Abstract
Background
Rhinosinusitis is a common, expensive disorder with a significant impact on patients' quality-of-life. Chronic sinus symptoms are associated with allergic rhinitis, asthma, and nasal polyposis. Saline nasal irrigation is an adjunctive therapy for rhinosinusitis and sinus symptoms. Prior studies suggest that HSNI may be effective for symptoms associated with allergy, asthma and nasal polyposis.
Objective
To assess the degree to which subjects using nasal irrigation for chronic sinus symptoms also reported improvements in symptoms related to allergy, asthma or nasal polyposis.
Design
Qualitative study using in-depth long interviews.
Participants
28 participants in a prior qualitative nasal irrigation study.
Intervention
Daily nasal irrigation.
Outcome
Qualitative transcripts
Results
Transcripts of interviews were systematically examined. Twelve of 21 subjects with allergic rhinitis spontaneously reported that HSNI improved symptoms. Two of seven subjects with asthma and one of two subjects with nasal polyposis reported a positive association between HSNI use and asthma or nasal polyposis symptoms. Transcript content was organized into themes which included: 1) HSNI resulted in improvement of allergic rhinitis and asthma symptoms, and 2) HSNI should be used for symptoms of allergic rhinitis.
Conclusions
This hypothesis generating study offers suggestive qualitative evidence that in patients with frequent rhinosinusitis and daily sinus symptoms, symptoms of concomitant allergic rhinitis, asthma or polyposis may also improve with HSNI. The parent studies offer strong evidence that HSNI is an effective adjunctive treatment for symptoms of chronic rhinosinusitis. Larger prospective studies are needed in patients with these diagnoses.
Keywords: nasal irrigation, allergic rhinitis, qualitative study
Introduction
Rhinosinusitis is a common, expensive disorder that has a significant impact on patients' quality-of-life (QOL).1 In a subset of patients, sinus symptoms can become chronic and are epidemiologically associated with asthma,2, 3 allergic rhinitis4, 5 and nasal polyposis,6 though the etiological relationships are not well understood. Each condition is associated with significant morbidity, cost, and impact on QOL.1, 7 Allergic rhinitis affects 20-40 million persons annually in the US,8 is responsible for 3.5 million lost work days, 2 million missed school days each year,9 and an estimated 28 million days of restricted activity or reduced productivity.10 Total costs of allergic rhinitis have been estimated at $250 million 1998 dollars ($291.6 million 2002 dollars).11 Overall health care costs for allergic rhinitis are rising at a rate of 12% per year.12 Treatment of allergic rhinitis is expensive and has significant side effects, resulting in expense of $3.8 billion dollars alone.13
Hypertonic saline nasal irrigation (HSNI) is a adjunctive therapy for rhinosinusitis and sinus symptoms.14 It flushes the nasal cavity, facilitating the evacuation of potentially allergen- and irritant-containing mucus (Fig. 1).15 It is commonly used therapy in Wisconsin; a recent study of 286 Family Physicians who use HSNI found that 95% use some form of nasal saline for a variety of conditions including chronic rhinosinusitis (91%), acute upper respiratory infections (80%), allergic rhinitis (70%), irritant rhinitis (48%) and URI-triggered asthma (9.1%)(Rabago unpublished data). Nasal saline irrigation has also been used for decades as post-operative care for endoscopic sinus surgery patients and as a complement to chronic nasal steroid use (personal communication with co-author, DB) in patients with allergic rhinitis. One study of nasal saline delivered as a spray reported that it may prevent viral upper respiratory infection16 but another reported that it may not lessen the severity or duration of active URI.17 HSNI was recently identified as “an important component in the management of most sino-nasal conditions” that is “effective and underutilized”.18 The Cochrane Collaboration has reported that HSNI is an effective adjunctive therapy for chronic rhinosinusitis symptoms.19
figure 1.
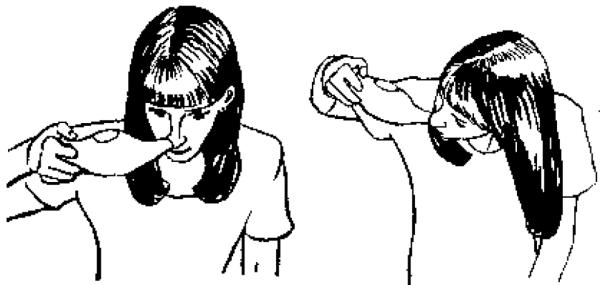
Nasal Irrigation Technique.
Ten randomized controlled trials (RCTs) suggest that HSNI is a safe, effective and tolerable therapy for rhinosinusitis and chronic sinus symptoms that results in improvement in disease related QOL scores and surrogate measures in adults and children.20-29 A recent study reported that nasal irrigation was effective in pediatric allergic rhinitis.30 In a closely monitored 6-month RCT28 and a 18-month follow-up study by the lead author (DR),31 subjects using daily 2% HSNI for chronic sinus symptoms reported improved QOL, high patient satisfaction, decreased antibiotic and nasal spray use and improved sinus symptoms. A subsequent qualitative study of 28 subjects confirmed these findings and described the overall experience of initiating and maintaining successful HSNI use;32 subjects felt empowered to self-treat and manage their sinus conditions, reported rapid and long-term QOL improvements, identified significant barriers to using HSNI and acknowledged positive aspects of HSNI training and patient education about in-home use in overcoming such barriers.
During the qualitative interviews,32 several subjects spontaneously reported that HSNI improved symptoms associated with their baseline allergic rhinitis, asthma or nasal polyposis. Because 1) epidemiological and pathophysiological relationships between these conditions and sinus symptoms exist, and 2) HSNI was incidentally noted to improve symptoms associated with these conditions, we speculated that HSNI might function as adjunctive therapy for these conditions.
Therefore we re-analyzed qualitative data to explore the research question `Do subjects using HSNI for chronic sinus symptoms, who also reported diagnoses of allergic rhinitis, asthma or nasal polyposis, spontaneously report improvements in symptoms related to allergic rhinitis, asthma or nasal polyposis?”
Methods
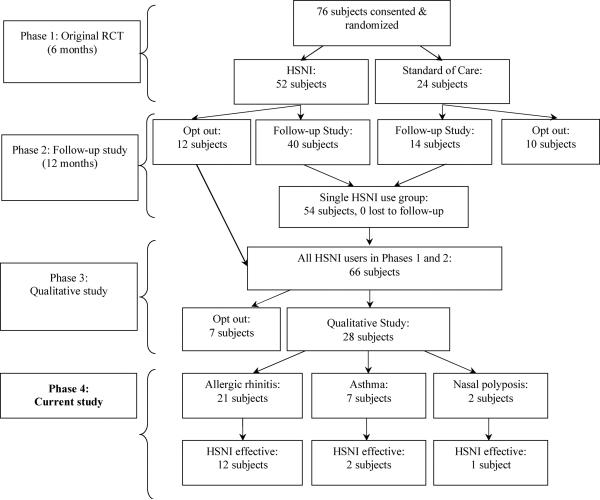
The methods of the parent RCT and follow up studies (Phases 1-3) were previously reported.28, 31, 32 The current study, Phase 4 (Fig. 2) was approved by the UW Human Subjects Committee. The inclusion criteria of Phase 1 were two or more episodes of acute rhinosinusitis, or one or more episodes of chronic rhinosinusitis per year for the prior two years, and a `moderate to severe' daily QOL burden associated with sinus symptoms. Intervention subjects in Phases 1 and 2 used 2% buffered saline solution daily for 18 months. (Fig. 1)28 In Phase 3, subjects participated in a qualitative study. In-depth long interview methodology suggests that a sample size of 20-30 subjects from a larger group of subjects with similar experiences captures all or nearly all relevant data.33 Accordingly, twenty-eight Phase 1 and 2 subjects were interviewed to determine themes associated with HSNI use (Table 1, Fig. 2). The inclusion criterion for the current study (Phase 4) was participation in the Phase 3 study.32 Phase 3 transcripts were reanalyzed for the current study from May to July 2006 using two methods. First, a computerized keyword search located descriptors of asthma, allergic rhinitis and nasal polyposis (Table 2). Second, each transcript was manually evaluated by inspection by the second author (EG) for phrases that were relevant to the conditions of interest. Quotations from the qualitative study describing the perceived effect of HSNI on allergic rhinitis, asthma or nasal polyposis symptoms were organized thematically.
Figure 2.
Subject participation in Phase 1 randomized controlled trial, Phase 2 follow-up study, Phase 3 qualitative study and the current study (Phase 4). Phase 4 subject number sums to greater than 28 because some subjects had more than one diagnosis.
Table 1.
Open-Ended Questions Used to Frame the Qualitative Interviews
| 1. What were your sinus problems like before using nasal irrigation and how did nasal irrigation affect you? |
| 2. Did you experience any problems from using nasal irrigation? |
| 3. How did you fit nasal irrigation into your life? |
| 4. Did you get any reactions about using nasal irrigation from those around you? |
| 5. How do you feel about nasal irrigation now? |
| 6. What was the informational meeting like for you? |
| 7. Is there anything else you'd like to tell us about your experience with nasal irrigation or this study? |
Table 2.
Key words used during computerized key word search of the interview transcripts.*
| KEY WORDS | ||
|---|---|---|
| Asthma | Allergic Rhinitis | Nasal Polyposis |
| asthma | allergic rhinitis | nasal polyposis |
| wheeze | allergy | nasal |
| short of breath | seasonal allergy | polyp |
| breathing problem | allergic | surgery |
| cough | runny nose | |
| inhaler | itchy eye | |
| albuterol | watery eye | |
| sneeze | ||
| pollen | ||
| mites | ||
| mold | ||
| smoke | ||
| decongestant | ||
| anti-histamine | ||
All words were searched with as a wildcard.
Results
Transcripts from the qualitative interviews of all 28 participants were examined. Subjects reported having allergic rhinitis (n=21), asthma (n=7) or nasal polyposis (n=3) at the beginning of Phase 1; 15 subjects reported associations between HSNI and the conditions of interest. Twelve out of 21 subjects (57%) with allergic rhinitis spontaneously reported improvements in their allergic rhinitis symptoms. Two out of seven (29%) subjects with asthma and 1 out of two (50%) subjects with nasal polyposis reported a positive association between HSNI use and asthma or nasal polyposis symptoms respectively. The age, gender and sinus-related quality-of-life scores of the 12 subjects with allergic rhinitis (Table 2) and of the 3 subjects with asthma or nasal polyposis were statistically similar to both the 28-member qualitative cohort and the cohort of all HSNI users in the parent study. All comments reflected a positive relationship between the subjects' use of HSNI and their perception of its effect on the conditions of interest (Table 3). Listed comments (Table 3) were distinct and separate from reports on sinus symptoms. The quotations illustrate participants' range of experience. The overall positive reaction to HSNI in the current study is consistent with that of the prior qualitative study from which this sample is drawn.32 The comments reflect subjects' experience with a debilitating condition (chronic sinus symptoms), who were introduced to a non-intuitive therapy whose mastery required work and insight (performing HSNI), who achieved therapeutic success (improved QOL, symptom scores) and who perceived a relationship between HSNI and their underlying conditions.
Table 3.
Baseline demographic and sinus-related outcome scores, and post-treatment scores, of all HSNI# users (n=66), all Phase 3 subjects (n=28), Phase 4 subjects with allergy symptoms (n=21) and Phase 4 subjects who noted relationship between HSNI use and their improved allergy symptoms (n=12).
| Characteristic | Phase 1 and 2 HSNI Users (N = 66) | Phase 3 Qualitative Study (N = 28) | Phase 4 Subjects w/ Allergy Symptoms (N = 21) | Phase 4 Subjects Reporting HSNI/Allergy Relationship (N = 12) |
|---|---|---|---|---|
| Age | 42.4 ± 1.3 | 44.8 ± 1.8 | 46.5 ± 2.0 | 44.2 ± 1.2 |
| Female | 48 (73%) | 19 (68%) | 15 (71%) | 9 (75%) |
| BaselineRSDI* | 58.8 ± 1.8 | 57.2 ± 2.9 | 52.5 ± 3.1 | 56.1 ± 4.9 |
| End RSDI | 77.9 ± 1.8 | 80.1 ± 2.9 | 76.8 ± 3.4 | 75.8 ± 5.5 |
| Baseline SIA** | 3.95 ± 0.12 | 4.02 ± 0.20 | 4.21 ± 0.24 | 3.96 ± 0.36 |
| End SIA | 2.36 ± 0.13 | 2.29 ± 0.18 | 2.33 ± 0.19 | 2.42 ± 0.26 |
HSNI: hypertonic saline nasal irrigation
RSDI: Rhino Sinusitis Disability Index.44 Using a 30-item validated multidimensional disease-specific assessment instrument, participants scored their sinus symptoms, 0= maximal impact of sinus symptoms on quality of life, 100=no impact.
SIA: Single-item Assessment. Using a 1-7 Likert scale where 1= “no impact” and 7= “maximal impact”, participants responded to the statement: “Please evaluate the overall severity of your sinus symptoms since enrolled in the study.”
The most dramatic set of comments were about the use of HSNI by subjects with allergic rhinitis. Of 21 subjects in the qualitative study with allergic rhinitis, 12 (57%) spontaneously reported improved allergy symptoms such as watery itchy eyes and rhinitis, and improved quality of life with use of HSNI. Two major themes were identified in relation to allergic rhinitis and HSNI use.
Symptom improvement
Subjects reported improvement of symptoms associated with allergic rhinitis with HSNI use. Most subjects did not differentiate allergy symptoms, referring to them collectively as “allergy symptoms”, though some identified rhinitis and watery, itchy eyes as specific symptoms. Participants reported that use of HSNI improved their allergy-related rhinitis symptoms separate from sinusitis symptoms, for example: “I am surprised that not only has my sinus incidence gone down but my whole allergy incidence has gone down, and “[nasal irrigation] helps with my sinus but it helps with my allergies as well.” They also reported improved QOL, noting: “just bringing [the allergen] in the house would trigger an allergic reaction and I would be miserable for days. But now [since nasal irrigation, that] doesn't even bother me,” and “…we did a lot of work in a basement with a lot of mold and [then] I actually had some bad allergic reactions. [Nasal irrigation] has helped a lot. Thinking back, my allergies aren't as bad using [nasal irrigation].” “It's such a big change when you can enjoy things that people take for granted” and “[nasal irrigation] literally changes a great aspect of my life.”
Two subjects related use of HSNI to asthma symptom improvement, one of whom reported: “…I noticed the neti pot helps with the [asthmatic] breathing too.” One subject commented on a possible preventive relationship between HSNI and nasal polyposis by stating “And then I …had [sinus surgery] again because my sinuses were so bad I was growing polyps in my nasal cavity…If I had [nasal irrigation] earlier I wouldn't have gone through what I have gone through…if I would have a way to prevent this outside of surgery I would have done anything.”
HSNI use recommendation
During the qualitative interview, subjects were asked to indicate the conditions for which HSNI could/should be recommended. Without prompting, eight out of 21 participants with allergies spontaneously indicated that HSNI should be used for allergy symptoms; typical comments were: “…I think if [patients complain] about their allergies that's enough [to use HSNI],” and “I think somebody who had a lot of…allergies [should use it]. It would see to me this would be the first line of attack for allergies.”
Discussion
This study investigated the relationship between HSNI use and symptoms of allergic rhinitis, asthma and nasal polyposis in adult subjects. More than half of subjects with self-reported chronic sinus symptoms and concurrent allergic rhinitis spontaneously reported positive effects of HSNI on allergy symptoms as distinct from chronic sinus symptoms, suggesting that HSNI may be effective adjunctive therapy for allergic rhinitis. This is the first study to report such a relationship in adults. The current study adds to prior data from the same cohort32 by suggesting that symptoms of both conditions are improved by HSNI. These results are consistent with the findings of a small but methodologically strong pediatric study;30 patients with laboratory confirmed, pollen-triggered allergic rhinitis reported that, compared to antihistamine alone, antihistamine plus HSNI resulted in significant improvement in allergy symptom scores and reduction in antihistamine use.30 These results are also consistent with current practice of Family Physicians in Wisconsin, many of whom use HSNI for allergic conditions (Rabago, unpublished data).
Two of seven subjects with asthma reported that HSNI improved asthma their symptoms. No study has formally tested HSNI as adjunctive treatment for asthma in patients with sinus disease. Epidemiological evidence suggests that the conditions are related; 80-90% of children and adolescents with asthma also have nasal symptoms, and half of all patients with asthma have radiographic evidence of sinusitis, though imaging results are non-specific.6 Whether the two conditions are causally linked is unclear, but in one study, aggressive treatment of sinusitis with HSNI with and without antibiotics resulted in significantly decreased bronchial hyperresponsiveness compared to baseline.3 In addition, some authors have hypothesized that systemic inflammatory processes underlying asthma and allergic rhinitis are similar.5 Studies of patients with both asthma and allergic rhinitis reported that effective treatment of allergic rhinitis results in reduced severity or frequency of asthma,34, 35 suggesting that HSNI may have a role as adjunctive therapy for allergy induced asthma.
One subject in the current study reported that nasal polyposis, a sequela of chronic rhinosinusitis, might have been prevented by nasal irrigation if used early enough. While speculative, at least 3 randomized controlled studies report symptomatic effectiveness of HSNI for chronic sinusitis or chronic sinus symptoms without documented polyposis.23, 24, 28 Given that polyposis is an extreme form of chronic sinus disease, and that HSNI may improve the function and health of the nasal mucosa, aggressive treatment with HSNI may inhibit progression of chronic rhinosinusitis to a polypoid form.
The mechanism of nasal irrigation's effect is not well understood and is likely multifaceted. Relating HSNI mechanistically to allergic rhinitis, asthma or polyposis is therefore somewhat speculative. However, nasal irrigation has been reported to have several physiological effects which individually or in concert may result in an improved ability of the nasal mucosa to reduce the pathologic effects of inflammatory mediators and other triggers of allergic rhinitis, asthma and other chronic mucosal reactions. These effects include: 1) direct cleansing effect by the saline as it thins and removes obstructive mucus and crusts;36-38 2) removal or reduction of inflammatory mediators such as histamine, prostaglandins, leukotriennes and eosinophil-released major basic protein;15, 39 3) improved mucociliary function in the presence of hypertonic saline40 and normal saline.41 Optimal tonicity and pH of the irrigating solution are unclear.42, 43
Limitations of this study include its small size, potential reporting bias given the prolonged contact with study personnel and recall bias. Details of subjects' views about the effects of HSNI on the conditions of interest are limited by the fact that subjects were not specifically queried about these conditions, but rather spontaneously reported their views. Diagnoses were not obtained objectively; subjects provided medical diagnoses and effect of HSNI on symptoms of particular diagnoses by self-report. Strengths include excellent training in the use of nasal irrigation (film, live demonstration, demonstrated proficiency), strong continuity with subjects through 3 prior studies of varied methodologies, demonstrated effectiveness of nasal irrigation in each of these studies using a variety of outcome measures, demonstrated high subject adherence and retention, and effective data collection throughout the study. Randomized controlled studies are needed to assess the clinical effect, side-effect profile and economic impact of HSNI in subjects with clear diagnoses of allergic rhinitis, asthma and nasal polyposis.
Conclusions
This hypothesis generating study suggests that patients with frequent rhinosinusitis, daily sinus symptoms and concurrent allergic rhinitis may benefit from adjunctive treatment with HSNI and that HSNI deserves further study as adjunctive treatment for this common condition, ideally in a population without other forms of rhinosinusitis. Given that HSNI is effective, safe, inexpensive and well-tolerated for symptoms of chronic rhinosinusitis, clinicians can feel comfortable recommending HSNI to their patients who also have allergic rhinitis. The relationship between HSNI and symptoms associated with URI induced asthma and nasal polyposis is unclear but is likewise deserving of further study in populations with these disorders.
Table 4.
Representative themes and quotations about HSNI and effects on symptoms of allergy rhinitis, asthma and nasal polyposis.
| 1. Allergy |
| Participants reported decrease in allergy symptoms and an increase in quality of life. |
| “I'm allergic to a lot of stuff, ragweed and pollen, and I live in a rural area where there is lots of farm and haying and that stuff goes on all summer and it was miserable for me. I am surprised that not only has my sinus incidence gone down but my whole allergy incidence has gone down. I don't know what it is but I feel like I have more tolerance to being outside. I don't have hay fever like I had before or in the runny nose and eyes and itching.” |
| “It [HSNI] literally changes a great aspect of my life. For instance, I couldn't mow my lawn because the grass, it would just kill me. And planting flowers, ...I love flowers. Planting my flowerbeds was just terrible, I would just have hay fever and then I'd be plugged up and then I'd have to go to the doctor and get more antibiotics.” |
| “[My kids would say] 'Mom I got you flowers' and just bringing [them] in the house would trigger an allergic reaction and I would be miserable for days. But now it doesn't even bother me. I am out there picking weeds and doing a lot of stuff. I have talked to my friends about this a lot because it's a big change for me. When you suffer for a chronic illness for so long and then you don't have problems with it anymore I think it's such a big relief and I can't explain it, it's such a big change where you can enjoy things that people take for granted.” |
| “We did a lot of work in a basement with a lot of mold and... a week or two after that...I actually had some bad allergic reactions and then I got an infection shortly after that too.(considering tossing this statement, I don't think it adds anything) [HSNI] has helped a lot. Thinking back, my allergies aren't as bad using the neti pot.” |
| “What I do find is that it helps with my allergies. It helps with my sinus but it helps with my allergies as well.” |
| “…with my allergies it's helped. I don't know if that's supposed to be or not but it's helped me cope with my allergies…” |
| Participants recommended HSNI for allergic symptoms when asked to name conditions for which HSNI might be useful. |
| aI think if [patients complain] about their allergies that's enough. There's enough other things out there that you can't help and [HSNI] seems to help.” |
| “I think somebody who had a lot of sinuses and allergies [should use it]. It would see to me this would be the first line of attack for allergies.” |
| “Allergies and sinus problems.” |
| 2. Asthma |
| Patients noted less frequent asthma symptoms. |
| “Whereas I use the Flovent after the fist couple of weeks, I was also using it [nasal irrigation] PRN and I use it twice a day now so that may be making some difference there too. I noticed the neti pot helps with the breathing....” |
| 3. Nasal Polyposis |
| One participant speculated that HSNI might have prevented the need for surgery for nasal polyposis. |
| “If I would have known about HSNI twelve years ago I [might] never have had my first sinus surgery...my sinuses were so bad I was growing polyps in my nasal cavity... If I had this [HSNI] earlier I [might not] have gone through what I have gone through.” |
| Legend: Bracketed words are the authors' interpretation of the subject's original intent; they are used to link ideas or abbreviate wordiness. |
References
- 1.Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis and other airway disorders. J Allergy Clin Immunol. 1999;103:408–414. doi: 10.1016/s0091-6749(99)70464-1. [DOI] [PubMed] [Google Scholar]
- 2.Vinuya Z. Upper airway disorders and asthma: a syndrome of airway inflammation. Annals of allergy, asthma, and immunology. 2002;88:8–15. doi: 10.1016/s1081-1206(10)62023-6. [DOI] [PubMed] [Google Scholar]
- 3.Tsao C, Chen L, Yeh K, et al. Concommitant chronic sinusitis treatment in children with mild asthma. The effect on hyperresponsiveness. Chest. 2003;123:757–764. doi: 10.1378/chest.123.3.757. [DOI] [PubMed] [Google Scholar]
- 4.Kirtsreesakul V, Naclerio RM. Role of allergy in rhinosinusitis. Curr Opin Allergy Clin Immunol. 2004;4(17):17–23. doi: 10.1097/00130832-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on athsma. The ARIA workshop/WHO. J Allergy Clin Immunol. 2001;108:S147–336. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 6.Fokkens W, Lund V, Bachert C, et al. EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy. 2005;60:583–601. doi: 10.1111/j.1398-9995.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- 7.Malone DC, Lawson KA, Smith DH, et al. A cost of illness study of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99(1):22–27. doi: 10.1016/s0091-6749(97)70296-3. [DOI] [PubMed] [Google Scholar]
- 8.Dykewicz MS, Fineman S. Diagnosis and management of rhinitis: parameter documents of the Joint Task Force on Practice Parameters in Allergy Asthma and Immunology. Executive summary of the Joint Task Force on Practice Parameters in Allergy Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81:S463–468. doi: 10.1016/S1081-1206(10)63152-3. [DOI] [PubMed] [Google Scholar]
- 9.Kay G. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. 2000;105:S622–627. doi: 10.1067/mai.2000.106153. [DOI] [PubMed] [Google Scholar]
- 10.Blaiss M. Costs of allergic rhinitis. In: Kaliner MA, editor. Current Review of Rhinitis. Current Medicine; Philadelphia: 2002. [Google Scholar]
- 11.Schoenwetter WF, Dupclay L, Appajosyula S, et al. Economic impact and quality of life burden of allergic rhinitis. Curr Med Res Opin. 2004;20:305–317. doi: 10.1185/030079903125003053. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt Associates L Survey on future health care expectations (on line) Online [was.hewitt.com] 2001
- 13.Ross RN. The cost of allergic rhinitis. Am J Man Care. 1996;2:285–290. [Google Scholar]
- 14.Kaliner MA, Osuguthorpe JD, Fireman P, et al. Sinusitis bench to bedside: current findings, future directions. J Allergy Clin Immunol. 1997;99:S829–847. [PubMed] [Google Scholar]
- 15.Ponikau JU, Sherris DA, Kephart DM, et al. Striking deposition of toxic eosinophil major basic protein in mucus: Implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116(2):362–369. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Tano L, Tano K. A daily nasal spray with saline prevents symptoms of rhinits. Acta Otolaryngol. 2004;124:1–4. doi: 10.1080/00016480410017657. [DOI] [PubMed] [Google Scholar]
- 17.Adam P, Stiffman M, Blake RL. A clinical trial of hypertonic saline nasal spray in subjects with common cold or rhinosinusitis. Arch Fam Med. 1998;7:39–43. doi: 10.1001/archfami.7.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Brown CL, Graham SM. Nasal irrigations: good or bad? Curr Opin Otolaryngology Head Neck Surg. 2004;12:1–13. doi: 10.1097/00020840-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Harvey R, Hannan S, Badia L, et al. Nasal saline for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007 July 18;3(CD006394) doi: 10.1002/14651858.CD006394.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Spector SL, Toshener D, Gay I, et al. Beneficial effects of propylene and polyethylene glycol and saline in the treatment of perennial rhinitis. Clinical Allergy. 1982;12:187–196. doi: 10.1111/j.1365-2222.1982.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 21.Heatley DG, McConnell KE, Kille TL, et al. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg. 2001;(125):44–48. doi: 10.1067/mhn.2001.115909. [DOI] [PubMed] [Google Scholar]
- 22.Tamooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope. 2000;(110):1189–1193. doi: 10.1097/00005537-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Taccariello M, Parikh A, Darby Y, et al. Nasal douching as a valuable adjunct in the management of chronic rhinosinusitis. Rhinology. 1999;(37):29–32. [PubMed] [Google Scholar]
- 24.Bachmann G, Hommel G, Michel O. Effect of irrigation of the nose with isotonic salt solution on patients with chronic paranasal sinus disease. Eur Arch Otorhinolaryngol. 2000;257:537–541. doi: 10.1007/s004050000271. [DOI] [PubMed] [Google Scholar]
- 25.Shoseyov D, Bibi H, Shai P, et al. Treatment with hypertonic saline versus normal saline wash of pediatric chronic sinusitis. J Allergy Clin Immunol. 1998;101:602–605. doi: 10.1016/S0091-6749(98)70166-6. [DOI] [PubMed] [Google Scholar]
- 26.Rabone SJ, Saraswati SB. Acceptance and effects of nasal lavage in volunteer woodworkers. Occupat Med. 1999;(49):365–369. doi: 10.1093/occmed/49.6.365. [DOI] [PubMed] [Google Scholar]
- 27.Holmstrom M, Rosen G, Walander L. Effect of nasal lavage on nasal symptoms and physiology in wood industry workers. Rhinology. 1997;(35):108–112. [PubMed] [Google Scholar]
- 28.Rabago D, Zgierska A, Mundt M, et al. Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: A randomized controlled trial. Journal of Family Practice. 2002;51(12):1049–1055. [PubMed] [Google Scholar]
- 29.Pynnonen MA, Mukerji SS, Kim HM, et al. Nasal Saline for Chronic Sinonasal Symptoms A Randomized Controlled Trial. Arch Otolaryngol Head Neck Surg. 2007;133(11):1115–1120. doi: 10.1001/archotol.133.11.1115. [DOI] [PubMed] [Google Scholar]
- 30.Garavello W, Romagnoli M, Sordo L, et al. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: a randomized study. Pediatric allergy and immunology. 2003;14:140–143. doi: 10.1034/j.1399-3038.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 31.Rabago D, Pasic T, Zgierska A, et al. The efficacy of hypertonic saline nasal irrigation for chronic sinonasal symptoms. Otolaryngol Head Neck Surg. 2005;133:3–8. doi: 10.1016/j.otohns.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Rabago D, Barrett B, Marchand L, et al. Qualitative aspects of nasal irrigation use by patients with chronic sinus disease in a multi-method study. Annals of Family Medicine. 2006;4:295–301. doi: 10.1370/afm.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crabtree BF, Miller WF. Doing qualitative research. Vol 3. Sage Publications; Thousand Oaks, CA: 1992. [Google Scholar]
- 34.Fireman P. Rhinitis and asthma connection: managment of coexisting upper airway allergic diseases and asthma. Allergy Asthma Proc. 2000;21:45–54. doi: 10.2500/108854100778248935. [DOI] [PubMed] [Google Scholar]
- 35.Simons FER. Allergic rhinobronchitis: the asthma-allergic rhinitis link. J Allergy Clin Immunol. 1999;104:534–540. doi: 10.1016/s0091-6749(99)70320-9. [DOI] [PubMed] [Google Scholar]
- 36.Ozsoylu S. Nose drops and the common cold. Eur J Pediatr. 1985;144:294. [Google Scholar]
- 37.Karadag A. Nasal saline for acute sinusitis. Pediatrics. 2002;109:165. doi: 10.1542/peds.109.1.165. [DOI] [PubMed] [Google Scholar]
- 38.Kurtaran H, Karadag A, Catal F, et al. A reappraisal of nasal saline solution use in chronic sinusitis. Chest. 2003;124:2036–2037. doi: 10.1378/chest.124.5.2036. [DOI] [PubMed] [Google Scholar]
- 39.Georgitis JW. Nasal hyperthermia and simple irrigation for perennial rhinitis. Changes in inflammatory mediators. Chest. 1994;106:1487–1492. doi: 10.1378/chest.106.5.1487. [DOI] [PubMed] [Google Scholar]
- 40.Talbot AR, Herr TM, Parsons DS. Mucocilliary clearance and buffered hypertonic saline solution. Laryngoscope. 1997;107:500–503. doi: 10.1097/00005537-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Boek WM, Graamans K, Natzijl H, et al. Nasal mucociliary transport: New evidence for a key role of ciliary beat frequency. Laryngoscope. 2002;112:570–573. doi: 10.1097/00005537-200203000-00029. [DOI] [PubMed] [Google Scholar]
- 42.Homer JJ, Dowley AC, Condon L, et al. The effect of hypertonicity on nasal mucociliary clearance. Clin. Otolaryngol. 2000;25:558–560. doi: 10.1046/j.1365-2273.2000.00420.x. [DOI] [PubMed] [Google Scholar]
- 43.Homer JJ, England RJ, Wilde AD, et al. The effect of pH of douching solution on mucociliary clearance. Clin Otolaryngol and Allied Sciences. 1999;24:312–315. doi: 10.1046/j.1365-2273.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 44.Benninger MS, Senior BA. The Development of the rhinosinusitis disability index. Arch Otolyryngol. Head Neck Surg. 1997 Nov.123(11):1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]