Abstract
Insulin-dependent diabetes mellitus (IDDM) is characterized by leukocyte invasion to the pancreatic tissues followed by immune destruction of the islets. Despite the important function of Th17 cells in other autoimmune disease models, their function in IDDM is relatively unclear. In this study, we found association of elevated Th17 cytokine expression with diabetes in NOD mice. To understand the function of Th17 cells in IDDM, we differentiated islet-reactive BDC2.5 TcR transgenic CD4+ cells in vitro into Th17 cells and transferred them into NOD.scid and neonate NOD mice. NOD.scid recipient mice developed rapid onset of diabetes with extensive insulitic lesions, whereas in newborn NOD mice, despite extensive insulitis, most recipient mice did not develop diabetes. Surprisingly, BDC2.5+ cells recovered from diabetic NOD.scid mice, in comparison with those from neonate NOD mice, showed predominant IFN-γ over IL-17 expression, indicating conversion of donor cells into Th1 cells. Moreover, diabetes progression in NOD.scid recipients was dependent on IFN-γ while anti-IL-17 treatment reduced insulitic inflammation. These results indicate that islet-reactive Th17 cells promote pancreatic inflammation, but only induce IDDM upon conversion into IFN-γ producers.
Keywords: Th17, islet inflammation, Type-one diabetes
Introduction
CD4+ helper T (Th) cells play essential pathogenic function in autoimmune diseases. After activation, antigen-specific Th cells differentiate into cytokine-secreting effector cells that have been historically classified into Th1 or Th2 cells [1]. Th1 cells make IFN-γ whereas Th2 cells produce IL-4, -5 and -13. Although Th1 cells were associated with diabetes in NOD mice, NOD mice lacking IFN-γ [2] , IFN-γ receptor [3] or IL-12 [4] developed T1D similarly to wild-type NOD mice. However, islet-reactive Th1 cells generated from BDC2.5 TcR transgenic T cells were reported to drive aggressive diabetes [5], possibly via IFN-γ induction of apoptosis of insulin-producing β cells [6]. Moreover, transfer of Th1 but not Th2 cells into neonatal NOD mice caused T1D [5] and BDC2.5 mice lacking IFN-γ receptor are resistant to cyclophosphamide-induced diabetes [6].
Recent studies have identified a new subset of Th cells, called Th17, which produce IL-17, IL-17F, IL-22 and IL-21 and mediate tissue inflammation [7, 8], There is growing evidence that Th17 cells are pathogenic in several autoimmune disease mouse models such as experimental allergic encephalomyelitis (EAE) and collage-induced arthritis (CIA) [9–12]. However, there is little information on Th17 or IL-17 in type 1 diabetes mellitus (T1DM). Recently Jain R et al, reported that treatment with a fusion protein consisting of IgG and GAD peptide 206–220 confers diabetes protection to hyperglycemic NOD mice, correlating with a reduced number of IL17-producing cells present in the spleen and induction of IFN-γ-producing cells [13].
To address the involvement of Th17 cells in T1D, we assessed IL-17 and IL-17F expression in NOD mice pancreas at different stages of T1D development and found that both cytokines were increased in expression in diabetic mice. In order to evaluate the function of Th17 cells, we differentiated islet-reactive BDC2.5 transgenic CD4+ cells into Th17 cells and transferred them into NOD.scid and newborn NOD mice. To our surprise, NOD.scid recipient mice developed full-blown diseases but newborn NOD recipients were mostly resistant. Although BDC2.5 Th17 cells consistently caused massive islet infiltration in both types of recipients, donor cells in NOD.scid mice predominantly expressed IFN-γ but not IL-17. A blocking antibody against IFN-γ inhibited diabetes in NOD.scid mice, while anti-IL-17 only reduced insulitic inflammation. Therefore, islet-reactive Th17 cells primarily function by promoting inflammation but their conversion to Th1 cells in lymphopenic hosts results in diabetes.
Results
Expression of IL-17 and IL-17F during diabetes progression in NOD mice
To determine whether Th17 cells play a role in diabetes development, we first assessed the expression of two characteristic cytokines produced by Th17 cells, IL-17 and IL-17F, in the pancreas of NOD mice. RNA was extracted from pancreas of two weeks old, 11 weeks old (non-diabetic mice), and recently detected diabetic NOD mice (16 to 25 weeks old), followed by real-time RT-PCR for IL-17 and IL-17F. We found that both genes were increased in mRNA expression in older mice and there was a significant expression of IL-17 and IL-17F in the pancreas of diabetic NOD mice (Figure 1A). Thus, IL-17 and IL-17F expression correlates with established insulitis and diabetes. These results prompted us to search for IL-17-producing cells in NOD mice. To detect IL-17 and IFN-γ by ICS, lymph node and spleen cells were activated with PMA and ionomycin for 5 h. Since IL-17 can be produced by other cell types, such as γδ T cells and macrophages [14], we analyzed the IL-17+ cells in the TCRβ+ and CD4+ population. IL-17+ cells were found in small quantities in young adults, pre-diabetic (16–18 week old) and diabetic NOD (16 to 23 weeks) mice but further increased by 2 to 3 times in the later especially in mesenteric lymph nodes (MLN), pancreatic lymph nodes (PLN) and spleen (Figure 1B and 1C). In contrast, IFN-γ-expressing cells were found predominantly in PLN of pre-diabetic and diabetic NOD mice (Figure 1B and 1C). The increase of IL-17-producing cells was not observed in control C57BL/6 mice (Figure 1C). Our results indicate that increased Th17 cells were associated with pathogenesis of T1D in NOD mice and suggest their functional involvement in this autoimmune disorder.
Figure 1. Diabetic NOD mice have elevated IL-17 and IL-17F expression.
A. mRNA from pancreas of diabetic, 11 weeks, and 2 weeks old mice were analyzed for the expression of IL-17 and IL17F mRNA by real-time RT-PCR. RNA was normalized to β-actin. B and C. Spleen and lymph nodes from 4 week and 1 year old C57BL/6 or NOD diabetic, pre-diabetic (15 to 17 weeks old) and young adults (6 weeks) were activated with PMA and Ionomycin for 5 hours and further analyzed IL-17 and IFN-γ by ICS. B. Plots for young and diabetic NOD mice. Shown are cells gated on CD4+. C. Averages of the percentage of IL-17 or IFN-γ positive cells gated from the CD4+Tcr-β+ cells (n= 6–8 mice per group analyzed in independent experiments). ILN: inguinal lymph nodes; CLN: cervical lymph nodes; MLN: mesenteric lymph nodes and PLN: pancreatic lymph nodes. P values were calculated with Student’s t-test
Polarized BDC2.5 Th17 cells cause diabetes in NOD.scid mice but only insulitis in newborn NOD mice
To analyze the capacity of Th17 cells to cause T1DM, we differentiated CD4+ T cells from BDC2.5 TcR transgenic mice into Th17 cells. CD4+ cells were cultured with the mimotope 63 (RTRPLWVRME) [15] under Th17 differentiation conditions (IL-6, TGF-β, IL-23, anti-IFN-γ and anti-IL-4) and irradiated antigen-presenting cells [16]. On day 3, a sample of the cells was restimulated with PMA and ionomycin or mimotope 63 and IL-17 and IFN-γ production was evaluated by ICS. Under these conditions, we would typically obtain 10 to 30% of IL-17-producing cells, 50% of IL-17F producing cells and between 0.5 to 5% of IFN-γ-producing cells (Figure 2A and data not shown). These cultured cells had 90 to 95% viability and did not required the addition of new media or more IL-23. On day 4, one million cells of this preparation were transferred into NOD.scid mice as reported previously [5, 17] and diabetes was monitored daily. 100 % incidence of diabetes was observed between day 6 and day 20 after transfer of BDC2.5 Th17 cells (Figure 2B and 2C). Disease incidence was dependent on the amount of BDC2.5 cells transferred and 0.5 million cells were the minimum amount of cells necessary to cause 100% disease (Figure 2C).
Figure 2. In vitro differentiated BDC2.5 Th17 cells can cause diabetes in NOD.scid mice, but only insulitis in young NOD mice.
A. CD4+ from BDC2.5 mice were differentiated to Th17, shown is the representative cytokine profile on day 3 after activation. B. Diabetes incidence when 1 million Th17 cells from A) were injected into NOD.scid mice. C. Diabetes incidence when different doses of Th17 cells were transferred into NOD.scid mice. Numbers represent millions of cells. D. Photograph of pancreas of NOD.scid mice that received Th17 cells sections. Tissues were stained with hematoxylin and eosin (HE). Doted lines indicate the periphery of the islet. The data is one representative experiment out of three independent experiments. E. Diabetes incidence when 1 million differentiated BDC2.5 Th17 cells were injected into 7-day-old NOD mice (Results derived from two independent experiments). F. Evalulation of insulitis of HE stained pancreas sections from NOD mice that received BDC2.5 cells on day 21 after transfer. f: female, m: male. G. Photograph of an islet that presented exocrine tissue infiltrates from E. H. IL-17 in serum measured by ELISA and insulitis in the pancreas were scored from newborn NOD mice transfused with BDC2.5 Th17 cells on day 6 after transfer (day 13). Results represent two independent experiments with 3 different litters with a total of 17 mice analyzed. White triangles represent mice injected with PBS and black diamonds mice that received BDC Th17 cells. Fit r2= 0.7424 , significance p= 0.001.
When we examined the islet lesions generated by Th17 cells by histology, we found that the leukocyte infiltrate was present in the islets but also covering extensive areas of the exocrine tissue (Figure 2D and supplementary figure 1), which are different from the lesion infiltrates reported for BDC2.5 or BDC2.5 Th1 cells [5, 18].
We then asked whether Th17 polarized BDC2.5 cells could cause disease in neonatal NOD mice with a full T cell repertoire with T regulatory cells, in which there is no influence of homeostatic expansion. One million of BDC2.5 Th17 cells were transferred into seven day-old NOD mice, similar Th1 cell dose has been reported to cause diabetes (31). To our surprise, only 20% of the recipient mice developed diabetes within 25 days (Figure 2E). However, when examined histologically, all the mice weer found to have developed insulitis (ranging from 20–70%) and perinsulitis (45% average) of the islets by day 25 (Figure 2F). Interestingly, the islet infiltrates generated by transferred cells also cover the exocrine tissue (Figure 2G). These results suggest that the Th17 cells can cause insulitis but are arrested in the islet environment in neonatal NOD mice.
To confirm that the transferred BDC2.5 Th17 cells were active and the cause of insulitis in the neonatal NOD recipient mice, we repeated the transfer experiment but this time, NOD recipient mice were sacrificed on day 6 after cell transfer. IL-17 was quantified in the serum of the NOD recipients and pancreata were evaluated for insulitis. We found that those animals with insulitis and perinsulitis at 6 days after transfer had high levels of IL-17 in the serum, when compared to control littermates that received PBS (Figure 2H).
To further ascertain that the transferred BDC2.5 Th17 cells migrate into pancreas, imunohistochemistry for IL-17F, CD4 and macrophages (F4/80 staining) were performed on frozen sections of pancreas of recipient NOD mice. Interestingly, IL-17F was detected only in the islets of NOD mice that developed diabetes (Supplementary figure 2). This population of IL-17F-producing cells were localized adjacent to macrophages and were in the perimeter of the islet structures (Supplementary figure 2, IL-17F).Thus, Th17 cells participate in the insulitic lesion and may be involved in the attraction and activation of macrophages in the islet. Another interesting observation of Th17 lesion of neonatal NOD mice was the presence of macrophages localized in the perimeter of the islets but never in the central area of the islet (Supplementary figure 2). Our results strongly suggest that Th17 cells might recruit and activate macrophages to the islets to promote tissue inflammation and active insulitis
IFN-γ is necessary for Th17-induced diabetes in NOD.scid mice
Since BDC2.5 Th17 cells caused significant diabetes in NOD.scid but not neonatal NOD mice, we further characterized the donor cells in different hosts. When BDC2.5 T cells recovered from the diabetic NOD.scid mice were analyzed for cytokine production, we found 8 % of IL-17 producing cells in almost every lymphoid organ; however, higher numbers of IFN-γ-producing BDC2.5 T cells were recovered, especially in PLN (Figure 3A). Moreover, the predominant cytokine in all the mice and organs analyzed was IFN-γ (Figure 3A graph). Thus, although BDC2.5 T cells differentiated into Th17 were transferred, some cells continued to produce IL-17 but a majority of cells produced IFN-γ (Figure 3A).
Figure 3. Characterization of donor cell cytokine patterns in NOD.scid and 7-day-old NOD mice.
A. Lymph node and spleen cells recovered from diabetic NOD.scid that received Th17 cells, were activated for 5 hours with mimotope 63 for further ICS of IFN-γ and IL-17. B. Average of IL-17, IFN-γ and double positive (DP) cells from four diabetic mice. ILN: inguinal lymph nodes; MLN: mesenteric lymph nodes and PLN: pancreatic lymph nodes. For this analysis diabetic mice were sacrificed one day after tested positive for glycosuria C. Average of IL-17, IFN-γ cells from 7 day old NOD mice infused with BDC2.5-Th17, analysis was done on day 25 after transferred (n=8).
In contrasts, when neonatal NOD recipient mice were analyzed, the numbers of IL-17-and IFN-γ–producing cells in all the secondary lymphoid organs were less than 3% and there was no dominance of IFN-γ production in the PLN (Figure 3B).
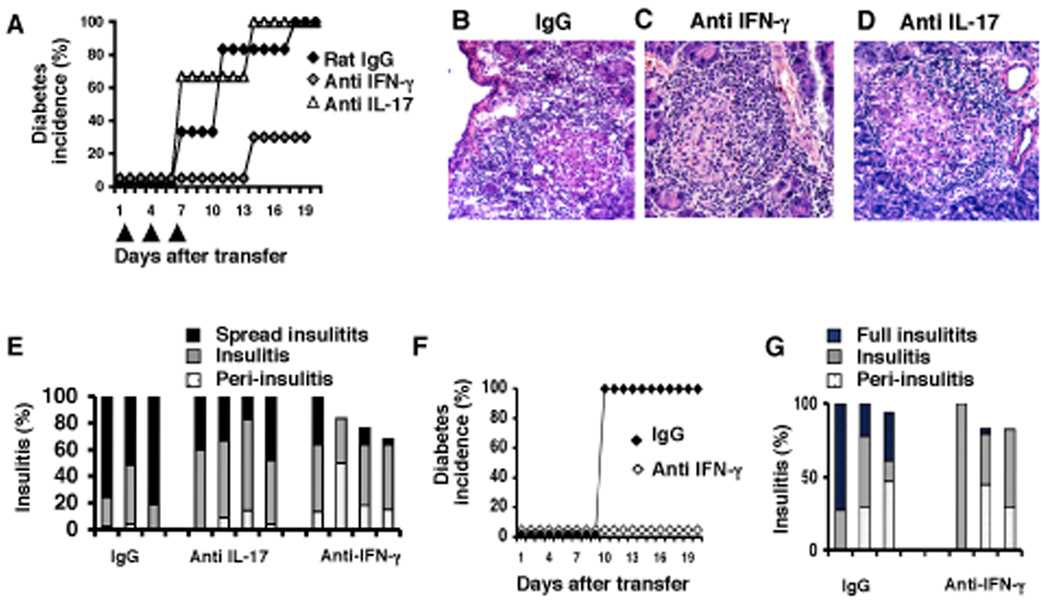
Since we found IFN-γ-producing cells in lymph nodes of NOD.scid mice that received Th17 cells, we then asked whether IL-17 or IFN-γ was more important to provoke diabetes. Blocking antibodies against IFN-γ or IL-17 or control rat IgG were administered to NOD.scid mice together with BDC2.5-Th17 differentiated cells, and continued the antibody administration every 4 days. We found that diabetes was significantly reduced in NOD.scid mice that received anti-IFN-γ when compared to the group-receiving rat IgG (Figure 4A). However, mice treated with anti-IL-17 blocking antibody developed similar diabetes as the group of mice treated with rat IgG (Figure 4A). This result indicates that transferred cells required IFN-γ to cause diabetes.
Figure 4. Th17 induce diabetes in NOD.scid is IFN-γ dependent.
A. Diabetes incidence when 1 million of Th17 differentiated BDC2.5 cells were injected into NOD.scid mice and treated with either Rat IgG, anti-IFN-γ and anti-IL-17 blocking antibodies on days 1, 4 and 7 after the transfer. B, C and D. Photographs of pancreas sections of NOD.scid mice that receive Th17 cells and blocking antibodies; tissues were stained with hematoxylin and eosin (HE). E. Evaluation of insulitis of pancreas sections from NOD.scid mice from A. P values for IgG vs anti IL-17: Insulitis = 0.0146, spread insulitis = 0. 0225, (Student’s t-test). F. Diabetes incidence when 1 million Th1-differentiated BDC2.5 cells were injected into NOD.scid mice and treated with either Rat IgG or anti-IFN-γ blocking antibodies on day 1, 4 and 7 after the transfer. G. Evaluation of insulitis of pancreas sections from NOD.scid mice from F.
Evaluation of the islet lesions from each group revealed that blockade of IL-17 reduced the extensive leukocyte infiltration observed in mice treated with rat IgG (Figure 4B, 4D and 4E) but not the total insulitis. The massive infiltration was only observed in 30% of the islets of mice treated with anti-IL-17 blocking antibody (Figure 4E), in comparison to 70% observed in the group treated with rat IgG (Figure 4E). As expected, insulitis in the mice treated with anti-IFN-γ blocking antibodies was limited to the islets and in the majority of these mice about 10–20% of islets were free of insulitis (Figure 4C and 4E). This let us to conclude that IL-17 produced by donor BDC2.5 cells does not directly participate in the destruction of islet β cells but is required to promote extensive inflammation in the islets. More importantly, IFN-γ was indispensable to cause islet destruction.
To compare Th17 cells with Th1 cells in their capacity to provoke disease, we analyzed NOD.scid mice that received BDC2.5 cells that were differentiated into Th1 cells. These mice developed diabetes as has been reported [5] with a similar onset as the mice that received polarized Th17 cells. Furthermore, the disease was prevented by blocking antibodies against IFN-γ, confirming that IFN-γ produced by Th1 cells participates in the destruction of the islet-β cells (Figure 4C, 4F, 4G). Some mice treated with anti-IFN-γ did not show full insulitis and in 20% of the islets of these mice there were no leukocyte infiltrates. Although both Th17 and Th1 cells can cause disease, the leukocyte infiltrates and the inflammatory process surrounding the islet appears different. Th1 cells promoted an islet-restricted lesion whereas the Th17 cells recruited a massive infiltrate that extended to the exocrine pancreas (Figure 4G, 4B and supplementary figure 1).
These results suggest a possible conversion of BDC2.5 T cells from Th17 to Th1 phenotype in the NOD.scid mice. To explore this possibility, we differentiated BDC2.5 T cells under Th17 conditions as described above and on day 3 the cells were stimulated with peptide 63 for 3 hours followed by staining for IFN-γ producers using a bi-specific capture system (Miltenyi Biotec) which was revealed with a fluorescently labeled antibody against IFN-γ (Figure 5A). CD4+ cells negative for IFN-γ were FACS-sorted and transferred into NOD.scid mice. A sample of the sorted cells was cultured for 5 hours with peptide 63 and irradiated APC and for analysis of IFN-γ, IL-17 and IL-17F expression. We observed that the purified cells did not produce IFN-γ but continued to produce IL-17 and IL-17F (Figure 5A). NOD. scid recipient mice developed diabetes on day 8 after the transfer (figure 5B) and on day 10 lymph nodes and spleen cells from these mice were stimulated with peptide 63 and evaluated for IL-17, IL-17F and IFN-γ production. Pancreatic lymph node and spleen cells predominantly produced IFN-γ and few cells expressed IL-17 (Figure 5C and 5D). Interestingly there was a 12.5% of IL-17F+ IL-17- cells in the spleen and only 2.3% in the PLN (Figure 5C and 5D). Nonetheless, the predominant population was IFN-γ-producing cells in all the animals analyzed. Therefore, these results indicate that fully differentiated Th17 cells can be converted to IFN-γ-producing cells in NOD.scid recipients to produce diabetes.
Figure 5. Th17 polarized cells convert to IFN-γ producing cells to provoke diabetes.
A. CD4+ from BDC2.5 mice were differentiated to Th17, on day 3 cells were activated with peptide 63 and irradiated APC for 3 hours and cells were stain with anti-mouse IFN-γ capture reagent and anti-IFN-γ detection antibody. Shown are the profiles of cells activated with peptide or PBS as control. Sorted cells are indicated with the arrow. A sample of sorted cells was further stimulated with peptide for 4 hours and analyzed for IL-17, IFN-γ and IL-17F. B. Diabetes incidence when sorted Th17 cells from A) were injected into NOD.scid mice. C. Lymph node and spleen cells recovered from diabetic NOD.scid that received purified Th17 cells, were activated for 5 hours with mimotope 63 for further analyzed for IFN-γ and IL-17 expression. D. Average of IL-17, IFN-γ and double positive (DP) (upper panel and IL-17F, IL-17 and DP cells (lower panel) from diabetic mice. ILN: inguinal lymph nodes; MLN: mesenteric lymph nodes and PLN: pancreatic lymph nodes. For this analysis diabetic mice were sacrificed two days after tested positive for glycosuria.
Discussion
Despite their prominent function of CD4- Th17 cells in mouse models for rheumatoid arthritis and multiple sclerosis, the role of Th17 cells in IDDM has been unclear. In this study, we found that IL-17 and IL-17F gene expression in pancreas of NOD mice correlated with diabetes. We show that BDC2.5 T cells that were polarized to Th17 cells in vitro induced T1D in lymphopenic NOD.scid mice and insulitis in neonatal NOD mice.
The onset and incidence Th17-induced disease in NOD.scid mice was similar to Th1-mediated diabetes reported before [5]. However, Th17-caused islet lesions were characterized by severe insulitis with destruction of exocrine tissues in contrast to the Th1 lesions that were localized in the islets. In newborn NOD recipient mice that developed diabetes, we found IL-17F+ CD4+ cells in the periphery of the islet where macrophages were also present. Our results strongly suggest that Th17 cells might recruit or activate macrophages to the islets to promote an uncontrolled inflammation that leads to profuse leucocyte infiltration inside the islet and very distinctively over the islet borders to the exocrine pancreas, thus facilitating diabetes development. It is interesting that we found detectable levels of IL-17 in the sera of NOD neonates that received Th17 cells and those mice were the ones establishing fastest insulitis. Our results also suggests that BDC2.5 Th17 cells in NOD neonates are a component of the disease onset and may complement Th1 cells, since the transfer of one million or less Th1 cells to NOD neonates [5, 17] or ConA activated BDC2.5 cells can induce full diabetes [19] . Since IL-17 and IL-17F seem to be elevated in mice with diseases, it would be of interest to explore levels of these cytokines in serum as an additional marker for T1D onset in humans.
Blockade of IL-17 does not prevent diabetes onset; however, we cannot discard the role of IL-17F and IL-22 in the disease process, both of which are produced by Th17 cells. An unexpected finding was that IFN-γ was indispensable for the Th17 induced diabetes process, and as described before, IFN-γ mediates β-cell destruction [6]. After diabetes onset, we found an increased number of BDC2.5 T cells producing IFN-γ in the PLN and MLN, which suggest a possible conversion from Th17 to Th1 phenotype of the diabetogenic T cells in the NOD.scid mice. Depletion of IFNγ producers before the transfer did not attenuate IFNγ expression in donor cells, thus supporting the idea of Th17 to Th1 conversion. At this stage, we can not discard the possibility that Th17 cells activate macrophages and dendritic cells in the islets that can further sustained Th1 differentiation or IFN-γ production by macrophages and NK T cells that will promote islet-β cell death in situ [2, 6, 20]. Our data differ from a recent report by Renu Jain that finds that blockade of IL-17 can prevent BDC2.5 Th17 mediated disease in NOD.scid mice when transferred 10 million Th17 polarized cells. However, Jain’s report does not analyze the blockade of IFN-γ in BDC2.5 Th17 mediated disease, or the BDC2.5 Th17 present in diabetic NOD.scid mice or when blockade of IL-17. Moreover, the focus of Jain R report is the vaccination with a fusion protein GAD 206–220- Ig where protection is mediated by IFN-γ producing cells and reduction of IL-17 cells in the spleen. The protective role of IFN-γ producing cells upon vaccination of NOD mice with BCG or CFA has previously been reported by Serreze et al [21] and it is not opposing to the pathogenic effect of IFN-γ in the initiation of disease as reported in an acute induction of diabetes by cyclophosphamide treatment [22]. Our report however does agree with Jain’s report on the pathogenic role of Th17 in NOD.scid mice and the fact that only the diabetic NOD mice that received Th17 have detectable levels of IL-17F in the pancreas and IL-17 in serum further confirms the role of Th17 during the destructive phase of diabetes.
One question that arises from our study is why Th17 cells are unable to cause disease in the NOD mice but can work in the lymphopenic model? It is possible that the Th17 cells are more susceptible to Treg suppression than Th1 cells in the islets and may not survive or stay long enough in the islet to promote diabetes. Another possibility is that in the newborn NOD mice Th17 cells cannot convert into Th1 cells to cause disease. In contrast, in the lymphopenic environment Th17 are not influenced by Tregs and can benefit from the rich cytokine environment that promotes homoestatic expansion. Katz and collaborators reported similar results using Th2 differentiated BDC2.5 cells to promote diabetes [17]. Therefore, in a lymphopenic condition, Th2 or Th17 cells could worsen islet-β cell destruction. On the other hand, since transferred Th17 BDC2.5 cells were more readily converted into a Th1-dominant phenotype in lymphopenic hosts, it is likely that these expanded or reprogrammed Th1 cells may mediate the diabetes development, a hypothesis supported by our results that the disease was ameliorated by anti-IFNγ. It would be interesting to investigate whether Th2 cells could further convert to Th1 in this same model in a similar fashion, which could indicate a dominant conversion to Th1 in lymphopenic conditions. Nonetheless, to our knowledge, this is the first time that conversion of Th17 cells into Th1 in lymphopenic mice is being reported. A recent report, on the use of polarized Th17 cells for therapy against solid melanoma showed that rejection of establish melanomas was dependent on IFN-γ production and irradiation of tumor bearing mice [23]. So far we know the role of TGF-β, IL-6, IL-21 and IL-1 for Th17 differentiation and of IL-23 to promote Th17 proliferation [7, 8]. IL-6 and TGF-β synergize to promote Th17, whereas TGF-β without inflammatory cytokines promotes inducible Treg (iTreg) differentiation [24]. Moreover, we recently reported that IL-6 treatment of iTreg and natural Treg promoted the conversion of these lineages into Th17 cells via down regulating Foxp3. Conversely, TGF-β induction of Foxp3 antagonizes RORγt and RORα function, which is required for Th17 differentiation [24]. These data indicate a greater plasticity of Th cells than previously expected. Future studies will shed more light on the exact combination of signals that promote the maintenance of Th17 phenotype, or facilitate the conversion to Th1 in vivo in a full versus empty immune compartments and help understand their pathological role in autoimmune disorders.
Then what is the function of IL-17 or Th17 cells in IDDM? Our transfer study indicates that Th17 cells induced different types of inflammation in the islets. Anti-IL-17 inhibited insulitis in NOD.scid and neonatal NOD mice. We think that Th17 cells may play an accessory role in IDDM by promoting inflammation, which may enhance the islet destruction by Th1 or CD8+ T cells. Nonetheless, our data indicate an involvement of Th17 cells in IDDM, which may be further investigated in human IDDM patients.
Material and Methods
Mice
NOD/LTJ (NOD/ShiLtJ) and NOD SCID (NOD.CB17-Prkdcscid/J) mice were purchase from The Jackson Laboratory (Bar Harbor, Maine). BDC2.5/NOD mice were kindly donated by Diane Mathis and Christophe Benoist. Mice were house and bred in the SPF animal facility at the M.D. Anderson Cancer Center and the experiments were performed using protocols approved by the Institutional Animal Care and Use Committee.
Quantitative real-time PCR
Total RNA was prepared from pancreas using TriZol reagent (Invitrogen). cDNAs were synthesized with Superscript reverse transcriptase and oligo (dT) primers (Invitrogen and gene expression was examined with a Bio-Rad iCycler Optical System using a iQ SYBR green real-time PCR kit (Bio-Rad). The data were normalized to a β-actin reference. The primers for IL-17 were: forward 5’-CTCCAGAAGGCCCTCAGACTAC- 3’, reverse 5’ GGGTCTTCATTGCGGTGG-3’; the primers for IL-17F were: forward 5’-CCCATGGGATTACAACATCACTC-3’, reverse 5’-CACTGGGCCTCAGCGTC-3’.
Th differentiation and T cell transfer for diabetes development
For Th17 differentiation, spleen and lymph node cells from BDC2.5 NOD mice were cultured with 0.5 µg/ml of peptide mimotope 63 RTRPLWVRME [15], 5 ng/ml of TGF-β, 10 ng/ml of IL-6 (Peprotec), 50 µg/ml of anti IFN-γ (XMG 1.2), 10 µg/ml of anti IL-4 (11B11) and 100 ng/ml of IL-23 (R&D Systems). For Th1 differentiation, the cells were cultured with 0.5 µg/ml of peptide 63, 10 µg/ml of anti IL-4 and 350 pg/ml of IL-12. On day 3, a sample of the culture was reactivated with 10 ng/ml PMA and 250 ng/ml of ionomycin or 0.5 µg/ml of peptide 63 and Golgi-Plug for 5 hours (BD Bioscience) to further process for intracellular staining for IL-17 and IFN-γ. On day 4, 1 million cells of BDC2.5-Th17 cells were transferred i.v. into NOD.scid or i.p. into 10 day-old NOD mice. When diabetes was induced with purified CD4+ cells from diabetic females, CD4+ cells were purified using anti CD4+ magnetic beads from Miltenyi following manufacturer instructions. All mice were monitored daily for urine glucose levels (Diastix; Bayer Pharmaceuticals) and high reads were confirmed by blood glucose measurements (Ascencia Elite; Bayer Pharmaceuticals). Diabetes was scored after three consecutive reads higher than 13.5 mM/L. For labeling of IFN-γ secreting cells, we use IFN-γ secretion assay detection kit (Miltenyi Biotec). On day 3 of the Th17 differentiation, T cells were harvest, washed and cultured with 1 µg/ml of peptide 63 and irradiated APC’s following manufacturers instructions for activation. Labeling with anti IFN-γ catch reagent and anti IFN-γ detection antibody was done as recommended by Miltenyi. CD4+ IFN-γ – cells were then sorted in a FACS Aria sorter (BD Bioscience) and further transferred into NOD.scid mice. A sample of the sorted cells was activated with 0.5 µg/ml of peptide 63, irradiated APC’s and Golgi stop for evaluation of IL-17, IFN-γ and IL-17F production by ICS.
Histology
Pancreata from NOD.scid, NOD/LTJ mice were fixed in 10% formalin, and embedded in paraffin. Six-micrometer sections were cut every 100 microns through the total tissue and Hematoxlin and Eosin staining was performed. 50 to 100 islets per mice were score for presence and type of insulitis. For IL-17F staining pancreata were frozen in tissue freezing medium (Triange Biomedical Sciences). Immuno-staining was carried out on 10-µm sections. Sections were fixed with acetone at −20°C for 10 minutes and then air dry. Sections were incubated with a 1:300 dilution of rabbit polyclonal anti IL-17 F antibody (tested in house), CD4, F4/80 and GR1. Normal rabbit IgG was used as isotype control.
Antibodies and flow cytometry
CD4 PercPCy5.5, TCR-β FITC, Vβ4 PE, IFN-γ APC and IL17 PE antibodies were from BD Biosciences. Policlonal antibody against IL-17F was conjugated with Alexa 647 (Invitrogen) following manufacturers instructions. All cell suspensions were incubated with anti-CD32/CD16 for 30 min at 4°C before staining with the antibody mixture. Intracellular staining (ICS) for IFN-γ and IL-17 was performed using BD Cytofix/Cytoperm kit following manufacturer instructions. Cells were analyzed on FACScalibur (BD Biosciences) and files were analyzed using Flowjo 8.4.3 (Tree Star, Inc).
Supplementary Material
Acknowledgements
We thank the entire Dong lab for their help and discussion and Pamela Grant for help with histology. This work is supported in part by grants from National Institute of Health and grants from the Center for Target Therapy of MD Anderson Cancer Center (to CD). SHC receives a postdoctoral fellowship from the Arthritis Foundation and CD receives a Cancer Research Institute Investigator award, an American Lung Association Career Investigator award, a Leukemia and Lymphoma Society Scholar award and a Trust Fellowship of the MD Anderson Cancer Center.
Abbreviations
- LN
lymph nodes
- PLN
pancreatic LN
- NOD
non-obese diabetic mice
Footnotes
Conflict of Interest
The authors have no commercial conflict of interest.
References
- 1.Dong C, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000;2:179–188. doi: 10.1186/ar85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 3.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes. 2000;49:2007–2011. doi: 10.2337/diabetes.49.12.2007. [DOI] [PubMed] [Google Scholar]
- 4.Trembleau S, Penna G, Gregori S, Chapman HD, Serreze DV, Magram J, Adorini L. Pancreas-infiltrating Th1 cells and diabetes develop in IL-12-deficient nonobese diabetic mice. J Immunol. 1999;163:2960–2968. [PubMed] [Google Scholar]
- 5.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, Benoist C, Mathis D. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 8.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 9.Dong G, Ye R, Shi W, Liu S, Wang T, Yang X, Yang N, Yu X. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl) 2003;116:543–548. [PubMed] [Google Scholar]
- 10.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med. 2008;205:207–218. doi: 10.1084/jem.20071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 16.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 17.Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J Exp Med. 1997;186:299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez A, Andre-Schmutz I, Carnaud C, Mathis D, Benoist C. Damage control, rather than unresponsiveness, effected by protective DX5+ T cells in autoimmune diabetes. Nat Immunol. 2001;2:1117–1125. doi: 10.1038/ni738. [DOI] [PubMed] [Google Scholar]
- 20.Sarvetnick N, Shizuru J, Liggitt D, Martin L, McIntyre B, Gregory A, Parslow T, Stewart T. Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature. 1990;346:844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- 21.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J Immunol. 2001;166:1352–1359. doi: 10.4049/jimmunol.166.2.1352. [DOI] [PubMed] [Google Scholar]
- 22.Matos M, Park R, Mathis D, Benoist C. Progression to islet destruction in a cyclophosphamide-induced transgenic model: a microarray overview. Diabetes. 2004;53:2310–2321. doi: 10.2337/diabetes.53.9.2310. [DOI] [PubMed] [Google Scholar]
- 23.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang Y-H, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.