Summary
Background
Endovascular treatment (angioplasty with or without stenting) is an alternative to carotid endarterectomy for carotid artery stenosis but there are scarce long-term efficacy data showing that it prevents stroke. We therefore report the long-term results of the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS).
Methods
Between March, 1992, and July, 1997, patients who presented at a participating centre with a confirmed stenosis of the internal carotid artery that was deemed equally suitable for either carotid endarterectomy or endovascular treatment were randomly assigned to either treatment in equal proportions by telephone or fax from the randomisation service at the Oxford Clinical Trials Unit, UK. Patients were seen by an independent neurologist at 1 and 6 months after treatment and then every year after randomisation for as long as possible, up to a maximum of 11 years. Major outcome events were transient ischaemic attack, non-disabling, disabling, and fatal stroke, myocardial infarction, and death from any other cause. Outcomes were adjudicated on by investigators who were masked to treatment. Analysis was by intention to treat. This study is registered, number ISRCTN 01425573.
Findings
504 patients with stenosis of the carotid artery (90% symptomatic) were randomly assigned to endovascular treatment (n=251) or surgery (n=253). Within 30 days of treatment, there were more minor strokes that lasted less than 7 days in the endovascular group (8 vs 1) but the number of other strokes in any territory or death was the same (25 vs 25). There were more cranial nerve palsies (22 vs 0) in the endarterectomy group than in the endovascular group. Median length of follow up in both groups was 5 years (IQR 2–6). By comparing endovascular treatment with endarterectomy after the 30-day post-treatment period, the 8-year incidence and hazard ratio (HR) at the end of follow-up for ipsilateral non-perioperative stroke was 11·3% versus 8·6% (HR 1·22, 95% CI 0·59–2·54); for ipsilateral non-perioperative stroke or TIA was 19·3% versus 17·2% (1·29, 0·78–2·14); and for any non-perioperative stroke was 21·1% versus 15·4% (1·66, 0·99–2·80).
Interpretation
More patients had stroke during follow-up in the endovascular group than in the surgical group, but the rate of ipsilateral non-perioperative stroke was low in both groups and none of the differences in the stroke outcome measures was significant. However, the study was underpowered and the confidence intervals were wide. More long-term data are needed from the on going stenting versus endarterectomy trials.
Funding
British Heart Foundation; UK National Health Service Management Executive; UK Stroke Association.
Introduction
Carotid endarterectomy became the mainstay of treatment for patients with symptomatic carotid artery stenosis after two randomised trials established the benefit of endarterectomy compared with medical treatment.1, 2 In recent years, endovascular treatments (first balloon angioplasty and then stenting) have been increasingly used as an alternative to endarterectomy, despite the paucity of evidence that endovascular treatment offers the same level of early safety and long-term effectiveness as surgery does. Several randomised trials have compared endovascular treatment with endarterectomy for carotid stenosis, but none have been of sufficient duration to report outcome after longer than 4 years.3
The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) is a randomised controlled trial to assess the safety and efficacy of endovascular treatment compared with endarterectomy for carotid stenosis. CAVATAS comprises three international multicentre randomised controlled trials, which started randomisation in 1992. CAVATAS-MED compared endovascular with medical treatment of carotid stenosis in patients who were not suitable for surgery; only a small number of patients were randomly assigned in this trial.4 CAVATAS-VER compared endovascular with medical treatment for symptomatic vertebral artery stenosis, and the long-term results were published in 2007.5 CAVATAS compared endovascular treatment (angioplasty with or without stenting) with surgery in patients with mainly symptomatic carotid artery stenosis, and the long-term results are reported here. We published the first report from CAVATAS—the safety outcome after 30 days of treatment with a maximum follow-up of 3 years—in 2001.6 Since then, two other multicentre randomised controlled trials that have compared carotid stenting with endarterectomy in patients with symptomatic carotid artery stenosis have published safety data and medium-term follow-up data, namely the Stent Protected Angioplasty versus Carotid Endarterectomy trial (SPACE), which had a follow-up period of 2 years, and the Endarterectomy versus Angioplasty in patients with Symptomatic Severe Carotid Stenosis trial (EVA-3S), which had a follow-up of 4 years.7, 8 However, the long-term efficacy of endovascular treatment compared with surgery after the first few years has not been published. We therefore report the final results of CAVATAS, including long-term data up to a maximum of 11 years' follow-up with the aim of assessing the long-term effectiveness of angioplasty and stenting compared with surgery in patients with symptomatic carotid artery stenosis.
Methods
Patients
The inclusion criteria for CAVATAS have been described previously.6 In brief, between March, 1992, and July, 1997, patients that were referred to the 22 trial centres in western Europe, Australia, or Canada with stenosis of the internal carotid artery that was deemed by the investigators to require treatment and was equally suitable for carotid endarterectomy or endovascular treatment were included in CAVATAS. Exclusion criteria included unwillingness to undergo one of the procedures, inability to give informed consent, and disabling stroke within the region supplied by the treated artery without useful recovery of function. There was no upper age limit for participation in the study. All patients provided written informed consent and each centre obtained local ethics committee approval.
Randomisation and masking
Patients were randomly assigned in equal proportions to endovascular treatment or endarterectomy in response to a telephone call or fax to the randomisation service at the Oxford Clinical Trials Unit, UK. Follow-up examinations were done by investigators who were neurologically trained but were not directly involved in the endovascular treatment or endarterectomy. All strokes and deaths were adjudicated masked to treatment allocation and based on the information provided by the individual centres, including brain scans and, where appropriate, death certificates.
Procedures
Participating centres used their own protocol to establish the presence of carotid stenosis, but in most cases ultrasound findings were confirmed by catheter angiography.
All patients enrolled before 1994 who received endovascular treatment had percutaneous balloon angioplasty. Stents suitable for the carotid artery became available during the course of the study. From 1994, stenting was allowed at the discretion of the intervening radiologist.
Patients were seen for follow-up at 1 and 6 months after treatment, and then every year after randomisation by an independent neurologist or stroke physician. There was no pre-determined total length of follow-up, but investigators were encouraged to continue follow-up for as long as they and their patients were willing to do so.
Outcome events reported to the central office were transient ischaemic attack (TIA), non-disabling, disabling, and fatal stroke, myocardial infarction, and death from any other cause. Amaurosis fugax was included in the category of TIA. Mortality and certified cause of death was confirmed from the General Register Office (GRO) in patients randomly assigned at UK centres. Stroke was defined as a clinical syndrome of acute onset of a focal neurological deficit that lasted longer than 24 h, was of vascular origin, and was classified as disabling if the patient required help from another person for more than 30 days as a result of the stroke. Stroke was classified as fatal if death was deemed to be a direct result of the stroke at any time after onset. Other stroke events were classified as non-disabling; non-disabling strokes were divided into those that lasted fewer than 7 days and those that lasted more than 7 days, to enable comparison with the European Carotid Surgery Trial (ECST), which only reported strokes that lasted more than 7 days.1 TIA was defined as an acute disturbance of focal neurological function with symptoms that lasted less than 24 h and was attributable to cerebrovascular disease. Death was classified as other vascular death if it was due to any cardiovascular-related illness other than stroke (including myocardial infarction and pulmonary embolism). Death caused by any other non-vascular-related illness was classified as non-vascular. If no information on the cause of death was available, the cause was classified as undetermined. Outcome events were classified as perioperative if they occurred at the time of treatment or within 30 days after treatment. In patients who did not have an intervention, the date of crossover to medical treatment was defined as the proxy-treatment date for the purpose of analysing the rate of events that occurred more than 30 days after treatment, which were defined as non-perioperative events.
The original study protocol defined the primary outcome measure as long-term period free from disabling stroke or death from the time of randomisation as an outcome that was relevant to the patient and health economics. In this paper, to assess the long-term efficacy of treatment to prevent stroke after treatment we compared the occurrence of non-perioperative TIA, stroke, or both, in various territories, excluding perioperative events that occurred within 30 days after treatment. Additionally, we assessed whether the use of stenting versus only balloon angioplasty and whether the baseline characteristics, including age dichotomised at the median age, influenced the rates of strokes that lasted more than 7 days or perioperative death. For each outcome measure, patients were counted only once; the first event was the event of interest, whenever this occurred. However, patients who had more than one outcome event were counted in each relevant outcome category.
Statistical analysis
All data were analysed by intention-to-treat with SPSS version 16.0 for Mac (Chicago, IL, USA) except where stated. The log-rank test was used to compare the survival experience free of events in the two treatment groups. A Cox proportional hazard model was used to obtain an estimate of the treatment effect (hazard ratio), with endarterectomy used as the reference group. The proportional hazards assumption was checked with a graphical log-minus-log method. Censoring was assumed to be non-informative. All outcome events up to the last available follow-up or death of the patient were included in the calculation of hazard ratios. However, because fewer than 50 patients were followed up beyond 8 years, Kaplan–Meier curves were only plotted up to 8 years after randomisation. Cox regression was used to test for treatment effect interaction within the subgroups. This study is registered, number ISRCTN 01425573.
Role of the funding source
The sponsors had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
505 patients with carotid artery stenosis who were suitable for either endovascular treatment or carotid endarterectomy were randomly assigned to endovascular treatment (n=252), or endarterectomy (n=253). Figure 1 shows the trial profile. One patient with carotid occlusion was randomly assigned to endovascular treatment in error and was excluded, leaving 504 patients for the analysis. 18 patients did not receive the treatment they were allocated to. The baseline characteristics were well balanced, and 90% of patients had symptoms within 6 months before randomisation (table 1).6
Figure 1.
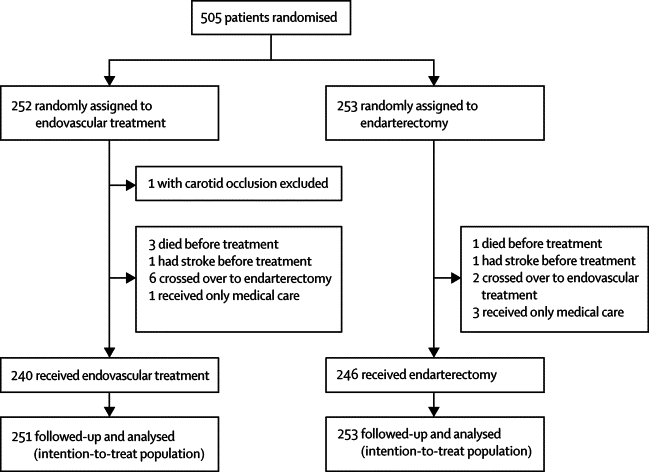
Trial profile
Table 1.
Patient characteristics at baseline
| Endovascular treatment (n=251) | Endarterectomy (n=253) | |||
|---|---|---|---|---|
| Age (years) | 68 (62–73) | 68 (62–73) | ||
| Sex | ||||
| Women | 77 (31%) | 75 (30%) | ||
| Men | 174 (69%) | 178 (70%) | ||
| Vascular risk factors | ||||
| Hypertension | 132 (53%) | 144 (58%) | ||
| Systolic blood pressure (mm Hg) | 151·8 (21·8) | 152·6 (20·1) | ||
| Diastolic blood pressure (mm Hg) | 83·5 (11·8) | 83·9 (10·7) | ||
| Diabetes mellitus | 35 (14%) | 32 (13%) | ||
| Cholesterol >6·5 mmol/L | 67 (34%) | 62 (32%) | ||
| Smoker (past or current) | 191 (77%) | 192 (78%) | ||
| History of cardiovascular or cerebrovascular disease | ||||
| Myocardial infarction | 43 (19%) | 40 (17%) | ||
| Atrial fibrillation | 12 (5%) | 12 (5%) | ||
| Peripheral vascular disease | 60 (24%) | 51 (20%) | ||
| Cerebrovascular symptoms >6 months before randomisation | 21 (8%) | 15 (6%) | ||
| Treatments at randomisation | ||||
| Antiplatelet | 216 (86%) | 230 (91%) | ||
| Warfarin | 23 (10%) | 28 (11%) | ||
| Cerebrovascular events within 6 months before randomisation | ||||
| Transient ischaemic attack | 94 (37%) | 98 (39%) | ||
| Amaurosis fugax | 60 (24%) | 63 (25%) | ||
| Hemisphere stroke | ||||
| Minor | 19 (8%) | 20 (8%) | ||
| Major (non-disabling) | 32 (13%) | 28 (11%) | ||
| Major (disabling) | 11 (4%) | 18 (7%) | ||
| Retinal infarct | 5 (2%) | 3 (1%) | ||
| Degree of symptomatic carotid stenosis* | ||||
| 60–69% | 15 (6%) | 16 (6%) | ||
| 70–79% | 34 (14%) | 40 (16%) | ||
| 80–89% | 91 (36%) | 92 (36%) | ||
| 90–99% | 109 (43%) | 98 (39%) | ||
| Contralateral carotid stenosis of 70–99% or occlusion* | 78 (31%) | 79 (32%) | ||
| Time from randomisation to treatment (days) | 20·0 (8·0–32·0) | 27·0 (13·5–41·0) | ||
| Follow-up (years) | 5 (2–6) | 5 (2–6) | ||
| Treatments, blood pressure, and smoking status during follow-up† | ||||
| Antiplatelet | 202 (92%) | 209 (90%) | ||
| 43 (86%) | 47 (90%) | |||
| Warfarin | 12 (6%) | 12 (5%) | ||
| 4 (8%) | 2 (4%) | |||
| Systolic blood pressure (mm Hg) | 151·0 (21·0) | 151·0 (23·4) | ||
| 153·9 (23·2) | 147·6 (26·5) | |||
| Diastolic blood pressure (mm Hg) | 83·0 (10·1) | 82·7 (10·2) | ||
| 78·3 (12·3) | 77·6 (14·0) | |||
| Smoker (past or current) | 48 (23%) | 50 (22%) | ||
| 5 (11%) | 12 (27%) | |||
Data are number (%), median (IQR), or mean (SD) unless otherwise indicated.
Measured by common carotid method.
The first line for each characteristic is taken from 1-year follow-up data and the second line from the 6-year follow-up data.
Follow-up data were available for up to 11 years after randomisation. The median length of follow-up in both groups was 5 years (IQR 2–6 years). The use of antithrombotic medications, control of blood pressure, and smoking rates were well matched in the two arms throughout the course of the trial (table 1). Data on the use of statins were not collected.
Table 2, Table 3 report the number of main outcome events. The rates of any stroke that lasted for more than 7 days or death within 30 days of treatment were well matched between the treatment groups (10% in each arm). There were more minor strokes that lasted fewer than 7 days within 30 days of treatment in the endovascular group than in the endarterectomy group (8 vs 1), but there were more cranial nerve palsies in the endarterectomy group than in the endovascular group (0 vs 22). Most cranial nerve palsies were transient, but the symptoms were still present in one patient 1 month after treatment and had resolved by the 6-month follow-up. The primary outcome measure of disabling stroke or death at any time after randomisation was recorded in 238 patients (endovascular n=117, surgery n=121; table 3). The 8-year cumulative incidence of disabling stroke or death estimated from the Kaplan–Meier survival analysis was 45·2% (SE 4·0%) in the endovascular treatment group and 50·4% (4·1%) in the endarterectomy group (figure 2). After 8 years (data not shown) the lines for each treatment came together and crossed, indicating an overall risk that was virtually identical in both groups (HR 1·02, 95% CI 0·79–1·32; table 4).
Table 2.
Outcome events within 30 days after first treatment
| Endovascular treatment (n=251) | Endarterectomy (n=253) | |
|---|---|---|
| Fatal stroke | 7 (3%) | 1 (0·3%) |
| Non-stroke death | 0 | 3 (2%) |
| Disabling stroke | 9 (4%) | 11 (4%) |
| Non-disabling stroke that lasted more than 7 days | 9 (4%) | 10 (4%) |
| Non-disabling stroke that lasted fewer than 7 days | 8 (3%) | 1 (0.3%) |
| Death or disabling stroke | 16 (6%) | 15 (6%) |
| Death or any stroke that lasted more than 7 days | 25 (10%) | 25 (10%) |
| Cranial nerve palsy* | 0 | 22 (9 %) |
| Haematoma that required surgery or extended stay in hospital† | 3 (1%) | 17 (7%) |
| Non-fatal myocardial infarction | 0 | 3 (1%) |
| Pulmonary embolus | 0 | 2 (1%) |
Data are number (%). Stroke refers to events in any territory.
p<0·0001.
p <0·002. Other differences not significant.
Table 3.
Major long-term outcome events
| Endovascular treatment (n=251)* | Endarterectomy (n=253)† | |||
|---|---|---|---|---|
| Disabling stroke or death | 117 | 121 | ||
| Stroke | 33 | 27 | ||
| Disabling | 18 | 21 | ||
| Fatal | 15 | 6 | ||
| Non-stroke death | 84 | 94 | ||
| Stroke that lasted >7 days or death | 134 | 131 | ||
| Stroke that lasted >7 days | 59 | 47 | ||
| Fatal | 14 | 6 | ||
| Disabling | 17 | 19 | ||
| Non-disabling | 28 | 22 | ||
| Non-stroke death | 75 | 84 | ||
| Perioperative | 0 | 3 | ||
| Non-perioperative | 75 | 81 | ||
| Any stroke or perioperative death | 67 | 51 | ||
| Any stroke | 67 | 48 | ||
| Fatal | 14 | 6 | ||
| Disabling | 17 | 19 | ||
| Non-disabling >7 days | 28 | 22 | ||
| Non-disabling <7 days | 8 | 1 | ||
| Perioperative death | 0 | 3 | ||
| Vascular non-stroke | 0 | 2 | ||
| Non-vascular | 0 | 1 | ||
| Non-perioperative stroke or TIA | 67 | 51 | ||
| Non-perioperative stroke | 31 | 18 | ||
| Fatal | 6 | 4 | ||
| Disabling | 7 | 5 | ||
| Non-disabling | 18 | 9 | ||
| Non-perioperative TIA | 36‡ | 33§ | ||
| Non-perioperative ipsilateral stroke or TIA | 34 | 27 | ||
| Non-perioperative ipsilateral stroke | 12 | 11 | ||
| Fatal | 1 | 1 | ||
| Disabling | 3 | 2 | ||
| Non-disabling | 8 | 8 | ||
| Non-perioperative ipsilateral TIA | 22¶ | 16‖ | ||
| Non-perioperative non-ipsilateral stroke | 24 | 13 | ||
| Fatal | 8 | 4 | ||
| Disabling | 5 | 6 | ||
| Non-disabling | 11 | 3 | ||
| Death | 112 | 113 | ||
| Stroke death | 16 | 6 | ||
| Vascular non-stroke death | 43 | 53 | ||
| Non-vascular | 44 | 46 | ||
| Undetermined | 9 | 8 | ||
Data are number of events. None of the differences were statistically significant. Indented lines below combined outcome measures indicate how many events contributed to the combined outcome measure. All non-perioperative strokes lasted for more than 7 days. TIA=transient ischaemic attack.
Total person-years of follow-up=1098.
Total person-years of follow-up=1083.
6 patients had a subsequent non-perioperative stroke (1 fatal, 2 disabling, 3 non-disabling).
5 patients had a subsequent non-perioperative stroke (1 fatal, 3 disabling, 1 non-disabling).
4 patients had a subsequent non-perioperative ipsilateral stroke (1 disabling, 3 non-disabling).
2 patients had a subsequent non-perioperative ipsilateral stroke (1 disabling, 1 non-disabling).
Figure 2.
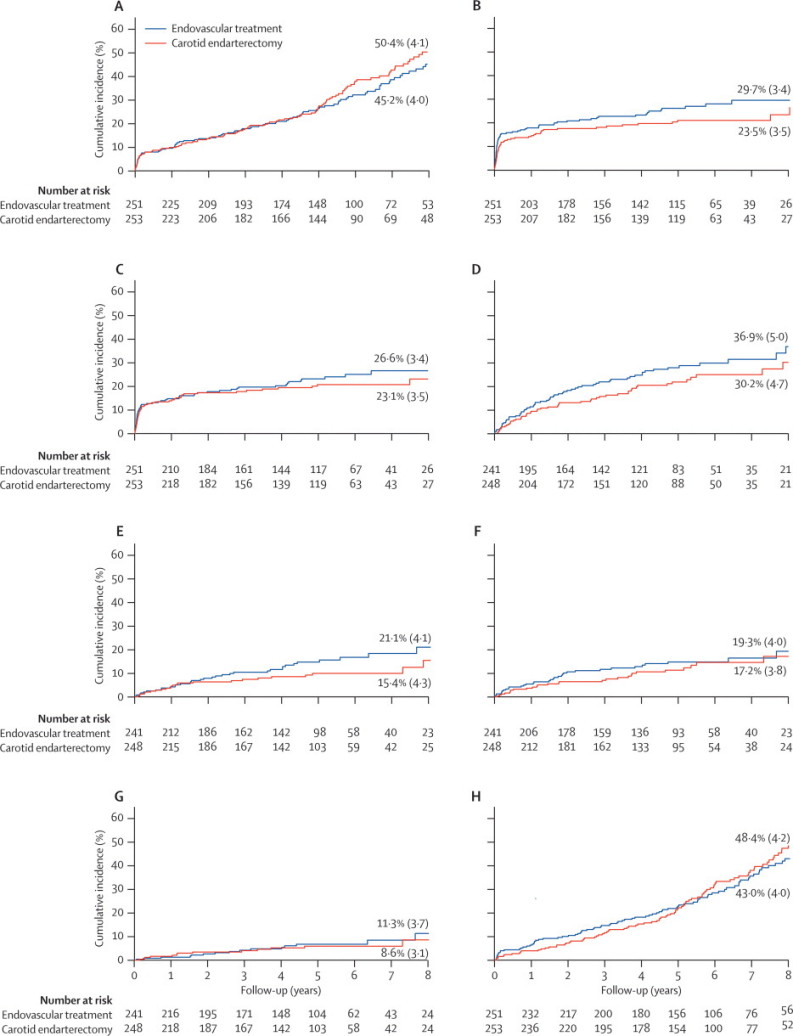
Kaplan–Meier estimates of cumulative incidence
The numbers above and below the lines refer to the 8-year incidence (SE) (%). (A) Disabling stroke or death (primary outcome measure). (B) Any stroke or perioperative death. (C) Stroke that lasted more than 7 days or perioperative death within 30 days of treatment. (D) Non-perioperative stroke or TIA. (E) Non-perioperative stroke. (F) Non-perioperative ipsilateral stroke or transient ischaemic attack. (G) Non-perioperative ipsilateral stroke. (H) Any cause of death. Except where stated, stroke refers to events in any territory. A, B, C, and H are analysed from date of randomisation; D and G are analysed from 30 days after treatment. No stroke that occurred more than 30 days after treatment lasted for fewer than 7 days.
Table 4.
Hazard ratios for all outcome measures
| Hazard ratio (95% CI) | |
|---|---|
| Disabling stroke or death | 1·02 (0·79–1·32) |
| Any stroke lasting > 7 days or death | 1·08 (0·97–3·73) |
| Any stroke or perioperative death | 1·35 (0·94–1·93) |
| Stroke lasting >7 days or perioperative death | 1·19 (0·82–1·72) |
| Non-perioperative stroke or TIA | 1·37 (0·95–1·97) |
| Non-perioperative stroke | 1·66 (0·99–2·80) |
| Non-perioperative ipsilateral stroke or TIA | 1·29 (0·78–2·14) |
| Non-perioperative ipsilateral stroke | 1·22 (0·59–2·54) |
| Non-perioperative, non-ipsilateral stroke | 1·08 (0·97–3·73) |
| Any cause of death | 1·07 (0·82–1·40) |
Data are hazard ratio (95% CI). Hazard ratios are calculated by intention-to-treat and are based on all available follow-up to a maximum of 11 years. TIA=transient ischaemic attack.
Any stroke or perioperative death were more frequent in the endovascular treatment group than they were in the surgery group. The estimated cumulative 8-year risk was 29·7% (SE 3·4%) after endovascular treatment and 23·5% (3·5%) after surgery (figure 2). The hazard ratio between the two treatments was not significantly different (HR 1·35, 0·94–1·93; table 4).
All the stroke outcome events recorded during follow-up longer than 30 days after treatment lasted more than 7 days. Only one of these events (a non-disabling stroke during endovascular treatment of restenosis in the endovascular arm) occurred as a result of carotid revascularisation. The estimated 8-year cumulative incidence of stroke that lasted for more than 7 days or death after randomisation, including perioperative events, was 54·4% (SE 4·0%) in the endarterectomy group and 52·9% (4·0%) in the endovascular treatment group. The overall risk difference was not significant (HR 1·08, 95% CI 0·85–1·38). Non-cerebrovascular death accounted for most of the deaths. By restricting the analysis to death due to perioperative events, there was no significant difference in the estimated 8-year occurrence of stroke that lasted more than 7 days or perioperative death in the endovascular group (26·6%, SE 3·4%) compared with the surgery group (23·1%, 3·5%; HR 1·19, 95% CI 0·82–1·72, figure 2 and table 4). The estimated 8-year cumulative occurrence of any cause of death was greater after carotid endarterectomy than it was after endovascular treatment (figure 2), but the lines for each treatment came together and crossed after 8 years (data not shown) and the overall risk was not significantly different (1·07, 0·82–1·40; table 4).
The combined outcome of non-perioperative stroke or TIA was more common in the endovascular group than it was in the endarterectomy group, but the difference was not significant (HR 1·37, 95% CI 0·95–1·97). The 8-year cumulative incidence was estimated as 36·9% (SE 5·0%) in the endovascular group and 30·2% (4·7%) in the endarterectomy group (figure 2). Non-perioperative stroke in any territory was also more common in the endovascular group than in the endarterectomy group, with the 8-year cumulative incidence in the endovascular group estimated as 21·1% (SE 4·1%) compared with 15·4% (4·3%) in the endarterectomy group; however, the difference was not significant (1·66, 0·99–2·80; figure 2 and table 4). When the analysis was restricted to ipsilateral events that occurred in the territory of the randomised vessel, the 8-year cumulative incidence of non-perioperative stroke or TIA was similar in the endovascular and surgery groups (19·3% [SE 4·0%] vs 17·2% [3·8%]; figure 2); the difference was not significant (1·29, 0·78–2·14; table 4). The difference was similar when the analysis was done by treatment received rather than by intention-to-treat (1·25, 0·76–2·06).
The estimated cumulative 8-year incidence of non-perioperative ipsilateral stroke was 11·3% (SE 3·7%) in the endovascular group and 8·6% (3·1%) in the endarterectomy group (figure 2). The overall risk difference was not significant when the data were analysed by intention-to-treat (1·22, 0·59–2·54; table 4) or by treatment received (1·10, 0·53–2·28). The cumulative incidence of non-perioperative stroke in a non-randomised vascular territory (contralateral or vertebrobasilar) at 8 years was also not significantly different (11·6% [SE 2·5%] vs 11·2% [4·2%]; 1·90, 0·97–3·73).
23% (n=55) of the patients randomly assigned to endovascular treatment had stenting. The remaining patients were treated with balloon angioplasty only. The 8-year cumulative occurrence of stroke that lasted for more than 7 days or perioperative death, analysed by treatment received, was 24% after balloon angioplasty, 34% after stenting, and 21% after endarterectomy. The higher risk in patients who had stenting compared with those treated by balloon angioplasty only was not significant (1·33, 0·72–2·46). This outcome included patients whose stroke occurred at the time of balloon insertion but before the stent was deployed. Excluding perioperative events, the risk of ipsilateral non-perioperative stroke was lower after stenting than it was after angioplasty alone, but the difference was not significant (0·66, 0·25–1·71).
Subgroup analysis to assess the influence of baseline variables on the long-term rate of any stroke that lasted more than 7 days or perioperative death showed no significant interaction with treatment effect of any of the variables tested (figure 3). In patients younger than 68 years, there was no significant difference in the rate of stroke or perioperative death between endovascular treatment and endarterectomy (1·05, 0·61–1·83). Although the HR was higher in patients aged 68 years or older (1·32, 0·79 to 2·20), this was not significant. Furthermore, the HR for endovascular treatment compared with endarterectomy was non-significantly greater in patients with ischaemic heart disease (1·88, 1·00–3·54).
Figure 3.
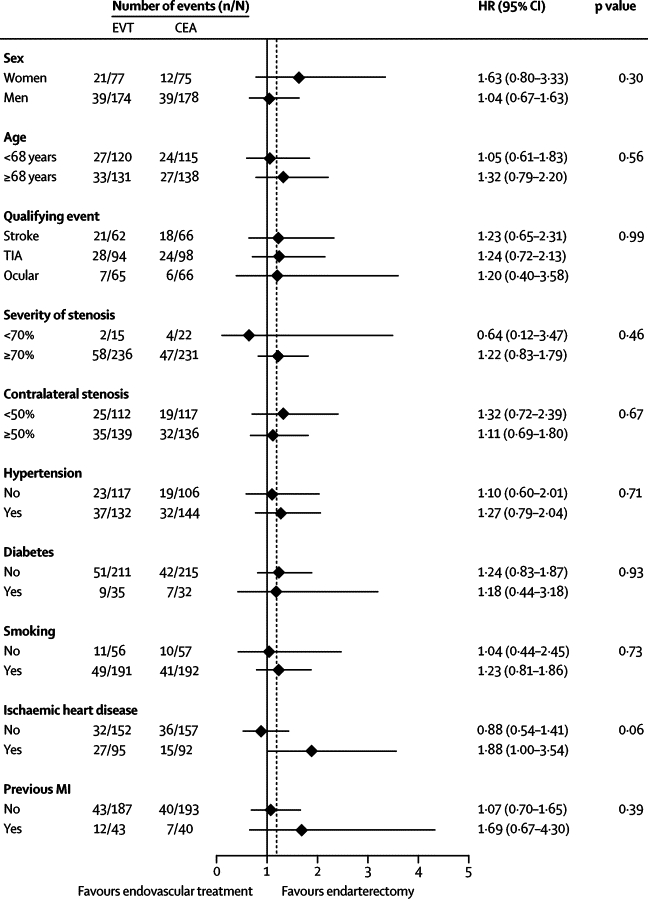
Subgroup analysis to compare the rates of the outcome event of stroke in any territory that lasted more than 7 days or perioperative death, according to various baseline characteristics
p values are associated with treatment–covariate interaction tests. The dotted vertical line is the hazard ratio in the overall population. Analyses are by intention to treat. n=number of events. N=number of patients in each group. EVT=endovascular treatment. CEA=carotid endarterectomy. HR=hazard ratio. TIA=transient ischaemic attack. MI=myocardial infarction.
Discussion
CAVATAS was a randomised controlled trial of endovascular treatment compared with endarterectomy for the treatment of mainly symptomatic carotid artery stenosis. CAVATAS was designed to provide data on two main questions regarding the endovascular treatment of atherosclerotic carotid artery stenosis. First, how safe is endovascular treatment compared with carotid endarterectomy in terms of 30-day outcome events; second, how do the two procedures compare in terms of their long-term effectiveness in preventing post-treatment stroke. The initial short-term data, published in 2001, showed similar rates of the major complications of stroke lasting more than 7 days or death, within 30 days of treatment, but the rates in both arms were unacceptably high (around 10%),6 which mandated further trials to compare stenting with endarterectomy for symptomatic carotid stenosis and determine whether the safety of treatment could be improved. Several of these have published their initial findings of outcome events within 30 days of the procedure.9, 10, 11, 12, 13 The most recent of these, EVA-3S and SPACE, did not establish the equivalence of carotid stenting with endarterectomy in terms of early safety. We concluded in our recent meta-analysis of all available randomised data that the results of the trials did not support a change in clinical practice away from recommending carotid endarterectomy as the treatment of choice for suitable carotid artery stenosis.3 Moreover, the long-term efficacy of endovascular treatment to prevent stroke, which is the main aim of the treatment of carotid stenosis, remained unclear.
Here, we have reported new data on the safety of the endovascular treatment. In the original analysis published in 2001,6 the steering committee prospectively decided that only strokes that lasted longer than 7 days would be included in the analysis of perioperative events occurring within 30 days of treatment, to match the European Carotid Surgery Trial (ECST), which also only reported strokes that lasted longer than 7 days. This criterion was also chosen to avoid ascertainment bias related to the concern that some short-lived events would be missed in patients who had surgery and were operated on under general anaesthesia and returned to surgical wards compared with patients who had endovascular treatment under local anaesthesia and were returned immediately to neurological wards. Our current finding that minor strokes with symptoms that lasted fewer than 7 days were reported more frequently in the 30 days after treatment in the endovascular arm than in the endarterectomy arm (8 vs 1), whereas there was no significant difference in the rate of stroke that lasted longer than 7 days, or death, could be seen as confirmation of an ascertainment bias in favour of the minor strokes, or could show additional hazards from endovascular treatment at the time. The number of minor strokes in the endovascular group was more than offset by the number of cranial nerve palsies after endarterectomy (8 minor strokes vs 22 cranial nerve palsies), one of which did not resolve until the 6 month follow-up.
The final results of CAVATAS reported here provide the first randomised data on the long-term outcome up to 11 years after endovascular treatment. Most events during follow-up (62%) were deaths, and the 5-year and 10-year risks of mortality were 22% and 75%, respectively. The long-term rate of ipsilateral stroke was similar to that recorded in the endarterectomy arms of previous trials: ECST reported a 10-year risk of ipsilateral stroke after carotid endarterectomy (excluding perioperative events) of 9·7%,14 which is similar to the 8-year risk of 8·6% after surgery and 11·3% after endovascular treatment reported here. The comparison of the primary outcome measure of long-term survival free of disabling stroke or death (including perioperative events) in CAVATAS showed no difference between endovascular treatment and endarterectomy (HR 1·02, 95% CI 0·79–1·32) and the rate after randomisation of any stroke that lasted longer than 7 days or perioperative death was also not significantly different (1·19, 0·82–1·72). There was also no significant difference in the rate of outcome events between the two arms if only events occurring more than 30 days after treatment are analysed, although there were more events in those patients allocated to endovascular treatment. EVA-3S and SPACE also found little difference in the rates of ipsilateral non-perioperative stroke more than 30 days after treatment when patients assigned to carotid stenting were compared with those assigned to endarterectomy over a shorter length of follow-up (maximums of 4 and 2 years, respectively).7, 8 The result of a meta-analysis of the data from all three trials suggested that both methods of treatment have similar efficacy at preventing long-term ipsilateral stroke after the treatment period (figure 4), but with wide confidence intervals (odds ratio 1·18, 95% CI 0·70–1·99). Further long-term data are needed from the large on going randomised stenting trials, to ascertain whether the small difference in favour of endarterectomy becomes significant or not with larger numbers of patients and outcome events.
Figure 4.
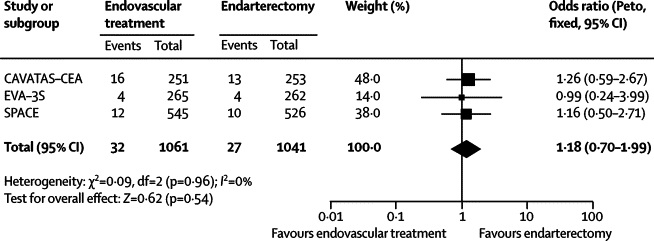
Meta-analysis of the main multicentre randomised controlled trials
Comparison of long-term benefit of endovascular treatment versus endarterectomy for symptomatic carotid artery stenosis in prevention of non-perioperative ipsilateral stroke. The summary estimate statistic is a Peto odds ratio (fixed-effect model), the centre of the diamond is the point estimate, and the ends of the line are the 95% CI. The I-square statistic gives an indication of heterogeneity, where 0% suggests that it might not be important. The value of I-squared depends on the magnitude and direction of effects and the strength of evidence for heterogeneity (p value from χ2 test).
CAVATAS was one of the earliest clinical trials of endovascular treatment, and most patients in the endovascular treatment group were treated with just angioplasty. Stenting was primarily used as a secondary procedure when balloon angioplasty alone produced an unsatisfactory result. Stents were used in 55 patients (26%), in whom endovascular treatment was deemed technically successful (balloon inflation or stent deployment across the lesion).6 Primary stenting has subsequently replaced balloon angioplasty as the radiological technique of choice for carotid stenosis. Additionally, the technical equipment used in endovascular treatment has improved since CAVATAS. However, there are no data from randomised trials to support the notion that stenting is superior to angioplasty alone. The exploratory subgroup analysis of patients treated with stenting compared with those treated with only balloon angioplasty showed no significant difference in the risk of stroke that lasted more than 7 days or perioperative death (1·33, 0·72–2·46), but this outcome included some patients whose stroke occurred as a result of full balloon deployment before a stent was inserted as a rescue procedure. The risk of ipsilateral non-perioperative stroke was lower after stenting than after angioplasty alone, but the number of events was small and the difference was not significant (0·66, 0·25–1·71).
One of the concerns about endovascular techniques is that angioplasty and stenting might be associated with a higher rate of restenosis than is endarterectomy. We report the results of analysis of the ultrasound follow-up from those centres in CAVATAS where ultrasound was available in a companion paper.15 The ultrasound data confirmed that severe restenosis (≥70%) was found significantly more commonly in patients after endovascular treatment than after endarterectomy, and the long-term risk of severe carotid restenosis or occlusion in CAVATAS was three times higher after endovascular treatment than after endarterectomy (adjusted HR 3·17, 1·89–5·32; p<0·0001). Severe carotid restenosis or occlusion after endovascular treatment or endarterectomy was also associated with an increased risk of recurrent ipsilateral cerebrovascular events (adjusted HR for the rate of ipsilateral non-perioperative stroke or TIA 2·18, 1·04–4·54; p=0·04) but the increase in recurrent ipsilateral stroke alone was not significant (1·67, 0·54–5·11; p=0·4).
Several tests were done to ascertain whether risk factors predicted outcome, regardless of treatment allocation. None of these subgroup analyses showed a significant interaction between baseline variables and treatment effect. In patients younger than 68 years there was no difference in risk of stroke that lasted more than 7 days or perioperative death according to treatment allocation (1·05, 0·61–1·83), but the hazard ratio for endovascular treatment in patients aged 68 years or older was higher than that for endarterectomy (1·32, 0·79–2·20). Although little emphasis can be placed on this finding, these results are consistent with the findings reported by the EVA-3S and SPACE investigators.7, 8 The subgroup analyses also suggest that patients with ischaemic heart disease might have a lower long-term rate of stroke after treatment with endarterectomy than after treatment with endovascular methods.
In conclusion, more patients in the endovascular group had a stroke during follow-up than did patients in the surgical group, but the rate of ipsilateral non-perioperative stroke was low in both groups and none of the differences in the stroke outcome measures reached statistical difference. However, the study was underpowered and the confidence intervals are wide. The low rate of long-term stroke longer than 30 days after endovascular treatment supports the use of endovascular treatment to prevent long-term stroke in patients in whom carotid endarterectomy is contraindicated or who prefer to risk the possibly greater hazard of endovascular treatment over surgery. However, the results do not support the use of endovascular treatment in preference to surgery in patients who are suitable for both treatments; there were also more minor strokes within 30 days of endovascular treatment and a greater incidence of restenosis. These results emphasise the need for further data from the short-term and long-term comparison of endovascular treatment with endarterectomy in trials that are on going, including the International Carotid Stenting Study (ICSS) and the Carotid Revascularisation Endarterectomy versus Stent Trial (CREST).16, 17, 18 There is a strong suggestion from the subgroup analysis of SPACE and EVA-3S, which is supported by the subgroup analysis in CAVATAS, that the small additional hazard of endovascular treatment applies only to older patients and that the two treatments might be equivalent in young patients. The Carotid Stenting Trialists Collaboration has recently been established to analyse the pooled data from the recent large trials, to establish more clearly in which subgroups endarterectomy is the treatment of choice and in which subgroups endovascular treatment has equivalent risks. Stenting avoids some of the minor complications of carotid endarterectomy and therefore would be the treatment of choice in any subgroups in which it could be shown to have equivalent safety and long-term outcome.
Acknowledgments
Acknowledgments
CAVATAS was funded by grants from the British Heart Foundation, the National Health Service Management Executive, and the Stroke Association, UK. The ultrasound laboratory at the central trial office was funded by the Wellcome Trust and the Neurosciences Research Foundation, UK. LHB was supported by a grant from the Swiss National Science Foundation (PBBSB-116873). JE and RLF were supported by a grant from the UK Medical Research Council. MMB's chair in stroke medicine is supported by the Reta Lila Weston Trust for Medical Research, UK. This work was done at University College London Hospitals/University College London, which received a proportion of its funding from the UK Department of Health NIHR Biomedical Research Centres funding scheme.
Contributors
JE wrote the first draft of the manuscript and performed the data analyses under the supervision of JD. JE and LHB adjudicated clinical outcome events. LHB reviewed the manuscript. RLF assisted with database queries and reviewed the manuscript. The remaining named authors contributed the majority of patients to the study and reviewed the manuscript. Collaborators at individual centres were given the opportunity to comment on a draft of the paper. MMB had the final responsibility for this analysis and the manuscript content as the chief investigator in CAVATAS.
CAVATAS collaborators
Organising Committee—JM Bland, MM Brown (principal investigator), T Buckenham, A Clifton, PAG Sandercock, RS Taylor. Data Monitoring Committee—R Collins, G Tognoni, CP Warlow (chairman). Trial Statistician—JM Bland. Trial Office Staff—T Bleakley, D Colquhoun, L Coward, F Crawley, P Dobinson, R Featherstone, S Holder, H Markus, DJH McCabe, A Pereira, J Rogers, L Silver (St George's Hospital Medical School and UCL Institute of Neurology, London, UK); J Burrett, J Crowther, M Dobson, B Hafner, J Heineman, C Hope, S Knight, A Naughten, A Radley, S Richards, D Smith, S Wenzel (Clinical Trial Service Unit, Oxford, UK). Clinical Audit Committee—M Harrison, J Ferro. Credentials Committee—J Beard, T Buckenham, MM Brown, A Clifton.
CAVATAS centres
Centre [number of patients randomised] (investigators).
Australia—Austin and Repatriation Medical Centre, Heidelberg [18] (CF Bladin, GA Donnan, G Fell, G Fitt, J Royle); Royal Melbourne Hospital [1] (S Davis, R Gerraty, P Mitchell); Royal Perth Hospital [21] (MA Goodman, GJ Hankey, MS Khangure, MM Lawrence-Brown, J Linto, W McAuliffe, FJ Prendergast, K Siennarine, EG Stewart-Wynne). Canada—Ottowa General Hospital [2] (S Grahovac, W Morrish, N Pageau, C E Pringle, D M Richard). Germany—Heinrich Heine University, Duesseldorf [4] (J Malms, L Reiher, M Siebler). Italy—Policlinico St Marco, Bergamo-Zingonia [13] (G Belloni, M Porta). Spain—Hospital Clinic I Provincial, Barcelona [4] (A Chamorro, N Vila [deceased], V Riambau, F Vazquez); Hospital Universitario Virgen del Rocio, Sevilla [16] (F Boza, JL Garcia Rodríguez, A Gil Peralta, A González, JR González Marcos, A Mayol Deya J Rauno). Switzerland—University Hospital, Basel [5] (EC Kirsch, PA Lyrer, JA Rem); Centre Hospitalier Universitaire Vaudois, Lausanne [1] (J Bogousslavsky, A Uske). United Kingdom—Royal Hallamshire and Northern General Hospitals, Sheffield [193] (JD Beard, TJ Cleveland, CDoyle, PA Gaines, A Sivaguru, GS Venables); London, Atkinson Morley's and St George's Hospitals [100] (MM Brown, T Buckenham, A Clifton, D Colquhoun, F Crawley, PW Leopold, T Loosemore, DJH McCabe, A Pereira, J Rogers, RS Taylor); Walton Centre, Liverpool [4] (TP Enevoldson, G Gilling-Smith, P Harris, T Nixon); London, King's College [21] (P Baskerville, T Cox, S Fraser, M Jeffrey, H Markus, J Molloy); London, Royal London [1] (P Butler, J Dick, F Frankel); Western General Hospital, Edinburgh [31] (A Bradbury, D Collie, JA Murie, CV Ruckley, PAG Sandercock, D Schultz, RJ Sellar, J Wardlaw); Withington Hospital, Manchester [7] (RJ Ashleigh, CN McCollum, P O'Neill); Newcastle General Hospital [14] (A Gholkar, AD Mendelow, TJ Walls); University Hospital of Wales, Cardiff [6] (H Angus-Leppan, S Halpin, J Hughes, I Lane, M Wiles, AM Wood); Gloucestershire Royal Hospital [19] (PA Birch, JJ Earnshaw, GN Fuller, B Heather, K Poskitt, AJ Tottle); Queens Medical Centre, Nottingham [18] (DT Hope, D Jefferson, N McConachie); Queen Elizabeth Neuroscience Centre, Birmingham [5] (M Duddy, MTE Heafield, RK Vohra).
Conflicts of interest
We have no conflicts of interest.
References
- 1.European Carotid Surgery Trialists' Collaborative Group Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 2.Barnett HJ, Taylor DW, Eliasziw M. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 3.Ederle J, Featherstone R, Brown M. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD000515.pub3. CD000515. [DOI] [PubMed] [Google Scholar]
- 4.Ederle J, Featherstone RL, Brown MM. Long-term outcome of endovascular treatment versus medical care for carotid artery stenosis in patients not suitable for surgery and randomised in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) Cerebrovasc Dis. 2009;28:1–7. doi: 10.1159/000215936. [DOI] [PubMed] [Google Scholar]
- 5.Coward LJ, McCabe DJ, Ederle J, Featherstone RL, Clifton A, Brown MM. Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke. 2007;38:1526–1530. doi: 10.1161/STROKEAHA.106.471862. [DOI] [PubMed] [Google Scholar]
- 6.Brown MM, Rogers J, Bland JM. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–1737. [PubMed] [Google Scholar]
- 7.Eckstein HH, Ringleb P, Allenberg JR. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 8.Mas JL, Trinquart L, Leys D. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892. doi: 10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 9.Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589–1595. doi: 10.1016/s0735-1097(01)01595-9. [DOI] [PubMed] [Google Scholar]
- 10.Yadav JS, Wholey MH, Kuntz RE. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 11.Mas JL, Chatellier G, Beyssen B. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 12.Ringleb PA, Allenberg J, Bruckmann H. 30-day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 13.Alberts MJ. Results of a multicenter prospective randomized trial of carotid artery stenting vs. carotid endarterectomy. Stroke. 2001;32:325. [Google Scholar]
- 14.Cunningham EJ, Bond R, Mehta Z, Mayberg MR, Warlow CP, Rothwell PM. Long-term durability of carotid endarterectomy for symptomatic stenosis and risk factors for late postoperative stroke. Stroke. 2002;33:2658–2663. doi: 10.1161/01.str.0000034397.72390.d3. [DOI] [PubMed] [Google Scholar]
- 15.Bonati LH, Ederle J, McCabe DJH, for the CAVATAS investigators Long-term risk of carotid restenosis in patients randomly assigned to endovascular treatment or endarterectomy in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): long-term follow-up of a randomised trial. Lancet Neurol. 2009 doi: 10.1016/S1474-4422(09)70227-3. published online August 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Featherstone RL, Brown MM, Coward LJ. International Carotid Stenting Study: protocol for a randomised clinical trial comparing carotid stenting with endarterectomy in symptomatic carotid artery stenosis. Cerebrovasc Dis. 2004;18:69–74. doi: 10.1159/000078753. [DOI] [PubMed] [Google Scholar]
- 17.Stringer RW., 2nd CREST (Carotid Revascularization Endarterectomy versus Stent Trial): background, design, and current status. Semin Vasc Surg. 2000;13:139–143. [PubMed] [Google Scholar]
- 18.Brown MM, Hacke W. Carotid artery stenting: need for randomised trials. Cerebrovasc Dis. 2004;18:57–61. doi: 10.1159/000078750. [DOI] [PubMed] [Google Scholar]


