Abstract
Objective
The type I interferon (IFN) pathway is activated in many patients with systemic lupus erythematosus (SLE), and high serum levels of IFN are associated with anti-SSA/Ro autoantibodies. To investigate the clinical features associated with type I IFN production in vivo, we compared serum IFN activity in individuals with anti-SSA/Ro antibodies who were asymptomatic with that in individuals with clinical manifestations of SLE or Sjögren's syndrome (SS).
Methods
Antibody-positive sera from 84 mothers of children with manifestations of neonatal lupus were studied for type I IFN activity, using a functional reporter cell assay. Maternal health status was characterized as asymptomatic, SS, SLE, pauci-SLE, or pauci-SS, based on a screening questionnaire, telephone interview, and review of medical records. The prefix “pauci-” indicates symptoms insufficient for a formal classification of the disease.
Results
Only 4% of asymptomatic mothers had high serum type I IFN activity, compared with 73% with pauci-SLE (P = 5.7 × 10−5), 35% with SLE (P = 0.011), and 32% of patients with SS (P = 0.032). One of the 4 patients with pauci-SS had high levels of IFN. The majority of patients for whom longitudinal data were available had stable type I IFN activity over time, and changes in IFN activity were not clearly accompanied by changes in the clinical diagnosis.
Conclusion
Patients with SLE, patients with pauci-SLE, and patients with SS are more likely to have high serum IFN activity than asymptomatic individuals with SSA/Ro autoantibodies, suggesting that these autoantibodies are insufficient for activation of the type I IFN pathway, and that disease-specific factors are important for type I IFN generation in vivo.
The type I interferon (IFN) system is activated in many patients with systemic lupus erythematosus (SLE) (1) or Sjögren's syndrome (SS) (2). Patients with SLE frequently have high serum levels of type I IFN (3), and type I IFN activity often correlates with measures of disease activity (4). In patients with SS, overexpression of type I IFN–induced messenger RNA (mRNA) has been demonstrated in salivary gland tissue, and some patients also have high serum levels of type I IFN (2,5). Anti-SSA/Ro and anti-SSB/La autoantibodies are frequently present in the serum of patients with SLE and patients with SS, and these autoantibodies are associated with high serum levels of type I IFN in patients with SLE (4,6). In vitro experiments show that sera containing anti–RNA binding protein antibodies, such as those targeting anti-SSA/Ro or anti-SSB/La, in combination with nucleic acid–containing necrotic or apoptotic cell debris can promote type I IFN production in plasmacytoid dendritic cells (7). These data suggest that immune complexes containing anti-SSA/Ro and/or anti-SSB/La antibodies may directly induce production of type I IFN.
Maternal antibodies to SSA/Ro and SSB/La are associated with the risk of neonatal lupus erythematosus (NLE); these antibodies are nearly universally present in the mother when isolated heart block is diagnosed in a fetus in utero (7). Fetal disease is independent of maternal disease, and in many cases autoantibodies are identified only because of the affected pregnancy. Mothers with these autoantibodies have a variety of clinical diagnoses, ranging from SLE or SS, and some are asymptomatic. Mothers who are initially asymptomatic can remain so or progress over time to SLE or SS (8). Given the potential role for anti-SSA/Ro or anti-SSB/La autoantibodies in the production of type I IFN in systemic autoimmune disease, serum levels of type I IFN were evaluated in mothers with varying clinical diagnoses, all of whom were enrolled in the US Research Registry for Neonatal Lupus (RRNL) (9).
Patients and Methods
Patients and samples
Serum and plasma samples were obtained from 84 mothers enrolled in the RRNL. Individuals enrolled in the RRNL have antibodies to SSA/Ro and/or SSB/La and at least 1 child with NLE. Clinical information was collected for each individual, using a questionnaire focusing on signs and symptoms of rheumatic diseases, telephone interviews, and a review of the medical records.
SS (probable or definite) was diagnosed in individuals meeting ≥3 of the revised criteria of the American-European Consensus Group (10). Pauci-SS was diagnosed in mothers with 1 of the following: dry eyes unrelated to the use of contact lenses, dry mouth, or parotid enlargement without objective evidence of keratoconjunctivitis sicca, xerostomia, or lymphocytic foci on salivary gland biopsy. SLE was diagnosed in individuals who fulfilled ≥4 of the American College of Rheumatology (ACR) revised criteria for a diagnosis of SLE (11), and pauci-SLE was diagnosed in individuals fulfilling <4 of the ACR criteria for a diagnosis of SLE in the absence of signs or symptoms of SS. Mothers with no symptoms of a rheumatic disease were classified as asymptomatic.
Followup clinical data were obtained using a standardized questionnaire, and a telephone interview and review of medical records were performed whenever a patient reported changes in her clinical status. The healthy donor samples were obtained from registries at the Hospital for Special Surgery, the Lupus Family Registry and Repository, and commercially from blood donors, as previously described (6).
Detection of antibodies to SSA/Ro and SSB/La
Determination of antibodies to SSA/Ro and SSB/La was performed by personnel at the clinical immunology laboratory of the Hospital for Joint Diseases, using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Diamedix, Miami, FL).
Stimulation of reporter cells with patient sera
The reporter cell assay for type I IFN activity was developed and validated in our laboratory (12). The reporter cells (WISH cells; American Type Culture Collection no. CCL-25) are an epithelium-derived cell line that is highly responsive to type I IFN. Cells were plated at a density of 5 × 105/ml and incubated with 50% patient plasma or serum for 6 hours. Recombinant interferon-α (IFNα) was used as a positive control, and normal sera and culture media were used as negative controls in each experiment. WISH cells do not express the Toll-like receptors (TLRs) TLR-3, TLR-7, TLR-8, or TLR-9 to any significant degree (data not shown), so immune complexes containing DNA or RNA in serum should not confound the assay by causing type I IFN generation in the WISH cells. Also, preincubation of WISH cells with cycloheximide does not decrease the IFN-induced gene response (12), suggesting that IFNα that is already present in the samples drives the IFNα-induced gene expression in the reporter cells.
The WISH assay was performed on 50 serum samples from patients with SLE who had previously been studied for IFN-induced gene expression in their peripheral blood mononuclear cells (PBMCs) (4), and serum IFN activity by WISH assay was highly correlated with IFN-induced gene expression in PBMCs (Spearman's r = 0.52, P = 0.0001; data not shown). (For further details regarding assay validation, see refs. 6 and 12.) The researcher performing the IFN assay (TBN) was blinded to the clinical information.
Synthesis of complementary DNA (cDNA) from total cellular mRNA
Total cellular mRNA was purified from WISH cell lysates using the TurboCapture 96 mRNA Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Complementary DNA was synthesized from total cellular mRNA using the oligo(dT) primer and SuperScript III R Reverse Transcriptase system (Invitrogen, Carlsbad, CA).
Real-time polymerase chain reaction (PCR) quantification of mRNA-derived cDNA
Ten microliters of a 1:40 dilution of the cDNA made from total cellular mRNA was then quantified using real-time PCR with the Bio-Rad SYBR Green fluorophore system (Richmond, CA). Forward and reverse primers were used for the genes MX1, PKR, and IFIT1, which are highly and specifically induced by type I IFN (13) (for MX1, forward TACCAGGACTACGAGATTG, reverse TGCCAGGAAGGTCTATTAG; for PKR, forward CTTCCATCTGACTCAGGTTT, reverse TGCTTCTGACGGTATGTATTA; for IFIT1, forward CTCCTTGGGTTCGTCTATAAATTG, reverse AGTCAGCAGCCAGTCTCAG). The housekeeping gene GAPDH was also quantified in each sample. Melt curves were analyzed to ensure the specificity of the amplified product, and standard curves were generated for each PCR experiment.
Real-time PCR data analysis
The amount of PCR product of the IFNα-induced gene was normalized to the amount of product for the housekeeping gene GAPDH in the same sample. The relative expression of each of the 3 tested IFN-induced genes was calculated as a fold increase compared with its expression in WISH cells cultured with media alone. The ability of SSA/Ro-positive patient serum to cause IFN-induced gene expression in the reporter cells was then compared with the mean and SD expression induced by healthy donor sera (n = 106). The number of SDs above the mean of healthy donors for each gene was calculated.
Data are expressed quantitatively as the sum of the number of SDs for each of the 3 genes above the mean values for healthy donors. The number of SDs above normal is used to quantify type I IFN activity instead of using raw relative expression data, because some genes are more highly induced than others, resulting in the more highly inducible genes being overrepresented in aggregate relative expression data. The relative expression values for each of the 3 genes individually showed a strong correlation with the combined sum of the number of SDs above the mean values for unrelated healthy donors (for IFIT1, r = 0.710; for MX1, r = 0.770; for PKR, r = 0.822 [P < 0.0001 for all]), confirming coordinate regulation of the 3 transcripts and that each measured gene was accurately represented in the SD analysis and was contributing to the combined sum of SD values (6). The 3 transcripts chosen for measurement in this study were selected to represent coordinate activation of the type I IFN pathway as would be expected after ligation of the type I IFN receptor, instead of measurement of only 1 IFN-induced transcript, which may not always accurately represent pathway activation.
Experimental samples were analyzed categorically using the following method, which was developed in our laboratory and validated in a cohort of patients with SLE (4,6). Patients were considered to have high type I IFN activity if either of the 2 following criteria were met: expression of 2 of the 3 tested IFN-induced genes was >1 SD above the mean for healthy donor sera, and expression of at least 1 gene was ≥2 SD above the mean for healthy donor sera; or expression of 1 IFN-induced gene was >4 SD above the mean for healthy donor sera. If neither criterion was met, the sample was considered to have low type I IFN activity. Categorical data were analyzed using 2-sided Fisher's exact test (sum of small P values method [observed ≥ expected]), and quantitative data were compared using the Mann-Whitney nonparametric t-test.
Results
Clinical characteristics of the mothers of children with NLE
The diagnostic classification of the mother was assigned at the time when blood was withdrawn, as detailed in Patients and Methods, and the clinical information is summarized in Table 1. All of the mothers of children with NLE had high-titer Ro/SSA antibodies, and 49% also had La/SSB antibodies.
Table 1.
Clinical characteristics of the mothers of children with NLE*
| Characteristic | Asymptomatic (n = 24) |
Pauci-SLE (n = 11) |
SLE (n = 26) |
Pauci-SS (n = 4) |
SS (n = 19) |
|---|---|---|---|---|---|
| Age at time of sample, mean ± SD years | 36 ± 16 | 32 ± 5 | 36 ± 8 | 32 ± 5 | 39 ± 7 |
| Age at birth of NLE child, mean ± SD years | 30 ± 6 | 32 ± 5 | 31 ± 4 | 32 ± 5 | 30 ± 4 |
| Self-reported ancestry | |||||
| European American | 17 (71) | 10 (91) | 20 (77) | 3 (75) | 15 (79) |
| African American | 2 (8) | 0 | 4 (15) | 1 (25) | 3 (16) |
| Hispanic | 1 (4) | 0 | 1 (4) | 0 | 1 (5) |
| Asian or Pacific | 3 (13) | 1 (9) | 0 | 0 | 0 |
| Unknown | 1 (4) | 0 | 1 (4) | 0 | 0 |
| Ro or Ro/La antibodies | |||||
| Ro antibodies | 18 (75) | 9 (82) | 9 (35) | 3 (75) | 4 (21) |
| Ro and La antibodies | 6 (25) | 2 (18) | 17 (65) | 1 (25) | 15 (79) |
| ACR SLE criteria | |||||
| Malar rash | – | 0 | 7 (27) | – | – |
| Discoid rash | – | 0 | 1 (4) | – | – |
| Photosensitivity | – | 11 (100) | 18 (73) | – | – |
| Oral ulcers | – | 0 | 12 (46) | – | – |
| Arthritis | – | 1 (9) | 21 (81) | – | – |
| Serositis | – | 0 | 13 (50) | – | – |
| Renal disorder | – | 0 | 5 (19) | – | – |
| Neurologic disorder | – | 0 | 1 (4) | – | – |
| Hematologic disorder | – | 1 (9) | 13 (50) | – | 4 (21) |
| Immunologic disorder† | 0 | 1 (13) | 14 (61) | – | 0 |
| Mean no. of SLE criteria met | – | 2.0 | 4.9 | – | – |
| European Consensus SS criteria | |||||
| Dry eyes | – | – | – | 2 (50) | 15 (79) |
| Dry mouth | – | – | – | 3 (75) | 17 (89) |
| Parotid enlargement | – | – | – | 1 (25) | 5 (26) |
| Positive Schirmer's test result | – | – | – | 0 | 4 (100)‡ |
| Positive lip biopsy result | – | – | – | 0 | 2 (100)§ |
| Nonspecific symptoms | |||||
| Arthralgias | – | 2 (18) | – | 2 (50) | 8 (42) |
| Raynaud's phenomenon | – | 1 (9) | 12 (46) | 0 | 7 (37) |
Except where indicated otherwise, values are the number (%). NLE = neonatal lupus erythematosus; ACR = American College of Rheumatology.
Data were available on 11 asymptomatic patients, 3 patients with pauci–systemic lupus erythematosus (pauci-SLE), 23 patients with SLE, and 10 patients with Sjögren's syndrome (SS).
Four patients were tested, and all 4 had results that were considered positive by standard criteria.
Data were available on 2 patients.
High serum levels of type I IFN in mothers with SS, SLE, or pauci-SLE
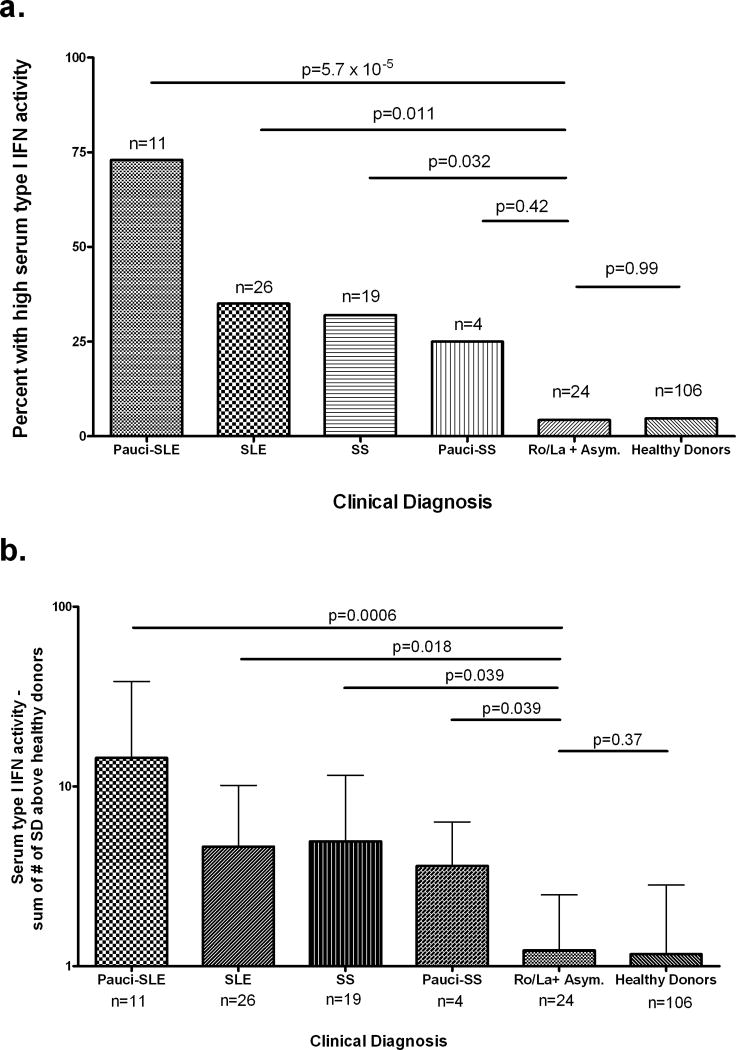
High serum levels of type I IFN were seen in 9 (35%) of 26 patients with SLE, 8 (73%) of 11 patients with pauci-SLE, 6 (32%) of 19 patients with SS, and 1 (25%) of 4 patients with pauci-SS. In contrast, only 1 (4%) of 24 asymptomatic mothers had high serum levels of type I IFN. As shown in Figure 1a, the proportion of individuals with high type I IFN activity was significantly greater in the groups with SLE, pauci-SLE, and SS compared with the group of asymptomatic mothers. When quantitative serum IFN activity levels were compared, all symptomatic patients had higher IFN activity when compared with the asymptomatic mothers, as shown in Figure 1b. Serum type I IFN activity in asymptomatic mothers of children with NLE was not significantly different from that in healthy controls, in either analysis. Each diagnostic category refers to symptoms and findings at the time the serum sample was obtained.
Figure 1.
a, Proportions of mothers of children with neonatal lupus erythematosus (NLE), stratified by clinical diagnosis, and healthy donors with high serum type I interferon (IFN) activity. n = total number of patients with the given diagnosis; if a patient had >1 sample available from different dates, the most recent sample and corresponding diagnosis were used. b, Quantitative serum type I IFN activity in mothers of children with NLE, stratified by clinical diagnosis, and healthy donors. Values are the mean and SD. P values were determined by Fisher's exact test. SLE = systemic lupus erythematosus; SS = Sjögren's syndrome; Asym. = asymptomatic.
Analysis of the entire group of mothers, independent of health status, showed a nonsignificant trend toward association of high IFN activity with positivity for both anti-SSA/Ro and anti-SSB/La as compared with those with anti-SSA/Ro alone (41.5% versus 23.3%; P = 0.1). The 1 asymptomatic mother with high IFN activity was positive for both anti-SSA/Ro and anti-SSB/La. There were no significant differences in the titer of either antibody between patients with high IFN activity and those with low IFN activity (for anti-SSA/Ro, P = 0.7; for anti-SSB/La, P = 0.5). Antibody titers for all of the subjects are shown in Figure 2.
Figure 2.
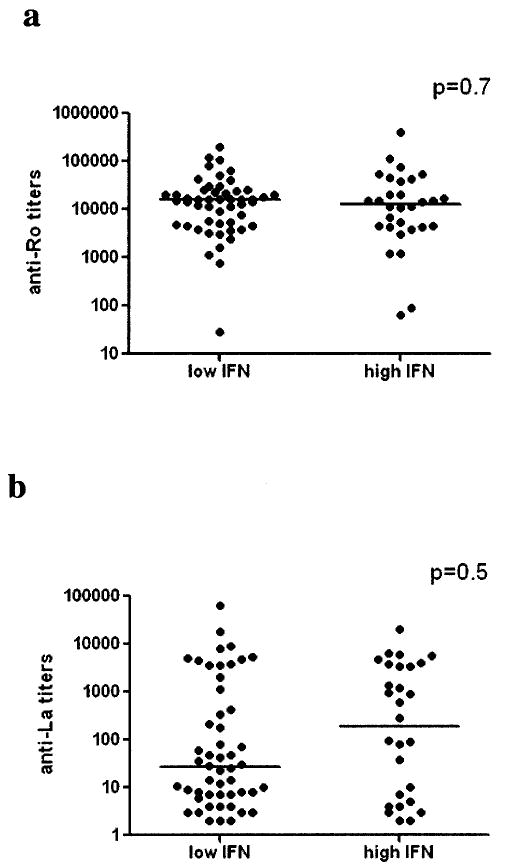
Titers of anti-SSA/Ro antibodies (a) and anti-SSB/La antibodies (b), as determined by enzyme-linked immunosorbent assay, in individuals with high versus low type I interferon (IFN) activity, regardless of health status. Autoantibodies were measured in serum samples that were obtained at the time of entry into the Research Registry for Neonatal Lupus. Although all individuals had positive test results for SSA/Ro and some had positive results for SSB/La, precise titer data for SSA/Ro were available on only 83 (99%) of 84 subjects, and precise SSB/La titer data were available on only 80 (95%) of 84 subjects in whom IFN activity was later quantified. Bars show the medians.
Longitudinal clinical followup
Longitudinal samples and followup data were available for 28 of the 84 mothers (mean ± SD followup 3.8 ± 3.3 years). Four (14%) of these 28 mothers had a change of diagnosis. One patient changed from having low IFN activity to having high IFN activity as her diagnosis changed from SS to SLE over a 9-year period. The other 3 patients had consistently low levels of IFN, despite changing diagnostic categories over a mean followup period of 6 years (1 changed from asymptomatic to SS, 1 changed from asymptomatic to pauci-SLE, and 1 changed from pauci-SS to SS). Of the 24 mothers with longitudinal samples whose diagnosis remained stable, 15 (63%) had equivalent type I IFN expression status over the followup period. Seven mothers with stable diagnoses (3 with SLE, 2 with SS, and 2 with pauci-SLE) changed from having low IFN expression to having high IFN expression during followup, and 2 mothers with stable diagnoses (1 asymptomatic and 1 with SS) changed from high to low IFN expression during followup.
Discussion
The finding that asymptomatic individuals with high-titer anti-SSA/Ro autoantibodies did not have high levels of serum type I IFN is intriguing. In vitro data demonstrating that sera containing these autoantibodies can promote type I IFN generation (6) led us to predict that most of the anti-SSA/Ro–positive mothers would have high type I IFN activity. However, this study suggests that anti-SSA/Ro antibodies alone are not sufficient for high levels of type I IFN in vivo, and that other factors related to the background disease processes in SLE and SS are important. Previous work by our group has demonstrated a heritable tendency toward high serum levels of type I IFN in both healthy and affected members of SLE families (6). A genetic susceptibility to high type I IFN expression may be required for a given individual to demonstrate high serum type I IFN activity in the presence of anti-SSA/Ro antibodies.
The frequent finding of high serum levels of type I IFN in patients with SS is interesting, because a previous study detected high serum levels of IFNα (using an ELISA) in only 3 of 38 patients with SS, despite finding increased type I IFN–induced gene expression in salivary glands (2). In previous work by our group, the functional assay used in this study was able to detect high serum levels of type I IFN in 10 of 17 patients with SS (14), and polyclonal anti-IFNα antibodies completely abrogated the type I IFN activity in these samples, suggesting that IFNα was the major type I IFN in the SS sera.
In this study, the patients with SLE by definition met more criteria for SLE than did the patients with pauci-SLE. However, there was no significant difference in type I IFN activity between these 2 groups, and there was a trend toward higher IFN activity in the pauci-SLE group. Although this observation is unexplained, it is possible that high serum levels of type I IFN are present early in SLE disease or may even precede disease in patients with SLE. In a microarray study, 29 (97%) of 30 pediatric patients with newly diagnosed SLE had evidence of type I IFN pathway activation (1), which exceeds the usual proportion of 50% of patients with high IFN activity seen in adult SLE populations (4,6). In some patients with pauci-SLE, SLE will develop at a later date (7), and high serum type I IFN activity could be a characteristic of early or developing SLE.
Interestingly, the patients with SS in the present study also had type I IFN activity very similar to that in the SLE and pauci-SLE groups. High serum levels of type I IFN may be a feature common to certain autoimmune disorders such as SLE and SS. Although anti-SSA/Ro and anti-SSB/La autoantibodies are common in both SLE and SS and may contribute to high serum type I IFN activity, this study demonstrates that disease-specific factors are also important. These factors are currently unknown, but genetic differences in the sensitivity of the type I IFN system (6), the presence or absence of HMGB-1 in the microenvironment resulting in differential responses to cellular debris (15), endogenous viruses, or other disease-specific inflammatory stimuli may have contributed to the large difference in serum type I IFN activity between symptomatic and asymptomatic individuals in this study. Although the longitudinal data are preliminary and inconclusive, extension of this study will determine whether serum IFN activity could predict disease progression in mothers of children with NLE.
Acknowledgments
Dr. Niewold's work was supported by the NIH (grant T32-AR-07517 and National Institute of Allergy and Infectious Diseases Clinical Research Loan Repayment grant AI-071651); he is also recipient of an Arthritis Foundation Post-Doctoral Fellowship. Dr. Buyon's work was supported by the Research Registry for Neonatal Lupus (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant N01-AR-42271) and a research grant from the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery. Dr. Crow's work was supported by research grants from the NIH (R01-AI-59893), the Alliance for Lupus Research, the Lupus Research Institute, and the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery.
Footnotes
Dr. Crow has applied for a patent for an interferon assay.
Author Contributions: Dr. Niewold had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Niewold, Rivera, Buyon, Crow.
Acquisition of data. Niewold, Rivera, Buyon.
Analysis and interpretation of data. Niewold, Rivera, Buyon, Crow.
Manuscript preparation. Niewold, Rivera, Buyon, Crow.
Statistical analysis. Niewold, Rivera, Buyon.
References
- 1.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, Eloranta ML, et al. Activation of the type I interferon system in primary Sjögren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–95. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 3.Kim T, Kanayama Y, Negoro N, Okamura M, Takeda T, Inoue T. Serum levels of interferons in patients with systemic lupus erythematosus. Clin Exp Immunol. 1987;70:562–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 5.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren's syndrome [published erratum appears in Proc Natl Acad Sci U S A 2006;103:5242] Proc Natl Acad Sci U S A. 2006;103:2770–5. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 8.Buyon JP. Neonatal lupus syndromes. 4th. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 9.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 10.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. European Study Group on Classification Criteria for Sjögren's Syndrome. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 12.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti–RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–16. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 13.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, et al. Coordinate overexpression of interferon-α–induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–67. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 14.Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-α pathway activation in patients with Sjögren's syndrome treated with etanercept. Arthritis Rheum. 2007;56:3995–4005. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]