Abstract
Mutations in connexins (Cxs), the constitutive protein subunits of gap junction (GJ) intercellular channels, are one of the most common human genetic defects that cause severe prelingual non-syndromic hearing impairments. Many subtypes of Cxs (e.g., Cxs 26, 29, 30, 31, 43) and pannexins (Panxs) are expressed in the cochlea where they contribute to the formation of a GJ-based intercellular communication network. Cx26 and Cx30 are the predominant cochlear Cxs and they co-assemble in most GJ plaques to form hybrid GJs. The cellular localization of specific Cx subtypes provides a basis for understanding the molecular structure of GJs and hemichannels in the cochlea. Information about the interactions among the various co-assembled Cx partners is critical to appreciate the functional consequences of various types of genetic mutations. In vitro studies of reconstituted GJs in cell lines have yielded surprisingly heterogeneous mechanisms of dysfunction caused by various Cx mutations. Availability of multiple lines of Cx-mutant mouse models has provided some insight into the pathogenesis processes in the cochlea of deaf mice. Here we summarize recent advances in understanding the structure and function of cochlear GJs and give a critical review of current findings obtained from both in vitro studies and mouse models on the mechanisms of Cx mutations that lead to cell death in the cochlea and hearing loss.
Keywords: inherited deafness, gap junction function, connexin mutations, review, deafness mechanism
1. Introduction
Gap junctions (GJs) are intercellular membrane channels that possess the unique feature of directly connecting the cytoplasm of neighboring cells. GJs connect cells electrically when they are open, acting like opened ion channels to generate high conductance pathways, a phenomenon at the basis of electrical synapses (Bennett and Zukin, 2004). Unique to GJs is their ability to allow small molecules (cut-off molecular weight at ~1,000 Daltons), such as second messengers (e.g., cAMP, IP3) and intracellular metabolites (e.g., glucose, ATP), to diffuse down their concentration gradients (Evans and Martin, 2002). GJs are formed by the juxtaposition of two hexameric structures called hemichannels (or connexons) at the GJ plaques, where a large number of GJs cluster at the cell-cell contact points. Before two hemichannels are aligned to form a whole GJ, they may perform functions independent of those carried out by GJs (Goodenough and Paul, 2003).
GJs are found in both invertebrates (Cruciani and Mikalsen, 2007) and vertebrates (Cruciani and Mikalsen, 2006; Evans and Martin, 2002). Invertebrate GJs are assembled from innexins (Phelan et al., 1998). Vertebrate GJs are formed by the assembly of six compatible connexin (Cx) subunits (Willecke et al., 2002). All Cx subtypes share a common topology that includes four transmembrane domains, two extracellular and one intracellular loop. Both amino and carboxyl termini of all Cxs are located on the cytoplasmic side of the membrane. Innexins and Cxs generally share little sequence similarity. However, another group of GJ subunits with homologies to the innexin family, called pannexins, are also found in the vertebrates (Baranova et al., 2004; Panchin et al., 2000). By allowing electrochemical as well as biochemical coupling between cells, GJs generally function to maintain tissue homeostasis and to allow fast intercellular electrical communication. Many fundamental biological processes require GJs (Lo, 1996) and the importance of these unique intercellular channels are demonstrated by the linkage of their mutations to a wide spectrum of human diseases, such as peripheral neuropathies (e.g. the X–linked Charcot-Marie-Tooth disease) (Bergoffen et al., 1993), various skin disorders (Richard, 2000), cataracts (White, 2002), oculodental dysplasia (Paznekas et al., 2003) and deafness (Chang et al., 2003; Rabionet et al., 2002).
In the cochlea, GJs were first revealed in the 1970s by ultrastructural observations (Forge, 1984; Iurato et al., 1977; Jahnke, 1975; Laciano et al., 1977) that suggested the existence of a “functional syncytium” among cochlear supporting cells. Intercellular electrical communication consistent with the existence of GJs was later demonstrated by patch-clamp recordings (Santos-Sacchi and Dallos, 1983; Zhao and Santos-Sacchi, 2000). Immunolabeling studies identified various types of Cxs in the cochlea as the molecular building blocks of GJs (Kikuchi et al., 1995; Lautermann et al., 1998; Tang et al., 2006; Xia et al., 2000). The essential role of GJs in the hearing process has been highlighted by a large number of genetic studies linking mutations (supplemental Table 1) in Cx genes to inherited deafness (Ballana et al., http://davinci.crg.es/deafness/). More than half of congenital deafness cases are caused by genetic mutations (Petit, 2006; Smith et al., 2005). Currently, at least 46 genes are known to cause hearing impairments in humans (Hilgert et al., 2008) and many more are suggested by animal studies. Strikingly, mutations in a single gene (GJB2, which codes for Cx26) account for a large proportion (up to 50%) of inherited prelingual non-syndromic deafness cases in almost all ethnic populations studied (supplemental Table 1). It is established that mutations in Cx genes are one of the most common forms of human genetic defects resulting in hearing losses in millions of patients with either autosomal dominant or recessive deafness (Chang et al., 2003; Denoyelle et al., 1997; Estivill et al., 1998; Kelsell et al., 1997). Carrier rate of various disease-causing Cx26 mutations is estimated to be 1–4% in many populations, which makes the GJB2 one of the most common disease-linked genes in humans (supplemental Table 1). In addition to GJB2, mutations in GJB6 (coding for Cx30) (Grifa et al., 1999) and GJB3 (coding for Cx31) (Liu et al., 2000; Xia et al., 1998) are known to cause hereditary deafness in humans. Other deafness-linked Cx candidates include GJB1 (coding for Cx32) (Bergoffen et al., 1993), GJE1 (Cx29) (Yang et al., 2007) and GJA1 (Cx43) (Liu et al., 2001).
Many of the Cx subtypes in the ~20 mammalian Cx genes (Sohl and Willecke, 2004) are expressed in the cochlea. Cx26 and Cx30 are the two predominant cochlear Cx subtypes in terms of their cellular distributions and reported mutational effects for human hearing. General reviews on GJ nomenclature (Sohl and Willecke, 2003), structure (Sosinsky and Nicholson, 2005; Yeager and Harris, 2007) and function (Evans and Martin, 2002; Laird, 2006; Nicholson, 2003)}, regulations of expressions (Laird, 2006; Oyamada et al., 2005; Saez et al., 2003)}, biophysical properties (Alexander and Goldberg, 2003; Goldberg et al., 2004) have been previously published. A few recent reviews on GJs in the cochlea are available (Martinez et al., 2009; Nickel and Forge, 2008; Zhao et al., 2006). This review focuses on the molecular structural basis of hemichannels and GJ-mediated intercellular communication network in the cochlea, as well as on the diverse mechanisms for deafness caused by various human Cx mutations (Table 1 and Fig. 2). A classification of Cx26 mutations based on in vitro functional studies of reconstituted GJs is presented (Table 1). Advances in understanding deafness mechanisms by studying multiple Cx-mutant mouse models (Table 2) and current theories about the mechanisms of deafness caused by Cx mutations are also critically reviewed.
Table 1.
Classifications of Cx26 mutations linked to deafness in human patients according to data obtained from in vitro studies.
Class I mutations are mutations preventing the formation of GJs. Class II comprises mutations that do not affect formation of GJs, but the mutated GJs display null functions. Class III refers to mutations specifically impaire the GJ-mediated biochemical coupling. Class IV consists of mutations causing a gain-of-function due to abnormal hemichannel openings. Mutations without reported functional effects on GJ functions (likely to represent polymorphism) are grouped in category V. Finally, class VI consists of mutations that have not been thoroughly studied in vitro. Detailed criteria for the classification are given in the text.
Figure 2.
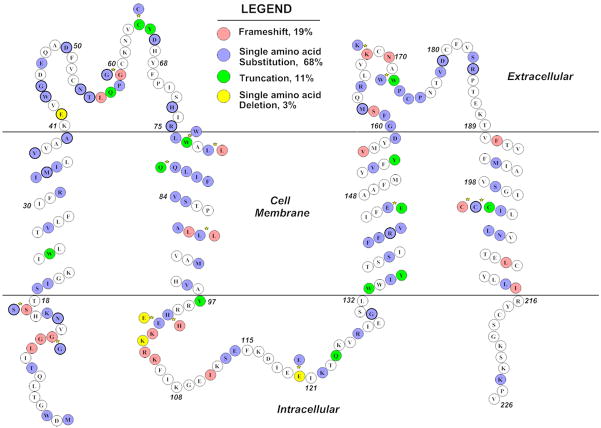
A diagram showing the location of reported human Cx26 mutations in the Cx26 protein that are linked to hearing loss. Four types of mutations (frame shift, truncation, single amino acid substitution or in-frame deletion) are color coded. Stars denote the location where more than one types of mutations are reported. References for these deafness-linked Cx26 mutations are given in the Table 1 and supplemental Table 1.
Table 2.
Comparison of results obtained from various Cx26 and Cx30 mutant mouse models
| Animal models | Cx30 null | cCx26 null | Cx26R75W-Kudo | Cx26R75W-Maeda |
|---|---|---|---|---|
| Reference of the first report | (Teubner et al., 2003) | (Cohen-Salmon et al., 2002) | (Kudo et al., 2003) | (Maeda et al., 2007) |
| Approach used | Targeted replacement of Gjb6 by LacZ/neo | Gjb2 is flanked by loxP. Excision of Gjb2 by otogelin-driven Cre expression from a BAC | hCx26 R75W is expressed under universal CAG promoter | pGJB2R75W-eGFP plasmid (CMV promoter) delivered by lipofection applied to the round window membrane |
| Time and location of Cx deletion | Germline deletion of Gjb6 | Gjb2 presumably is deleted at E10 in sensory epithelium of the cochlea | The dominant- negative Gjb2 mutant is presumably expressed before the first meiotic division. Detailed cellular pattern of expression is unknown | The dominant- negative Gjb2 mutant is expressed in adult cochlea. Many cochlear cells expressed the mutant Cx as detected by the GFP immunolabeling |
| Inner and outer hair cell loss | Hair cell losses begin at the third week postnatally. and increase gradually with age. Outer hair cells are affected first and more severely | Gross morphology of inner hair cells appears to be normal in most animals. Outer hair cell loss starts at P15. The two most internal rows are affected first | Inner hair cells are present but show changed shape. Outer hair cells are present at P14 but show shape changes and they degenerated at the seventh week | No hair cell loss and hearing loss is transient. Auditory sensitivities recover in 5 days after introducing mutant |
| Vestibular morphology | Vestibular hair cell loss specifically in the saccule is observed (Qu et al., 2007) | Normal up at least to P60 | Normal by functional assessment | No data reported |
| Supporting cell loss | Not degenerated | Initial damage observed at P15 | Initial damage observed at P14 | Not affected |
| Is there SG neuron loss? | No description provided. | No SG neuron degeneration observed | Degeneration of SG neurons in basal turn noted at seventh week postnatally | |
| Is the opening of the tunnel of Corti affected? | No | No | Yes (Inoshita et al., 2008) | |
| Hearing threshold elevation | At P17–18, click ABR threshold elevation is about is 50 dB. Adult mice show no ABRs at >100 dB | About 30 dB elevation at the most sensitive frequencies on average. | Greater than 100 dB threshold elevation | About 15–20 dB threshold elevation as assessed by click ABR |
| EP value | At P13/P14: 0±4 vs. 74±9mV in control mice. In adults: 3±3 vs. 148±15 mV in controls | At P12–13: 56±12 vs. 58±12mV in control mice. In adults: 38±14 vs. 110±12mV in controls | In adults: 87±2.5 vs. 97.4±7.1 mV in control mice | |
| Endolymphatic K+ concentration | At P13/P14: 100±39 vs. 102±24 mM in control mice. In adults: 44±19 vs. 148±15mM | In adult: 85±21 vs. 153±7mM in the control mice | ||
| Morphology changes in the organ of Corti, spiral limbus, stria vascularis, firbrocytes in the lateral wall | No gross morphological changes in stria vascularis, lateral wall is observed. No displacement of Reissner’s membrane observed | Disruption of the reticular lamina, missing of some interdental cells. Gross cochlea structure appear to be normal | No gross changes in gross cochlear morphology observed. No opening of the tunnel of Corti. The Nuel’s space is absent. Microtubule abnormality in Inner pillar cells. |
2. Molecular structural basis of GJ networks in the cochlea
Cx26 and Cx30 are the two major Cx subtypes in the cochlea that co-assemble to form GJs
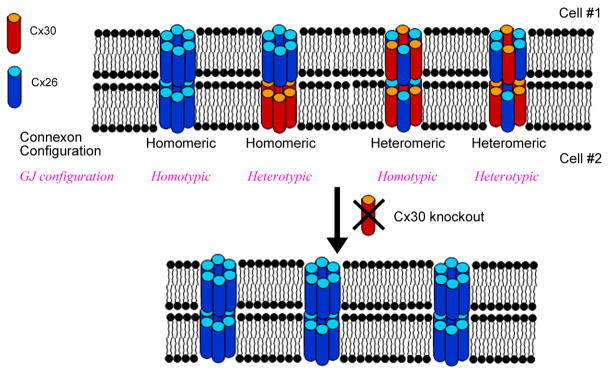
The molecular composition of GJs determines their unitary conductance, gating and rectification properties, and influence permeability and intracellular trafficking of hemichannels (Elfgang et al., 1995; Niessen et al., 2000; Rackauskas et al., 2007a; Rackauskas et al., 2007b; White and Bruzzone, 1996). Furthermore, defining the molecular assembly of cochlear GJs is essential for understanding functional consequences of Cx mutations. For example, Cx26 and Cx30 are colocalized in most cochlear GJ plaques and coimmunoprecipitation of the two Cxs suggest they coassemble in cochlear GJs (Ahmad et al., 2003; Forge et al., 2003a). Fig. 1 illustrates some of the possible molecular configurations of GJs when two Cxs are coassembled. If most GJs are heteromeric in the cochlea, a direct functional implication is that cochlear GJs are not necessarily eliminated by null expression of either Cx26 or Cx30 individually (Fig. 1). The functional properties and the number of remaining homomeric GJs, however, may differ significantly from that of the native Cx26/Cx30 hybrid GJ channels (Sun et. al., 2005; Jagger and Forge, 2006; Yum et. al., 2007).
Figure 1.
A diagram showing molecular configurations of co-assembled cochlear GJs from Cx26 and Cx30 in the cell membrane and the predicted effect of genetic knockout of GJB6. Cxs26 and 30 normally co-assemble into GJs in the cochlea. Connexons are called homomeric or heteromeric, respectively, depending on whether a single or more than one Cx subtype are used as building blocks. A heterotypic GJ channel is formed by the docking of two different connexons whereas a homotypic channel is constituted from the same connexons. The ablation of a single Cx subtype may eliminate GJs in regions where it is the only Cx expressed. However, significantly amounts of GJs may remain in areas where co-asssembly of Cxs dominates, although disruption of the expression of one Cx subtype is likely to affect biophysical properties of GJs that are highly dependant on the composition Cxs. The number of GJs may also be reduced as a gene dosage effect.
At the mRNA level, gene transcription profiles of Cxs in the cochlea have been investigated by low-density gene array (Ahmad et al., 2003) and in situ hybridization (Buniello et al., 2004) approaches. Dot-blot analyses (Ahmad et al., 2003) revealed the presence of Cxs 26, 29, 30, 31 and 43. In addition, mRNA of Cx30.2, Cx37 and Cx46 was detected in the cochlea by in situ hybridization (Buniello et al., 2004). However, mRNA transcript levels do not necessarily faithfully reflect protein expressions (Nelson and Keller, 2007). Therefore, we will mainly examine expressions of Cxs in the cochlea based on immunolabeling and western blot results in the following sections.
Cx26 and Cx30
Immunolabeling of Cx26 (Kikuchi et al., 1995) and Cx30 (Lautermann et al., 1998) in the cochlea of adult rats localized the Cxs in the spiral limbus, the spiral ligaments, the supporting cells of the organ of Corti and the stria vascularis. Although the low-resolution images of co-immunolabeling did not show colocalization at the level of single GJ plaque (Lautermann et al., 1998), the results suggested a similar cellular expression pattern for Cx26 and Cx30 in the cochlea. Higher resolution images, at the level of a single GJ plaque observed either at optical (Ahmad et al., 2003; Forge et al., 2003a; Sun et al., 2005) or electron (Forge et al., 2003a) microscope levels, were later obtained. Results demonstrate extensive co-localization of Cx26 and Cx30 immunoreactivities in most (>85%) cochlear GJ plaques (Sun et al., 2005). In the adult mouse cochlea, the only region that showed minimal Cx26 and Cx30 colocalization (5%) was in the Deiters’ cells, where expression of Cx30 dominated. Direct interactions of the two Cxs have been demonstrated by co-immunoprecipitation (Ahmad et al., 2003; Forge et al., 2003a), further supporting that Cx26 and Cx30 are co-assembled in the same GJs. General co-localization of Cx26 and Cx30 in cochlear GJs seems to be a universal phenomenon found in many animal species, including rats (Lautermann et al., 1999), mice (Sun et al., 2005), guinea pigs (Zhao and Yu, 2006), as well as in humans (Liu et al., 2009). These two predominant cochlear Cxs also co-localize in the saccule, utricle, and cristae of the vestibular organs, where Cx26 and Cx30 are found in most GJ plaques in supporting cells and connective tissue cells (Qu et al., 2007).
During the development, embryologic expressions of Cx26 and Cx30 in the human cochlea were detected as early as 11 weeks of gestation (Kammen-Jolly et al., 2001) and adult level was reached at week 20 (Kammen-Jolly et al., 2001; Lautermann et al., 1999). In the prenatal mouse cochlea, these Cxs are sparsely expressed and the early pattern differed significantly from that of the adult (Lautermann et al., 1999; Sun et al., 2005). Both Cxs are detected as early as E14.5 (14.5 days of gestation) (Sun et al., 2005) and the immunoreactivities of the two Cxs are generally found in the same cochlear regions at all developmental stages. From E14.5 until early postnatal days, both Cx26 and Cx30 expressions are absent from the sensory epithelia of the developing organ of Corti. They are found in cells of the nascent spiral limbus and in part of the lateral wall. Before the onset of hearing, strong expressions of the two Cxs are found in the spiral limbus and in a band of fibrocytes adjacent to the basal cells of the stria vascularis (Sun et al., 2005). Expression in supporting cells gradually intensifies, giving a dynamic pattern during postnatal development of the organ of Corti. At the onset of hearing, Cx26 and Cx30 expressions in the lateral wall quickly changes from primarily a band of cells bordering the stria vascularis to almost all the cells above the spiral ligament. Studies agree that Cx26 and Cx30 are not expressed in both inner and outer hair cells, nor in marginal cells of the stria vascularis (Ahmad et al., 2003; Forge et al., 2003a; Frenz and Van De Water, 2000; Kikuchi et al., 1995; Lautermann et al., 1998; Liu and Zhao, 2008; Zhao and Yu, 2006). Whether intermediate cells of the stria vascularis express functional level of Cx26 and Cx30 is still controversial. Compared to their expression levels in basal cells of the stria vascularis, a majority of studies (Ahmad et al., 2003; Forge et al., 2003a; Wangemann et al., 2004; Xia et al., 1999) found a significantly lower, if any, expression of Cx26 and Cx30 in the intermediate cells, although a positive labeling result was also reported (Liu and Zhao, 2008).
Cx29
Cx29 is mostly expressed by myelinated glial cells (e.g., oligodendrocytes, Bergmann astroglia cells and Schwann cells), but not by astrocytes (Altevogt et al., 2002; Eiberger et al., 2006; Kleopa et al., 2004; Nagy et al., 2003). In the cochlea, Cx29 mRNA expression was first detected by cDNA dot-blot hybridization (Ahmad et al., 2003). Immunolabeling of Cx29 in wild-type (WT) mice and localization of the LacZ reporter gene in Cx29 null mice indicate that Cx29 is highly expressed in the cochlear Schwann cells ensheathing the afferent fibers of the eighth nerve up to the glial junction (Eiberger et al., 2006; Tang et al., 2006). After the afferent auditory fibers enter the brainstem, they are surrounded by astrocytes which are not labelled by Cx29 antibody. Low levels of Cx29 in the stria vascularis were also detected by immunolabeling (Eiberger et al., 2006; Tang et al., 2006).
Functional examinations of the Cx29 null mice (Tang et al., 2006) indicate that the absence of the Cx29 gene, with a penetrance of about 50%, causes a delay in the maturation of hearing. Auditory thresholds of about half of the Cx29 null mice tested at 3 weeks postnatally were at least two standard deviations above the averaged results of Cx29 WT littermate controls. By 6 weeks of age, however, most of the Cx29 null mice tested (13 out of 16) showed hearing thresholds not statistically different from WT controls. Hearing thresholds measured by another group from Cx29 null mice at 4–10 weeks postnatally also show no difference comparing to WT animals (Eiberger et al., 2006). However, as the Cx29 null mice mature to 6 months, they display early loss of high-frequency sensitivities. An elevated susceptibility to noise at high frequencies (12, 18 & 24 kHz) is also observed. Cochlear morphology examined at the electron microscopic level show specific demyelination of the soma, but not the fibers, of the spiral ganglion neurons. Tang et al. (Tang et al., 2006) suggests that Cx29 is a candidate gene to study auditory neuropathies. Currently, few studies have screened human mutations in the Cx29 gene. Interestingly, one study in Taiwan reported Cx29 mutations in some non-syndromic deaf patients (Yang et al., 2007).
Cx31
Cx31 is one of the earliest Cx genes expressed in the embryo (Dahl et al., 1996). At adult stages, its expressions are found in the skin (Hoh et al., 1991), cochlea (Xia et al., 2000), peripheral auditory nerve (Lopez-Bigas et al., 2001), seminiferous epithelium of rat testes (Mok et al., 1999) and placenta (Plum et al., 2001).
Although genetic data linking mutations in Cx31 to deafness are strong, its cellular expression in the rodent cochlea is still controversial. Two studies, one observing the expression of the LacZ reporter gene that replaces Cx31 gene in Cx31 null mice (Plum et al., 2001) and the other using immunolabeling (Lautermann et al., 1998), failed to detect Cx31 expression in the cochlea. On the other hand, Cx31 mRNA transcripts have been detected in the cochlea by cDNA macroarray hybridization (Ahmad et al., 2003), in situ hybridization (Lopez-Bigas et al., 2002) and RT-PCR amplifications (Forge et al., 2003a; Xia et al., 2000). However, cellular patterns of Cx31 in the cochlea, as detected by immunolabeling, show poor consensus among published results. Cx31 was localized among type II fibrocytes below the spiral prominence where both Cx26 and Cx30 appear to be weakly expressed (Forge et al., 2003a). Other studies also found Cx31 in fibrocytes of the spiral ligament and spiral limbus (Xia et al., 2000), and in supporting cells of the organ of Corti (Liu et al., 2008).
Human mutations in Cx31 have been linked to the skin disorder erythrokeratodermia variabilis (Richard et al., 1998a) as well as to autosomal dominant (Xia et al., 1998) and recessive (Liu et al., 2000; Uyguner et al., 2003) non-syndromic deafness. However, these phenotypes did not correlate well with the corresponding mouse model. Cx31 null mice displayed a transiently abnormal placental development and a reduced viability (60%) of homozygote embryos, but neither epidermis nor auditory malfunctions were observed in the surviving mice (Plum et al., 2001). Human genetic studies also revealed an interaction between Cx26 and Cx31. GJB3 mutations occurring in compound heterozygosity with the GJB2 mutations have been identified in three unrelated Chinese families. Direct interaction of Cx26 with Cx31 has been shown by coimmunoprecipitation, supporting the idea of an interaction between these two Cxs that results in hearing loss in human digenic heterozygotes (Liu et al., 2008).
Cx32
Cx32 is generally expressed in oligodendrocytes and Schwann cells. It is believed to contribute to the myelination process and to participate in the K+ buffering during neuronal activities. Genetic mutations in GJB1 were the first to be associated with a human disease, the X-linked Charcot-Marie-Tooth disease, which is a demyelinating neuropathy (Bergoffen et al., 1993).
Cx32 expression has been studied during cochlear development by in situ hybridization (Lopez-Bigas et al., 2002) and by immunocytochemistry from the adult cochlea (Tang et al., 2006). Cx32 mRNA transcripts have been detected as early as E12 in the otocyst. At neonatal stages until P7 (one week after birth), expression was widespread in the cochlea. Labeling obtained from cochleae older than P13 restricted Cx32 to type II and IV fibrocytes of the spiral ligament. In the adult cochlea, however, no Cx32 transcripts were detected (Lopez-Bigas et al., 2002). These findings were corroborated by another study (Forge et al., 2003a) in which RT-PCR amplifications from mouse mature cochleae (6–8 week-old) and immunoblots failed to detect Cx32 expression. In the adult cochlea Tang et al. (Tang et al., 2006) reported Cx32 expression in astrocytes located outside the glial juncture, which are the cells surrounding the central portion of the auditory nerve fibers in the brainstem. In support of a minor role played by Cx32 in auditory functions, no severe hearing loss in Gjb1−/− mice was reported (Scherer et al., 1998).
Cx43
Cx43 is widely expressed in the human body (Laird, 2006). In the mouse cochlea, its expression has been investigated by cDNA dot-blot hybridization (Ahmad et al., 2003), RT-PCR amplification and western blotting (Forge et al., 2003a). Its cellular localization has been studied by immunolabeling (Lautermann et al., 1998) and by the LacZ reporter gene expression in Cx43 null mice (Cohen-Salmon et al., 2004b). By localizing the LacZ reporter expression pattern, Cohen-Salmon et al. showed that Cx43 is expressed in the cochlea as early as E15.5. During early development, Cx43 expression is more widespread than in mature stage, with staining observed in fibrocytes in the later wall, mesenchymal cells below the basilar membrane, and capillaries in the stria vascularis. In the adult cochlea, Cx43 is localized to the cochlear bony shell only (Cohen-Salmon et al., 2004b). Inconsistent results are reported by other groups about the Cx43 expression in the cochlea (Lautermann et al., 1998; Liu et al., 2001; Suzuki et al., 2003). Liu et al. (Liu et al., 2001) reported that mutations in GJA1 are linked to deafness in the African American population. However, later studies showed that the reported mutations (L11F, V24A) may be located in GJA1 pseudogene on chromosome 5 (Paznekas et al., 2003).
Cx45
Cx45 expression has not been detected by RT-PCR amplifications in mature mouse cochleae or by western blotting (Forge et al., 2003a). However, Cx45 expression has been reported by studying the expression of the LacZ reporter gene in Cx45 null mice (Cohen-Salmon et al., 2004a). During the development, expression of LacZ reporter was detected as early as E17.5. One day later, all cochlear cells, apart from the hair cells, were labelled (Cohen-Salmon et al., 2004a). Starting at P4, LacZ reporter expression increased in capillaries. By P8, expression remained only in capillaries and mesenchymal cells lining the basilar membrane.
Pannexins
Three subtypes of Panxs (Panx1, Panx2 and Panx3) have been reported (Panchin et al., 2000). Functional expression of Panxs in Xenopus oocytes indicated that at least some Panxs can form functional intercellular GJ channels and hemichannels (e.g., homotypic Panx1 and heterotypic Panx1/Panx2) (Bruzzone et al., 2003a; Bruzzone et al., 2005). In the cochlea, Panxs 1 and 2 have been detected by immunoblots and RT-PCR amplifications. Immunolabeling localized Panx1 to the inner and outer sulcus cells, as well as to the Claudius cells. Additionally, both Panxs are expressed in the spiral ganglion and Scarpa’s ganglion neurons (Tang et al., 2008). A more widespread cochlear expression of Panx1 and Panx2 has been reported and the expression of Panx3 has also been detected in the cochlear bone by a recent study (Wang et al., 2009).
3. Functional classifications of various types of deafness-linked Cx mutations
So far, more than 100 mutations associated with human deafness have been identified in the coding region of the Cx26 gene (Ballana et al., 2005; Chang et al., 2003; White et al., 1998). Deafness linked mutations in the regulatory region of Cx26 have also been reported (Wilch et al., 2006). The locations of some of the reported human Cx26 mutations are summarized in Fig. 2. Classifications of Cx mutations may be based on structural alterations (e.g., truncation and frame shift vs. single amino acid substitution). Truncation and frame-shift mutations (e.g., 35delG, E147X) which only produce partial Cx26 protein, represents about 28% of Cx26 mutants illustrated in Fig. 2. Most of the Cx26 mutations (~79% shown in Fig. 2) belong to the category of point mutations (e.g., R75Q, L214P, delE42) that are produced by a single base substitution or in-frame deletion in the Cx26 coding sequence. It is interesting to note that all autosomal dominantly inherited Cx26 mutations found so far are linked to Cx26 point mutations. Many of them also cause skin disorders (Lee et al., 2008). In addition, at least four human Cx30 point mutations, including T5M (Grifa et al., 1999), 63delG, G11R, A88V (Common et al., 2002; Lamartine et al., 2000; Xia et al., 1998) and two large deletion mutations in Gjb6 (del Castillo et al., 2002; Lerer et al., 2001; Pallares-Ruiz et al., 2002) have been linked to deafness. Since most functional studies focused on Cx26 and Cx30 mutations, this section will present a summary of their functional effects based mainly on results obtained from in vitro approaches.
The first step of in vitro functional studies is to reconstitute WT or mutant GJs in a heterologous system by either injecting mRNA into oocytes, or by transfecting cell lines lacking endogenous GJs (e.g., HEK293 or Hela cells). By transfecting cells with plasmid constructs containing the Cx coding sequence fused in frame to that of the enhanced green fluorescent protein (eGFP) or any other fluorescent proteins, homomeric and hybrid GJ plaques can be directly identified in vitro (Sun et al., 2005). GJ functions can then be assessed by hemichannel dye loading and single cell dye injection assays, and by double-electrode patch-clamp recording and optical recording methods (Guo et al., 2008; Hernandez et al., 2007; Sun et al., 2005; Yum et al., 2007; Zhang et al., 2005; Zhao et al., 2005). Large numbers of in vitro studies (Table 1) suggest that the effects of various Cx26 mutations can be classified into at least four distinct mechanisms according to their effects on GJ functions.
I. Mutations preventing the formation of GJs in the cell membrane
The life cycle of Cxs includes protein synthesis, trafficking/targeting to plasma membrane, membrane insertion and assembly into connexins, and degradation (Laird, 2006). Cx mutations belonging to this category may cause dysfunction in any of the steps, or premature degradation of Cxs before they reach the cell membrane. Potentially, this type of Cx26 mutations could also affect binding of Cx26 with other intracellular partners that normally interact with the Cx protein subunit.
II. Mutations resulting in GJ formation with null functions
Both intercellular ionic and biochemical coupling are lost for Cx26 mutants belonging to this category, although they still form GJs in the cell membrane. Most mutants in this group also lose hemichannel activities. However, mutations at the two extracellular loops of Cxs may specifically affect the docking/alignment of two connexons. Thus, hemichannel permeability may be intact.
III. Mutations resulting in a specific loss of intercellular biochemical coupling
A subgroup of structurally-mild Cx26 mutations, most of them located in the second transmembrane domain, selectively affect the permeation of molecules larger than simple ions. Although in vitro studies identified a specific loss of GJ-mediated permeability to inositol 1,4,5-trisphosphate (IP3) (Beltramello et al., 2005; Zhang et al., 2005), whether it is a major molecule required for the in vivo function of cochlear GJs is unclear. Using Cx30 null mice, Chang et al. showed a dramatic reduction of GJ-mediated glucose transportation and elevated free radical concentrations in cochlear supporting cells (Chang et al., 2008). Similar deficiency in GJ-mediated biochemical coupling could happen in the cochlea of Cx26 mutant mice, although this hypothesis has not been directly tested yet. Since glucose is the major energy source for cellular metabolic activities, a chronic shortage of glucose in the organ of Corti where microcirculation is generally poor may have extensive damaging effects on cell survival and functions.
IV. Mutations causing a gain-of-function effect: abnormal hemichannel opening at resting state
One deafness-linked Cx26 mutation, G45E, has been linked to a fatal form of keratitis–ichthyosis–deafness syndrome (Griffith et al., 2006; Janecke et al., 2005). Morphological examinations revealed that this mutation disrupts cochlear differentiation and causes dysplasia of the cochlear and saccular neuroepithelium (Griffith et al., 2006). G45 (Fig. 2) is located in the first extracellular loop, next to an aspartic acid, a previously reported Ca++ binding site for the hemichannels (Gomez-Hernandez et al., 2003). The G45E mutation changes the charge of the amino acid side chain from neutral to negative, therefore it is likely to affect Ca++ binding to the hemichannels. Stong et al. (Stong et al., 2006) reported that G45E mutation resulted in apoptosis and cell death within 24 hours of transfection. Increasing the extracellular Ca++ concentration ([Ca++]o) rescued the transfected cells in a dose-dependent manner. Dye loading assay suggest that the Cx26 G45E mutation causes leaky GJ hemichannels when cells are bathed in normal [Ca++]o, which overloads the cellular homeostatic mechanisms and ultimately leads to cell death. Other Cx26 mutations in this category are also reported by other groups (Gerido et al., 2007; Lee et al., 2008; Matos et al., 2008). The Cx26 mutants belonging to this category usually show dramatic phenotypes including death (Griffith et al., 2006; Janecke et al., 2005). Interestingly, a mutation in Cx32 that results in the formation of leaky hemichannels has been found to be responsible for a severe type of neuropathy due to imbalanced ions and metabolites (Liang et al., 2005). Evidence of the presence of functional hemichannels within the cochlea is suggested by membrane-impermeable fluorescent dye uptake assays carried on dissociated cochlear cells and in acute or cultured preparation of the cochlear epithelium (Zhao et al., 2005; Anselmi et al., 2008). These studies suggest that one of the functions of cochlear hemichannels is to release ATP into the extracellular space, which could modulate the electromotility of outer hair cells and therefore exert a control on hearing sensitivity.
The fifth category of Cx26 mutants (Table 1) can form functional GJs, but no apparent impairment in intercellular coupling is detected by in vitro assays. They are likely to represent polymorphism in the Cx26 coding region. The Cx26 mutations in the sixth category are reported human mutations apparently linked to deafness, but they are not tested by thorough in vitro studies yet. Finally, mutations in the non-coding region of Cx26 are also linked to deafness in patients (Wilch et al., 2006).
4. Mouse models of Cx mutations display diverse pathogenesis processes in the cochlea
In vitro studies suggest that deafness-linked Cx mutations can be classified into two general categories, loss-of-function (categories I, II &III in Table 1) and gain-of-function (category IV in Table 1) mutations. Four Cx mutant mouse models are generated either by targeted deletion of Cx genes that results in null expression (Cohen-Salmon et al., 2002; Teubner et al., 2003) or by expression of a dominant-negative Cx26 mutant protein (R75W) (Kudo et al., 2003; Maeda et al., 2007). These animal models are appropriate for studying in vivo effects of Cx mutations belonging to categories I & II (Table 1). Currently, mouse models for investigating Cx26 mutants belonging to categories III & IV are not yet available. Genetic deletion of Gjb6 is achieved by replacing the Cx30 gene with a reporter gene LacZ and a neo resistance cassette (Teubner et al., 2003). Germline deletion of Gjb2 is embryonically lethal due to ~60% reduction in GJ-mediated glucose transfer across the placenta (Gabriel et al., 1998), The problem is circumvented by utilizing a Cre-loxP system (Cohen-Salmon et al., 2002; Kudo et al., 2003). In another study, transient expression of the dominant-negative Cx26 R75W mutant protein in the cochlea of adult mice was achieved by lipofection through the round window route (Maeda et al., 2007). Table 2 summarizes major findings obtained from these animal models. All mouse models show significant hearing loss. The most severe threshold elevations are displayed by Cx30 null and Cx26 R75W mice, with hearing thresholds measured at over 100 dB SPL in adult mice (Kudo et al., 2003; Teubner et al., 2003). Only a 15–20 dB threshold increase is detected transiently by click ABR in the model reported by Maeda et al. (Maeda et al., 2007). In general, none of the mouse models display obvious endolymphatic hydrops and degeneration of stria vascularis when observed at the level of light microscope. Hair cell and supporting cell loss after the time of hearing onset are observed in all mouse models (Cohen-Salmon et al., 2002; Kudo et al., 2003; Teubner et al., 2003).
No gross developmental defects in cochlear morphology are detected in both Cx26 and Cx30 mutant mice, suggesting that the two cochlear GJs do not play essential roles in cochlear development. One interesting exception is that the opening of the tunnel of Corti and the Nuel’s space, which normally happens around P9 just before the onset of hearing, is absent in the Cx26R75W mutant mice (Inoshita et al., 2008; Kudo et al., 2003). Another surprising difference is the effect on the EP generation between the Cx26 and Cx30 mutant mice. Normally, the EP starts to develop around P5 in mice and reaches adult-like value at P11 to P20 depending on the location in the cochlea (Sadanaga and Morimitsu, 1995). The EP develops normally either initially in one model (Cohen-Salmon et al., 2002) or even reaches normal level in the adult stage in the Cx26 R75W mice (Kudo et al., 2003). In contrast, EP is never developed in the Cx30 null mice (Teubner et al., 2003). Normal EP suggests that GJ-mediated ionic coupling is not affected by the Cx26 mutations, which is not consistent with the K+ recycling hypothesis for the Cx26 mutations (Kikuchi et al., 1995).
In the study by Cohen-Salmon et al. (Cohen-Salmon et al., 2002), null expression of Cx26 in the cochlea is targeted specifically to the epithelial GJ system. In contrast, the expression of R75W dominant-negative Cx26 mutant is driven by a ubiquitous CAG promoter (Kudo et al., 2003), which theoretically results in the mutant expression in the whole cochlea including cells that normally do not express Cxs. The precise cellular pattern of the mutant expression, however, is unknown. In general, the phenotypes (in terms of both hearing loss and morphological deteriorations) displayed by Cx26 R75W mice are more severe than those shown by the conditional Cx26 null mice, in which a floxed Gjb2 is specifically deleted by Otog-driven Cre. The deafness phenotypes displayed in human patients are extremely heterogeneous. Although in most cases deafness caused by Cx26 mutations is congenital, some patients may not show hearing impairments until a few months after birth (Orzan and Murgia, 2007; Pagarkar et al., 2006). Some of the phenotypic differences in various mouse models may be due to technical approaches used in different studies to generate mutant mice, or caused by heterogeneous deafness mechanisms. The surprisingly different effects on the EP generation observed between the Cx26 and Cx30 null mice also hint that the underlying deafness mechanisms may not necessarily be the same, despite the observations that Cx26 and Cx30 are coassembled in most cochlear GJs (Sun et al., 2005). Until more Cx mutant mouse models are generated and validated by independent methods, clear answers to these questions will not be possible. In addition, mouse models for Cx mutants in categories III and IV are not currently available. More Cx26 mutant mouse models are certainly needed for further understanding molecular mechanisms of the most common form of inherited deafness in humans.
5. Current theories on mechanisms of deafness caused by Cx26 and Cx30 mutations
The extracellular fluid in the endolymphatic space of the cochlea has a high K+ concentration ([K+]o) that is similar to normal intracellular [K+]o found in most cells. The ~160 mM of extracellular [K+]o and ~+80 mV positive potential in the endolymph (endolymphatic potential, or EP) give an unusual electrochemical environment on the apical side of hair cells that is essential for the sensitive mechanical transduction of hair cells (Wangemann, 2002). Clinical phenotypes of deafness caused by most Cx mutations are non-syndromic (Chang et al., 2003). Expressions of both Cx26 and Cx30, however, are widespread in the body (Sohl and Willecke, 2004). In order to explain the distinctive phenotype of deafness, investigators generally link GJ dysfunctions to disturbance of the unique endocochlear environment. Many theories focus on possible scenarios about how the maintenance of high concentration of K+ in the scala media and/or endolymphatic potential (EP) could be disrupted. A more recent hypothesis (Chang et al., 2008) considers the contribution of cochlear GJ network to homeostasis of the avascular sensory epithelium of the organ of Corti. Chang et al. found that GJs in the cochlear supporting cells play a vital role in maintaining biochemical coupling and delivering glucose to these cells.
a) Disruption of cochlear K+ recycling theory
During the auditory transduction, endolymphatic K+ enters hair cells through mechanotransducton channels. The intracellular K+ concentration is balanced by the exit through hair cells’ basolateral K+ channels (e.g. Kcnq4) (Wangemann, 2002). Since high extracellular K+ is generally considered toxic and data support that K+ is recycled back to the endolymph (Konishi et al., 1978; Sterkers et al., 1982), the K+ ions around the base of hair cells are believed to be quickly absorbed by cochlear supporting cells and recycled back to the endolymph (Kikuchi et al., 1995; Spicer and Schulte, 1996). Because no local mechanisms of returning K+ ions back to the endolymph seem to exist in the cochlear supporting cells and the apparent source of generating high [K+]o of the endolymph is in the stria vascularis, K+ ions are thought to be transported through a relatively long route, first along the epithelial cell GJ network to the spiral ligament, then through the connective tissue GJ system in the lateral wall and finally are moved by the stria vascularis back to the endolymphatic space (Kikuchi et al., 1995; Zhao et al., 2006). However, direct measurements of current flux show an alternative route for K+ recycling. A standing current in the perilymph of the scala tympani can be measured exiting the basilar membrane and flowing towards the spiral ligament in the scala tympani (Zidanic and Brownell, 1990). The current suggest that K+ ions in the scala tympani are absorbed back to the connective tissue GJ system in the lateral wall, which bypasses the epithelial GJ system located in the sensory epithelium. In support of the K+ route through the epithelial GJ network, however, targeted deletion of Cx26 specifically in the sensory epithelia of the cochlea clearly shows that GJ coupling in the cochlear supporting cells is required for normal hearing (Cohen-Salmon et al., 2002). In addition, the driving force and the active membrane mechanisms that move K+ laterally through the GJs of the sensory epithelium and then upward through the lateral wall towards the stria vascularis are unclear. Immunolabeling studies provide indirect support that some necessary active mechanisms (e.g., ion pumps and transporters) exist along the proposed recycling route (Crouch et al., 1997; Schulte and Adams, 1989; Spicer and Schulte, 1996).
Contrary to the notion that mutation of cochlear GJs disrupts K+ recycling, recent data indicate that the intercellular conduit provided by GJs is not significantly disrupted by the absence of either Cx26 or Cx30 individually. Immunolabeling data obtained from the cochlea of conditional Cx26 (Cohen-Salmon et al., 2002) and Cx30 (Chang et al., 2008; Teubner et al., 2003) null mice support the existence of homomeric GJs in the cochlea of mutant mice. Double-electrode patch clamp recordings made from Cx30 null mice demonstrated that the ionic coupling among the cochlear supporting cells is indistinguishable from that of WT animals (Chang et al., 2008), suggesting that the GJ-mediated intercellular K+ movement is not significantly affected in the epithelial GJ system of the Cx30 null mice. Additionally, Cx26 mutations specifically affecting biochemical coupling (e.g., V84L, V95M, and A88S) are sufficient to cause deafness in humans (Beltramello et al., 2005; Zhang et al., 2005), indicating that loss of GJ-mediated transfer of molecules larger than K+ ions may underlie the deafness mechanism. The most direct evidence against a disruption of K+ recycling as the basis for Cx-mutation-linked deafness is the finding that the EP is normal in the deaf Cx26R75W mutant mice (Kudo et al., 2003).
b) Endothelial barrier breakage theory
In Cx30 null mice, the time course of cell death in the organ of Corti substantially lags that of hearing loss, indicating that hair cell loss is not directly responsible for deafness. One major finding is that the EP is never formed in these mutant mice (Teubner et al., 2003). It is well known that loss of EP directly results in deafness (Flagella et al., 1999; Gow et al., 2004; Kitajiri et al., 2004; Marcus et al., 2002). To investigate mechanism for the failure in EP generation, Cohen-Salmon et al. (Cohen-Salmon et al., 2007) reported that ion channels and transporters required for EP generation (e.g., KCNQ1, KCNE1, KCNJ10, and H+/K+-ATPase) and tight junctions that enclose the intrastrial fluid space appeared to be normal in the Cx30 null mice. Further examination at the electron microscope level showed that the endothelial barrier of the capillaries in the stria vascularis was disrupted before the EP is developed. Conceptually, damaged endothelial cells lining the microvessels in the stria vascularis could provide a short-circuit leak conductance to overload the EP generation machinery in the stria vascularis. The electric shunt is believed to be sufficient to account for the total loss of EP in Cx30 null mice. However, neither Cx26 nor Cx30 are expressed in the endothelial cells in the stria vascularis (Cohen-Salmon et al., 2007). It is therefore unclear how GJs dysfunctions can result in damages to the endothelial barrier, although a marker for endothelial dysfunction (an increase in homocysteine) has been found. Moreover, the report of a normal EP in deaf Cx26R75W mutant mice (Kudo et al., 2003) indicates that this electric shunt theory may not be generalized to explain deafness caused by all Cx mutations. Apparently, more experiments are needed to test the relatively new theory of deafness caused by a breakdown of endothelial barrier in the stria vascularis.
c) Deficiency in GJ-facilitated metabolite transportation theory
The sensory epithelium of the organ of Corti is an avascular organ where direct microcirculation to hair cells and supporting cells is lacking. In contrast, GJs co-assembled from Cx26 and Cx30 are highly expressed in the non-sensory cells of this region (Ahmad et al., 2003; Forge et al., 2003a; Kikuchi et al., 1995; Sun et al., 2005). Recent studies directly demonstrated that glucose from the cardiovascular circulation could reach the cochlear supporting cells (Chang et al., 2008), fibrocytes in the lateral wall (Suzuki et al., 2008) and spiral limbus (Matsunami et al., 2006) in a GJ-dependent manner. Chang et al. reported (Chang et al., 2008) that GJ-mediated intercellular diffusion of many fluorescent tracers among cochlear supporting cells of Cx30 null mice, including a fluorescent analogue of glucose (2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose, or 2-NBDG), is dramatically reduced. In contrast, ionic coupling among the same group of cells measured directly by two-electrode patch-clamp recordings show no change of the intercellular conductance by the deletion of the Gjb6 gene. These results suggest that a chronic shortage of glucose, but not an eradication of the pathway for K+ recycling, exists in the cochlea of Cx30 null mice.
In general, O2 consumed by mitochondria is reduced fully to water and only about 2% of electrons leak out of the oxidative chain to generate superoxide anions (O2−) and H2O2. Deficiency in glucose supply exacerbates ATP exhaustion and increases the generation of reactive oxygen species (ROS) (Moley and Mueckler, 2000). Co-assembly of Cxs in cochlear GJs indicates that a total elimination of GJ-mediated intercellular biochemical and ionic coupling is an unlikely consequence (Fig. 1). Decreased glucose transportation through GJs and increased ROS production are directly detected in cochlear supporting cells of Cx30 null mice. Based on these results, Chang et al. (Chang et al., 2008) proposed that deafness linked to loss-of-function Cx mutations is caused by a reduction in the efficiency in delivering energetic metabolites (e.g., glucose) through the GJ intercellular network, especially in cochlear regions where microcirculation is poor (e.g., the organ of Corti). The further speculate that the accumulated damaging effects to the cellular homeostasis become destructive when large amount of ROS is generated that ultimately lead to cell death and cochlear dysfunction.
6. Conclusions and perspectives
It is clear that we are only at the beginning stage of revealing the molecular mechanisms of deafness caused by Cx mutations. Evidences support that Cx26, Cx30 and perhaps Cx31 are the major Cx subtypes present in both epithelial and connective tissue GJ networks in the cochlea. Other Cxs and Panxs (Cx29, Cx43, Panx1 and Panx2) are either expressed in cochlear Schwann cells, neuronal cells, or capillary cells that do not play a direct role in the auditory transduction or EP generation. Co-immunolabeling and co-immunoprecipitation data support that the combinations of Cx26 and Cx30 (Ahmad et al., 2003; Forge et al., 2003b; Yum et al., 2007), Cx26 and Cx31 (Liu et al., 2008) are co-assembled in cochlear GJs. As pointed out earlier, a direct functional implication draw from these structural studies is that targeted deletion of one Cx gene is unlikely to totally eliminate GJ-mediated ionic coupling in the cochlea. In addition, co-assembly of cochlear GJs complicates interpretations of the inheritance patterns of Cx mutational effects. Studies have reported that at least some Cx26 recessive mutations have transdominant effects on Cx30 (Marziano et al., 2003). This transdominant effect on Cx30 is, however, unlikely to be universally true for all Cx26 mutations. More investigations are certainly needed to understand the meanings of genetic dominant and recessive inheritance patterns under the context of heteromeric GJs. Although most studies show that Cx26 and Cx30 generally co-localize in the cochlea, the relative proportion of GJs that exist in each molecular configurations (Fig. 1), their precise locations in the cochlea and whether the proportion is dynamically regulated during development or after injury are unclear. If Cx26 and Cx30 are not expressed in a synchronized manner during certain development stages or after stress/injuries, it is possible that a local elimination of GJ-mediated intercellular communication may occur temporally or spatially. Thus, further investigations into the temporal and spatial expression patterns of various subtypes of Cxs and their protein interactions are indispensible information for advancing the studies on the function of cochlear GJs. Other urgently-needed investigations concern the regulatory mechanisms of Gjb2 and Gjb6 gene expression and their interactions. Some recent in vitro studies have begun to address these issues (Ortolano et al., 2008). Cx26 over-expression in Cx30 null mice completely rescues hearing in these deaf mice (Ahmad et al., 2007), suggesting a novel therapeutic strategy for Cx30 null expression patients. However, the translation of the finding into clinical applications is not possible until the genetic regulatory mechanisms of Cx26 and Cx30 are fully understood and a safe and effective pharmacological intervention method is found.
Development of better mouse models will greatly help further testing the new theories about deafness mechanisms caused by Cx mutations (Cohen-Salmon et al., 2007; Martinez et al., 2009) and various aspects of the glucose deficiency hypothesis (Chang et al., 2008). The efforts should help to answer whether Cx26 play any significant roles in cochlear development, which is uniquely demonstrated by Cx26 R75W mutant mice (Inoshita et al., 2008). According to the hypothesis proposed by Chang et al. (Chang et al., 2008), reduction in glycolysis due to glucose shortage should decrease concentration of ATP and mitochondrial ROS production should increase in the cochlea of Cx mutant mice. Cell death should first occur in the most vulnerable regions of the sensory epithelium, particularly at the onset of hearing when cellular energy demand rapidly increases. All these aspects need to be tested experimentally. It is interesting to note that a recent paper proposed a similar general scheme in GJ-connected astroglia metabolic network in the brain to efficiently delivers energetic metabolites from blood vessels to distal neurons in an activity-dependent manner (Rouach et al., 2008). The observations that Cx-mutation-linked defects tend to happen in areas where either microcirculation is poor (e.g., lens in the eyes (White and Paul, 1999)) or transportation of metabolites via GJ intercellular coupling is demanding (e.g., placenta (Gabriel et al., 1998)) suggest a unified theory that dysfunctions of GJ-mediated metabolite transfer underlie many pathogenesis processes.
Supplementary Material
Acknowledgments
Studies in Lin laboratory at Emory University are supported by grants to XL from NIDCD (RO1-DC006483 and R21-DC008353) and Woodruff foundation. W. Tang received grant supports from NIDCD (R21-DC008672) and deafness research foundation. Shoeb Ahmad received a RO3 grant from NIDCD (RO3-DC008693). We thank Mike Kelly for carefully reading the manuscript and offering many helpful suggestions.
Abbreviations
- 2-NBDG
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose
- Cx
connexin
- eGFP
enhanced green fluorescent protein
- EP
endolymphatic potential
- GJ
gap junction
- IP3
inositol 1,4,5-trisphosphate
- Panxs
pannexins
- ROS
reactive oxygen species
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad S, Chen S, Sun J, Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun. 2003;307:362–8. doi: 10.1016/s0006-291x(03)01166-5. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Tang W, Chang Q, Qu Y, Hibshman J, Li Y, Sohl G, Willecke K, Chen P, Lin X. Restoration of connexin26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc Natl Acad Sci U S A. 2007;104:1337–41. doi: 10.1073/pnas.0606855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–58. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci. 2002;22:6458–70. doi: 10.1523/JNEUROSCI.22-15-06458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–5. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadi T, Gronskov K, Sand A, Pampanos A, Brondum-Nielsen K, Petersen MB. Mutation analysis of the GJB2 (connexin 26) gene by DGGE in Greek patients with sensorineural deafness. Hum Mutat. 2000;16:7–12. doi: 10.1002/1098-1004(200007)16:1<7::AID-HUMU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Arita K, Akiyama M, Aizawa T, Umetsu Y, Segawa I, Goto M, Sawamura D, Demura M, Kawano K, Shimizu H. A novel N14Y mutation in Connexin26 in keratitis-ichthyosis-deafness syndrome: analyses of altered gap junctional communication and molecular structure of N terminus of mutated Connexin26. Am J Pathol. 2006;169:416–23. doi: 10.2353/ajpath.2006.051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballana E, Ventayol M, Rabionet R, Gasparini P, Estivill X. Connexins and deafness homepage. 2005 http://davinci.crg.es/deafness/
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–16. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Batissoco AC, Abreu-Silva RS, Braga MC, Lezirovitz K, Della-Rosa V, Alfredo T, Jr, Otto PA, Mingroni-Netto RC. Prevalence of GJB2 (connexin-26) and GJB6 (connexin-30) mutations in a cohort of 300 Brazilian hearing-impaired individuals: implications for diagnosis and genetic counseling. Ear Hear. 2009;30:1–7. doi: 10.1097/AUD.0b013e31819144ad. [DOI] [PubMed] [Google Scholar]
- Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue MM, Chandrasekhar A, Nicholson BJ, Sosinsky GE. Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem. 2006;281:7994–8009. doi: 10.1074/jbc.M506533200. [DOI] [PubMed] [Google Scholar]
- Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol. 2005;7:63–9. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–42. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- Bicego M, Beltramello M, Melchionda S, Carella M, Piazza V, Zelante L, Bukauskas FF, Arslan E, Cama E, Pantano S, Bruzzone R, D’Andrea P, Mammano F. Pathogenetic role of the deafness-related M34T mutation of Cx26. Hum Mol Genet. 2006;15:2569–87. doi: 10.1093/hmg/ddl184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CW, Levy ML, Flaitz CM, Reid BS, Manolidis S, Hebert AA, Bender MM, Heilstedt HA, Plunkett KS, Fang P, Roa BB, Chung P, Tang HY, Richard G, Alford RL. A novel GJB2 (connexin 26) mutation, F142L, in a patient with unusual mucocutaneous findings and deafness. J Invest Dermatol. 2003;121:1221–3. doi: 10.1046/j.1523-1747.2003.12550_4.x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Gomes D, Denoyelle E, Duval N, Perea J, Veronesi V, Weil D, Petit C, Gabellec MM, D’Andrea P, White TW. Functional analysis of a dominant mutation of human connexin26 associated with nonsyndromic deafness. Cell Commun Adhes. 2001;8:425–31. doi: 10.3109/15419060109080765. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003a;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, Veronesi V, Gomes D, Bicego M, Duval N, Marlin S, Petit C, D’Andrea P, White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003b;533:79–88. doi: 10.1016/s0014-5793(02)03755-9. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–43. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Buniello A, Montanaro D, Volinia S, Gasparini P, Marigo V. An expression atlas of connexin genes in the mouse. Genomics. 2004;83:812–20. doi: 10.1016/j.ygeno.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Chang EH, Van Camp G, Smith RJ. The role of connexins in human disease. Ear Hear. 2003;24:314–23. doi: 10.1097/01.AUD.0000079801.55588.13. [DOI] [PubMed] [Google Scholar]
- Chang Q, Tang W, Ahmad S, Zhou B, Lin X. Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin30 null mice. PLoS ONE. 2008;3:e4088. doi: 10.1371/journal.pone.0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Deng Y, Bao X, Reuss L, Altenberg GA. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. Faseb J. 2005;19:1516–8. doi: 10.1096/fj.04-3491fje. [DOI] [PubMed] [Google Scholar]
- Choung YH, Moon SK, Park HJ. Functional study of GJB2 in hereditary hearing loss. Laryngoscope. 2002;112:1667–71. doi: 10.1097/00005537-200209000-00026. [DOI] [PubMed] [Google Scholar]
- Christiani TV, Alexandrino F, de Oliveira CA, Amantini RC, Bevilacqua MC, Filho OA, Porto P, Sartorato EL. Molecular study in Brazilian cochlear implant recipients. Am J Med Genet A. 2007;143A:1580–2. doi: 10.1002/ajmg.a.31778. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–11. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, Maxeiner S, Kruger O, Theis M, Willecke K, Petit C. Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell Tissue Res. 2004a;316:15–22. doi: 10.1007/s00441-004-0861-2. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Maxeiner S, Kruger O, Theis M, Willecke K, Petit C. Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear [In Process Citation] Cell Tissue Res. 2004b;316:15–22. doi: 10.1007/s00441-004-0861-2. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A. 2007;104:6229–34. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Common JE, Becker D, Di WL, Leigh IM, O’Toole EA, Kelsell DP. Functional studies of human skin disease- and deafness-associated connexin 30 mutations. Biochem Biophys Res Commun. 2002;298:651–6. doi: 10.1016/s0006-291x(02)02517-2. [DOI] [PubMed] [Google Scholar]
- Crouch JJ, Sakaguchi N, Lytle C, Schulte BA. Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J Histochem Cytochem. 1997;45:773–8. doi: 10.1177/002215549704500601. [DOI] [PubMed] [Google Scholar]
- Cruciani V, Mikalsen SO. The vertebrate connexin family. Cell Mol Life Sci. 2006;63:1125–40. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani V, Mikalsen SO. Evolutionary selection pressure and family relationships among connexin genes. Biol Chem. 2007;388:253–64. doi: 10.1515/BC.2007.028. [DOI] [PubMed] [Google Scholar]
- D’Andrea P, Veronesi V, Bicego M, Melchionda S, Zelante L, Di Iorio E, Bruzzone R, Gasparini P. Hearing loss: frequency and functional studies of the most common connexin26 alleles. Biochem Biophys Res Commun. 2002;296:685–91. doi: 10.1016/s0006-291x(02)00891-4. [DOI] [PubMed] [Google Scholar]
- Dahl E, Winterhager E, Reuss B, Traub O, Butterweck A, Willecke K. Expression of the gap junction proteins connexin31 and connexin43 correlates with communication compartments in extraembryonic tissues and in the gastrulating mouse embryo, respectively. J Cell Sci. 1996;109(Pt 1):191–7. doi: 10.1242/jcs.109.1.191. [DOI] [PubMed] [Google Scholar]
- de Zwart-Storm EA, Hamm H, Stoevesandt J, Steijlen PM, Martin PE, van Geel M, van Steensel MA. A novel missense mutation in GJB2 disturbs gap junction protein transport and causes focal palmoplantar keratoderma with deafness. J Med Genet. 2008a;45:161–6. doi: 10.1136/jmg.2007.052332. [DOI] [PubMed] [Google Scholar]
- de Zwart-Storm EA, van Geel M, van Neer PA, Steijlen PM, Martin PE, van Steensel MA. A novel missense mutation in the second extracellular domain of GJB2, p.Ser183Phe, causes a syndrome of focal palmoplantar keratoderma with deafness. Am J Pathol. 2008b;173:1113–9. doi: 10.2353/ajpath.2008.080049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Castillo I, Villamar M, Moreno-Pelayo MA, del Castillo FJ, Alvarez A, Telleria D, Menendez I, Moreno F. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N Engl J Med. 2002;346:243–9. doi: 10.1056/NEJMoa012052. [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, Dode C, Marlin S, Boulila-ElGaied A, Grati M, Ayadi H, BenArab S, Bitoun P, Lina-Granade G, Godet J, Mustapha M, Loiselet J, El-Zir E, Aubois A, Joannard A, Petit C, et al. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum Mol Genet. 1997;6:2173–7. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- Di WL, Gu Y, Common JE, Aasen T, O’Toole EA, Kelsell DP, Zicha D. Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J Cell Sci. 2005;118:1505–14. doi: 10.1242/jcs.01733. [DOI] [PubMed] [Google Scholar]
- Eiberger J, Kibschull M, Strenzke N, Schober A, Bussow H, Wessig C, Djahed S, Reucher H, Koch DA, Lautermann J, Moser T, Winterhager E, Willecke K. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia. 2006;53:601–11. doi: 10.1002/glia.20315. [DOI] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–17. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill X, Fortina P, Surrey S, Rabionet R, Melchionda S, D’Agruma L, Mansfield E, Rappaport E, Govea N, Mila M, Zelante L, Gasparini P. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet. 1998;351:394–8. doi: 10.1016/S0140-6736(97)11124-2. [DOI] [PubMed] [Google Scholar]
- Evans WH, Ahmad S, Diez J, George CH, Kendall JM, Martin PE. Trafficking pathways leading to the formation of gap junctions. Novartis Found Symp. 1999;219:44–54. doi: 10.1002/9780470515587.ch4. discussion 54–9. [DOI] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol. 2002;19:121–36. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–55. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- Forge A. Gap junctions in the stria vascularis and effects of ethacrynic acid. Hear Res. 1984;13:189–200. doi: 10.1016/0378-5955(84)90108-4. [DOI] [PubMed] [Google Scholar]
- Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003a;467:207–31. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- Forge A, Marziano NK, Casalotti SO, Becker DL, Jagger D. The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell Commun Adhes. 2003b;10:341–6. doi: 10.1080/cac.10.4-6.341.346. [DOI] [PubMed] [Google Scholar]
- Frei K, Lucas T, Ramsebner R, Schofer C, Baumgartner WD, Weipoltshammer K, Erginel-Unaltuna N, Wachtler FJ, Kirschhofer K. A novel connexin 26 mutation associated with autosomal recessive sensorineural deafness. Audiol Neurootol. 2004;9:47–50. doi: 10.1159/000074186. [DOI] [PubMed] [Google Scholar]
- Frenz CM, Van De Water TR. Immunolocalization of connexin 26 in the developing mouse cochlea. Brain Res Brain Res Rev. 2000;32:172–80. doi: 10.1016/s0165-0173(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Gabriel HD, Jung D, Butzler C, Temme A, Traub O, Winterhager E, Willecke K. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol. 1998;140:1453–61. doi: 10.1083/jcb.140.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerido DA, DeRosa AM, Richard G, White TW. Aberrant hemichannel properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol. 2007;293:C337–45. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez JM, de Miguel M, Larrosa B, Gonzalez D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci U S A. 2003;100:16030–5. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D, Paul D. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–94. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DC, Kachar B. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci. 2004;24:7051–62. doi: 10.1523/JNEUROSCI.1640-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifa A, Wagner CA, D’Ambrosio L, Melchionda S, Bernardi F, Lopez-Bigas N, Rabionet R, Arbones M, Monica MD, Estivill X, Zelante L, Lang F, Gasparini P. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nat Genet. 1999;23:16–8. doi: 10.1038/12612. [DOI] [PubMed] [Google Scholar]
- Griffith AJ, Chowdhry AA, Kurima K, Hood LJ, Keats B, Berlin CI, Morell RJ, Friedman TB. Autosomal recessive nonsyndromic neurosensory deafness at DFNB1 not associated with the compound-heterozygous GJB2 (connexin 26) genotype M34T/167delT. Am J Hum Genet. 2000;67:745–9. doi: 10.1086/303045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith AJ, Yang Y, Pryor SP, Park HJ, Jabs EW, Nadol JB, Jr, Russell LJ, Wasserman DI, Richard G, Adams JC, Merchant SN. Cochleosaccular dysplasia associated with a connexin 26 mutation in keratitis-ichthyosis-deafness syndrome. Laryngoscope. 2006;116:1404–8. doi: 10.1097/01.mlg.0000224549.75161.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualandi E, Ravani A, Berto A, Burdo S, Trevisi P, Ferlini A, Martini A, Calzolari E. Occurrence of del(GIB6-D13S1830) mutation in Italian non-syndromic hearing loss patients carrying a single GJB2 mutated allele. Acta Otolaryngol Suppl. 2004;552:29–34. doi: 10.1080/03655230410017166. [DOI] [PubMed] [Google Scholar]
- Gualandi F, Ravani A, Berto A, Sensi A, Trabanelli C, Falciano F, Trevisi P, Mazzoli M, Tibiletti MG, Cristofari E, Burdo S, Ferlini A, Martini A, Calzolari E. Exploring the clinical and epidemiological complexity of GJB2-linked deafness. Am J Med Genet. 2002;112:38–45. doi: 10.1002/ajmg.10621. [DOI] [PubMed] [Google Scholar]
- Guo YM, Chen S, Shetty P, Zheng G, Lin R, Li WH. Imaging dynamic cell-cell junctional coupling in vivo using Trojan-LAMP. Nat Methods. 2008;5:835–841. doi: 10.1038/nmeth.1238. [DOI] [PubMed] [Google Scholar]
- Haack B, Schmalisch K, Palmada M, Bohmer C, Kohlschmidt N, Keilmann A, Zechner U, Limberger A, Beckert S, Zenner HP, Lang F, Kupka S. Deficient membrane integration of the novel p.N14D-GJB2 mutant associated with non-syndromic hearing impairment. Hum Mutat. 2006;27:1158–9. doi: 10.1002/humu.9464. [DOI] [PubMed] [Google Scholar]
- Hernandez VH, Bortolozzi M, Pertegato V, Beltramello M, Giarin M, Zaccolo M, Pantano S, Mammano F. Unitary permeability of gap junction channels to second messengers measured by FRET microscopy. Nat Methods. 2007;4:353–8. doi: 10.1038/nmeth1031. [DOI] [PubMed] [Google Scholar]
- Hilgert N, Smith RJ, Van Camp G. Forty-six genes causing nonsyndromic hearing impairment: Which ones should be analyzed in DNA diagnostics? Mutat Res. 2008 Aug 29; doi: 10.1016/j.mrrev.2008.08.002. epublication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh JH, John SA, Revel JP. Molecular cloning and characterization of a new member of the gap junction gene family, connexin-31. J Biol Chem. 1991;266:6524–31. [PubMed] [Google Scholar]
- Inoshita A, Iizuka T, Okamura HO, Minekawa A, Kojima K, Furukawa M, Kusunoki T, Ikeda K. Postnatal development of the organ of Corti in dominant-negative Gjb2 transgenic mice. Neuroscience. 2008;156:1039–47. doi: 10.1016/j.neuroscience.2008.08.027. [DOI] [PubMed] [Google Scholar]
- Iurato S, Franke KD, Luciano L, Wermbter G, Pannese F, Reale E. The junctional complexes among the cells of the organ of Corti as revealed by freeze-fracturing. Adv Otorhinolaryngol. 1977;22:76–80. doi: 10.1159/000399490. [DOI] [PubMed] [Google Scholar]
- Jagger DJ, Forge A. Compartmentalized and signal-selective gap junctional coupling in the hearing cochlea. J Neurosci. 2006;26:1260–8. doi: 10.1523/JNEUROSCI.4278-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke K. The fine structure of freeze-fractured intercellular junctions in the guinea pig inner ear. Acta Otolaryngol Suppl. 1975;336:1–40. [PubMed] [Google Scholar]
- Janecke AR, Hennies HC, Gunther B, Gansl G, Smolle J, Messmer EM, Utermann G, Rittinger O. GJB2 mutations in keratitis-ichthyosis-deafness syndrome including its fatal form. Am J Med Genet A. 2005;133A:128–31. doi: 10.1002/ajmg.a.30515. [DOI] [PubMed] [Google Scholar]
- Kalay E, Caylan R, Kremer H, de Brouwer AP, Karaguzel A. GJB2 mutations in Turkish patients with ARNSHL: prevalence and two novel mutations. Hear Res. 2005;203:88–93. doi: 10.1016/j.heares.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Kammen-Jolly K, Ichiki H, Scholtz AW, Gsenger M, Kreczy A, Schrott-Fischer A. Connexin 26 in human fetal development of the inner ear. Hear Res. 2001;160:15–21. doi: 10.1016/s0378-5955(01)00310-0. [DOI] [PubMed] [Google Scholar]
- Kelley PM, Harris DJ, Comer BC, Askew JW, Fowler T, Smith SD, Kimberling WJ. Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet. 1998;62:792–9. doi: 10.1086/301807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–3. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 1995;191:101–18. doi: 10.1007/BF00186783. [DOI] [PubMed] [Google Scholar]
- Kitajiri S, Miyamoto T, Mineharu A, Sonoda N, Furuse K, Hata M, Sasaki H, Mori Y, Kubota T, Ito J, Furuse M, Tsukita S. Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J Cell Sci. 2004;117:5087–96. doi: 10.1242/jcs.01393. [DOI] [PubMed] [Google Scholar]
- Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS. Unique distributions of the gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia. 2004;47:346–57. doi: 10.1002/glia.20043. [DOI] [PubMed] [Google Scholar]
- Konishi T, Hamrick PE, Walsh PJ. Ion transport in guinea pig cochlea.I. Potassium and sodium transport. Acta Otolaryngol. 1978;86:22–34. doi: 10.3109/00016487809124717. [DOI] [PubMed] [Google Scholar]
- Kudo T, Ikeda K, Kure S, Matsubara Y, Oshima T, Watanabe K, Kawase T, Narisawa K, Takasaka T. Novel mutations in the connexin 26 gene (GJB2) responsible for childhood deafness in the Japanese population. Am J Med Genet. 2000;90:141–5. doi: 10.1002/(sici)1096-8628(20000117)90:2<141::aid-ajmg10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kure S, Ikeda K, Xia AP, Katori Y, Suzuki M, Kojima K, Ichinohe A, Suzuki Y, Aoki Y, Kobayashi T, Matsubara Y. Transgenic expression of a dominant-negative connexin26 causes degeneration of the organ of Corti and non-syndromic deafness. Hum Mol Genet. 2003;12:995–1004. doi: 10.1093/hmg/ddg116. [DOI] [PubMed] [Google Scholar]
- Kupka S, Mirghomizadeh F, Haug T, Braun S, Leistenschneider P, Schmitz-Salue C, Arold R, Blin N, Zenner HP, Pfister M. Mutational analysis of the connexin26 gene in sporadic cases of moderate to profound deafness. HNO. 2000;48:671–4. doi: 10.1007/s001060050637. [DOI] [PubMed] [Google Scholar]
- Kupka S, Braun S, Aberle S, Haack B, Ebauer M, Zeissler U, Zenner HP, Blin N, Pfister M. Frequencies of GJB2 mutations in German control individuals and patients showing sporadic non-syndromic hearing impairment. Hum Mutat. 2002;20:77–8. doi: 10.1002/humu.9044. [DOI] [PubMed] [Google Scholar]
- Laciano L, Franke K, Iurato S, Reale E. Freeze-fracture study of the cell junctions in the utricle and saccule. Acta Otolaryngol. 1977;83:79–84. doi: 10.3109/00016487709128816. [DOI] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–43. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaitre G, Hand C, Hayflick SJ, Zonana J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der Kaloustian V, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet. 2000;26:142–4. doi: 10.1038/79851. [DOI] [PubMed] [Google Scholar]
- Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998;294:415–20. doi: 10.1007/s004410051192. [DOI] [PubMed] [Google Scholar]
- Lautermann J, Frank HG, Jahnke K, Traub O, Winterhager E. Developmental expression patterns of connexin26 and -30 in the rat cochlea. Dev Genet. 1999;25:306–11. doi: 10.1002/(SICI)1520-6408(1999)25:4<306::AID-DVG4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Lee JR, Derosa AM, White TW. Connexin Mutations Causing Skin Disease and Deafness Increase Hemichannel Activity and Cell Death when Expressed in Xenopus Oocytes. J Invest Dermatol. 2008 Nov 11; doi: 10.1038/jid.2008.335. epublication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer I, Sagi M, Ben-Neriah Z, Wang T, Levi H, Abeliovich D. A deletion mutation in GJB6 cooperating with a GJB2 mutation in trans in non-syndromic deafness: A novel founder mutation in Ashkenazi Jews. Hum Mutat. 2001;18:460. doi: 10.1002/humu.1222. [DOI] [PubMed] [Google Scholar]
- Leshinsky-Silver E, Berman Z, Vinkler C, Yannov-Sharav M, Lev D. A novel missense mutation in the Connexin 26 gene associated with autosomal recessive sensorineural deafness. Hear Res. 2005;202:258–61. doi: 10.1016/j.heares.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liang GS, de Miguel M, Gomez-Hernandez JM, Glass JD, Scherer SS, Mintz M, Barrio LC, Fischbeck KH. Severe neuropathy with leaky connexin32 hemichannels. Ann Neurol. 2005;57:749–54. doi: 10.1002/ana.20459. [DOI] [PubMed] [Google Scholar]
- Liu W, Boström M, Kinnefors A, Rask-Andersen H. Unique Expression of Connexins in the Human Cochlea. Hear Res. 2009 doi: 10.1016/j.heares.2009.01.010. in press. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xia XJ, Xu LR, Pandya A, Liang CY, Blanton SH, Brown SD, Steel KP, Nance WE. Mutations in connexin31 underlie recessive as well as dominant non-syndromic hearing loss. Hum Mol Genet. 2000;9:63–7. doi: 10.1093/hmg/9.1.63. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xia XJ, Adams J, Chen ZY, Welch KO, Tekin M, Ouyang XM, Kristiansen A, Pandya A, Balkany T, Arnos KS, Nance WE. Mutations in GJA1 (connexin 43) are associated with non-syndromic autosomal recessive deafness. Hum Mol Genet. 2001;10:2945–51. doi: 10.1093/hmg/10.25.2945. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Yuan Y, Yan D, Ding EH, Ouyang XM, Fei Y, Tang W, Yuan H, Chang Q, Du LL, Zhang X, Wang G, Ahmad S, Kang DY, Lin X, Dai P. Digenic inheritance of non-syndromic deafness caused by mutations at the gap junction proteins Cx26 and Cx31. Hum Genet. 2008;125:53–62. doi: 10.1007/s00439-008-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Zhao HB. Cellular characterization of Connexin26 and Connnexin30 expression in the cochlear lateral wall. Cell Tissue Res. 2008;333:395–403. doi: 10.1007/s00441-008-0641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CW. The role of gap junction membrane channels in development. J Bioenerg Biomembr. 1996;28:379–85. doi: 10.1007/BF02110114. [DOI] [PubMed] [Google Scholar]
- Lopez-Bigas N, Olive M, Rabionet R, Ben-David O, Martinez-Matos JA, Bravo O, Banchs I, Volpini V, Gasparini P, Avraham KB, Ferrer I, Arbones ML, Estivill X. Connexin 31 (GJB3) is expressed in the peripheral and auditory nerves and causes neuropathy and hearing impairment. Hum Mol Genet. 2001;10:947–52. doi: 10.1093/hmg/10.9.947. [DOI] [PubMed] [Google Scholar]
- Lopez-Bigas N, Arbones ML, Estivill X, Simonneau L. Expression profiles of the connexin genes, Gjb1 and Gjb3, in the developing mouse cochlea. Mech Dev. 2002;119(Suppl 1):S111–5. doi: 10.1016/s0925-4773(03)00102-3. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Kawasaki A, Nishizaki K, Smith RJ. Cochlear expression of a dominant-negative GJB2R75W construct delivered through the round window membrane in mice. Neurosci Res. 2007;58:250–4. doi: 10.1016/j.neures.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Mani RS, Ganapathy A, Jalvi R, Srikumari Srisailapathy CR, Malhotra V, Chadha S, Agarwal A, Ramesh A, Rangasayee RR, Anand A. Functional consequences of novel connexin 26 mutations associated with hereditary hearing loss. Eur J Hum Genet. 2008 Oct 23; doi: 10.1038/ejhg.2008.179. epublication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D, Wu T, Wangemann P, Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol. 2002;282:pC403–7. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- Marlin S, Feldmann D, Blons H, Loundon N, Rouillon I, Albert S, Chauvin P, Garabedian EN, Couderc R, Odent S, Joannard A, Schmerber S, Delobel B, Leman J, Journel H, Catros H, Lemarechal C, Dollfus H, Eliot MM, Delaunoy JL, David A, Calais C, Drouin-Garraud V, Obstoy MF, Goizet C, Duriez F, Fellmann F, Helias J, Vigneron J, Montaut B, Matin-Coignard D, Faivre L, Baumann C, Lewin P, Petit C, Denoyelle F. GJB2 and GJB6 mutations: genotypic and phenotypic correlations in a large cohort of hearing-impaired patients. Arch Otolaryngol Head Neck Surg. 2005;131:481–7. doi: 10.1001/archotol.131.6.481. [DOI] [PubMed] [Google Scholar]
- Martin PE, Coleman SL, Casalotti SO, Forge A, Evans WH. Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal heriditary deafness. Hum Mol Genet. 1999;8:2369–76. doi: 10.1093/hmg/8.13.2369. [DOI] [PubMed] [Google Scholar]
- Martinez AD, Acuna R, Figueroa V, Maripillan J, Nicholson B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid Redox Signal. 2009;11:309–22. doi: 10.1089/ars.2008.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marziano NK, Casalotti SO, Portelli AE, Becker DL, Forge A. Mutations in the gene for connexin 26 (GJB2) that cause hearing loss have a dominant negative effect on connexin 30. Hum Mol Genet. 2003;12:805–12. doi: 10.1093/hmg/ddg076. [DOI] [PubMed] [Google Scholar]
- Matos TD, Caria H, Simoes-Teixeira H, Aasen T, Dias O, Andrea M, Kelsell DP, Fialho G. A novel M163L mutation in connexin 26 causing cell death and associated with autosomal dominant hearing loss. Hear Res. 2008;240:87–92. doi: 10.1016/j.heares.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Matsunami T, Suzuki T, Hisa Y, Takata K, Takamatsu T, Oyamada M. Gap junctions mediate glucose transport between GLUT1-positive and -negative cells in the spiral limbus of the rat cochlea. Cell Commun Adhes. 2006;13:93–102. doi: 10.1080/15419060600631805. [DOI] [PubMed] [Google Scholar]
- Melchionda S, Bicego M, Marciano E, Franze A, Morgutti M, Bortone G, Zelante L, Carella M, D’Andrea P. Functional characterization of a novel Cx26 (T55N) mutation associated to non-syndromic hearing loss. Biochem Biophys Res Commun. 2005;337:799–805. doi: 10.1016/j.bbrc.2005.09.116. [DOI] [PubMed] [Google Scholar]
- Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet. 2004;115:191–9. doi: 10.1007/s00439-004-1142-6. [DOI] [PubMed] [Google Scholar]
- Mese G, Valiunas V, Brink PR, White TW. Connexin26 deafness associated mutations show altered permeability to large cationic molecules. Am J Physiol Cell Physiol. 2008;295:C966–74. doi: 10.1152/ajpcell.00008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok BW, Yeung WS, Luk JM. Differential expression of gap-junction gene connexin 31 in seminiferous epithelium of rat testes. FEBS Lett. 1999;453:243–8. doi: 10.1016/s0014-5793(99)00714-0. [DOI] [PubMed] [Google Scholar]
- Moley KH, Mueckler MM. Glucose transport and apoptosis. Apoptosis. 2000;5:99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- Montgomery JR, White TW, Martin BL, Turner ML, Holland SM. A novel connexin 26 gene mutation associated with features of the keratitis-ichthyosis-deafness syndrome and the follicular occlusion triad. J Am Acad Dermatol. 2004;51:377–82. doi: 10.1016/j.jaad.2003.12.042. [DOI] [PubMed] [Google Scholar]
- Murgia A, Orzan E, Polli R, Martella M, Vinanzi C, Leonardi E, Arslan E, Zacchello F. Cx26 deafness: mutation analysis and clinical variability. J Med Genet. 1999;36:829–32. [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Ionescu AV, Lynn BD, Rash JE. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia. 2003;44:205–18. doi: 10.1002/glia.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P, Keller J. RNA in Brain Disease: No Longer Just “The Messenger in the Middle”. Journal of Neuropathology & Experimental Neurology. 2007;66:461–468. doi: 10.1097/01.jnen.0000240474.27791.f3. [DOI] [PubMed] [Google Scholar]
- Nicholson BJ. Gap junctions - from cell to molecule. J Cell Sci. 2003;116:4479–81. doi: 10.1242/jcs.00821. [DOI] [PubMed] [Google Scholar]
- Nickel R, Forge A. Gap junctions and connexins in the inner ear: their roles in homeostasis and deafness. Curr Opin Otolaryngol Head Neck Surg. 2008;16:452–7. doi: 10.1097/MOO.0b013e32830e20b0. [DOI] [PubMed] [Google Scholar]
- Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci. 2000;113:1365–72. doi: 10.1242/jcs.113.8.1365. [DOI] [PubMed] [Google Scholar]
- Ortolano S, Di Pasquale G, Crispino G, Anselmi F, Mammano F, Chiorini JA. Coordinated control of connexin 26 and connexin 30 at the regulatory and functional level in the inner ear. Proc Natl Acad Sci U S A. 2008;105:18776–81. doi: 10.1073/pnas.0800831105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzan E, Murgia A. Connexin 26 deafness is not always congenital. Int J Pediatr Otorhinolaryngol. 2007;71:501–7. doi: 10.1016/j.ijporl.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Oshima A, Doi T, Mitsuoka K, Maeda S, Fujiyoshi Y. Roles of Met-34, Cys-64, and Arg-75 in the assembly of human connexin 26. Implication for key amino acid residues for channel formation and function. J Biol Chem. 2003;278:1807–16. doi: 10.1074/jbc.M207713200. [DOI] [PubMed] [Google Scholar]
- Oyamada M, Oyamada Y, Takamatsu T. Regulation of connexin expression. Biochim Biophys Acta. 2005;1719:6–23. doi: 10.1016/j.bbamem.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Pagarkar W, Bitner-Glindzicz M, Knight J, Sirimanna T. Late postnatal onset of hearing loss due to GJB2 mutations. Int J Pediatr Otorhinolaryngol. 2006;70:1119–24. doi: 10.1016/j.ijporl.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Pallares-Ruiz N, Blanchet P, Mondain M, Claustres M, Roux AF. A large deletion including most of GJB6 in recessive non syndromic deafness: a digenic effect? Eur J Hum Genet. 2002;10:72–6. doi: 10.1038/sj.ejhg.5200762. [DOI] [PubMed] [Google Scholar]
- Palmada M, Schmalisch K, Bohmer C, Schug N, Pfister M, Lang F, Blin N. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiol Dis. 2006;22:112–8. doi: 10.1016/j.nbd.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–4. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–18. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C. From deafness genes to hearing mechanisms: harmony and counterpoint. Trends Mol Med. 2006;12:57–64. doi: 10.1016/j.molmed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Phelan P, Bacon JP, Davies JA, Stebbings LA, Todman MG, Avery L, Baines RA, Barnes TM, Ford C, Hekimi S, Lee R, Shaw JE, Starich TA, Curtin KD, Sun YA, Wyman RJ. Innexins: a family of invertebrate gap-junction proteins. Trends Genet. 1998;14:348–9. doi: 10.1016/s0168-9525(98)01547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza V, Beltramello M, Menniti M, Colao E, Malatesta P, Argento R, Chiarella G, Gallo LV, Catalano M, Perrotti N, Mammano F, Cassandro E. Functional analysis of R75Q mutation in the gene coding for Connexin 26 identified in a family with nonsyndromic hearing loss. Clin Genet. 2005;68:161–6. doi: 10.1111/j.1399-0004.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- Plum A, Winterhager E, Pesch J, Lautermann J, Hallas G, Rosentreter B, Traub O, Herberhold C, Willecke K. Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev Biol. 2001;231:334–47. doi: 10.1006/dbio.2000.0148. [DOI] [PubMed] [Google Scholar]
- Prasad S, Cucci RA, Green GE, Smith RJ. Genetic testing for hereditary hearing loss: connexin 26 (GJB2) allele variants and two novel deafness-causing mutations (R32C and 645–648delTAGA) Hum Mutat. 2000;16:502–8. doi: 10.1002/1098-1004(200012)16:6<502::AID-HUMU7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Primignani P, Castorina P, Sironi F, Curcio C, Ambrosetti U, Coviello DA. A novel dominant missense mutation--D179N--in the GJB2 gene (Connexin 26) associated with non-syndromic hearing loss. Clin Genet. 2003;63:516–21. doi: 10.1034/j.1399-0004.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- Primignani P, Trotta L, Castorina P, Lalatta F, Cuda D, Murri A, Ambrosetti U, Cesarani A, Curcio C, Coviello D, Travi M. A new de novo missense mutation in connexin 26 in a sporadic case of nonsyndromic deafness. Laryngoscope. 2007;117:821–4. doi: 10.1097/MLG.0b013e31803330d9. [DOI] [PubMed] [Google Scholar]
- Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys. 2000;381:181–90. doi: 10.1006/abbi.2000.1989. [DOI] [PubMed] [Google Scholar]
- Qu Y, Tang W, Dahlke I, Ding D, Salvi R, Sohl G, Willecke K, Chen P, Lin X. Analysis of connexin subunits required for the survival of vestibular hair cells. J Comp Neurol. 2007;504:499–507. doi: 10.1002/cne.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabionet R, Lopez-Bigas N, Arbones ML, Estivill X. Connexin mutations in hearing loss, dermatological and neurological disorders. Trends Mol Med. 2002;8:205–12. doi: 10.1016/s1471-4914(02)02327-4. [DOI] [PubMed] [Google Scholar]
- Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J. 2007a;92:1952–65. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackauskas M, Verselis VK, Bukauskas FF. Permeability of homotypic and heterotypic gap junction channels formed of cardiac connexins mCx30.2, Cx40, Cx43, and Cx45. Am J Physiol Heart Circ Physiol. 2007b;293:H1729–36. doi: 10.1152/ajpheart.00234.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH, Jr, DiGiovanna JJ, Compton JG, Bale SJ. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet. 1998a;20:366–9. doi: 10.1038/3840. [DOI] [PubMed] [Google Scholar]
- Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ. Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet. 1998b;103:393–9. doi: 10.1007/s004390050839. [DOI] [PubMed] [Google Scholar]
- Richard G. Connexins: a connection with the skin. Exp Dermatol. 2000;9:77–96. doi: 10.1034/j.1600-0625.2000.009002077.x. [DOI] [PubMed] [Google Scholar]
- Richard G, Rouan F, Willoughby CE, Brown N, Chung P, Ryynanen M, Jabs EW, Bale SJ, DiGiovanna JJ, Uitto J, Russell L. Missense mutations in GJB2 encoding connexin-26 cause the ectodermal dysplasia keratitis-ichthyosis-deafness syndrome. Am J Hum Genet. 2002;70:1341–8. doi: 10.1086/339986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G. trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J Cell Sci. 2001;114:2105–13. doi: 10.1242/jcs.114.11.2105. [DOI] [PubMed] [Google Scholar]
- Sadanaga M, Morimitsu T. Development of endocochlear potential and its negative component in mouse cochlea. Hear Res. 1995;89:155–61. doi: 10.1016/0378-5955(95)00133-x. [DOI] [PubMed] [Google Scholar]
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Dallos P. Intercellular communication in the supporting cells of the organ of Corti. Hearing Research. 1983;9:317–326. doi: 10.1016/0378-5955(83)90034-5. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia. 1998;24:8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schulte BA, Adams JC. Distribution of immunoreactive Na+, K+-ATPase in gerbil cochlea. Journal of Histochemistry & Cytochemistry. 1989;37:127–134. doi: 10.1177/37.2.2536055. [DOI] [PubMed] [Google Scholar]
- Skerrett IM, Di WL, Kasperek EM, Kelsell DP, Nicholson BJ. Aberrant gating, but a normal expression pattern, underlies the recessive phenotype of the deafness mutant Connexin26M34T. FASEB J. 2004;18:860–2. doi: 10.1096/fj.03-0763fje. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Bale JF, Jr, White KR. Sensorineural hearing loss in children. Lancet. 2005;365:879–90. doi: 10.1016/S0140-6736(05)71047-3. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes. 2003;10:173–80. doi: 10.1080/cac.10.4-6.173.180. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–32. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Sosinsky GE, Nicholson BJ. Structural organization of gap junction channels. Biochim Biophys Acta. 2005;1711:99–125. doi: 10.1016/j.bbamem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res. 1996;100:80–100. doi: 10.1016/0378-5955(96)00106-2. [DOI] [PubMed] [Google Scholar]
- Sterkers O, Saumon G, Tran Ba Huy P, Amiel C. Evidence for a perilymphatic origin of the endolymph: application to the pathophysiology of Meniere’s disease. Am J Otolaryngol. 1982;3:367–75. doi: 10.1016/s0196-0709(82)80012-4. [DOI] [PubMed] [Google Scholar]
- Stong BC, Chang Q, Ahmad S, Lin X. A novel mechanism for connexin 26 mutation linked deafness: cell death caused by leaky gap junction hemichannels. Laryngoscope. 2006;116:2205–10. doi: 10.1097/01.mlg.0000241944.77192.d2. [DOI] [PubMed] [Google Scholar]
- Sun J, Ahmad S, Chen S, Tang W, Zhang Y, Chen P, Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol. 2005;288:C613–23. doi: 10.1152/ajpcell.00341.2004. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takamatsu T, Oyamada M. Expression of gap junction protein connexin43 in the adult rat cochlea: comparison with connexin26. J Histochem Cytochem. 2003;51:903–12. doi: 10.1177/002215540305100705. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Matsunami T, Hisa Y, Takata K, Takamatsu T, Oyamada M. Roles of gap junctions in glucose transport from glucose transporter 1-positive to -negative cells in the lateral wall of the rat cochlea. Histochem Cell Biol. 2009;131:89–102. doi: 10.1007/s00418-008-0502-z. [DOI] [PubMed] [Google Scholar]
- Tamayo ML, Olarte M, Gelvez N, Gomez M, Frias JL, Bernal JE, Florez S, Medina D. Molecular studies in the GJB2 gene (Cx26) among a deaf population from Bogota, Colombia: Results of a screening program. Int J Pediatr Otorhinolaryngol. 2009;73:97–101. doi: 10.1016/j.ijporl.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Tang W, Zhang Y, Chang Q, Ahmad S, Dahlke I, Yi H, Chen P, Paul DL, Lin X. Connexin29 is highly expressed in cochlear Schwann cells, and it is required for the normal development and function of the auditory nerve of mice. J Neurosci. 2006;26:1991–9. doi: 10.1523/JNEUROSCI.5055-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Ahmad S, Shestopalov VI, Lin X. Pannexins are new molecular candidates for assembling gap junctions in the cochlea. Neuroreport. 2008;19:1253–7. doi: 10.1097/WNR.0b013e32830891f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin M, Duman T, Bogoclu G, Incesulu A, Cin S, Akar N. Moderate hearing loss and pseudodominant inheritance due to L90P/35delG mutations in the GJB2 (connexin 26) gene. Genet Couns. 2003;14:379–86. [PubMed] [Google Scholar]
- Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- Thomas T, Telford D, Laird DW. Functional domain mapping and selective trans-dominant effects exhibited by Cx26 disease-causing mutations. J Biol Chem. 2004;279:19157–68. doi: 10.1074/jbc.M314117200. [DOI] [PubMed] [Google Scholar]
- Thonnissen E, Rabionet R, Arbones ML, Estivill X, Willecke K, Ott T. Human connexin26 (GJB2) deafness mutations affect the function of gap junction channels at different levels of protein expression. Hum Genet. 2002;111:190–7. doi: 10.1007/s00439-002-0750-2. [DOI] [PubMed] [Google Scholar]
- Uyguner O, Emiroglu M, Uzumcu A, Hafiz G, Ghanbari A, Baserer N, Yuksel-Apak M, Wollnik B. Frequencies of gap- and tight-junction mutations in Turkish families with autosomal-recessive non-syndromic hearing loss. Clin Genet. 2003;64:65–9. doi: 10.1034/j.1399-0004.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- Wang HL, Chang WT, Li AH, Yeh TH, Wu CY, Chen MS, Huang PC. Functional analysis of connexin-26 mutants associated with hereditary recessive deafness. J Neurochem. 2003;84:735–42. doi: 10.1046/j.1471-4159.2003.01555.x. [DOI] [PubMed] [Google Scholar]
- Wang XH, Streeter M, Liu YP, Zhao HB. Identification and characterization of pannexin expression in the mammalian cochlea. J Comp Neurol. 2009;512:336–46. doi: 10.1002/cne.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J Bioenerg Biomembr. 1996;28:339–50. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- White TW, Deans MR, Kelsell DP, Paul DL. Connexin mutations in deafness. Nature. 1998;394:630–1. doi: 10.1038/29202. [DOI] [PubMed] [Google Scholar]
- White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–20. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- Wilch E, Zhu M, Burkhart KB, Regier M, Elfenbein JL, Fisher RA, Friderici KH. Expression of GJB2 and GJB6 is reduced in a novel DFNB1 allele. Am J Hum Genet. 2006;79:174–9. doi: 10.1086/505333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–37. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Xia A, Kikuchi T, Hozawa K, Katori Y, Takasaka T. Expression of connexin 26 and Na, K-ATPase in the developing mouse cochlear lateral wall: functional implications. Brain Res. 1999;846:106–11. doi: 10.1016/s0006-8993(99)01996-4. [DOI] [PubMed] [Google Scholar]
- Xia AP, Ikeda K, Katori Y, Oshima T, Kikuchi T, Takasaka T. Expression of connexin 31 in the developing mouse cochlea. Neuroreport. 2000;11:2449–53. doi: 10.1097/00001756-200008030-00022. [DOI] [PubMed] [Google Scholar]
- Xia JH, Liu CY, Tang BS, Pan Q, Huang L, Dai HP, Zhang BR, Xie W, Hu DX, Zheng D, Shi XL, Wang DA, Xia K, Yu KP, Liao XD, Feng Y, Yang YF, Xiao JY, Xie DH, Huang JZ. Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet. 1998;20:370–3. doi: 10.1038/3845. [DOI] [PubMed] [Google Scholar]
- Xiao ZA, Xie DH. GJB2 (Cx26) gene mutations in Chinese patients with congenital sensorineural deafness and a report of one novel mutation. Chin Med J (Engl) 2004;117:1797–801. [PubMed] [Google Scholar]
- Yang JJ, Huang SH, Chou KH, Liao PJ, Su CC, Li SY. Identification of mutations in members of the connexin gene family as a cause of nonsyndromic deafness in Taiwan. Audiol Neurootol. 2007;12:198–208. doi: 10.1159/000099024. [DOI] [PubMed] [Google Scholar]
- Yeager M, Harris AL. Gap junction channel structure in the early 21st century: facts and fantasies. Curr Opin Cell Biol. 2007;19:521–8. doi: 10.1016/j.ceb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto S, Hashiguchi T, Chen X, Ohtake N, Tomitaka A, Akamatsu H, Matsunaga K, Shiraishi S, Miura H, Adachi J, Kanzaki T. Novel mutations in GJB2 encoding connexin-26 in Japanese patients with keratitis-ichthyosis-deafness syndrome. Br J Dermatol. 2003;148:649–53. doi: 10.1046/j.1365-2133.2003.05245.x. [DOI] [PubMed] [Google Scholar]
- Yuge I, Ohtsuka A, Matsunaga T, Usami S. Identification of 605ins46, a novel GJB2 mutation in a Japanese family. Auris Nasus Larynx. 2002;29:379–82. doi: 10.1016/s0385-8146(02)00055-x. [DOI] [PubMed] [Google Scholar]
- Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Scherer SS. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol. 2007;293:C1032–48. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]
- Zelante L, Gasparini P, Estivill X, Melchionda S, D’Agruma L, Govea N, Mila M, Monica MD, Lutfi J, Shohat M, Mansfield E, Delgrosso K, Rappaport E, Surrey S, Fortina P. Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet. 1997;6:1605–9. doi: 10.1093/hmg/6.9.1605. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P, Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci U S A. 2005;102:15201–6. doi: 10.1073/pnas.0501859102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Santos-Sacchi J. Voltage gating of gap junctions in cochlear supporting cells: evidence for nonhomotypic channels. J Membr Biol. 2000;175:17–24. doi: 10.1007/s002320001051. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A. 2005;102:18724–9. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol. 2006;209:177–86. doi: 10.1007/s00232-005-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Yu N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J Comp Neurol. 2006;499:506–18. doi: 10.1002/cne.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidanic M, Brownell WE. Fine structure of the intracochlear potential field. I. The silent current. Biophys J. 1990;57:1253–68. doi: 10.1016/S0006-3495(90)82644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.