Abstract
Purpose
Chick eyes compensate for defocus imposed by positive or negative spectacle lenses. Glucagon may signal the sign of defocus. Do insulin (or IGF-1) and glucagon act oppositely in controlling eye growth, as they do in metabolic pathways and in control of retinal neurogenesis?
Methods
Chicks, wearing either lenses or diffusers or neither over both eyes, were injected with glucagon, a glucagon antagonist, insulin, or IGF-1 in one eye (saline in other eye). Alternatively, chicks without lenses received insulin plus glucagon in one eye, and either glucagon or insulin in the fellow eye. Ocular dimensions, refractive errors and glycosaminoglycan synthesis were measured over 2-4 days.
Results
Glucagon attenuated the myopic response to negative lenses or diffusers by slowing ocular elongation and thickening the choroid; in contrast, with positive lenses, it increased ocular elongation to normal levels and reduced choroidal thickening, as did a glucagon antagonist. Insulin prevented the hyperopic response to positive lenses by speeding ocular elongation and thinning the choroid. In eyes without lenses, both insulin and IGF-1 speeded, and glucagon slowed, ocular elongation, but either glucagon or insulin increased the rate of thickening of the crystalline lens. When injected together, insulin blocked choroidal thickening by glucagon, at a dose that did not, by itself, thin the choroid.
Conclusions
Glucagon and insulin (or IGF-1) cause generally opposite modulations of eye-growth, with glucagon mostly increasing choroidal thickness and insulin mostly increasing ocular elongation. These effects are mutually inhibitory and depend on the visual input.
Keywords: emmetropization; glucagon; insulin; myopia; hyperopia; choroid; IGF, insulin-like growth factor
Introduction
Interest in the control of eye growth has been mobilized in recent years by two factors: the increasing prevalence of myopia among the educated and the evidence from animal research that eyes use defocus to modulate their rate of elongation, resulting in the eye-length being matched to the focal length of the optics. In every species studied, postnatal ocular growth is modulated by visual input with the result that the eye grows toward emmetropia--the condition of distant images’ being focused on the photoreceptors. When myopic defocus (image in front of the photoreceptors) is imposed by a positive lens, the rate of ocular elongation slows and the choroid thickens, thus pushing the retina forward toward the focal plane; conversely, when hyperopic defocus (image behind the photoreceptors) is imposed by a negative lens, ocular elongation is accelerated and the choroid thins, pulling the retina back toward the focal plane.1,2
One implication of this visual modulation of eye-growth is that clinical myopia may not be a disease, but a consequence of a malfunctioning of the homeostatic control of eye growth by vision. Because the eye compensates by growing in opposite directions when an image is defocused by being in front of versus behind the photoreceptors, one can look for the potential molecular signals that underlie this homeostatic regulation by examining signals that change in opposite directions when positive vs. negative lenses are worn. One such signal is glucagon.
Glucagon, which acts systemically as a hormone to increase glucose levels through gluconeogenesis and glycogenolysis, acts in the avian retina as a neuromodulator. It is a promising candidate for signaling the sign of blur for several reasons: (a) Retinal glucagonergic amacrine cells show a bi-directional response to positive and negative lenses in that expression of the immediate early gene ZENK increases after wearing positive lenses and decreases after wearing negative lenses3,4. (b) The amount of retinal proglucagon mRNA is increased after wearing positive lenses for one day, and decreased (although not significantly) after wearing negative lenses for one day.5 (c) Intravitreal injection of glucagon inhibits development toward myopia in eyes wearing diffusers6 or negative lenses7, by decreasing the rate of ocular elongation and, in eyes wearing diffusers, by increasing choroidal thickness (refs. 6 and 7 and Zhu X., et al. IOVS 2001;42:ARVO Abstract 318). (d) Treatment of eyes wearing positive lenses with a glucagon antagonist increases the rate of ocular elongation6,7 and inhibits recovery from form-deprivation myopia6. (e) In tissue culture, glucagon increases choroidal thickness and decreases scleral glycosaminoglycan (GAG) synthesis in eyecups consisting of the retinal pigment epithelium (RPE), choroid, and sclera (Zhu X., et al. IOVS 2005;46:ARVO E-Abstract 3338).
In the retina, as in systemic metabolic pathways, insulin has effects opposite to those of glucagon: Insulin increases, whereas glucagon decreases, the proliferation of neural progenitor cells at the margin of the postnatal chick retina.8,9 Form deprivation, which increases ocular elongation, also increases the rate of proliferation of these neural stem cells.8 Moreover, insulin has also been shown to modulate the production10, and, together with FGF, the regeneration of ganglion cells after toxin-induced cell loss11, and to activate a neurogenic program in Müller glia to dedifferentiate, proliferate, and generate new neurons12.
With respect to eye growth, two recent abstracts show that intravitreal injection of insulin has effects opposite to those of positive lenses on refraction and axial length (Feldkaemper M.P. et al. IOVS 2007;48:ARVO E-Abstract 5924; Zhu X., et al. IOVS 2007;48:ARVO E-Abstract 5925). In addition, the first abstract showed that insulin enhanced the effect of positive lenses on ZENK expression in chick retina.
The role of insulin in emmetropization has been the subject of some speculation, based on the notion that consumption of foods with a high glycemic index might affect sensitivity to insulin or insulin-like growth factor-1 (IGF-1), which in turn could lead to myopia.13,14 IGF-1 is a peptide hormone that promotes cell proliferation and differentiation throughout the body15 and has a high degree of homology with insulin16. The receptors for IGF-1 and insulin receptors are also similar17, resulting in insulin and IGF-1 cross-reacting with receptors for each other18.
In the present study, we ask which of the changes in ocular components that accompany emmetropization or lens-compensation are affected by glucagon and its antagonist, and by insulin and IGF-1, and whether glucagon and insulin influence each other’s actions. We found that glucagon had generally opposite effects on development toward myopia and hyperopia: As expected, it inhibited development toward myopia primarily by causing choroidal expansion and secondarily by decreasing the rate of ocular elongation; unexpectedly, it partially inhibited development toward hyperopia primarily by increasing the rate of ocular elongation and secondarily by reducing choroidal expansion. Insulin and IGF-1 acted in the opposite direction as glucagon, increasing the rate of ocular elongation and decreasing choroidal thickness in eyes wearing positive lenses, but both insulin and glucagon increased the rate of thickening of the crystalline lens. When glucagon and insulin were combined, a subthreshold dose of insulin prevented a suprathreshold dose of glucagon from thickening the choroid. Some of these results have previously been presented in a preliminary form (Zhu X., et al. IOVS 2001;42:ARVO Abstract 318; Zhu X., et al. IOVS 2007;48:ARVO E-Abstract 5925).
Materials And Methods
Animals
White Leghorn chicks were obtained from either Cornell University (Cornell K-strain; Ithaca, NY) or Truslow Farms (Hyline-W98-strain; Chestertown, MD, only groups 2 and 12, see Table 1). Chicks were housed in a heated, sound-attenuated chamber (76 × 61 cm), with a 14:10 light:dark cycle. One- or two-week-old chicks were used in experiments that lasted 2 to 4 days. Care and use of animals adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Table 1. Treatment Protocols and Mean Changes over Course of Experiment in Experimental (X) and Fellow (N) Eyes (± SEM).
Summary of protocols and results
| Exp. group | drug | dose | concentration (in vitreous) | age | lens or diffuser (both eyes) | n | expt. duration (day) | Δ Ocular length (μm) |
Δ Choroidal Thickness (μm) |
Δ Vitreous Chamber Depth (μm) |
Δ Refractive Error (D) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | N | X | N | X | N | X | N | |||||||||
| 1 | 1 | glucagon (synthetic) | 2nmol | 6 μM | 1w | diffuser | 5 | 4 | 111 ± 15* | 267 ± 34 | 175 ± 45** | -33 ± 15 | -347 ± 67** | 134 ± 50 | 0.7 ± 1.3** | -3.4 ± 0.7 |
| 2 | 2 nmol† | 6 μM | 1w | -7 D | 6 | 2 | -36 ± 53* | 57 ± 29 | 50 ± 35** | -123 ± 24 | -159 ± 50*** | 135 ± 35 | 1.2 ± 1.2* | -2.0 ± 1.2 | ||
| 3 | 2 nmol | 6 μM | 1w | -15 D | 7 | 4 | 98 ± 42 | 182 ± 39 | -7 ± 54 | -14 ± 18 | -103 ± 68* | 144 ± 39 | 0.1 ± 1.1 | 0.0 ± 1.2 | ||
| 4 | 2 nmol | 6 μM | 2w | +10 D | 5 | 3 | 131 ± 19* | 11 ± 30 | 74 ± 13 | 157 ± 70 | -108 ± 19 | -227 ± 66 | 2.9 ± 0.5 | 6.9 ± 1.7 | ||
| 5 | 2 nmol | 6 μM | 2w | +15 D | 4 | 3 | 215 ± 37 | 57 ± 35 | 141 ± 91 | 75 ± 78 | -121 ± 107 | -108 ± 67 | 3.5 ± 1.8 | 6.3 ± 2.0 | ||
| 6 | glucagon (procine) | 0.02 nmol | 0.06 μM | 1w | none | 7 | 3 | 60 ± 39* | 101 ± 31 | -91 ± 24 | -71 ± 15 | 39 ± 27 | 68 ± 21 | |||
| 7 | 0.02 nmol‡ | 0.06 μM | 1w | none | 7 | 3 | 217 ± 24 | 193 ± 17 | 34 ± 30 | 5 ± 12 | -28 ± 45 | -1 ± 22 | ||||
| 8 | 0.2 nmol | 0.6 μM | 1w | none | 5 | 3 | 5 ± 24*** | 120 ± 25 | 62 ± 42* | -46 ± 20 | -236 ± 39** | 57 ± 32 | ||||
| 9 | 2 nmol | 6 μM | 1w | none | 4 | 3 | 57 ± 34 | 86 ± 30 | 120 ± 54** | -47 ± 35 | -285 ± 51** | 40 ± 45 | ||||
| 10 | 2 nmol‡ | 6 μM | 1w | none | 5 | 3 | 220 ± 18 | 165 ± 35 | 268 ± 63** | 39 ± 32 | -351 ± 68** | -7 ± 29 | ||||
| 2 | 11 | glucagon antagonist | 26 pmol† | 1 μM | 1w | +7 D | 12 | 2 | -32 ± 21** | -91 ± 18 | 136 ± 16* | 92 ± 12 | -229 ± 19 | -238 ± 27 | 6.1 ± 0.6 | 6.2 ± 0.8 |
| 12 | 26 pmol | 1 μM | 1w | +15 D | 4 | 4 | 36 ± 19** | -120 ± 34 | 154 ± 79 | 196 ± 46 | -219 ± 96* | -397 ± 56 | 10.7 ± 1.3 | 13.2 ± 1.4 | ||
| 13 | 26 pmol | 1 μM | 1w | none | 6 | 2 | 120 ± 61 | 45 ± 24 | -76 ± 23 | -89 ± 12 | 31 ± 90 | 48 ± 18 | -0.6 ± 0.5 | 0.0 ± 0.6 | ||
| 14 | 305 pmol | 11 μM | 2w | +10 D | 6 | 2 | 119 ± 37** | -37 ± 15 | 160 ± 31 | 88 ± 40 | -127 ± 37 | -141 ± 42 | 4.6 ± 0.8 | 5.7 ± 0.6 | ||
| 15 | 305 pmol | 11 μM | 2w | +15 D | 3 | 4 | 356 ± 41* | 51 ± 28 | 173 ± 65 | 208 ± 58 | -58 ± 41 | -250 ± 77 | 4.4 ± 1.2 | 9.9 ± 1.1 | ||
| 16 | 305 pmol§ | 11 μM | 2w | +10 D | 5 | 2 | 60 ± 40 | 20 ± 23 | 64 ± 58 | 43 ± 31 | -58 ± 44 | -68 ± 33 | 3.7 ± 1.1 | 3.9 ± 0.8 | ||
| 17 | 305 pmol§ | 11 μM | 2w | +15 D | 3 | 2 | 146 ± 32 | 10 ± 40 | -143 ± 32** | 157 ± 35 | 183 ± 61* | -200 ± 28 | -1.1 ± 1.7 | 4.7 ± 0.5 | ||
| 3 | 18 | insuline | 0.001 U (6.8 pmol) | 0.02 μM | 1w | +10 D | 4 | 3 | -32 ± 20 | -104 ± 13 | -53 ± 18* | 73 ± 33 | -90 ± 20* | -254 ± 26 | ||
| 19 | 0.01 U (68 pmol) | 0.2 μM | 1w | +10 D | 8 | 3 | 382 ± 96** | 49 ± 33 | -27 ± 36 | -3 ± 43 | 103 ± 78 | -20 ± 52 | ||||
| 20 | 0.1 U (680 pmol) | 2.4 μM | 1w | +10 D | 9 | 3 | 352 ± 94** | -104 ± 24 | -75 ± 27* | 43 ± 36 | 216 ± 70*** | -175 ± 41 | ||||
| 21 | 0.001 U (6.8 pmol) | 0.02 μM | 1w | none | 5 | 3 | 173 ± 41 | 93 ± 86 | -4 ± 10 | -47 ± 21 | -11 ± 38 | 30 ± 54 | ||||
| 22 | 0.01 U (68 pmol) | 0.2 μM | 1w | none | 5 | 3 | 379 ± 96* | 57 ± 16 | -13 ± 34 | -23 ± 30 | 101 ± 82 | 9 ± 28 | ||||
| 23 | 0.1 U (680 pmol)‡# | 2.4 μM | 1w | none | 5 | 3 | 661 ± 28*** | 230 ± 28 | 21 ± 49 | -30 ± 14 | 231 ± 57** | 5 ± 33 | ||||
| 4 | 24 | IGF-1 | 0.04 pmol‡ | 0.1 nM | 1w | none | 8 | 3 | 216 ± 20 | 207 ± 22 | -5 ± 19 | 40 ± 17 | 42 ± 23* | -7 ± 22 | ||
| 25 | 0.4 pmol‡ | 1 nM | 1w | none | 5 | 3 | 195 ± 28 | 156 ± 18 | -2 ± 10 | -46 ± 19 | 8 ± 24 | 18 ± 15 | ||||
| 26 | 15 pmol‡ | 56 nM | 1w | none | 8 | 3 | 579 ± 54*** | 209 ± 32 | 154 ± 44* | 30 ± 11 | 41 ± 48** | -6 ± 24 | ||||
| 5 | X: glucagon plus | 0.02 nmol‡ | 0.06 μM | |||||||||||||
| 27 | insulin | 0.01 U (68 pmol)‡ | 0.2 μM | 1w | none | 8 | 3 | 569 ± 66 | 495 ± 60 | 88 ± 41 | 53 ± 41 | 60 ± 60 | 67 ± 41 | |||
| N: insulin | 0.01 U (68 pmol)‡ | 0.2 μM | ||||||||||||||
| X: glucagon plus | 2 nmol | 6 μM | ||||||||||||||
| 28 | insulin | 0.01 U (68 pmol) | 0.2 μM | 1w | none | 7 | 3 | 207 ± 34*** | 73 ± 22 | -11 ± 22* | 148 ± 48 | 53 ± 33* | 287 ± 55 | |||
| N: glucagon | 2 nmol | 6 μM | ||||||||||||||
| X: glucagon plus | 2 nmol | 6 μM | ||||||||||||||
| 29 | insulin | 0.01 U (68 pmol) | 0.2 μM | 1w | none | 5 | 3 | 280 ± 46* | 481 ± 57 | -3 ± 66 | -40 ± 16 | -68 ± 80** | 207 ± 49 | |||
| N: insulin | 0.01 U (68 pmol) | 0.2 μM | ||||||||||||||
For Experiments 1-4, drugs listed were injected in the experimental (X) eyes, and PBS was injected into the fellow (N) eyes, except for group 23 in Expt. 3, in which heat-denatured insulin was injected in the fellow eyes. The concentration in the syringe was 13 times the concentration in vitreous chamber, except for the following groups: Groups 7 and 24-27, in which the concentration in the syringe was 130 times the concentration in vitreous chamber, and groups 10 and 23, in which the concentration in the syringe was 35 and 62 times the concentration in vitreous chamber, respectively.
p < 0.05
p < 0.01
p < 0.001 (paired, 1-tailed Student t-tests, unless specified otherwise).
injected once at the beginning of the experiment
injections with 35-gauge needle
chicks wore positive lenses for 2 days prior to the injections
heat-denatured insulin injected into the fellow eyes.
Lenses and diffusers
We used PMMA plastic lenses (12 mm diameter and with a back optic radius of 7 mm) or glass lenses (not conspicuously curved) of +15, +10, +8, +7, -7, and -15 D, and we used white, plastic diffusers (with a narrow slit in front so that chicks could locate food and water). In all experiments, chicks either wore diffusers or lenses binocularly or had no devices on their eyes. Each lens or diffuser was glued between a rigid plastic ring and a Velcro ring and attached to a mating Velcro ring glued to the feathers around the chicks’ eyes. Lenses were cleaned at least twice a day.
Measurements
Measurements of refractive error and ocular dimensions were conducted with chicks anaesthetized with 1.5% of isoflurane. Refractive error was measured using a modified Hartinger refractometer.19 A-scan ultrasonography was used to measure internal ocular dimensions.20 Ocular length was defined as the sum of anterior chamber depth, lens thickness, vitreous chamber depth, and the thicknesses of the retina, choroid, and sclera. This measurement, like measurement by calipers, is different from “axial length” as used clinically--the distance from the anterior surface of the cornea to that of the retina--which is influenced by choroidal thickness as well as ocular length.
Intravitreal injection
Intravitreal injections were made daily (unless specified otherwise), immediately after each ultrasound measurement while the chicks were under anesthesia and the lenses or diffusers removed. The injections were made through the conjunctiva with an entry site at 12 o’clock about 3 mm above the corneal limbus. The needle was pointed toward the posterior pole at about 45 degrees to avoid damaging the lens; eyes that developed cataracts or vitreous infections were not used. We either injected 20 μL through a 26-gauge needle (Hamilton Company, Reno, NV) or 2 to 7.4 μL through a 35-gauge needle (NanoFil syringe, World Precision Instruments, Inc., Sarasota, FL); see Table 1 for details. The smaller needle and volume eliminated a growth-retarding effect we observed with the larger needle and volume.
Measurement of Proteoglycan (GAG) Synthesis
Since the rate of synthesis of glycosaminoglycans (GAGs) in the extracellular matrix of the sclera and choroid has been shown to parallel the changes in the rate of ocular elongation21 and in choroidal thickness22, respectively, we measured these parameters as indicators of the remodeling of the sclera and choroid. For groups 6, 7, 9 and 10 in Experiment 1, and all groups in Experiments 3 through 5, at the end of each experiment, the newly synthesized choroidal and scleral GAGs were measured as previously described.23 In brief, 7-mm choroidal and scleral punches were made from the posterior pole and kept in CO2-independent Medium (Gibco, Carlsbad, CA) on ice for 1.5-2 hr until incubation for 3 hr at 37°C in L-15 medium (Millipore Corporation, Phillipsburg, NJ) with radioactive Na2 35SO4(40 μCi/ml). The tissues were collected and cultured under the same conditions for all these experiments. To assay the newly synthesized GAGs, the tissues were digested overnight at 57°C with proteinase K (protease type XXVIII, Sigma-Aldrich, St. Louis, MO), and centrifuged at 13,000 rpm for 15 min. GAGs were precipitated overnight with cetylpyridinium chloride (Sigma-Aldrich) and unlabeled chondroitin sulfate. The precipitate was captured on Whatman GF/F filters, dried overnight and measured in a liquid scintillation counter. Because the cartilaginous layer of the chick sclera incorporates thirty-times more sulfate than the fibrous sclera, as shown when the layers are incubated separately23,24, this measurement is dominated by the GAG synthesis of the cartilaginous sclera.
Protocols
Treatment protocols are summarized in Table 1.
Pharmacological agents
For glucagon injections, synthetic glucagon (90% purity; Sigma) was used in groups 1 through 5, glucagon extracted from porcine pancreas (80% purity, Sigma) was used in groups 6-10 and 27-29. (Glucagon from both sources has the same chemical structure.) A glucagon antagonist (des-His1-(Glu9)-glucagon-amide, purity 97%, Sigma) was used in Exp. 2. For insulin injections, insulin isolated from bovine pancreas (93% purity, 27 USP units/mg, Sigma) was used. For IGF-1 injections, IGF-1 (human recombinant, 97% purity, Sigma) was used in Exp. 4. The total dose and the concentration in the vitreous chamber (correcting for percentage purity) of each drug injected are given in Table 1.*
To control for effects of insulin not due to its biological activity, some chicks had native insulin injected daily in the experimental eyes, and the same concentration and volume of heat-denatured insulin injected in the fellow eyes for 3 days (group 23, Table 1). The insulin was denatured by being maintained at 65°C for 4 hr before injection. Because denaturation usually makes proteins more easily precipitatable, we assessed the degree of denaturation by centrifugation at 5000 g for 10 min and comparing the absorbance of the post-heating supernatant at 280 nm with that of the unheated insulin solution. We found that the absorbance was reduced from 0.92 to 0.11 by denaturation, suggesting that approximately 88% had been denatured.
In Experiments 1 to 4, drugs (in 2 to 20 μL of solution) and PBS were injected daily (unless specified otherwise) into experimental and fellow eyes, respectively. In Experiment 5, glucagon plus insulin were injected in one eye, with the same dose of either glucagon or insulin injected in the other eye. Ocular dimensions were measured with ultrasound daily (except for group 4 in which the birds were not measured on day 2) during each experiment, as was refractive error in groups 1-5 and 11-17. At the end of the experiment, the synthesis of choroidal and scleral GAGs was measured (except for groups 1-5 and 8 in Exp. 1, and those in Exp. 2). Unless specified otherwise, injections were 20 μL made with a 26-gauge needle.
Experiment 1. Glucagon injections
Chicks, wearing either diffusers or spectacle lenses over both eyes or with no devices, had glucagon injected into one eye (PBS into the fellow eye) daily, except for group 2, which was injected only once (see Table 1 for details). Three doses of glucagon (0.02, 0.2, and 2 nmol) were injected with either a 26-gauge needle or a 35-gauge needle (groups 7 and 10).
Experiment 2. Glucagon antagonist injections
Chicks, wearing either positive lenses over both eyes or no lenses, had the glucagon antagonist, des-His1-(Glu9)-glucagon-amide, injected into one eye daily (same volume of PBS into the fellow eye), except for group 11, which was injected only once (see Table 1). To study the effect of the glucagon antagonist on the maintenance of thickening in an already thickened choroid, two groups of birds wore binocular either +10 or +15 D lenses for 2 days before the injection of the glucagon antagonist started (groups 16 and 17).
Experiment 3. Insulin injections
To study the effect of insulin on normal eye growth or on hyperopia caused by positive lens-wear, chicks, either wearing no lenses or binocular +10 D lenses, had insulin injected daily in the experimental eyes with a 26-gauge needle, and the same volume of PBS in the fellow eyes for 3 days. The doses of insulin were chosen to ensure maximal binding of insulin to its receptors in chick retina (IC50 = 0.4 - 0.7 nM26). To control for non-biological effects of insulin, in another group, active and heat-denatured insulin of the same concentration and volume were injected in the experimental and fellow eyes, respectively, with a 35-gauge needle (group 23).
Experiment 4. IGF-1 injections
To test the effect of IGF-1 on normal eye growth, chicks without any devices had IGF-1 injected daily in the experimental eyes and the same volume of PBS in the fellow eyes for 3 days with a 35-gauge needle. We chose doses of IGF-1 so that the vitreous chamber concentration, 56 nM or 469 ng/ml, would be within the physiological range of concentrations in rat plasma27 and ensure maximal binding to the IGF-1 receptor (half-maximal binding at 3.5 nM in chick retina26).
Experiment 5. Interaction of glucagon and insulin effects
To study whether glucagon and insulin oppose each other’s effects or simply act in opposite directions, we took advantage of the fact that 2 nmol of glucagon has no effect on ocular elongation, but causes choroidal expansion, whereas 68 pmol of insulin has no effect on the choroid, but strongly stimulates ocular elongation. Thus we injected glucagon (0.02 or 2 nmol) and insulin (68 pmol or 0.01 U) into one eye, either sequentially (10 min apart, group 27, since the order of injection did not make a difference, the data from these birds were pooled) or simultaneously (groups 28-29), and the same amount of either insulin or glucagon alone into the fellow eye on the same schedule.
Statistics
We analyzed our results in four ways unless otherwise specified: (1) We presented measured values (of ocular parameters) each day in the experimental and fellow eyes in line-graphs, and compared the interocular difference (the difference of the measured values between paired eyes, X-N) within a group on different days with respect to day 0 using Analysis of Variance (ANOVA) with Bonferroni post hoc tests. (2) We presented the change in the experimental and fellow eyes over the course of the experiment (ΔX and ΔN) in bar-graphs, and used paired, Student t-tests to compare changes in paired eyes. (3) We analyzed the rate of GAG synthesis by dividing the values from the scintillation counter for the drug-treated eye by those for the fellow eye (X/N) and evaluated the resulting ratio by a one-sample t-test, comparing it to a value of 1. Since the counts were in a range of thousands for choroids and tens of thousands for scleras, and the counts from the background were generally within 50, the background counts were not subtracted from each sample. (4) We compared the relative change (change in the experimental eye over the course of the experiment minus the change in the fellow eye, ΔX-ΔN), by ANOVA with Bonferroni post hoc tests to compare different drug doses. All these comparisons are shown in the figures, and explained in the captions. In the text we only give the means and whether or not the comparisons were significant.
Results
In brief, we found that glucagon and insulin have opposite effects in the modulation of eye growth: Glucagon inhibited growth toward myopia by thickening the choroid and slowing ocular elongation, whereas insulin inhibited compensation for positive lenses by speeding ocular elongation and thinning the choroid. Both insulin and IGF-1 increased the rate of ocular elongation in eyes not wearing lenses. Furthermore, insulin, at a dose too low to affect choroidal thickness by itself, blocked glucagon from thickening the choroid. Changes in ocular length, choroidal thickness, vitreous chamber depth, and refractive error in experimental and fellow eyes over the course of experiments are summarized in Table 1; details of statistical tests are given in figures.
Experiment 1. Treatment with glucagon
Glucagon increased choroidal thickness and lens thickness and decreased the rate of ocular elongation in negative lens-wearing eyes, and had the opposite effects in positive lens-wearing eyes. Extracted and synthetic glucagon had similar effects.
Diffusers and negative lenses
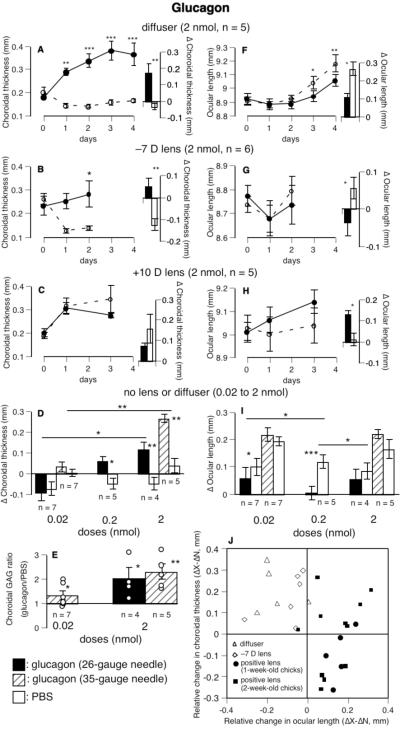
In eyes wearing diffusers, glucagon reversed the normal choroidal thinning, resulting in robust choroidal thickening in diffuser-wearing eyes even by the first day (Fig. 1A, interocular difference, p < 0.01). Glucagon also inhibited the increased ocular elongation resulting from wearing diffusers, although it acted more slowly with the effect being significant after 3 days of injections (Fig. 1F) Changes over 4 days in glucagon-injected eyes were half as great as those in fellow eyes (111 μm vs. 267 μm, glucagon vs. PBS, p < 0.05, group 1, bar chart in Fig. 1F). The dose used was approximately 9 times the EC50 reported by Vessey et al.28
FIGURE 1.
The effects of glucagon on ocular length and the thickness and GAG synthesis of the choroid. Glucagon increased choroidal thickness (A, B, D, and J) and decreased the rate of ocular elongation (F, G, I, and J) in eyes wearing diffusers, -7 D lenses, or no devices. The opposite effect was found in eyes wearing positive lenses (C, H, and J). Glucagon also increased the rate of choroidal GAG synthesis (E) in eyes without lenses or diffusers. Panel J shows that, in eyes wearing positive lenses, glucagon (2 nmol) increased the rate of ocular elongation in nearly all birds and reduced choroidal thickening in half of them. Panel J includes 7 birds (five 1-week-old and two 2-week-old) from pilot experiments not shown in Table 1. Bar-charts show the mean changes and SEMs over the course of the experiments shown in the line-graphs, in which solid and interrupted lines show the time course of the experimental and fellow eyes, respectively. For choroidal GAG synthesis, the ratios of the scintillation counts in paired eyes (glucagon/PBS) of individual chicks (circles) are superimposed on the mean (bars) and SEM of each group. Analysis of Variance (ANOVA) with Bonferroni post hoc tests were used to test the statistical significance of interocular difference (X-N) among different days within a group with respect to day 0 (asterisks on line-graphs), and of relative changes (ΔX-ΔN) among the different doses (asterisks over horizontal lines in panels D and I). For ocular length and choroidal thickness, asterisks over bars indicate paired, 1-tailed Student t-tests comparing changes in experimental and fellow eyes, except for panels C and H, in which paired, 2-tailed Student t-tests were used. For GAG synthesis, one-sample t-tests were used to compare the ratio of the counts from paired eyes (glucagon/PBS, or X/N) with a value of 1. *p < 0.05, **p < 0.01, ***p < 0.001.
In eyes wearing negative lenses, even a single injection of glucagon caused the choroids to expand significantly by 50 μm, whereas the choroids of the PBS-injected fellow eyes also wearing -7 D lenses thinned by 123 μm (bar chart in Fig. 1B, p < 0.01, group 2). It also reduced the increased ocular elongation caused by wearing -7 D lenses for 2 days (glucagon vs. PBS, -36 μm vs. 57 μm, p < 0.05, group 2, Fig. 1G). In eyes wearing -15 D lenses, four days of daily injections of glucagon caused the rate of ocular elongation to be half of that in the fellow eyes (98 μm vs. 182 μm, p > 0.05, group 3, Table 1), but without choroidal thickening. In both of these experiments, the fellow eyes grew less than would have been expected for untreated eyes, presumably because of the daily injections with a 26-gauge needle.
As a result, glucagon caused a hyperopic shift (an average of 3.7 D) in eyes wearing either diffusers or -7 D lenses, relative to the fellow eyes (groups 1 and 2, Table 1).
Furthermore, glucagon increased the rate of lens thickening 2- to 4-fold in eyes wearing diffusers or lenses (Fig. 2A; diffusers: 284 μm vs. 164 μm, p < 0.01; -7 D lenses: 140 μm vs. 77 μm, p < 0.05; -15 D lenses: 233 μm vs. 65 μm, p < 0.001).
FIGURE 2.
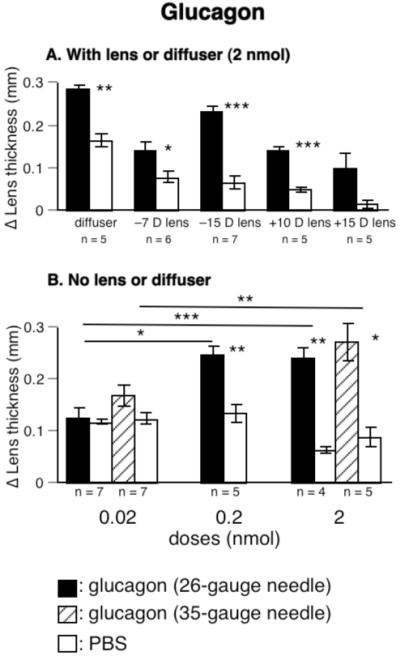
The effect of glucagon on lens thickness. A. Glucagon increased lens thickness in eyes wearing diffusers or negative lenses. B. Glucagon increased lens thickness in a dose-dependent fashion in eyes not wearing lenses. Asterisks over horizontal lines show comparisons of relative changes (ΔX-ΔN) that were significant by ANOVA with Bonferroni post hoc test. Asterisks over bars show significant difference in changes in experimental and fellow eyes from paired, 2-tailed Student t-tests.
Positive lenses
In eyes wearing positive lenses, however, glucagon (2 nmol) acted in the opposite direction: First, ocular elongation was less inhibited in the glucagon-injected eyes than in the fellow eyes in nearly all birds (increase in ocular length, for +10 D lenses: 131 μm vs. 11 μm, for +15 D lenses: 215 μm vs. 57 μm, glucagon vs. PBS, p < 0.05, One Sample Sign Test, data pooled, groups 4 and 5 in Table 1 and bar charts in Figs. 1H and 1J). Second, in eyes wearing positive lenses, the choroids were not further thickened by glucagon, whereas they were thickened by glucagon in all other groups (for relative change, glucagon with positive lenses vs. glucagon without lenses, p < 0.001, by either an unpaired, 2-tailed Student t-test or a Mann-Whitney U test, bar chart in Fig. 1C and Fig. 1J). Notice in Fig. 1J that, in contrast to the glucagon-treated eyes wearing negative lenses or diffusers, which all thickened their choroids and slowed their elongation more than their fellow eyes did, the glucagon-treated eyes wearing positive lenses nearly all increased their elongation. As a consequence, glucagon reduced the compensatory hyperopia in eyes wearing positive lenses (for birds wearing +10 D lenses, glucagon vs. PBS, 2.9 D vs. 6.9 D, p > 0.05, group 4, Table 1; for birds wearing +15 D lenses, 3.5 D vs. 6.3 D, p > 0.05, group 5).
Glucagon also increased lens thickness in eyes wearing positive lenses (changes in lens thickness for birds wearing +10 D lenses, glucagon vs. PBS,139 μm vs. 48 μm, p < 0.001, group 4; for +15 D lens-wear, 100 μm vs. 14 μm, p > 0.05, group 5, Fig. 2A).
No lenses or diffusers
In eyes without lenses or diffusers, glucagon thickened choroids in a dose-dependent fashion (Fig. 1D, groups 6-10). Because daily injections of PBS with a 26-gauge needle, but not a 35-gauge needle, thinned the choroids (26- vs. 35-gauge needles, -58 μm vs. 19 μm, pooled data from fellow eyes; p < 0.001), we repeated the highest and lowest doses (0.02 and 2 nmol) with the finer needle; in both cases the differences in the effects of these two doses of glucagon were significant (p < 0.01 for 35-gauge needle, p < 0.05 for 26-gauge needle). Glucagon also reduced the rate of ocular elongation, at least at the 0.2 nmol dose (Fig. 1I, groups 6 through 10).
Furthermore, the rate of choroidal GAG synthesis was increased by 30% after 3 days of the lowest dose of glucagon (p < 0.05, Fig. 1E) and approximately doubled at the highest dose (p < 0.05 for 26-gauge needle, p < 0.01 for 35-gauge needle).
Experiment 2. Treatment with the glucagon antagonist
The glucagon antagonist reduced development toward hyperopia by reducing the inhibitory effect of wearing positive lenses on ocular elongation.
Ocular length
The glucagon antagonist reduced, and at the higher dose, eliminated the inhibition of ocular elongation caused by wearing positive lenses (Fig. 3A, the dotted line in Fig. 3A shows the daily growth of 70 μm seen in normal eyes).
FIGURE 3.
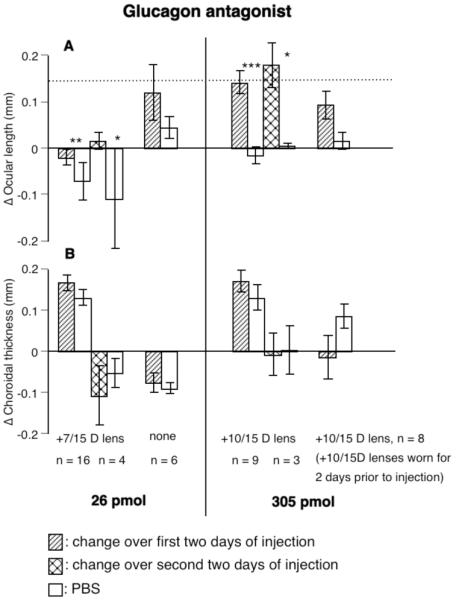
The effects of a glucagon antagonist on changes in paired eyes (ΔX and ΔN) in ocular length (A) and choroidal thickness (B). The glucagon antagonist, at the lower dose, reduced the inhibition of ocular elongation caused by positive lenses and, at the higher dose, returned the rate of ocular elongation to normal or above (dotted line is normal elongation over 2 days without injections). The glucagon antagonist tended to reduced choroidal thickness only when the chicks had worn positive lenses for 2 days prior to the start of the injections (B, right-most bar). Statistics as in Fig. 1. Left-hand bars of each pair (change over first two days) are data pooled from eyes wearing +7 or +15 D lenses and injected with 26 pmol (groups 11 & 12) or from eyes wearing +10 or +15 D lenses and injected with 305 pmol (groups 14 & 15). The right-most bars are data pooled from eyes wearing +10 or +15 D lenses 2 days prior to the start of injections (groups 16 and 17).
Choroidal thickness
The glucagon antagonist generally had no effect on choroidal thickness, except that it blocked further thickening of the choroid in eyes that had worn either +10 or +15 D lenses for 2 days before the injections began (choroids in these eyes were already thick prior to the injection, mean, 365 μm for group 16, and 344 μm for group 17, p > 0.05, Fig. 3B).
Refractive error
Consistent with its effect on choroidal thickness, the glucagon antagonist significantly reduced development towards hyperopia in chicks injected during the 4 days of wearing positive lenses (in 6 out of 7 birds, mean difference = -3.8 D, p <0.05, paired t-test, groups 12 and 15) but not in chicks in which the injections started after 2 of the 4 days of positive lens-wear (6 out of 8 birds, mean difference = -2.3 D, p>0.05, paired t-test, groups 16 and 17).
Experiment 3. Treatment with insulin
In eyes not wearing lenses, insulin had an effect opposite to that of glucagon, in that it increased the rate of ocular elongation; and in eyes wearing +10 D lenses, it increased ocular elongation and scleral GAG synthesis and decreased choroidal thickness.
Ocular Length and scleral GAG synthesis
When combined with positive lens-wear, which normally stops the eye from elongating, insulin, at the two higher doses, caused the eye to elongate at a rate considerably greater than normal eyes (0.01 U, 382 μm vs. 49 μm, p < 0.001; 0.1 U, 352 μm vs. -104 μm, p < 0.001; if the absolute change in the experimental eyes alone was used, the middle dose had significantly greater effect than the lowest dose, p < 0.05, Fig. 4A).
FIGURE 4.
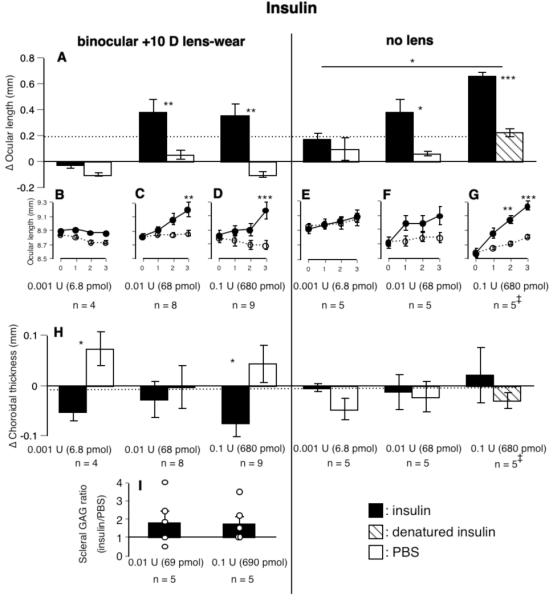
The effects of insulin on changes in paired eyes in ocular length, scleral GAG synthesis, and choroidal thickness. (B-G) Ocular length (measured values) as a function of time (in days) are shown for each dose under the bar-chart showing the changes in paired eyes over the 3-day duration of the experiments (all y-axes are identical). Insulin increased the rate of ocular elongation in eyes wearing positive lenses (panels on left) and in eyes not wearing lenses (panels on right), as well as increasing scleral GAG synthesis in eyes wearing positive lenses (I). (H) Insulin also reduced choroidal thickness in eyes wearing positive lenses. Statistics as in Fig. 1. The dotted line shows the normal rate of ocular elongation (A) and choroidal thickness (H) over 3 days without injections. ‡: injections with a 35-gauge needle.
In keeping with this stimulatory effect on ocular elongation, insulin also increased scleral GAG synthesis by about 100% in eyes wearing positive lenses (Fig. 4I).
In eyes not wearing positive lenses, insulin caused a 2- to 6-fold increased rate of elongation (0.001 U, 173 μm vs. 93 μm; 0.01 U, 379 μm vs. 57 μm, p < 0.05; 0.1 U, 661 μm vs. 230 μm, p < 0.001, Fig. 4A, groups 21-23). Among the doses tested, the highest dose showed a greater effect than the lowest dose (relative change, p < 0.05; if the absolute change in the experimental eyes alone was used, the middle dose also had significantly greater effect than the lowest dose, p < 0.05). Injecting the fellow eye (with a 35-gauge needle) with denatured insulin instead of PBS did not cause any obvious increase in ocular elongation in the fellow eye (Fig. 4A, right-most bar).
Choroidal Thickness
Because acceleration of ocular elongation is nearly always associated with choroidal thinning29, it is in keeping with the stimulatory effect of insulin on ocular elongation that insulin reversed the choroidal thickening in eyes wearing positive lenses (0.001 U, experimental eye vs. fellow eye: -53 μm vs. 73 μm, p < 0.05; for 0.1 U, -75 μm vs. 43 μm, p < 0.05, Fig. 4H). Insulin had no effect on choroidal thickness of eyes that were not wearing lenses (Fig. 4H).
Anterior chamber depth
Insulin increased anterior chamber depth significantly in eyes both with and without lenses (Fig. 5A). In eyes not wearing lenses, this stimulation was clearly dose-dependent, in that at 0.1 U, insulin increased anterior chamber depth significantly more than at 0.001 U (p < 0.05 for relative change; if the absolute change in the experimental eye alone was used, the highest dose of insulin also increased anterior chamber depth significantly more than at 0.01 U, p < 0.001). At the two higher doses, the anterior chambers of the eyes injected with insulin were deeper than the fellow eyes (for 0.01 U: 52 μm vs. -53 μm, p > 0.05; for 0.1 U: 273 μm vs. 39 μm, p < 0.001). At the highest dose, insulin caused the anterior chamber to deepen by 23%, whereas the ocular length increased by only 8%. In eyes wearing positive lenses, the anterior chambers were 8-10% deeper at the two higher doses (0.01 U: 100 μm vs. 11 μm; 0.1 U: 126 μm vs. -3 μm; p < 0.05 for both concentrations, Fig. 5A), whereas the eyes were only 4% longer. The responses were not dose-dependent. This increase in anterior chamber depth is much too small to account for the increased ocular length caused by insulin (Figs. 4A and 5A).
FIGURE 5.
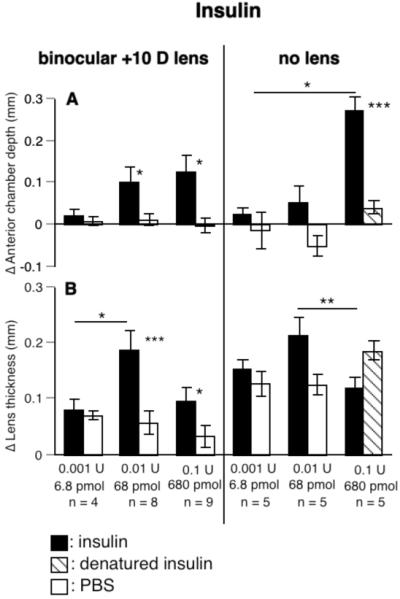
The effects of insulin on anterior chamber depth (A) and lens thickness (B). Insulin increased anterior chamber depth (A) and lens thickness (B) in a dose-dependent fashion. Statistics as in Fig. 2.
Lens thickness
Insulin also increased lens thickness, at least in eyes wearing positive lenses (changes in lens thickness of eyes wearing positive lenses, for 0.01 U: 188 μm vs. 57 μm, p < 0.001; for 0.1 U: 96 μm vs. 32 μm, p < 0.05; changes in eyes without lenses, 0.01 U: 214 μm vs. 125 μm, p > 0.05, Fig. 5B). This effect seemed to be maximal at the intermediate dose tested, significantly more than either 0.001 U for eyes wearing positive lenses (p < 0.05) or 0.1 U for eyes without lenses (p < 0.01).
Experiment 4. Treatment with IGF-1
Similar to insulin, IGF-1 also increased the rate of ocular elongation and the anterior chamber depth. Unlike choroids in insulin-injected eyes, choroids in the IGF-1-injected eyes thickened.
Ocular length
At the highest dose tested (15 pmol), IGF-1 caused a 3-fold increase in the rate of ocular elongation (IGF-1 vs. PBS, 579 μm vs. 209 μm, p < 0.001, group 26, Fig. 6A), significantly more than at 2 lower doses (0.04 and 0.4 pmol, p < 0.001).
FIGURE 6.
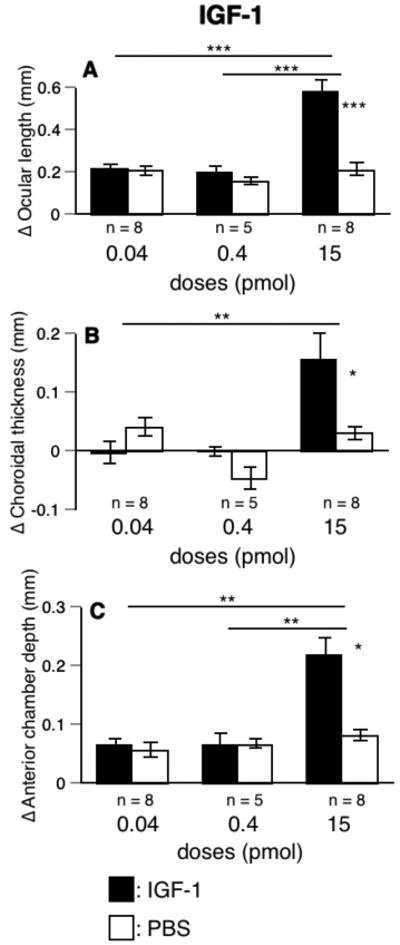
The effects of IGF-1 on changes in ocular length, choroidal thickness, and anterior chamber depth. IGF-1 at the highest dose increased the rate of ocular elongation (A), choroidal thickness (B), and anterior chamber depth (C) in eyes not wearing lenses. Statistics as in Figs. 1 and 2.
Choroidal thickness
In contrast to choroids in eyes injected with insulin, the choroids in eyes injected with the highest dose of IGF-1 thickened significantly more than the choroids in the PBS-injected eyes (154 μm vs. 30 μm, p < 0.05, Fig. 6B), significantly more than at the lowest dose (0.04 pmol, p < 0.01).
Anterior chamber depth
At the highest dose, IGF-1 increased anterior chamber depth (15 pmol, IGF-1 vs. PBS: 219 μm vs. 81 μm, p < 0.05, Fig. 6C), significantly more than at the two lower doses (14.7 pmol vs. 0.04 or 0.4 pmol, p < 0.01). The highest dose of IGF-1 caused the anterior chamber depth to deepen by 11%, whereas the ocular length increased by only 6%. Again, the increase in anterior chamber depth is much too small to account for the increase in ocular length caused by IGF-1 (Figs. 6A and 6C).
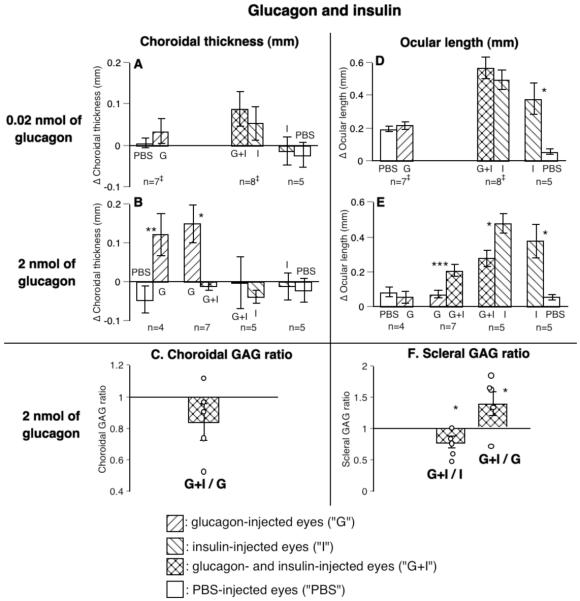
Experiment 5. The interaction between glucagon and insulin
We were interested in whether or not glucagon and insulin act independently. Thus we injected glucagon (at a dose that did not reduce the rate of ocular elongation, Fig. 1I) together with insulin (at a dose that increased the rate of ocular elongation, Figs. 4A and 4H) into one eye (considered as the experimental eye, X) and the same dose of either glucagon or insulin alone into the fellow eye (N, groups 27-29, Table 1). Thus we had three groups of birds with the combined injections, with the fellow eye receiving either glucagon or insulin. If their combined effects were the resultant of their separate effects, it would argue that glucagon and insulin act independently; if one drug, at a subthreshold level, inhibited the effect of the other one, it would argue that it acts through the pathway by which the effect of the other one is mediated.
Although insulin by itself had no effect on choroidal thickness in eyes not wearing lenses, it completely inhibited the strong effect of glucagon. Conversely, the same dose of glucagon, which by itself had no effect on ocular length, reduced the strong stimulatory effect of insulin.
Choroidal thickness and GAG synthesis
The most striking result of injecting glucagon and insulin in the same eyes was that although insulin (at 0.01 U, 68 pmol) alone did not reduce choroidal thickness, when injected with glucagon, insulin completely blocked the choroidal thickening effect of glucagon at 2 nmol (combination vs. glucagon: -11 μm vs. 148 μm, p < 0.05, group 28; combination vs. insulin: -3 μm vs. -40 μm, p > 0.05, group 29, Fig. 7B). Because the lower dose of glucagon (0.02 nmol) did not thicken the choroid, insulin could not reduce its effect (group 27, Fig. 7A).
FIGURE 7.
The interaction between glucagon- and insulin-dependent mechanisms on choroidal thickness (A, B), choroidal GAG synthesis (C), ocular length (D, E), and scleral GAG synthesis (F). Although insulin alone (at 0.01 U, 68 pmol) did not reduce choroidal thickness, it completely blocked the increase in both choroidal thickness (B: compare G vs. G+I) and choroidal GAG synthesis (C) caused by glucagon. Conversely, although glucagon by itself did not affect normal ocular elongation at either dose used, at the high dose it reduced the effect of insulin on the rate of ocular elongation by half (E: compare G+I vs. I). Glucagon also decreased the effect of insulin the rate of scleral GAG synthesis (F: compare G+I vs. I). Statistics as in Fig. 1. ‡: eyes injected with a 35-gauge needle.
Insulin also reduced by 15% the stimulatory effect of glucagon on the rate of choroidal GAG synthesis in 4 out of 5 birds, compared to the effect in eyes injected with glucagon alone (p > 0.05, Fig. 7C).
Ocular length and scleral GAG synthesis
Glucagon, at a dose that did not affect normal eye growth (2 nmol), decreased the stimulatory effect of insulin on the rate of ocular elongation by half (glucagon alone vs. combination: 73 μm vs. 207 μm, p < 0.001, group 28; combination vs. insulin alone: 280 μm vs. 481 μm, p < 0.05, group 29, Fig. 7E). The combination of the lower dose of glucagon (0.02 nmol) and insulin caused a small increase in the rate of ocular elongation compared with that in the insulin-injected eyes (combination vs. insulin alone: 569 μm vs. 495 μm, Fig. 7D, group 27).
As mentioned above, insulin (at 0.01 U, 68 pmol) caused a doubling of scleral GAG synthesis (Fig. 4I), whereas glucagon had no effect. The combination of glucagon (2 nmol) and insulin reduced this stimulatory effect of insulin on the rate of scleral GAG synthesis in 4 out of 5 birds (mean reduction: combination/insulin, 25%, p < 0.05, left bar in Fig. 7F, group 29), but did not eliminate it, since eyes injected with both had a higher rate of scleral GAG synthesis in 4 out of 5 birds compared with that in the fellow eyes injected with glucagon alone (mean increase, combination/glucagon, 45%, p < 0.05, right bar in Fig. 7F, group 28).
Discussion
Do glucagon and insulin mimic the effects of positive and negative lenses?
A prominent feature of the control of eye growth by vision is that the control is bidirectional, in that wearing positive spectacle lenses has effects opposite to those of wearing negative lenses, relative to normal eyes. In light of the facts that the visual modulation of eye growth persists in eyes detached from the brain from optic nerve section30,31 and that retinal neurons have been identified that show opposite responses to positive and negative lenses3, it is tempting to suppose that the retina exports chemical signals that direct the choroid and sclera to change in compensatory directions. One can further suppose that, because the levels of glucagon mRNA increase with positive lenses and exogenous glucagon inhibits the response to negative lenses, perhaps increased glucagon is the signal to halt ocular elongation and to thicken the choroid, while either decreased glucagon or an increase in another molecule does the opposite. Our results from treating eyes with glucagon, a glucagon antagonist, insulin or IGF-1 partially support this simple scenario. We find, as others6,7 have, that glucagon inhibits ocular elongation and thickens the choroid, whereas insulin and IGF-1 tend to do the opposite (see summary in Table 2). However, when examined in detail, our results are not supportive of the simple notion of a STOP and a GO signal that regulates compensation for spectacle lenses.
Table 2. Summary of Drug Effects.
A table summarizing the effects of the drugs used on the rate of ocular elongation and on choroidal thickness. Bold arrows show stronger effects.
| Drugs | Devices | Ocular length | Choroidal thickness |
|---|---|---|---|
| glucagon | None | ? | |
| Diffusers | |||
| Negative lenses | |||
| Positive lenses | |||
| glucagon antagonist | none | - | |
| Positive lenses | - | ||
| insulin | None | - | |
| Positive lenses | |||
| IGF-1 | None | ||
We found that glucagon countered the changes associated with myopia from wearing negative lenses or diffusers by slowing ocular elongation and thickening the choroid; Vessey et al. had similar results6, and Feldkaemper and Schaeffel found that a glucagon agonist slowed the ocular elongation7. We, like others, found that glucagon or a glucagon agonist injected into the eye reduced the rate of ocular elongation and caused the choroid to thicken, thereby opposing the changes that makes eyes myopic as a result of form-deprivation or wearing negative lenses6,7. Conversely, in eyes wearing positive lenses, we, like others6,7, found that an antagonist to glucagon partially restored ocular elongation in the direction of normal levels. These results are consistent with an inhibitory action of glucagon on ocular growth. However, we did not find a thinning of the choroid by the antagonist, although the hyperopia caused by compensation for the positive lenses was reduced by the antagonist, as Feldkaemper and Schaeffel also found7.
More provocatively, we found that glucagon reversed its action in eyes wearing positive lenses: instead of inhibiting the rate of ocular elongation, glucagon restored the normal rate despite the positive lenses and reduced the choroidal expansion that positive lenses normally produce. (The effect on ocular elongation was also found by Feldkaemper and Schaeffel using a glucagon agonist.7) We have no persuasive argument for why this should be the case. Perhaps endogenous glucagon levels are high enough when positive lenses are worn that either the glucagon receptors reverse their action when fully occupied or the number of receptors is down-regulated or another lower affinity receptor is involved. Furthermore, at the dose (2 nmol) that strongly inhibited ocular elongation resulting from wearing diffusers or negative lenses, glucagon had little if any effect on eyes not wearing lenses or diffusers, evidence also consistent with conjecture that when endogenous glucagon (perhaps at higher levels in normal than in eyes wearing negative lenses or diffusers) is added to the exogenous glucagon, something different happens. Whatever the mechanism of action, the opposite action of glucagon in eyes wearing opposite signs of lenses suggests the possibility that the action of glucagon, rather than being a consistent “stop” signal, is modulated by the presence and sign of defocus.
When chicks wear lenses for only a fraction of the day, we find that positive lenses have a greater effect on the rate of ocular elongation than on choroidal thickness, whereas the opposite is true for negative lenses.32 Contrary to what would be expected if glucagon were acting much like a positive lens, we find that glucagon acts more on the choroid thickness of eyes wearing negative lenses or diffusers, whereas the glucagon antagonist acts more by inhibiting ocular elongation, with no effect on choroid thickness (unless the eyes had been already wearing positive lenses when the injections started). This conclusion must be taken with caution, because the pharmacology of the glucagon antagonist in the avian eye is poorly understood.
The situation is similar with respect to insulin. In birds not wearing lenses or diffusers, insulin as much as triples the rate of ocular elongation, as one would expect by a GO signal, antagonizing the effect of glucagon. (Feldkaemper et al. find a much smaller increase in axial length, probably attributable to increased depth of anterior chamber.33) However, insulin increases ocular elongation without thinning the choroid, a situation that could not be achieved by lens-wear. Only when eyes wear positive lenses, which on their own would cause choroidal thickening, does insulin thin the choroid, as well as accelerating ocular elongation. (Feldkaemper et al. find a much larger acceleration of ocular elongation with positive lenses.33)
With IGF-1, the discrepancy from expectations is even greater in that the drug increases ocular elongation together with thickening, rather than thinning, the choroid. Unfortunately, little is known about the similarity of avian physiological responses to IGF-1 and insulin.
Finally, with either insulin or IGF-1, the depth of the anterior chamber increases greatly, and with either insulin or glucagon, the lens thickens, both changes not seen with short periods of lens-wear. Feldkaemper et al. report similar findings for insulin33 and Vessey et al. report similar findings for glucagon28. The finding that glucagon and insulin both increase lens thickness also argues that the peptidergic control of lens growth may differ from that controlling ocular elongation and choroidal thickness.
All in all, although the general direction of effects of glucagon is like that of positive lenses, and the general direction of effects of insulin and IGF-1 is like that of negative lenses, there are too many differences in their actions to give one confidence that the of the various visually modulated components of eye growth is mediated by two simple signals.
Role of glucagon
The findings discussed above complicate a simple view of the role of glucagon. In particular, the finding that both glucagon and its antagonist restore normal ocular elongation in the eyes wearing positive lenses is puzzling. When we examine the responses of individual animals (Fig. 1J), we find that in the animals wearing positive lenses the glucagon-treated eye was longer than the fellow eye (which also wore a positive lens) and had a thinned choroid. Considering the individual animals, all the glucagon-treated eyes had a greater rate of ocular elongation, and half showed the usual association of decreased choroidal thickness with increased ocular elongation. A possible explanation for this dissociation is that glucagon inhibits ocular elongation only within a range of concentrations; when high doses of exogenous glucagon are added to high levels of endogenous glucagon resulting from the positive lens-wear, this may be out of the inhibitory range and therefore the action of glucagon is reversed, perhaps because desensitization of the glucagon receptors may remove a tonic inhibition of insulin action by glucagon. Our results from animals not wearing lenses hint in this direction to the extent that high levels of glucagon do not inhibit ocular elongation (Fig. 1I).
Fischer et al. have recently shown that the effect of glucagon is exerted much more on the equatorial diameter than on the ocular length.34 They suggest that glucagon secreted by two populations of neurons near the retinal margin (bullwhip and mini-bullwhip cells) acts on the adjacent RPE, choroid and sclera, all of which express glucagon receptor mRNA, thereby inhibiting growth. These findings suggest that there might be a way for the glucagon secreted by the retina to act on the choroid and sclera, despite the barrier of the RPE. The findings that killing the bullwhip and mini-bullwhip neurons exerts an effect mostly on the equatorial diameter of the eye and that giving glucagon can reverse this effect suggest that the normal action of glucagon might be at the equator, and that perhaps glucagon has privileged access to the choroid and sclera there. If the growth at the equator of the eye were isotropic, one would expect a great inhibition of equatorial growth and a small inhibition of axial growth, the latter being what we find. It may be the case that the effects we see on the choroidal thickness come about from glucagon entering the choroid at the equator of the eye and being circulated within the choroid to the posterior pole, where our measurements took place. On the other hand, this conjecture goes against our findings that myopia, localized to part of the retina, results in local expansion of the choroid35. Fischer et al. also show by in situ hybridization a higher level of the glucagon receptor in the fibrous sclera than in the cartilaginous sclera. In conditions that stimulate ocular elongation, such as by form-deprivation or negative lenses, the fibrous sclera grows less (as shown by decreased thickness36,37 and decreased GAG synthesis23). The physiological state of the fibrous sclera has been shown to modulate the cartilaginous sclera23. One is tempted to conjecture that, if a principal action of glucagon is on the sclera, it may stimulate the growth of the fibrous sclera and perhaps, according to the hypothesis of Kusakari38, inhibit transdifferentiation of fibroblasts into chondrocytes, thereby decreasing ocular elongation. The signaling pathways controlling eye growth in mammals are almost certainly different from those in chicks insofar as glucagonergic amacrine cells have not been shown to exist in the mammalian retina39.
Possible receptors mediating responses to injected insulin
Although our findings of opposite actions of insulin and glucagon are consistent with the opposite actions of insulin and glucagon in other biological contexts, it is an open question whether the insulin we injected is acting on insulin receptors rather than IGF receptors, one of which is activated by insulin, although less effectively than by IGF-1. In our results, the effective dose of IGF-1 was somewhat lower than that of insulin, especially on the ocular length, but the difference is not great enough to allow one to discriminate the receptor involved. Although the fact that insulin acts in the opposite direction as glucagon in promoting the proliferation of retinal progenitor cells8,9 originally provoked this study, we do not regard this as pointing to insulin’s being the active molecule with respect to control of eye-growth, especially because it has been shown that IGF-1 is secreted by photoreceptors and acts to increase the number of rod progenitor cells in fish40.
Although receptors for insulin and IGF-1 have been found in chick retina26 and sclera41, and proinsulin has been found in chick embryo retina42, it is not clear whether insulin synthesis occurs in the retina of post-hatching chicks.
Several lines of evidence favor IGF-I’s playing a role in the control of eye-growth. First, the level of mRNA expression for the IGF-I receptor in the RPE has been shown to be modulated in opposite directions by having chicks wear negative vs. positive lenses (Zhang Z. et al. IOVS 2007;48:ARVO E-Abstract 4417). Second, the sclera of chicks contains a layer of cartilage, and IGF-I profoundly affects the growth of cartilage, in general. In particular, IGF-I has been shown to increase proteoglycan synthesis in chick sclera (Luebke A. et al. IOVS 2003;44:ARVO E-abstract 414). Third, retinoic acid, the synthesis of which is changed in opposite directions by positive and negative lenses in both the retina and choroid, has been shown to affect proteoglycan synthesis in the chick sclera.43 In other systems, the activities of chondrocytes are modulated in opposite directions by retinoic acid and IGF-I.44 One mechanism by which retinoic acid might modulate scleral IGF-I levels is by changing the level of IGF- binding-proteins.45,46
Relationship of glucagon and insulin effects
In eyes wearing neither lenses nor diffusers, insulin increases the rate of ocular elongation, with no effect on choroidal thickness, whereas glucagon thickens the choroid with little effect on the rate of ocular elongation. One can ask, then, whether these actions are the normal functions of these two molecules, that is, acting separately with different effects. To illuminate this question, we treated eyes with combinations of the two drugs. We found that glucagon, at a dose that had no effect on ocular elongation, greatly reduced the stimulation of ocular elongation by insulin and, conversely, insulin, having no effect on choroidal thickness, completely eliminated the choroidal thickening caused by glucagon. We interpret these results as showing that the actions of glucagon and insulin are not independent, but must act together on some tissue to determine both the effects on the rate of ocular elongation and choroidal thickness. The most likely site of interaction is the retina, not only because both molecules act on retinal neurons8,9, but, more directly, because Feldkaemper et al. have shown that insulin injections block the rise in glucagon mRNA expression caused by wearing positive lenses and that they also block the rise in the fraction of glucagonergic amacrine cells that express the immediate early gene ZENK (egr-1)33. However, the interaction could as well occur at the level of the RPE, for which there is evidence of receptors for glucagon47, insulin26 and IGF-1 (ref. 40 and Zhang Z. et al. IOVS 2007;48:ARVO E-Abstract 4417), and the level of glucagon receptor mRNA is higher in the RPE than in the retina48. We have preliminary evidence that insulin has a greater effect on the choroidal thickness of in vitro eye-cups lacking the retina if the RPE is present (Sheng C.K., et al. IOVS 2008;49:ARVO E-Abstract 1734). Furthermore, expression of mRNA for glucagon receptors has been found in the choroid and sclera34,48, so these are also possible sites of interaction.
Relation of choroidal and ocular length changes
Both in normal chicks and in those wearing lenses, increased or decreased rate of elongation of the eye is associated with thinning or thickening of the choroid, respectively. Are changes in choroidal thickness and ocular elongation independent, or are they linked? On the side of independence, having chicks wear lenses briefly twice a day can affect one component without affecting the other32, and having chicks wear very weak diffusers over positive lenses can block the effect of the positive lenses on the choroidal thickening but not on the ocular length inhibition49,50. On the side of the two components being linked, a variety of manipulations that cause inhibition of ocular elongation all cause choroidal thickening, although in some cases the thickening lasts for only a few hours29, suggesting that our findings of ocular inhibition without choroidal thickening mentioned above may have been due to the choroidal thickness’s not having been measured at the right time.
Our present results, taken together, tend to support the linkage of choroidal thickening to inhibition of ocular elongation, at least in birds wearing lenses (see Table 2). Specifically, we find that treatment with glucagon causes the choroid to thicken in parallel with slowed ocular elongation when the birds wore negative lenses or diffusers. When birds wearing positive lenses were treated with the glucagon antagonist, both the inhibition of ocular elongation and the choroidal thickening were blocked, although the choroid was affected only during the second two days of treatment. As for insulin, when it was administered to eyes wearing positive lenses, it both thinned the choroids and accelerated the rate of ocular elongation, the normal linkage. However, as discussed above, when glucagon, insulin or IGF-1 was administered without lenses’ being worn, the coupling of choroidal thickness with rate of ocular elongation was lost. We interpret these findings as indicating that coupling of choroidal thickness and ocular elongation depends on the eye’s being in a state of compensating for defocus and that, under these conditions, the coupling tends to be maintained despite the effect of the drugs.
Possible mechanisms of action of glucagon and insulin
As mentioned at the start of the Discussion, the simplest view of how glucagon and insulin might control emmetropization would be that insulin stimulates the eye to elongate and the choroid to thin, thus acting like a negative lens, whereas glucagon does the reverse, slowing the elongation and causing the choroid to thicken, thus acting like a positive lens. We conclude that the situation is considerably more complex.
We conjecture that, whether glucagon and insulin act at the retina, RPE, or choroid, their end-effect is to change the physiological state of the choroid, which, in turn, modulates both choroidal thickness and scleral growth, the latter being manifested as a change in the rate of ocular elongation. The evidence in support of the choroid’s modulating the sclera comes from in vitro studies in which choroids from eyes with accelerated or decelerated rates of elongation were co-cultured with pieces of sclera from normal eyes. In this situation, the scleras increased or decreased their rate of proteoglycan synthesis, respectively, implying that the physiological state of the choroid determines the rate of growth of the sclera and, therefore, the rate of elongation of the eye23, a finding recently replicated51. Furthermore, the choroid synthesizes and releases retinoic acid, the amount of which is increased in eyes wearing positive lenses and decreased in eyes wearing negative lenses; the retinoic acid produced by choroids of eyes wearing positive lenses inhibits proteoglycan synthesis in scleral explants.43
In our view, in addition to the accepted functions of the choroid as a source of oxygen and nutrients for the outer retina, as a lymphatic drainage for the eye and as an effector to move the retina forward and back toward the focal plane, the choroid also acts as a secretory tissue modulating the growth of the sclera and hence of the eye in response to signals from the retina, two of which are likely to be glucagon and insulin or IGF-1.
Acknowledgments
Supported by National Institute of Health Grants EY-02727 and RR-03060 Commercial relationships policy: N.
Footnotes
The volume of the vitreous chamber was estimated to be 260 μL, assuming the vitreous chamber to be a segment of a sphere with a diameter of 13 mm and a height of 4 mm.25
References
- 1.Wildsoet CF. Active emmetropization--evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–90. [PubMed] [Google Scholar]
- 2.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999;2:706–12. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- 4.Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthal Vis Sci. 2002;43:246–52. [PubMed] [Google Scholar]
- 5.Feldkaemper MP, Wang HY, Schaeffel F. Changes in retinal and choroidal gene expression during development of refractive errors in chicks. Invest Ophthal Vis Sci. 2000;41:1623–8. [PubMed] [Google Scholar]
- 6.Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthal Vis Sci. 2005;46:3922–31. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19:755–66. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- 8.Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- 9.Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J Neurosci. 2005;25:10157–66. doi: 10.1523/JNEUROSCI.3247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–91. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- 11.Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251:367–79. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- 12.Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J Neurosci. 2002;22:9387–98. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordain L, Eaton SB, Brand Miller J, Lindeberg S, Jensen C. An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta Ophthalmol Scand. 2002;80:125–35. doi: 10.1034/j.1600-0420.2002.800203.x. [DOI] [PubMed] [Google Scholar]
- 14.Cordain L, Eades MR, Eades MD. Hyperinsulinemic diseases of civilization: more than just Syndrome X. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:95–112. doi: 10.1016/s1095-6433(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 15.Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-I and central nervous system development. Horm Metab Res. 1999;31:120–5. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- 16.Rechler M, Nissley S. Insulin-like growth factors. In: Sport M, Roberts A, editors. Handbook of experimental pharmacology. Springer-Verlag; Heidelberg: 1990. pp. 263–367. [Google Scholar]
- 17.Chernausek SD, Jacobs S, Van Wyk JJ. Structural similarities between human receptors for somatomedin C and insulin: analysis by affinity labeling. Biochemistry. 1981;20:7345–50. doi: 10.1021/bi00529a004. [DOI] [PubMed] [Google Scholar]
- 18.Van Wyk JJ, Smith EP. Insulin-like growth factors and skeletal growth: possibilities for therapeutic interventions. J Clin Endocrinol Metab. 1999;84:4349–54. doi: 10.1210/jcem.84.12.6201. [DOI] [PubMed] [Google Scholar]
- 19.Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–63. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 20.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–81. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 21.Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11:767–82. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- 22.Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16:320–6. doi: 10.1076/ceyr.16.4.320.10697. published erratum appears in Curr Eye Res 1997 Jun;16(6):624-5. [DOI] [PubMed] [Google Scholar]
- 23.Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthal Vis Sci. 1997;38:1726–39. [PubMed] [Google Scholar]
- 24.Gentle A, Truong HT, McBrien NA. Glycosaminoglycan synthesis in the separate layers of the chick sclera during myopic eye growth: comparison with mammals. Curr Eye Res. 2001;23:179–84. doi: 10.1076/ceyr.23.3.179.5466. [DOI] [PubMed] [Google Scholar]
- 25.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–7. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 26.Waldbillig RJ, Arnold DR, Fletcher RT, Chader GJ. Insulin and IGF-I binding in developing chick neural retina and pigment epithelium: a characterization of binding and structural differences. Exp Eye Res. 1991;53:13–22. doi: 10.1016/0014-4835(91)90139-6. [DOI] [PubMed] [Google Scholar]
- 27.Prins T, Fodor M, Delemarre-van de Waal HA. Pituitary mRNA expression of the growth hormone axis in the 1-year-old intrauterine growth restricted rat. J Neuroendocrinol. 2006;18:611–20. doi: 10.1111/j.1365-2826.2006.01451.x. [DOI] [PubMed] [Google Scholar]
- 28.Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthal Vis Sci. 2005;46:3932–42. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- 29.Nickla DL. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp Eye Res. 2007;84:951–9. doi: 10.1016/j.exer.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987;6:993–9. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- 31.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–94. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 32.Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42:2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 33.Feldkaemper M, Neacsu I, Schaeffel F. Insulin acts as a powerful stimulator of axial myopia in chicks. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-1702. in press. [DOI] [PubMed] [Google Scholar]
- 34.Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008;317:196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 35.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form-deprivation myopia. Curr Eye Res. 1990;9:1157–65. doi: 10.3109/02713689009003472. [DOI] [PubMed] [Google Scholar]
- 37.Kusakari T, Sato T, Tokoro T. Regional scleral changes in form-deprivation myopia in chicks. Exp Eye Res. 1997;64:465–76. doi: 10.1006/exer.1996.0242. [DOI] [PubMed] [Google Scholar]
- 38.Kusakari T, Sato T, Tokoro T. Visual deprivation stimulates the exchange of the fibrous sclera into the cartilaginous sclera in chicks. Exp Eye Res. 2001;73:533–46. doi: 10.1006/exer.2001.1064. [DOI] [PubMed] [Google Scholar]
- 39.Mathis U, Schaeffel F. Glucagon-related peptides in the mouse retina and the effects of deprivation of form vision. Graefes Arch Clin Exp Ophthalmol. 2007;245:267–75. doi: 10.1007/s00417-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 40.Zygar CA, Colbert S, Yang D, Fernald RD. IGF-1 produced by cone photoreceptors regulates rod progenitor proliferation in the teleost retina. Brain Res Dev Brain Res. 2005;154:91–100. doi: 10.1016/j.devbrainres.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Waldbillig RJ, Arnold DR, Fletcher RT, Chader GJ. Insulin and IGF-1 binding in chick sclera. Invest Ophthal Vis Sci. 1990;31:1015–22. [PubMed] [Google Scholar]
- 42.Alarcon C, Serna J, Perez-Villamil B, de Pablo F. Synthesis and differentially regulated processing of proinsulin in developing chick pancreas, liver and neuroretina. FEBS Lett. 1998;436:361–6. doi: 10.1016/s0014-5793(98)01168-5. [DOI] [PubMed] [Google Scholar]
- 43.Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–27. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- 44.Kondo S, Cha SH, Xie WF, Sandell LJ. Cytokine regulation of cartilage-derived retinoic acid-sensitive protein (CD-RAP) in primary articular chondrocytes: suppression by IL-1, bfGF, TGFbeta and stimulation by IGF-1. J Orthop Res. 2001;19:712–9. doi: 10.1016/S0736-0266(00)00068-1. [DOI] [PubMed] [Google Scholar]
- 45.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 46.Wong CK, Lai T, Holly JM, Wheeler MH, Stewart CE, Farndon JR. The effects of retinoic acid on the insulin-like growth factor axis in primary tissue culture from hyperparathyroidism. World J Surg. 2006;30:714–20. doi: 10.1007/s00268-005-0340-2. [DOI] [PubMed] [Google Scholar]
- 47.Koh SM, Chader GJ. Elevation of Intracellular Cyclic AMP and Stimulation of Adenylate Cyclase Activity by Vasoactive Intestinal Peptide and Glucagon in the Retinal Pigment Epithelium. Journal of Neurochemistry. 1984;43:1522–1526. doi: 10.1111/j.1471-4159.1984.tb06072.x. [DOI] [PubMed] [Google Scholar]
- 48.Buck C, Schaeffel F, Simon P, Feldkaemper M. Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken. Invest Ophthal Vis Sci. 2004;45:402–9. doi: 10.1167/iovs.03-0789. [DOI] [PubMed] [Google Scholar]
- 49.McLean RC, Wallman J. Severe astigmatic blur does not interfere with spectacle lens compensation. Invest Ophthal Vis Sci. 2003;44:449–57. doi: 10.1167/iovs.01-0670. [DOI] [PubMed] [Google Scholar]
- 50.Park T, Winawer J, Wallman J. Further evidence that chicks use the sign of blur in spectacle lens compensation. Vision Res. 2003;43:1519–1531. doi: 10.1016/s0042-6989(03)00180-9. [DOI] [PubMed] [Google Scholar]
- 51.Rada JA, Palmer L. Choroidal regulation of scleral glycosaminoglycan synthesis during recovery from induced myopia. Invest Ophthal Vis Sci. 2007;48:2957–66. doi: 10.1167/iovs.06-1051. [DOI] [PubMed] [Google Scholar]