurel et al., 2008), the relative contributions of these pathways to leaf hydraulic conductance are not well characterized. Changes in hydraulic conductance can take place in the apoplastic and transmembrane pathways (Salleo et al., 2001; Zwieniecki, 2001). In the transmembrane pathway, the cell membrane water permeability can be rapidly modulated by the abundance and activity of aquaporins (Tyerman et al., 2002; Maurel et al., 2008).
Aquaporins in plants constitute a large and divergent family with 35 members identified in Arabidopsis thaliana (Johanson et al., 2001), 33 members in Oryza sativa (Sakurai et al., 2005), and 36 members in Zea mays (Chaumont et al., 2001). Based on sequence similarities, plant aquaporins are typically divided into four subfamilies: plasma membrane intrinsic proteins (PIP), tonoplast intrinsic proteins (TIP), noduline-26 like intrinsic proteins (NIP), and small basic intrinsic proteins (SIP) (Johanson et al., 2001; Zardoya, 2005; Danielson and Johanson, 2008). Three new aquaporin subfamilies have also been described recently in Physcomitrella patens, designated GlpF-like intrinsic proteins (GIP), hybrid intrinsic proteins (HIP), and X intrinsic proteins (XIP) (Danielson and Johanson, 2008). The XIP subfamily appears to be found in a range of dicotyledonous taxa (Danielson and Johanson, 2008). The PIP subfamily can be further divided into two groups: PIP1, with low water permeability, and PIP2 with high water permeability, as determined by heterologous expression and swelling assays using Xenopus oocytes (Chaumont et al., 2001; Katsuhara et al., 2002). Heterotetramerization of PIP1 and PIP2 aquaporin isoforms may enhance the water transport activity of aquaporins (Fetter et al., 2004).
The contribution of aquaporins to leaf water transport is not well understood. Aquaporin localization in leaves (Kaldenhoff et al., 1995; Robinson et al., 1996; Sarda et al., 1997; Frangne et al., 2001), the accumulation of apoplastic dyes in minor veins of transpiring leaves (Canny, 1995), temperature responses (Sack et al., 2004), and the chemical inhibition of leaf hydraulic conductance (Nardini et al., 2005) argue for a role of aquaporins in the bulk leaf water transport. In addition, in detached walnut (Juglans regia) leaves, the irradiance-dependent increase of leaf hydraulic conductance during HPFM measurements (when leaves are filled with water under constant pressure) was correlated with increased expression of two PIP2 aquaporin genes (Cochard et al., 2007). However, other studies suggest that leaf aquaporins may not always play a dominant role in leaf water transport. For example, leaf hydraulic conductance was similar in wild-type Arabidopsis and double antisense plants with reduced expression of PIP1 and PIP2 aquaporins (Martre et al., 2002). Also in Arabidopsis, protoplasts isolated from leaves with high rates of transpiration showed reduced osmotic water permeability while the opposite was true for the protoplasts from leaves with reduced transpiration (Morillon and Chrispeels, 2001). In tobacco, leaf hydraulic conductance was similar in wild-type and transgenic plants constitutively overexpressing PIP2;5 and PIP1;4 aquaporins under high and low irradiance conditions (Lee et al., 2009). In addition, cell pressure-probe studies showed that cell hydraulic conductivity decreased in the leaves of plants in response to high irradiance, probably due to the inhibition of aquaporin-mediated water transport (Kim and Steudle, 2007, 2008; Lee et al., 2008, 2009). Clearly, more studies are needed to clarify the role of aquaporins in leaf water transport.
In leaves, water flows through several order veins before leaving the xylem and entering the mesophyll. In larger veins the xylem and phloem are surrounded by parenchyma cells and by supporting cells such as collenchyma and sclerenchyma. The smallest veins are enclosed in a tightly packed layer of cells called the bundle sheath which sometimes may have lignified or suberized cell walls. Analogous to the endodermis in roots, the bundle-sheath cells may constitute a barrier that prevents water loss from the xylem (Van Fleet, 1950; Sack and Holbrook, 2006). In transpiring leaves, apoplastic dye crystals may accumulate in leaf minor veins suggesting the movement of water through the bundle-sheath cells (Canny, 1990). In a recent study (Salleo et al., 2003), Phloxin B apoplastic dye was infiltrated under positive pressure through the petioles inside leaves to show that, depending on a plant species, major veins may be leaky or water is transported to increasing order veins before exiting the xylem.
It has previously been shown that the irradiance-dependent increase of leaf lamina hydraulic conductance in bur oak leaves, as measured by the HPFM method, could be modulated by various classes of chemical inhibitors, suggesting that the cell-to-cell transport may play a role in this response (Voicu et al., 2008). The main objective of the present study was to examine the contribution of aquaporin gene expression at the level of transcript abundance to water transport in bur oak leaves exposed to low and high irradiance. The apoplastic fluorescent tracer dye, trisodium 3-hydroxy-5,8,10-pyrenetrisulphonate (PTS3) was used to test the relative contributions of the apoplastic versus the cell-to-cell pathway (Voicu and Zwiazek, 2004). Consistent with the previous work (Cochard et al., 2007; Voicu et al., 2008) it was expected that, if high irradiance increases the expression of aquaporins in excised leaves, a similar response would be found in the sun-exposed leaves collected from trees.
Materials and methods
Plant material
Oak (Quercus macrocarpa Michx.) leaves were collected from two mature trees growing at the University of Alberta North Campus, Edmonton, AB, Canada. After excision, the leaf petioles were quickly placed in distilled water and the leaves were brought to the laboratory. The petioles were re-cut under water, the leaves connected to the high pressure flow meter (HPFM, Dynamax Inc., Houston, TX, USA), and their hydraulic conductance measured as described below. Ten leaves per treatment were sampled from one tree (July 2007) and three leaves per treatment were sampled from the second tree (August 2007), about three weeks apart.
In July 2007, several attached, intact leaves from one of the above trees were covered at about 18.00 h with aluminium foil. The next morning at about 10.00 h, the leaves were quickly excised from the tree branch and rapidly frozen in liquid nitrogen. At the same time, several sun leaves were also sampled as above from the same tree branch. At the time of sampling, the sun leaves were either in full sunlight (between 1300 and 1750 μmol m−2 s−1 photosynthetic photon flux, PPF), or shaded by other leaves (PPF between 50 and 250 μmol m−2 s−1). The leaves were shaded due to the angle of the sun and it was not recorded for how long they were shaded. For RNA extraction, all leaf samples were stored at –80 °C.
Leaf hydraulic conductance measurements
Leaf hydraulic conductance (Kleaf) was measured using the HPFM as previously described by Voicu et al. (2008). Leaves were connected to the HPFM through their petioles using compression couplings, and were perfused with water at constant pressure ranging from 350-450 kPa. A computer recorded the water flow (Q, kg s−1) and the applied pressure (P, MPa) and computed Kleaf as Q/P every 2 s, saving mean values every 60 s. The leaves were immersed in distilled water during Kleaf determination to minimize temperature fluctuations at the leaf level. The flow stabilized after 30 min at ambient laboratory light (typically less than 15 μmol m−2 s−1 PPF). Subsequently, a subset of leaves were illuminated at about 1200 μmol m−2 s−1 PPF supplied by white LED lamps (BL-300 series, Lamina Ceramics, Westampton, NJ, USA), while control leaves were kept at ambient laboratory light. After 30 min of differential light treatments, the leaf blade was cut and the leaf area (A, cm2) measured with a leaf area meter (LI-3100, Li-Cor Biosciences, Lincoln, NE, USA). Leaf blades were then quickly frozen in liquid nitrogen and stored at –80 °C. Following leaf blade detachment, the flow usually stabilized within 1 min and then was recorded for an additional 6 min. Petiole hydraulic conductance (KP) was calculated as the mean of the last six readings.
Leaf lamina hydraulic conductance (Klam) was determined as Klam=(1/(1/Kleaf–1/KP))/A (Sack et al., 2002).
RNA extraction
Frozen leaf tissue was ground to a fine powder in liquid N2 using a prechilled mortar and pestle. The ground tissue was stored at –80 °C. Total RNA was extracted using the protocol of Chang et al. (1993) with modifications as described in Pavy et al. (2008). The pellet was dried and resuspended in RNase-free water. The RNA concentration was quantified by measuring the absorbance at 260 nm. The quality of RNA preparation was checked by calculating the ratio of A260/A280 nm. The integrity of RNA was checked on a 1% agarose gel containing ethidium bromide (0.001% v/v). Extracted RNA was stored at –80 °C.
Isolation of sequences encoding putative aquaporins
Total RNA (3 μg) was reverse-transcribed using the SuperscriptII Reverse Transcriptase kit, according to the manufacturer's instructions (final reaction volume 21 μl) (Invitrogen, San Diego, CA, USA). The resulting single-stranded cDNA was used for amplification of aquaporin cDNAs using PCR. Degenerate primers were designed against conserved regions from known plant aquaporin cDNA sequences using Vector NTI (Invitrogen). The following primers were used: for the PIP1 group 5′-CTTGGKCYTTYGGTGGYATGA-3′ (forward) and CTTGAADGGRATGGCYCTGA (reverse); for the PIP2 group 5′-AAGGAYTAYSWYGAYCCWCC-3′ (forward) and TBGYYGTAGATVACAGCAGC (reverse) (IDT, Coralville, IA, USA). EF1-α was cloned using the following primers: 5′-GCTGCTGAGATGAACAAGAG-3′ (forward) and 5′-AACGACCCAATGGAGGATAC-3′ (reverse). PCR was performed using the following conditions: 94 °C for 4 min; followed by 35 cycles of 94 °C for 30 s, 55 °C (PIP1) or 56 °C (PIP2) for 45 s, 72 °C for 60 s; and the last elongation step of 72 °C for 7 min. The 50 μl PCR reaction contained 1× ThermoPol Reaction Buffer and 2.5 U of 5 U μl−1 Taq DNA Polymerase (New England BioLabs, Beverly, MA, USA), 0.2–0.5 μM primers, 0.2 mM dNTPs (Fermentas, Burlington, ON, Canada), and 1 μl of the above cDNA preparation. PCR products were checked on a 1% agarose gel. For the PIP1 reaction, only one band was detected, which corresponded to the expected size of 580 bp. For the PIP2 reaction, the band corresponding to the expected size of 680 bp was excised from the gel. PCR products were purified using the QIAquik PCR purification or gel extraction kits (Qiagen, Chatsworth, CA, USA).
Purified PCR products were ligated into the pGEM-T plasmid according to the manufacturer's protocol (Promega, Madison, WI, USA). The vector to insert ratio was 1:1.5–3. Recombinant plasmids were used to transform E. coli DH5α cells using standard protocols (Sambrook and Russell, 2001). The presence of inserts was verified by PCR using SP6 and T7 universal primers, essentially as described above. Plasmids were purified from cultures grown overnight in liquid LB using GeneJET Plasmid Miniprep Kit (Fermentas). Plasmid preparations were visualized by separating on a 1% agarose gel containing ethidium bromide. The concentration and purity of plasmid preparations were determined by reading the absorbance at 260 nm, and by calculating A260/A280, respectively. Plasmids were stored at –20 °C.
Sequencing was carried out using the BigDye Terminator v3.1 Cycle Sequencing kit and the ABI 3730 DNA sequencer from Applied Biosystems (Foster City, CA, USA).
Multiple sequence alignment and phylogenetic analysis
Cloned DNA sequences were translated into deduced amino acid sequences using VectorNTI. ClustalW was used to generate multiple sequence alignments of the Q. macrocarpa sequences with aquaporin sequences from Arabidopsis thaliana, Juglans regia, and Populus tremula×P. tremuloides, as follows: AtPIP1;1 (CAB71073), AtPIP1;2 (AAC28529), AtPIP1;3 (AAF81320), AtPIP1;4 (AAF02783), AtPIP1;5 (CAA20461), AtPIP2;1 (CAB67649), AtPIP2;2 (AAD18142), AtPIP2;3 (AAD18141), AtPIP2;4 (BAB09839), AtPIP2;5 (CAB41102), AtPIP2;6 (AAC79629), AtPIP2;7 (CAA17774), AtPIP2;8 (AAC64216), JrPIP2;1 (AAO39007), JrPIP2;2 (AAO39008), PttPIP1;2 (CAH60718), PttPIP1;3 (CAH60719), PttPIP2;1 (CAH60720), PttPIP2;2 (CAH60721), PttPIP2;3 (CAH60722), PttPIP2;4 (CAH60723), PttPIP2;5 (CAH60724). Per cent similarity between different proteins was calculated as 1–distance, according to the Mega4 manual (Tamura et al., 2007). The alignments were visually inspected and manually adjusted as required. Maximum parsimony phylogenetic trees were constructed in Mega4 using both the complete sequence of PIP proteins from Arabidopsis, Juglans, and Populus and the partial PIP sequences corresponding to the cDNAs isolated from bur oak, as well as only the region common to all sequences. As a means to test the robustness of the tree architecture obtained using maximum parsimony, these trees were compared to trees obtained by the Neighbor–Joining method, with the reliability of the resulting Neighbor–Joining trees tested using the interior branch test of phylogeny with 1000 replicates.
Transmembrane helices were predicted with the online TopPred program (http://mobyle.pasteur.fr/cgi-bin/MobylePortal/portal.py?form = toppred), which predicts transmembrane regions using hydrophobicity analysis according to Claros and von Heijne (1994).
Quantitative real-time PCR (QRT-PCR)
To test the hypotheses that aquaporin expression will increase in the leaves which show a high irradiance-induced increase in hydraulic conductance and in the leaves exposed to full sunlight while on a tree crown, QRT-PCR experiments were performed. Plant material was collected and total RNA was extracted as described above. For each sample, 6 μg of DNaseI-treated total RNA was reverse transcribed using M-MuLV reverse transcriptase, and the cDNA was treated with RNaseH to remove the RNA using the manufacturer's protocol (New England Biolabs), with a final reaction volume of 20 μl. The cDNA was diluted 1:10, and 2.5 μl of the dilution was used for each QRT-PCR reaction. QRT-PCR analysis of the putative aquaporin genes was carried out in 96-well plates using the Applied Biosystems 7500 Fast Real-Time PCR System. The primers used for QRT-PCR were designed using Primer Express 3 (Applied Biosystems). The following gene-specific primers (5′–3′) were used: for QmPIP1;1 gene AAA AAG GTG GAG CCA ACT TCG (forward) and ACA TGG GAG TCC CTA GCG C (reverse); QmPIP2;2 gene GTG GTG GCG CCA ACT CTC (forward) and AGG AAC GTG TGA ATC TCT AGC TTT C (reverse); for QmPIP2;3 gene TAA TGG TGG TGG TGC TAA CCT TG (forward) and CGT GCG CTT CTC TTT GGG (reverse); for QmPIP2;1 gene GGT GGA GCC AAC GGG C (forward) and GAA CAT GGG AAT CCC TTG CA (reverse); for EF1-α gene CTC ACG GGT CTG TCC ATC CT (forward) and TGT GCC GTC CTC ATT ATT GAC T (reverse). Specificity of the amplification was confirmed by melting curve analysis for the PCR products at the end of each QRT-PCR run. For the quantitative PCR reaction, 2.5 μl of the cDNA dilution was used in a total volume of 10 μl containing 0.4 μM of each forward and reverse gene-specific primer, 0.2 mM dNTPs, 0.25× SYBR Green, 0.1× ROX, and 0.3 U Platinum Taq (Invitrogen). The cycling conditions were: Step 1, 95 °C for 2 min; Step 2, 40 cycles of 95 °C for 15 s and 60 °C for 60 s; Step 3, 95 °C for 15 s, 60 °C for 60 s, 95 °C for 15 s, and 60 °C for 15 s. Data collection were carried out at the end of each round in Step 2. Quantification of transcript abundance was performed using standard curves generated with a dilution series of amplicons produced from each of the aquaporin cDNAs, using plasmid inserts as template and SP6 and T7 universal primers for amplification in PCR reactions as described in previous sections. The five different amplicons were pooled prior to making a serial dilution series spanning from 4×107 to 4×101 copies for each amplicon. EF1-α was used as the reference gene. The amplifications were done on three to four independent samples for each treatment (biological replicates), and triplicate reactions were carried out for each sample (technical replicates). All samples that were to be compared statistically were assayed on the same 96-well plate. Therefore, each 96-well plate was divided into two standard curves (for the PIP and EF1-α genes) occupying 20 wells, two wells as blanks, and six wells for each sample (three for the PIP gene and three for the EF1-α gene). By assaying all biological replicates of an experiment for PIP expression and EF1-α expression on the same 96-well plate, the effect of plate-to-plate variation, that would have been encountered if multiple 96-well plates were used to accommodate additional biological replicates, was minimized. The amount of each amplicon (expressed as copy number) was determined using its corresponding standard curve. The relative abundance of each investigated gene was calculated by dividing the copy number of the transcript of interest with the copy number of EF1-α transcript. The mean values of the technical replicates were used for statistical analysis.
Relative apoplastic water flow and HgCl2 effects, pH changes in bur oak leaves
Changes in apoplastic water flux through leaves were investigated using a fluorescent tracer dye, trisodium 3-hydroxy-5,8,10-pyrenetrisulphonate (PTS3) (Hanson et al., 1985; Voicu and Zwiazek, 2004). The dye is membrane impermeable and does not bind to cell walls (Hanson et al., 1985; Moon et al., 1986). In leaves, water moving out of the xylem can flow either around the cells or enter the symplast at the bundle-sheath level. Since PTS3 is confined to the apoplast, it may be used to assess the relative contribution of apoplastic versus cell-to-cell pathways for water movement in leaves. Therefore, if irradiance increases the aquaporin activity in leaves and the contribution of the cell-to-cell pathway increases, a decline in the dye concentration from ambient light to high irradiance would be expected. Using pressure chambers (PMS Instruments, Corvallis, OR, USA), leaves were infused with a solution of 0.02% (w/v) PTS3 under 0.4 MPa pressure. The first droplets coming out of the stomata were wiped with paper towels, then leaves were enclosed in plastic bags, placed on the bench under ambient light (PPF <15 μmol m−2 s−1), and the and the solution was collected in the bags. After about 0.5 ml had been collected (about 1.5 h), the leaves were wiped dry with paper towels, enclosed in new plastic bags, and placed under high irradiance (PPF of about 1200 μmol m−2 s−1) provided by LED lamps as described above and a new fraction collected for about 30 min. Then the tertiary veins, secondary veins, and leaf blade were successively severed and the corresponding exudates collected in order to investigate if accumulation of PTS3 had occurred in the leaf veins and if there are apoplastic pH changes as water moves through the leaves. A small amount of the infusion solution was also collected. The pH of the different fractions collected was then measured with a micro pH probe (Jenco Electronics, Taipei, Taiwan). The concentration of PTS3 was determined using a Sequoia-Turner 450 fluorometer (Apple Scientific, Chesterland, OH, USA) using an excitation wavelength of 405 nm and an emission wavelength of 515 nm. Concentrations of PTS3 in different fractions were calculated from a standard curve prepared from known PTS3 concentrations.
To measure changes in apoplastic flow induced by mercury treatment, two solutions were forced inside different leaves: control solution consisting only of 0.02% PTS3, and 0.1 mM HgCl2 in 0.02% PTS3. The leaves were enclosed in plastic bags and placed under the high irradiance source as described above. The leaf exudates were collected every hour for 3 h. Before a new exudate was collected, leaves were wiped dry with paper towels and enclosed in new plastic bags. It was expected that if water moves mostly through the cell-to-cell pathway under high irradiance, mercury treatment would block the aquaporins and cause an increase in the dye concentration of the exudate, since more water would now move mostly through the apoplast.
Statistical analysis
Analysis of variance was performed using the GLM or Mixed procedure of SAS (Version 9.1, SAS Institute Inc., Cary, NC, USA). Data were checked to meet the normality and homogeneity of variances assumptions.
Lamina hydraulic conductance from different trees, PTS3 concentration, HgCl2 effects, and pH changes in bur oak leaves apoplast were analysed using a repeated measures analysis of variance. The Variance-Covariance Matrix chosen for each model was an iterative process and based on the Schwarz's Bayesian Criterion (SAS 2000). Petiole hydraulic conductivity was analysed with a t test. Transcript levels of different genes in leaves with Klam determined were analysed using the sampled tree as a random factor. For the QmPIP2;2 gene, the data were log transformed to meet the ANOVA assumptions. Data for QmPIP2;1 gene were analysed using ANOVA with unequal variance followed by mean separation with Tukey adjustment. For all different statistical models, Least Square Means (lsmeans) and standard errors were calculated for treatments and the lsmeans separated using a pdiff option. All differences were tested at the P ≤0.05 level. For the log transformed data mean values of the raw data were used for plotting.
Results
Cloning of new PIP genes expressed in Q. macrocarpa leaves
The above approach produced four unique sequences that showed high similarity to PIP genes after a BLAST analysis (Altschul et al., 1997; http://www.ncbi.nlm.nih.gov/BLAST). These partial cDNA clones have lengths between 578 bp and 677 bp, corresponding to 192–225 amino acids, respectively. The deduced amino acid sequence of these partial cDNA clones corresponded to approximately two-thirds to four-fifths of the full coding region of the Arabidopsis PIPs. A multiple alignment of the deduced amino acid sequences of Q. macrocarpa and aquaporins identified from other plant species indicated that one of the four sequences exhibits greater sequence similarity to the PIP1 class of aquaporins, while the other three sequences exhibit greater sequence similarity to the PIP2 class. Accordingly, these cDNAs were named QmPIP1;1, QmPIP2;1, QmPIP2;2, and QmPIP2;3, with accession numbers FJ495147, FJ495155, FJ495161, and FJ495163, respectively.
Structure and phylogenetic analysis of PIP genes from Q. macrocarpa
The deduced amino acid sequences of the identified cDNAs from Q. macrocarpa included features typical of MIP proteins. The partial sequences obtained in this study contained residues conserved in other PIP proteins, as well as the repeated NPA motif, a typical MIP family signature (Fig. 1). Hydrophobicity analysis of the putative partial polypeptides as determined by TopPred, indicated five membrane spanning putative α-helices (Fig. 1). All of the identified putative aquaporin amino acid sequences contain the four amino acid residues of the ar/R (aromatic/Arg) filter, thought to govern the water transport specificity of aquaporin-type MIP (Forrest and Bhave, 2008; Wallace and Roberts, 2004). They also contain histidine residues shown to be involved in pH sensing in other aquaporins (Luu and Maurel, 2005).
Fig. 1.
Multiple sequence alignment of PIP sequences from Q. macrocarpa, A. thaliana, and P. tremula×P. tremuloides. Predicted amino acid sequences were aligned using ClustalW. Identical residues in all sequences are shaded in black, while similar residues are shaded in grey. The conserved NPA motives are shaded in green. The ar/R selectivity filter residues are shaded in magenta. Histidine residues involved in pH sensing are shaded in blue. Predicted transmembrane helices are shown in brown boxes.
At the amino acid level, the Q. macrocarpa sequences share a high percentage similarity with different aquaporin proteins. The predicted amino acid sequence of QmPIP2;2 gene has the highest sequence similarity with PttPIP2;4 (87.9%), AtPIP2;5 (85.3%), JrPIP2;1 (85.3%), and JrPIP2;2 (85.3%). For the QmPIP2;1 sequence the highest similarity is shared with JrPIP2;1 (90.2%), JrPIP2;2 (90.2%), PttPIP2;5 (88.8%), PttPIP2;4 (86%), PttPIP2;3 (84.4%), AtPIP2;1 (84.4%), and AtPIP2;2 (84.4%). The QmPIP2;3 sequence has the highest amino acid similarity with AtPIP2;8 (88.9%), AtPIP2;7 (88.5%), PttPIP2;1 (87.6%), and PttPIP2;2 (87.6%). The QmPIP1;1 sequence has the highest sequence similarity with AtPIP1;3 (90.6%), AtPIP1;5 (90.6%), AtPIP1;1 (89.6%), and Ptt1;2 (89.6%). Among them, the deduced amino acid sequences of these partial cDNAs share between 77.5% and 88.5% sequence similarity. At the nucleotide level, the percentage similarity varies from 71.3% to 80.4%. Bur oak QmPIP2;1 and QmPIP2;2 also show a longer A loop region.
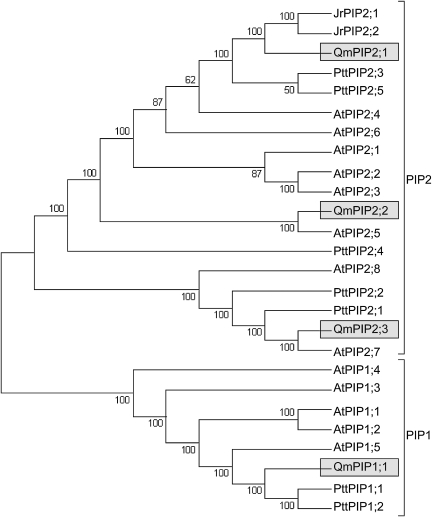
Phylogenetic analysis of the Q. macrocarpa PIP partial sequences was performed together with selected characterized aquaporin sequences from A. thaliana, J. regia, and P. tremula×P. tremuloides hybrid (Fig. 2). Figure 2 depicts a consensus maximum parsimony tree (of 212 trees) obtained using the portion of sequence common to all sequences. The maximum parsimony consensus tree (of eight trees) obtained using full-length sequences for Arabidopsis, Juglans, and Populus together with the partial Q. macrocarpa sequences showed identical topology and similar branch support to the tree obtained using only the common sequence region (data not shown). Neighbor–Joining trees obtained using either full-length sequences for Arabidopsis, Juglans, and Populus or the common sequence region also showed very similar topology (data not shown). The maximum parsimony tree showed clustering of the encoded proteins with different aquaporin subgroups, verifying that QmPIP1;1 belongs to the PIP1 class, while QmPIP2;1, QmPIP2;2, and QmPIP2;3 belong to the PIP2 class. According to the phylogenetic analysis, QmPIP1;1 is a potential orthologue of one Arabidopsis (AtPIP1;5) and two hybrid poplar (PttPIP1;1 and PttPIP1;2) aquaporins; QmPIP2;1 is a potential orthologue of AtPIP2;4, PttPIP2;3, PttPIP2;5, JrPIP2;1, and JrPIP2;2; QmPIP2;2 is a potential orthologue of AtPIP2;5; and QmPIP2;3 is a putative orthologue of AtPIP2;7, PttPIP2;1, and PttPIP2;2.
Fig. 2.
Phylogenetic relationship between QmPIP deduced amino acid sequences and sequences of A. thaliana, P. tremula×P. tremuloides, and J. regia. Sequences were aligned using ClustalW, and the region common to all sequences was selected for analysis by maximum parsimony. The maximum parsimony tree was obtained using Mega4. The percentage of parsimonious trees in which the associated taxa clustered together are shown next to the branches.
Klam light response and transcript level of putative leaf aquaporins
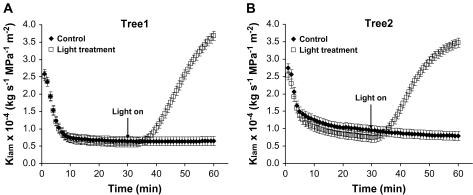
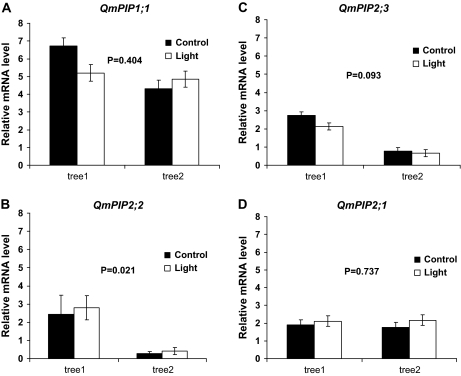
Lamina hydraulic conductance increased significantly in leaves from both trees upon exposure to high irradiance (Fig. 3A, B). For the same leaves, there were no significant differences in the transcript level of putative aquaporin genes between the treatments (Fig. 4). However, there were some significant differences (P=0.021) between trees in terms of the expression level of putative aquaporin genes (Fig. 4). As required before assessing gene expression, the suitability of EF1-α as a reference gene was assessed by performing ANOVA with the EF1-α CT values of each sample. There was no significant difference in the mean CT value of EF1-α between light treated (22.38±0.41) or control leaves (22.44±0.41) (P=0.894).
Fig. 3.
Time-course of lamina hydraulic conductance (Klam) of leaves from two bur oak trees: (A) tree 1 (n=10), and (B) tree 2 (n=3). Control leaves were exposed only to ambient laboratory lights. Light treated leaves were exposed for 30 min to high irradiance, following low irradiance exposure. Values are least square means. Mean standard error bars are also shown. At the end of Klam determination, leaves were quickly frozen and used for gene expression analysis.
Fig. 4.
Relative expression level of putative aquaporin genes QmPIP1;1 (A), QmPIP2;2 (B), QmPIP2;3 (C), and QmPIP2;1 (D), between the leaves with light-induced high hydraulic conductance (light treatment) and leaves with low hydraulic conductance (control), shown separately for each tree. Mean values ±standard errors are shown (n=3). The P values assess the statistical difference (P ≤0.05) between trees.
Aquaporin expression and the light responses within a tree crown
Different putative aquaporin genes showed different expression profiles with the light regime. The EF1-α was also used as a reference gene for this experiment since there were no significant difference in the mean CT value of EF1-α among shade-exposed (23.74±0.32), light-exposed (23.89±0.32), and dark-treated leaves (23.85±0.32) (P=0.947).
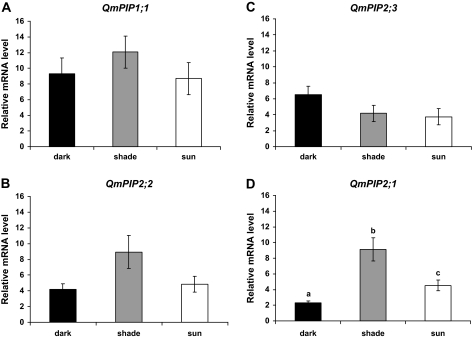
There was no significant difference in transcript abundance for QmPIP1;1 and QmPIP2;3 as a function of the different treatments (Fig. 5A, C). For QmPIP2;2, although the differences were not significant at the 0.05 level, there was a tendency for an increasing level of expression in the shade-exposed leaves (overall ANOVA P=0.073; dark-shade P=0.078; light-shade P=0.159; and light-dark P=0.888) (Fig. 5C). Different light regimes significantly influenced the expression of QmPIP2;1. The highest level of expression was found in shade-exposed leaves, followed by light exposed leaves, and dark-treated leaves (Fig. 5D).
Fig. 5.
Relative expression level of putative aquaporin genes QmPIP1;1 (A), QmPIP2;2 (B), QmPIP2;3 (C), and QmPIP2;1 (D), in leaves exposed to different light levels in situ (full sunlight, sun-adapted; shade, shade-adapted; and covered with aluminium foil, dark-adapted leaves). Mean values ±standard errors are shown (n=4). Bars with different letters represent significant (P ≤0.05) differences.
Changes in pH, PTS3 concentration and HgCl2 effects in leaf exudates
When leaves were exposed to high irradiance, the pH of the leaf exudate significantly decreased (P=0.04) (Table 1). The pH increased in the exudates collected after tertiary veins and secondary veins were severed. When the leaf blade was removed, the pH of the petiole exudates was similar to the pH of the infusion solution (Table1).
Table 1.
pH and PTS3 concentration changes in 0.02% PTS3 forced though bur oak leaves
| Treatment | pH | % PTS3×100 |
| Infusion solution | 5.07±0.13 a | 2.06±0.04 a |
| Ambient light leaf exudates | 5.09±0.19 a | 1.66±0.24 b |
| High irradiance leaf exudates | 4.72±0.17 b | 1.63±0.30 b |
| Tertiary veins exudates | 4.74±0.53 ab | 1.76±0.41 ab |
| Secondary veins exudates | 5.04±0.41 ab | 1.68±0.45 ab |
| Petiole | 5.24±0.20 a | 1.73±0.35 ab |
| ANOVA P value | 0.014 | 0.003 |
The leaves were exposed to ambient light (PPF <15 μmol m−2 s−1) followed by high irradiance (1000 μmol m−2 s−1 PPF), then tertiary veins and secondary veins were successively severed. At the termination of the experiment, the leaf blade was detached and the petiole exudates collected. The pH and % PTS3 of the infusion solution are presented. ANOVA P values for differences among different treatments are also given. The values represent least square means ±SD (n=5–6). The numbers followed by different letters represent significant differences (P ≤0.05) after Tukey adjustment.
The concentration of the apoplastic dye PTS3 significantly decreased (P=0.002) by 20% in leaf exudates collected in ambient light and did not change in the exudates collected in high irradiance (Table 1). When the leaves were subjected to high irradiance, the PTS3 concentration remained significantly lower than the concentration of the infusion solution. The concentration of PTS3 increased slightly in the exudates collected after vein severance (Table 1). However, the concentration of PTS3 in ambient light or high irradiance was not significantly different that that of vein exudates (Table 1).
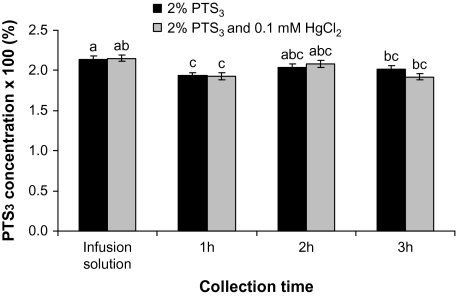
When leaves were pressure-infiltrated with a solution of PTS3 in combination with 0.1 mM HgCl2, the concentration of PTS3 in leaf exudates after 1 h significantly decreased by about 10% in both PTS3 (P=0.048) and PTS3+HgCl2 (P=0.03) treatments (Fig. 6). However, there were no differences between treatments over time, while the PTS3 concentrations in both treatments increased after 2–3 h from the beginning of the treatment (Fig. 6).
Fig. 6.
Changes in the concentration of a solution of 0.02% PTS3 pressure-infiltrated into oak leaves, alone or in combination with 0.1 mM HgCl2. Least square means ±SE are presented (n=6). Bars with different letters represent significant (P ≤0.05) differences after Tukey adjustment.
Discussion
The present study identified four putative aquaporin genes from bur oak leaves, and characterized the response of these genes to light at the level of transcript abundance. Contrary to our hypothesis, the transcript abundance corresponding to these four genes was not correlated with the irradiance-dependent increase in the lamina hydraulic conductance during the HPFM measurements. The experiment using leaves exposed to full sunlight and shade, showed that at least one aquaporin, QmPIP2;1, was differentially expressed when the leaves were exposed in situ to different light regimes. The study also showed that light induced changes in the pH of the apoplast and that there does not appear to be a significant shift to the cell-to-cell mediated water transport in bur oak leaves exposed to high irradiance.
The cDNA sequences identified from the bur oak leaves belong to the PIP subfamily of aquaporins, three to the PIP2 group and two to the PIP1 group. The presence of the highly conserved NPA motif and the ar/R selectivity filter residues (Fig. 1) suggest that the identified PIPs are likely to be functional aquaporins (Wallace and Roberts, 2004; Forrest and Bhave, 2008). The ar/R filter is thought to govern the water transport specificity of aquaporin-type MIP (Wallace and Roberts, 2004; Forrest and Bhave, 2008). In addition, the amino acid sequences contain the histidine residue involved in pH sensing (Luu and Maurel, 2005). Most of the putative orthologues of QmPIP2;1 and QmPIP2;2 suggested by phylogenetic analysis, with the exception of PttPIP2;3 and AtPIP2;5 have been shown to be highly conductive to water when expressed in oocytes (Sakr et al., 2003; Marjanovic et al., 2005). In addition, QmPIP2;1 and QmPIP2;2 also show a longer loop A region, which was suggested to increase water transport capacities (Marjanovic et al., 2005). All plant PIP2 proteins tested so far exhibit high water-channel activity, while PIP1 proteins have either low or no water-channel activity (Chaumont et al., 2005). However, PIP1 and PIP2 may form heterotetramers which may further enhance their water transport activities (Maurel et al., 2008).
The contribution of aquaporins to water transport in leaves is not very well understood. The rapid irradiance-dependant increase of leaf hydraulic conductance is thought to involve increased aquaporin activity (Tyree et al., 2005; Voicu et al., 2008). However, in the present study, there was no change in the relative expression level of four putative aquaporin genes between leaves with light-induced high hydraulic conductance (high irradiance) and those with low hydraulic conductance (low irradiance) (Figs 3, 4). By contrast, Cochard et al. (2007) showed a large increase in the JrPIP2;1 and JrPIP2;2 transcript levels in walnut leaves with light-induced high hydraulic conductance measured by the HPFM technique. There are several possible explanations for this apparent discrepancy. For example, although in our study the putative aquaporin genes were isolated from leaves of bur oak, it is possible that there are other aquaporins that are also expressed in leaves. Further, in Arabidopsis leaves, all of the PIP genes are expressed but at different levels (Alexandersson et al., 2005). Consequently, it is possible that one or more other leaf-expressed aquaporins contributes to leaf hydraulic conductance. It is also possible that the expression of aquaporins involved in regulating leaf hydraulic conductance is controlled at the post-translational rather than transcriptional level, since aquaporin activity can be regulated by post-translational modifications such as phosphorylation, protonation, and heterotetramer formation (Maurel et al., 2008). Finally, there may be species-specific differences in aquaporin expression and activity. In the present study, the difference in the aquaporin expression levels between the two different trees may be related to the different microenvironmental conditions of the sampled trees or to the time difference between sampling of trees (about 3 weeks).
During the HPFM measurements of Klam water is pushed under constant pressure inside detached leaves and quickly floods leaf air spaces. An infiltrated leaf apoplast resulted in cytoplasmic acidification and apoplastic alkalinization (Felle, 2006) and may decrease the rate of water transport through aquaporins by cytosolic proton gating (Tournaire-Roux et al., 2003). In the present study, a small acidification has been detected in response to high irradiance in leaf exudates and that possibly started in tertiary veins (Table 1). Light-induced acidification of the leaf apoplast has previously been reported (Mühling et al., 1995; Shabala and Newman, 1999; Stahlberg and Van Volkenburgh, 1999) and shown to be associated with leaf veins (Shabala et al., 2002). In addition, changes in apoplastic pH may accompany changes in leaf hydraulic conductance (Aasamaa and Sõber, 2001). Therefore, while leaf infiltration may cause cytosolic acidification and, subsequently, aquaporin blockage by cytosolic protons, high irradiance may promote the pumping of protons out of the cytosol and activation of the aquaporin activity. This may be reflected in an increased Klam under high irradiance. At the same time, pH decreases in leaves may also increase the rate of water flow out of leaf veins through pit membranes (Zwieniecki, 2001). While only a change in the pH of leaf exudates was detected, the influence of apoplastic pH on leaf hydraulic conductance should be investigated further.
The contribution of gene expression of putative aquaporins to water transport in bur oak in sun leaves that were exposed to different light levels in situ (full sunlight, shaded, and covered leaves) was investigated further. To our knowledge, this study is the first to report expression of aquaporin genes in leaves growing and harvested under natural field conditions. The relative transcript level of the QmPIP2;1 gene increased significantly in shaded leaves as opposed to the leaves kept in darkness or in full sunlight (Fig. 5D), contrary to our original hypothesis that aquaporin expression will increase in full sunlight. Transcript levels corresponding to QmPIP2;2 also showed a similar trend (Fig. 5B). Our results are in agreement with those from Arabidopsis showing that protoplasts isolated from leaves with high levels of transpiration had reduced osmotic water permeability, while the opposite was true for the protoplasts isolated from the leaves with reduced transpiration (Morillon and Chrispeels, 2001). In addition, cell-pressure-probe measurements of leaf midrib cells showed that, in intact plants, there is a decrease of leaf cell hydraulic conductivity under high transpirational demand induced by high irradiance (Kim and Steudle, 2007, 2008; Lee et al., 2008, 2009). Our previous study showed that, in bur oak, the light-induced enhancement of Klam saturates at around 150 μmol m−2 s−1 PPF which corresponds to shade-like conditions (Voicu et al., 2008). Therefore, at higher irradiances, as experienced in the field and beyond those that are typically achievable in growth chambers, aquaporin transcripts and activity are likely to be down-regulated. When stomata partially closed in shaded leaves, there was a moderate enhancement in leaf water potential experienced by Q. macrocarpa leaves within the tree crown, (Knapp, 1992; Hamerlynck and Knapp, 1994). This may trigger aquaporin up-regulation which may allow for a faster leaf rehydration and restoration of the water homeostasis.
The relative expression levels of the PIP genes appear to be different in the leaves that were measured with the HPFM (Fig. 4) as compared with the leaves for which no HPFM measurements were performed (Fig. 5). Since the leaves for both experiments were sampled only two days apart, the mRNA content may have decreased after leaf excision and during HPFM measurements as a result of hypoxia being imposed on the leaf.
In the present study, when the concentration of the fluorescent apoplastic tracer PTS3 was assessed, it showed no difference among different experimental treatments that were expected either to enhance (high irradiance versus ambient light – decline in PTS3 concentration; Table 1) or reduce (HgCl2 versus distilled water – increase in PTS3 concentration; Fig. 6) the rate of water flow through the cell-to-cell pathway. This is not consistent with an increase in the cell-to-cell pathway as suggested for the Klam light response (Cochard et al., 2007; Voicu et al., 2008). Mercury reduced the bur oak Klam irradiance response in our previous study (Voicu et al., 2008), but in the present study, the PTS3 concentration was not affected by mercury (Fig. 6). The use of PTS3 as a quantitative apoplastic tracer has been criticized because, in roots, PTS3 is mainly retained by the endodermis and around 0.3% of the initial concentration is recovered in the xylem when the roots were pressure-infiltrated with PTS3 (Zimmermann and Steudle, 1998; Zimmermann et al., 2000; Voicu and Zwiazek, 2004). However, it has previously been found that, in detached aspen roots, an increase in the relative PTS3 concentration was correlated with an overall reduced water flow and a concomitant decline in the abundance of aquaporin proteins caused by a treatment with cycloheximide (Voicu and Zwiazek, 2004). Therefore, in the present study, PTS3 was used to assess the relative contributions of the apoplastic and cell-to-cell pathways. However, it was noticed that there was very little difference in the PTS3 concentration of the infusion solution and that of leaf exudates. When tertiary or secondary veins were cut open or when the lamina was detached, the increase in PTS3 concentration was small, indicating that there was probably no high accumulation of the dye in the apoplast of leaf xylem. Since some non-specific binding of PTS3 in the leaf may have occurred, the leaf treatment applied in the present study did not result in any difference in the PTS3 concentration. Under the conditions of the present study the dye moved relatively fast past the veins xylem. The possibility that PTS3 has accumulated in leaf minor veins (Canny, 1995) and moved by diffusion outward cannot be discounted. By the time the leaf exudates were collected in ambient light (which took about 1.5 h), the diffusion may have equalized the PTS3 concentration in veins with that of leaf exudates. However, the diffusion of PTS3 out of veins through the cell walls is probably a very slow process in ambient light; when light intensity of 10 μmol m−2 s−1 PPF was used, it took about 8 h for the dye to reach the abaxial epidermis (Fitzgerald and Allaway, 1991). In one instance when leaves were viewed under an epifluorescent microscope, PTS3 moved very fast even across the suberized bundle-sheath tissues (Eastman et al., 1988). In the present study, leaves experienced similar conditions during PTS3 applications as during the HPFM measurements of Klam. Therefore, it is possible that, during the HPFM measurements of Klam, the cell-to-cell pathway of water movement is largely bypassed (Sack et al., 2002), or that the main pathway for water movement in leaves exposed to high irradiance is through the apoplast (Kim and Steudle, 2007; Lee et al., 2008, 2009). In the latter case, the rapid change of Klam under high irradiance may be accomplished by the increase in water permeability of the pit membranes at the bundle-sheath–xylem interface or of cell-walls as determined by changes in the ionic composition of the xylem sap (Zwieniecki, 2001). Light may induce large ionic fluxes in the vascular bundles of the leaf (Shabala et al., 2002) and xylem sap ionic composition and pH may increase or decrease the xylem hydraulic conductance through the swelling and shrinking of the pectin hydrogel of the pit membranes (Zwieniecki, 2001). In twigs, the effect appears to be mediated by phloem (Zwieniecki et al., 2004). However, the involvement of phloem or bundle-sheath cells in regulating the water permeability of the surrounding apoplast should be further tested.
In conclusion, the putative aquaporin genes that were identified in the present study probably do not play a role in the light responses of hydraulic conductance at the transcript level as measured by the HPFM technique and at irradiance levels greater than 1000 μmol m−2 s−1 PPF. However, at least one of these aquaporins could be important in regulating water homeostasis in leaves under varying light conditions. These results and those of the apoplastic fluorescent tracer experiments demonstrate that the pathways for water movement in leaves are far from being understood and require more follow-up studies. Future experiments should investigate the interplay among aquaporin transcripts and protein levels in leaves from different species exposed to a combination of different water potentials, air relative humidity and light intensities.
Acknowledgments
Funding for this study was provided by the Alberta Ingenuity Endowment Fund and a Natural Sciences and Engineering Research Council of Canada Discovery Grant to JJZ. We thank Adriana Almeida-Rodriguez and Walid El Kayal for helpful assistance during aquaporin isolation and cloning and the anonymous reviewers of the manuscript for helpful suggestions.
References
- Aasamaa K, Sõber A. Hydraulic conductance and stomatal sensitivity to changes of leaf water status in six deciduous tree species. Biologia Plantarum. 2001;44:65–73. [Google Scholar]
- Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P. Whole gene family expression and drought stress regulation of aquaporins. Plant Molecular Biology. 2005;59:469–484. doi: 10.1007/s11103-005-0352-1. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canny MJ. What becomes of the transpiration stream? New Phytologist. 1990;114:341–368. doi: 10.1111/j.1469-8137.1990.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Canny MJ. Apoplastic water and solute movement: new rules for an old space. Annual Review of Plant Physiology. 1995;46:215–236. [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11:1572–9818. [Google Scholar]
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiology. 2001;125:1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Moshelion M, Daniels MJ. Regulation of plant aquaporin activity. Biology of the Cell. 2005;97:749–764. doi: 10.1042/BC20040133. [DOI] [PubMed] [Google Scholar]
- Claros MG, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Computer Applications in the Biosciences. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- Cochard H, Venisse JS, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology. 2007;143:122–133. doi: 10.1104/pp.106.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson JA, Johanson U. Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biology. 2008;8:45. doi: 10.1186/1471-2229-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman PAK, Peterson CA, Dengler NG. Suberized bundle sheaths in grasses (Poaceae) of different photosynthetic types. II. Apoplastic permeability. Protoplasma. 1988;142:112–126. [Google Scholar]
- Felle HH. Apoplastic pH during low-oxygen stress in barley. Annals of Botany. 2006;98:1085–1093. doi: 10.1093/aob/mcl193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. Interactions between plasma membrane aquaporins modulate their water channel activity. The Plant Cell. 2004;16:215–228. doi: 10.1105/tpc.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald MA, Allaway WG. Apoplastic and symplastic pathways in the leaf of the grey mangrove Avicennia marina (Forsk.) Vierh. New Phytologist. 1991;119:217–226. doi: 10.1111/j.1469-8137.1991.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Forrest KL, Bhave M. The PIP and TIP aquaporins in wheat form a large and diverse family with unique gene structures and functionally important features. Functional and Integrative Genomics. 2008;8:115–133. doi: 10.1007/s10142-007-0065-4. [DOI] [PubMed] [Google Scholar]
- Frangne N, Maeshima M, Schaffner AR, Mandel T, Martinoia E, Bonnemain JL. Expression and distribution of a vaculoar aquaporin in young and mature leaf tissues of Brassica napus in relation to water fluxes. Planta. 2001;212:270–278. doi: 10.1007/s004250000390. [DOI] [PubMed] [Google Scholar]
- Hamerlynck E, Knapp AK. Stomatal responses to variable sunlight in bur oak (Quercus macrocarpa Michx.) leaves with different photosynthetic capacities. International Journal of Plant Sciences. 1994;155:583–587. [Google Scholar]
- Hanson PJ, Sucoff EI, Markhart AH., III Quantifying apoplastic flux through red pine root systems using trisodium, 3-hydroxy-5,8,10-pyrenetrisulfonate. Plant Physiology. 1985;77:21–24. doi: 10.1104/pp.77.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiology. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Kolling A, Meyers J, Karmann U, Ruppel G, Richter G. The blue light-responsive AthH2 gene of Arabidopsis thaliana is primarily expressed in expanding as well as in differentiating cells and encodes a putative channel protein of the plasmalemma. The Plant Journal. 1995;7:87–95. doi: 10.1046/j.1365-313x.1995.07010087.x. [DOI] [PubMed] [Google Scholar]
- Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K. Functional analysis of water channels in barley roots. Plant and Cell Physiology. 2002;43:885–893. doi: 10.1093/pcp/pcf102. [DOI] [PubMed] [Google Scholar]
- Kim YX, Steudle E. Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. Journal of Experimental Botany. 2007;58:4119–4129. doi: 10.1093/jxb/erm270. [DOI] [PubMed] [Google Scholar]
- Kim YX, Steudle E. Gating of aquaporins by light and reactive oxygen species in leaf parenchyma cells of the midrib of Zea mays. Journal of Experimental Botany. 2008;60:547–556. doi: 10.1093/jxb/ern299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AK. Leaf gas exchange in Quercus macrocarpa (Fagaceae): rapid stomatal responses to variable sunlight in a tree growth form. American Journal of Botany. 1992;79:599–604. [Google Scholar]
- Lee SH, Chung GC, Zwiazek JJ. Effects of irradiance on cell water relations in leaf bundle sheath cells of wild-type and transgenic tobacco (Nicotiana tabacum) plants overexpressing aquaporins. Plant Science. 2009;176:248–255. [Google Scholar]
- Lee SH, Zwiazek JJ, Chung GC. Light-induced transpiration alters cell water relations in figleaf gourd (Cucurbita ficifolia) seedlings exposed to low root temperatures. Physiologia Plantarum. 2008;133:354–362. doi: 10.1111/j.1399-3054.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- Luu DT, Maurel C. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant, Cell and Environment. 2005;28:85–96. [Google Scholar]
- Marjanovic Z, Uehlein N, Kaldenhoff R, Zwiazek JJ, Weiss M, Hampp R, Nehls U. Aquaporins in poplar: what a difference a symbiont makes! Planta. 2005;222:258–268. doi: 10.1007/s00425-005-1539-z. [DOI] [PubMed] [Google Scholar]
- Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology. 2002;130:2101–2110. doi: 10.1104/pp.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- Moon GJ, Clough BF, Peterson CA, Allaway WG. Apoplastic and symplastic pathways in Avicennia marina (Forsk.) Vierh. roots revealed by fluorescent tracer dyes. Functional Plant Biology. 1986;13:637–648. [Google Scholar]
- Morillon R, Chrispeels MJ. The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proceedings of the National Academy of Sciences, USA. 2001;98:14138–14143. doi: 10.1073/pnas.231471998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühling KH, Plieth C, Hansen UP, Sattelmacher B. Apoplastic pH of intact leaves of Vicia faba as influenced by light. Journal of Experimental Botany. 1995;46:377–382. [Google Scholar]
- Nardini A, Salleo S, Andri S. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv. Margot) Plant, Cell and Environment. 2005;28:750–759. [Google Scholar]
- Pavy N, Boyle B, Nelson C, et al. Identification of conserved core xylem gene sets: conifer cDNA microarray development, transcript profiling and computational analyses. New Phytologist. 2008;180:766–786. doi: 10.1111/j.1469-8137.2008.02615.x. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Sieber H, Kammerloher W, Schaeffner AR. PIP1 aquaporins are concentrated in plasmalemmasomes of Arabidopsis thaliana mesophyll. Plant Physiology. 1996;111:645–649. doi: 10.1104/pp.111.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Melcher PJ, Zwieniecki MA, Holbrook NM. The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. Journal of Experimental Botany. 2002;53:2177–2184. doi: 10.1093/jxb/erf069. [DOI] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology. 2004;134:1824–1833. doi: 10.1104/pp.103.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sakr S, Alves G, Morillon R, Maurel K, Decourteix M, Guilliot A, Fleurat-Lessard P, Julien JL, Chrispeels MJ. Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiology. 2003;133:630–641. doi: 10.1104/pp.103.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant and Cell Physiology. 2005;46:1568–1577. doi: 10.1093/pcp/pci172. [DOI] [PubMed] [Google Scholar]
- Salleo S, Lo Gullo MA, Raimondo F, Nardini A. Vulnerability to cavitation of leaf minor veins: any impact on leaf gas exchange? Plant, Cell and Environment. 2001;24:851–859. [Google Scholar]
- Salleo S, Raimondo F, Trifilò P, Nardini A. Axial-to-radial water permeability of leaf major veins: a possible determinant of the impact of vein embolism on leaf hydraulics? Plant, Cell and Environnment. 2003;26:1749–1758. [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sarda X, Tousch D, Ferrare K, Legrand E, Dupuis JM, Casse-Delbart F, Lamaze T. Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. The Plant Journal. 1997;12:1103–1111. doi: 10.1046/j.1365-313x.1997.12051103.x. [DOI] [PubMed] [Google Scholar]
- Shabala S, Schimanski LJ, Koutoulis A. Heterogeneity in bean leaf mesophyll tissue and ion flux profiles: leaf electrophysiological characteristics correlate with the anatomical structure. Annals of Botany. 2002;89:221–226. doi: 10.1093/aob/mcf029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S, Newman I. Light-induced changes in hydrogen, calcium, potassium, and chloride ion fluxes and concentrations from the mesophyll and epidermal tissues of bean leaves. Understanding the ionic basis of light-induced bioelectrogenesis. Plant Physiology. 1999;119:1115–1124. doi: 10.1104/pp.119.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlberg R, Van Volkenburgh E. The effect of light on membrane potential, apoplastic pH and cell expansion in leaves of Pisum sativum L. var. Argenteum. Planta. 1999;208:188–195. [Google Scholar]
- Steudle E. Water uptake by roots: effects of water deficit. Journal of Experimental Botany. 2000;51:1531–1542. doi: 10.1093/jexbot/51.350.1531. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D, Bligny R, Maurel C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425:393–397. doi: 10.1038/nature01853. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Niemietz CM, Bramley H. Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant, Cell and Environment. 2002;25:173–194. doi: 10.1046/j.0016-8025.2001.00791.x. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Nardini A, Salleo S, Sack L, El Omari B. The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: any role for stomatal response? Journal of Experimental Botany. 2005;56:737–744. doi: 10.1093/jxb/eri045. [DOI] [PubMed] [Google Scholar]
- Van Fleet DS. The cell forms, and their common substance reactions, in the parenchyma-vascular boundary. Bulletin of the Torrey Botanical Club. 1950;77:340–353. [Google Scholar]
- Voicu MC, Zwiazek JJ. Cycloheximide inhibits root water flow and stomatal conductance in aspen (Populus tremuloides) seedlings. Plant, Cell and Environment. 2004;27:199–208. [Google Scholar]
- Voicu MC, Zwiazek JJ, Tyree MT. Light response of hydraulic conductance in bur oak (Quercus macrocarpa) leaves. Tree Physiology. 2008;28:1007–1015. doi: 10.1093/treephys/28.7.1007. [DOI] [PubMed] [Google Scholar]
- Wallace IS, Roberts DM. Homology modeling of representative subfamilies of Arabidopsis major intrinsic proteins. Classification based on the aromatic/arginine selectivity filter. Plant Physiology. 2004;135:1059–1068. doi: 10.1104/pp.103.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardoya R. Phylogeny and evolution of the major intrinsic protein family. Biology of the Cell. 2005;97:397–414. doi: 10.1042/BC20040134. [DOI] [PubMed] [Google Scholar]
- Zimmermann HM, Hartmann K, Schreiber L, Steudle E. Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.) Planta. 2000;210:302–311. doi: 10.1007/PL00008138. [DOI] [PubMed] [Google Scholar]
- Zimmermann HM, Steudle E. Apoplastic transport across young maize roots: effect of the exodermis. Planta. 1998;210:302–311. [Google Scholar]
- Zwieniecki MA. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Melcher PJ, Feild TS, Holbrook NM. A potential role for xylem-phloem interactions in the hydraulic architecture of trees: effects of phloem girdling on xylem hydraulic conductance. Tree Physiology. 2004;24:911–917. doi: 10.1093/treephys/24.8.911. [DOI] [PubMed] [Google Scholar]