Abstract
When viewing a stimulus that has multiple plausible real-world interpretations, perception alternates between these interpretations every few seconds. Alternations can be halted by intermittently removing the stimulus from view. The same interpretation dominates over many successive presentations, and perception stabilizes. Here we study perception during long sessions of such intermittent presentation. We demonstrate that, rather than causing truly stable perception, intermittent presentation gives rise to a perceptual alternation cycle with its own characteristics and dependencies, different from those during continuous presentation. Alternations during intermittent viewing typically occur once every few minutes—much less frequently than the seconds-scale alternations during continuous viewing. Strikingly, alternations during intermittent viewing occur at fairly regular intervals, making for a surprisingly periodic alternation cycle. The duration of this cycle becomes longer as the blank duration between presentations is increased, reaching dozens of minutes in some cases. We interpret our findings in terms of a mathematical model that describes a neural network with competition between alternative interpretations. Network sensitivities depend on prior dominance, thus providing a memory for past perception. Slow changes in sensitivity produce both perceptual stabilization and the regular but infrequent alternations, meaning that the same memory traces are responsible for both. This model provides a good description of psychophysical findings, and offers several indications regarding their neural basis.
Keywords: binocular vision, computational modeling, memory, perceptual organization, ambiguous images, binocular rivalry
Introduction
Hermann von Helmholtz argued that perception is based on a process he called unconscious inference that, according to him, involved the unconscious formation of hypotheses as to what external objects may have given rise to an observed pattern of nervous stimulation (Von Helmholtz, 1867). Few paradigms illustrate this point as convincingly as that of ambiguous perception. Here an observer is presented with a particular visual pattern that has two (or more) plausible external-world interpretations (Figure 1). As a result perception alternates back and forth between these alternative interpretations, as if the brain were unable to settle on one definitive hypothesis to account for the observed pattern of stimulation (Blake & Logothetis, 2002; Hohwy, Roepstorff, & Friston, 2008; Leopold & Logothetis, 1999).
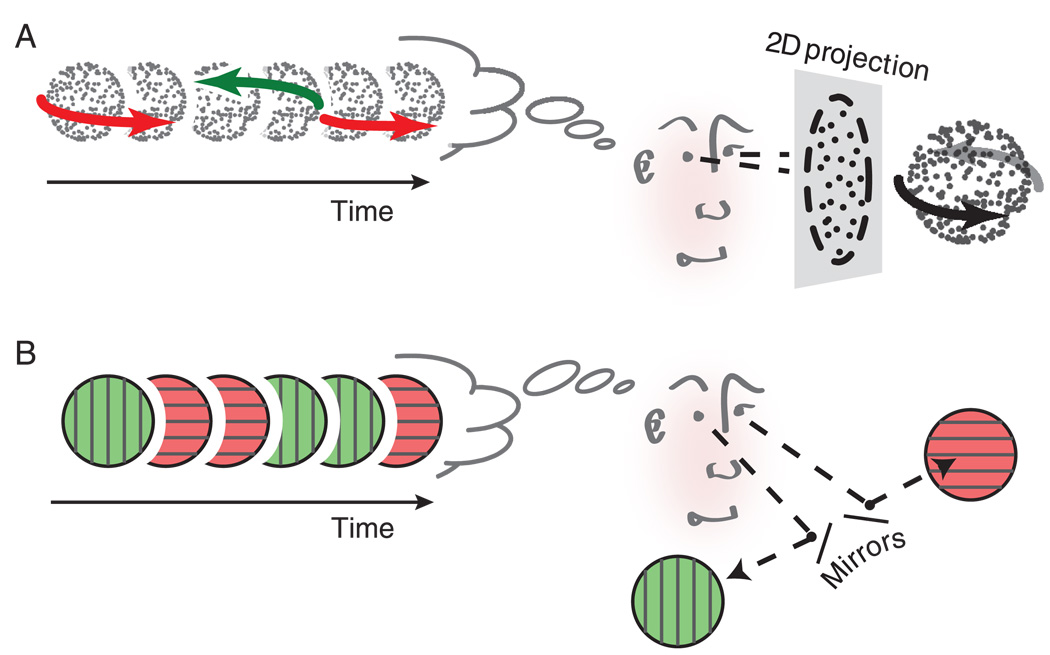
Figure 1.
Schematic illustration of the ambiguous stimuli used in this study. (A) Structure-from-motion rivalry. Viewing a two-dimensional projection of a transparent structure rotating in depth can cause perceptual alternations between two opposite rotation directions. (B) Binocular rivalry. When the left and right eye view incompatible images in the same region of space, perception typically alternates between exclusive visibility of either of the two images in isolation.
As one watches an ambiguous display the rate of perceptual alternations may be modulated by stimulus parameters (e.g. Brouwer & van Ee, 2006; Klink, van Ee, & van Wezel, 2008; Levelt, 1966) and also by factors such as attention (Chong, Tadin, & Blake, 2005; Meng & Tong, 2004; van Ee, van Dam, & Brouwer, 2005). However, alternations cannot be arrested during continuous viewing, despite mental effort to hold one interpretation dominant. Ironically, perception of an ambiguous stimulus can be stabilized simply by periodically removing that stimulus from view. In other words, an ambiguous stimulus shown for brief consecutive presentations, not continuously, is generally perceived in the same configuration for many presentations in a row (Leopold, Wilke, Maier, & Logothetis, 2002; Orbach, Ehrlich, & Heath, 1963; Pearson & Brascamp, 2008). In terms of Helmholtz’ idea of unconscious inference, it seems that when an image reappears its prior perception is a main factor constraining the generation of perceptual hypotheses (Maloney, Dal Martello, Sahm, & Spillmann, 2005).
Perceptual stability during intermittent presentation contrasts with the inherent instability observed during continuous viewing, and a major challenge for research using the intermittent presentation paradigm has been to study and explain this lack of alternations (Brascamp et al., 2008; Chen & He, 2004; Grossmann & Dobbins, 2006; Klink, van Ee, Nijs, Brouwer, Noest, & van Wezel, 2008; Leopold et al., 2002; Maier, Wilke, Logothetis, & Leopold, 2003; Noest, van Ee, Nijs, & van Wezel, 2007; Orbach et al., 1963; Pastukhov & Braun, 2008; Pearson & Clifford, 2004; Wilson, 2007). These efforts have culminated in several computational models designed to explain perceptual stability in the face of stimulus ambiguity (Brascamp et al., 2008; Noest et al., 2007; Wilson, 2007).
In this study we investigate perception of intermittent ambiguous stimuli, but we do not focus on the stabilization phenomenon. Although infrequent, perceptual alternations during intermittent viewing do occur. Given the existence of several theories consistent with a lack of alternations during intermittent viewing, we wondered why they occur at all. Thus, here we study the characteristics of these alternations and investigate what might cause them.
By way of preview, we have found that perceptual alternations during intermittent viewing occur at regular, fairly predictable intervals. This shows up as a slow and periodic cycle of alternating perception during intermittent viewing. The period length of this cycle can be over ten minutes (hundreds of presentations), in stark contrast to the brisk cycle during continuous viewing, where a given perceptual state lasts no longer than a few seconds. We furthermore observe that the period of this slow cycle becomes longer as the blank duration between consecutive presentations is increased.
We demonstrate that these and other known characteristics of perception of intermittent ambiguous stimuli are consistent with a dynamic network model of perceptual ambiguity (Brascamp et al., 2008; Noest et al., 2007). The model provides a plausible account of the computations underlying the perceptual time course and allows several inferences regarding its neural origin.
Experiment 1: A periodic alternation cycle during intermittent viewing
Our first experiment was inspired by the observation that perceptual stabilization, although strikingly robust, does not extend indefinitely. After, say, a minute of stabilized perception, a period of repeated dominance of one interpretation may give way to a period of repeated dominance of the alternative interpretation. This is an interesting observation, because perceptual stabilization involves a facilitatory effect of past perceptual dominance on subsequent perception (Pearson & Clifford, 2005), and this facilitatory trace is known to grow stronger over multiple presentations (Brascamp et al., 2008; Pastukhov & Braun, 2008). That is, the more a given percept has dominated in the past, the higher the probability that it will gain dominance again on a subsequent presentation. Given this ever-growing facilitation during stabilized perception, why should perception in this paradigm alternate at all?
Possible answers to this question can be broadly categorized into two groups. In one class of scenarios, alternations are caused by random fluctuations in brain activity (noise), which cause the previously unperceived interpretation to gain perceptual dominance in spite of a counteracting stabilizing force. Indeed, noise has been implicated as a primary determinant of alternations in perception of ambiguous stimuli under continuous viewing (Brascamp, van Ee, Noest, Jacobs, & van den Berg, 2006; Kim, Grabowecky, & Suzuki, 2006; Moreno-Bote, Rinzel, & Rubin, 2007). Alternatively, it is possible that the alternations have a deterministic origin. This latter option would arguably be more interesting, as it would mean that stabilization and alternations during intermittent viewing are both an intrinsic part of the dynamics that govern this system.
One way to make this first distinction is to look for periodicity in the occurrence of perceptual alternations during intermittent viewing. If alternations are caused by noise, they should occur at random intervals and display no periodicity. If alternations are part of the deterministic behavior, however, they may occur at more regular intervals.
In our first experiment observers reported perception of an ambiguous stimulus presented intermittently, and we analyzed the resulting perceptual alternation cycle.
Methods
We investigated both binocular rivalry and structure-from-motion ambiguity (Figure 1). For binocular rivalry we used square wave gratings (0.67 Michelsen contrast; 3.3 periods per deg, 50% duty cycle), viewed within a square aperture (side 1.4 deg). Stimuli were surrounded by a white fusion square (62 cd/m2, side 1.5 deg). Background luminance was 31 cd/m2, as was the mean stimulus luminance. Our structure-from-motion stimulus was an orthographic projection of a sphere rotating around a vertical axis (radius 1.2 deg, rotation speed 0.17 revolutions/s). It consisted of 250 white dots on a black background, presented with a red plus sign at fixation. Stimuli were generated on an Apple G5 computer using OpenGL within Apple’s Xcode development environment, and presented on a gamma-corrected CRT monitor at 1600 × 1200 dpi and 85 Hz.
Stimuli were presented intermittently during sessions that lasted 30 minutes. Observers were instructed to report their percept via key presses on every stimulus appearance. In the case of mixed percepts observers were instructed to choose the most salient one. The duration of individual stimulus presentations was 0.6 s for both stimuli. Every observer completed three sessions, each with a different blank duration separating consecutive presentations.
For binocular rivalry all observers started with a session using the default blank duration of 1.6 s; for the sphere all started with a session with 0.8 s blank intervals. The blank durations for the two remaining sessions of an observer were chosen individually for each observer and only after the session with the default blank duration had been completed. In choosing the other two blank durations we aimed to preclude sessions containing no alternations and sessions with a highly unstable perceptual time course. The values chosen depended on the observer’s personal alternation rate at the default blank duration, relying on our preliminary observation that a reduction in blank duration could increase this alternation rate. For example, if an observer of the sphere perceived only two alternations per half hour at the default blank duration of 0.8 s, we would tune the blank duration downward in subsequent sessions, thus aiming for more alternations. If, instead, that observer perceived many alternations at the default blank duration, we would tune the blank duration upward. All blank durations applied in this way fell between 450 ms and 2.8 s (median 1.6 s).
A total of seven observers (five naive to the purpose of the study) participated in the sessions with the ambiguous sphere. The same seven observers, supplemented with an additional three naive ones, participated in the sessions using binocular rivalry. Experiments were conducted in agreement with the ethics and safety guidelines of Utrecht University and Vanderbilt University.
One of our main points of interest was whether the perceptual alternation cycle during intermittent viewing displayed periodicity. To investigate this issue we analyzed power spectra of the reported perceptual time courses (Figure 2B and Figure 3B). The expected values and error margins on the power curves, under the assumption of no periodicity, were obtained as follows. For a given time series of perceptual reports during a session of intermittent viewing we calculated the average probability of a perceptual alternation occurring from any one presentation to the next. Taking this as a fixed probability we generated 500 artificial time series that were subsequently used to calculate the expected values and error margins given a fixed alternation probability (i.e. no periodicity). We used the same procedure to calculate the expected value and error margin of the highest peak of the power spectrum. These calculations allowed us to assess whether the observed power spectra were more peaked than could be expected by chance, and thus whether the observed alternations displayed significant periodicity. All power spectra were normalized by dividing by the total area under the curve.
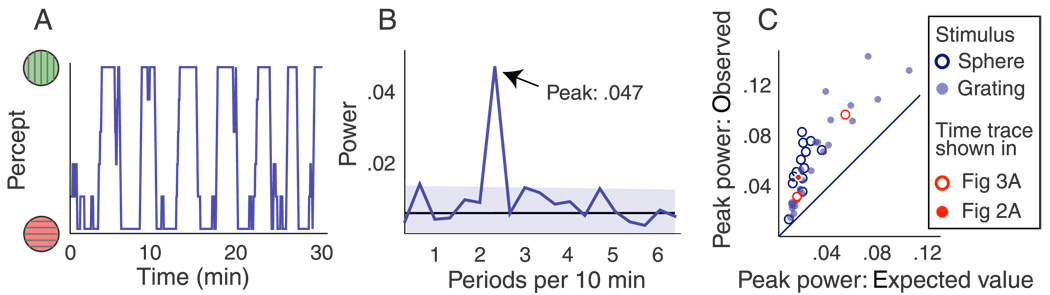
Figure 2.
Alternations during stabilized intermittent viewing are infrequent and periodic. (A) Perception of a binocular rivalry stimulus during a half-hour session (box car filtered with a window size of 5 presentations to enhance clarity). There is a distinct impression of periodicity in the alternations. (B) Section of the associated power spectrum (based on non-smoothed data). The spectrum is significantly peaked, indicating periodicity. Because power was normalized by dividing by the total area under the curve, it has no unit in this panel. (C) Summary of all data. Each point corresponds to one observer and condition. In every case the power spectrum had a higher peak value (y-axis) than expected assuming random alternations (x-axis). This confirms that alternations during stabilized intermittent viewing occur periodically. The three individual data points that correspond to the raw data shown in Figure 2A and Figure 3A are marked in red in this panel.
Figure 3.
Relation between oscillation frequency and blank duration. (A) Perception of an ambiguous sphere during a half-hour session (box car filtered with a window size of 5 presentations to enhance clarity). Perception appears to oscillate faster in the condition with a shorter blank duration (left). (B) Sections of the power spectra corresponding to panel A. The power peaks at a higher frequency in the condition with a shorter blank duration (left). Because power was normalized by dividing by the total area under the curve, it has no unit in this panel. (C) Data for all observers. Increasing the blank duration (x-axis) caused perception to oscillate at a lower frequency (y-axis), for both rivalry (n = 6) and the sphere (n = 4). Error bars are standard errors of the mean. In this analysis we normalized oscillation frequencies for each observer separately by dividing by the frequency observed at the shortest blank duration. This compensated for the broad inter-observer variability in oscillation frequency that we observed. Before normalizing, all frequencies lay between 1 and 7 periods per half hour.
Results
Alternations during intermittent viewing occur at long, regular intervals
Figure 2 shows the main findings of this experiment. Panel A shows box car smoothed data from one typical session during which a binocular rivalry stimulus (Figure 1B) was shown for 0.6 s presentations, separated by 2 s blanks. Perception as reported by the observer is indicated on the y-axis, against time on the x-axis. Although the line itself is continuous, it connects points corresponding to perception on separate presentations. Note that our observers did not indicate periods of mixed dominance of the two percepts, and that intermediate percept values apparent at some points in this figure are due to data smoothing (see figure caption).
Visual inspection of the time traces reveals a striking impression of alternations that tend to occur at regular intervals, separated by minutes-long stretches of perceptual stabilization. Such regularity can be demonstrated more formally by inspecting the power spectrum of this time series. The blue curve in panel B shows part of the power spectrum of the (unsmoothed) data shown in panel A. The distinct peak suggests that alternations did tend to occur at regular intervals in this session. In this case the peak is located at a frequency of just over 2 periods per 10 minutes, or about 14 alternations per half-hour session. This is equivalent to an average percept duration of about two minutes. The black line and shaded area indicate the power expected in the absence of periodicity, plus or minus two standard deviations (see Methods). The peak lies significantly above the expected value (p < .01; Monte Carlo procedure described in Methods), thus confirming the presence of periodicity in this session.
Panel C summarizes data from all sessions, using either the ambiguous sphere (open circles; 7 observers) or binocular rivalry (solid circles; 10 observers), and using a total range of blank durations from 0.45 s to 2.8 s. The figure displays the observed peak value in the power spectrum as a function of the highest value of the curve that is expected in the absence of periodicity (see Methods). The line y = x thus indicates a lack of periodicity. All points lie above their expected value ( p < .01 for each point but two; Monte Carlo procedure described in Methods), indicating that alternations tended to occur at regular intervals throughout all our sessions.
The data point in Figure 2C that corresponds to the raw time trace of Figure 2A is marked in red in this panel, as are the two points that correspond to the time traces in Figure 3A. These points fall nicely in the middle of the range of data points shown here. Indeed, the raw time traces in Figure 2A and Figure 3A are typical of the data obtained in this experiment.
Apart from the widely spaced alternations that cause this low-frequency periodicity, the time trace of Figure 2A also shows incidental brief excursions back and forth, but these have little effect on the overall slow cycle.
Oscillation frequency depends on blank duration
The periodicity observed in Figure 2 implies that alternations during intermittent viewing are not attributable to random fluctuations but, instead, are deterministic in origin.
One may wonder whether these periodic alternations of the stabilized percept are affected by the specifics of the visual stimulation regime, or whether instead they might involve some autonomous internal oscillator that operates independently of visual input (e.g. Carter & Pettigrew, 2003). We therefore investigated whether changing the rate of intermittent presentation could influence the slow oscillation of the stabilized percept. Each observer ran three sessions, each with a different blank duration between consecutive presentations. It is known that percept alternations during intermittent viewing become more numerous as the blank duration between presentations is reduced (e.g. Orbach, Zucker, & Olson, 1966). Does the frequency of the minute-scale oscillations we observe here also depend on this duration?
Figure 3A shows smoothed perceptual reports for one observer of the ambiguous sphere. We display only data of his two extreme-most blank durations, namely 0.8 s and 2.6 s (his data for 1.6 s were intermediate). In the shorter blank duration condition (left) there are more perceptual alternations than during the longer blank duration condition (right). Moreover, irrespective of the number of incidental excursions back and forth, the frequency of the minute-scale oscillation is higher with the short blank durations than with the longer intervals. Panel B shows this more explicitly by displaying the power spectra associated with the time courses in panel A (unsmoothed). At the short blank duration the spectrum peaks at a frequency of just over 1 period per 10 minutes (with a secondary peak at a slightly higher frequency). At the longer blank duration it peaks at a lower frequency, near half a period per 10 minutes. We will refer to the location of the peak in the power spectrum as the ‘oscillation frequency.’
Panel C summarizes how the oscillation frequency varied with blank duration for all observers. For both binocular rivalry and the ambiguous sphere the oscillation frequency decreases as the blank interval becomes longer, confirming the example of panels A and B (p < .01 for both stimuli; one-sided t-test comparing all observers’ slopes of oscillation frequency vs. blank duration with 0). These results indicate that an increase in blank duration is accompanied by a slowing down of the oscillation cycle.
Experiment 2: Comparison to continuous presentation
To what extent does the perceptual alternation cycle during intermittent viewing resemble the one observed during continuous viewing? Similarities between the alternation cycles in both paradigms could indicate a common neural basis. To make a direct comparison between the two paradigms we performed an additional experiment in which our observers tracked perception of a binocular rivalry stimulus during continuous presentation.
Methods
The ten observers of binocular rivalry in the previous experiment participated in an additional experiment in which the same binocular rivalry stimulus was presented continuously. Sessions lasted three minutes, and observers pressed and held the key corresponding to the dominant percept. During mixed perception of both eyes’ images simultaneously they released all keys.
In our comparison between intermittent and continuous presentation we compared the results from this experiment with those obtained at the default blank duration of 1.6 s in the previous experiment, as that was the only blank duration common to all ten observers.
Results
One hallmark characteristic of the alternation cycle during continuous presentation is the distribution of dominance durations. This distribution is invariably unimodal and right-skewed (e.g. Levelt, 1967). Figure 4A shows this distribution as obtained in our continuous viewing sessions. Its shape is as expected on the basis of previous findings.
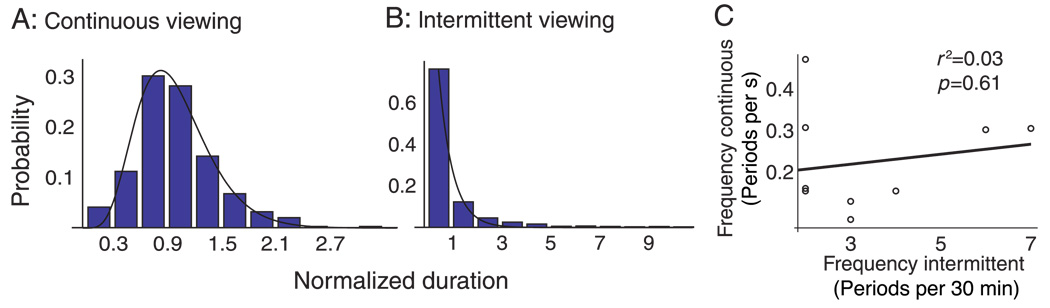
Figure 4.
Comparison between the alternation cycles during intermittent presentation and during continuous presentation. (A) and (B) The distribution of percept durations during continuous presentation is unimodal and right-skewed. During intermittent viewing this distribution is monotonically decreasing, with an exceptionally slowly decreasing tail. The histograms were obtained by first normalizing percept durations per observer by dividing by the mean, and then pooling data over observers. The durations are therefore dimensionless. (C) Inter-observer variation in percept durations during intermittent viewing is not obviously correlated with inter-observer variation during continuous viewing.
We investigated whether the intermittent viewing paradigm produces a similar distribution of percept durations. Figure 4B shows the probability histogram obtained during intermittent viewing of a binocular rivalry stimulus. Here the x-axis depicts the duration of individual dominance episodes in terms of (normalized) number of presentations. The shape of the distribution is qualitatively different from that obtained during continuous presentation: instead of peaking at an intermediate duration, this distribution is monotonically decreasing.
The lack of a peak at intermediate durations in Figure 4B is not due to the choice of bin size in this particular histogram. The data in this panel were normalized by dividing by the average percept duration per observer and thus have no unit; however, in the raw data we can investigate percept durations in terms of the number of presentations per dominance episode (perception rarely alternates within our 0.6 s presentations). In those raw data it turns out that dominance periods of one single presentation are most common, followed by those of two presentations, then three—monotonically decreasing up to about ten presentations, where dominance periods become increasingly rare. Thus, regardless of bin size, the distribution of percept durations during intermittent viewing does not show a peak like the one during continuous viewing does.
Although the distribution in Figure 4B somewhat resembles an exponential distribution, it may be noted that it has a much slower falloff toward the right. The high bars near the y-axis in Figure 4B reflect mainly the many short dominance durations that are scored during brief periods of unstable perception, which typically occur between two periods of opposite stabilized perception. During these unstable periods, visible even in the smoothed time traces of Figure 2A and Figure 3A, perception often erratically goes back and forth every few presentations before re-stabilizing. In contrast, during stabilized episodes the same percept often dominates for tens to hundreds of presentations in a row. Those stabilized episodes thus provide observations far beyond the right-ward edge of Figure 4B, and well beyond the range of durations that would be predicted by the relatively rapid falloff of an exponential distribution. This indicates that the distribution at hand has a rightward tail that decreases much more slowly than exponentially.
As a second comparison between intermittent and continuous presentation, we examined inter-observer variability in the frequency of perceptual alternations. There is a considerable spread among observers in the average perceptual alternation frequency, which provides a handle to compare various aspects of ambiguous perception (Carter & Pettigrew, 2003; Pastukhov & Braun, 2007; Pearson, Tadin, & Blake, 2007; Pressnitzer & Hupé, 2006). In our case the reasoning is as follows: if the alternation frequency of an observer during intermittent viewing is correlated with their alternation frequency during continuous viewing, this suggests that their neural bases may partially overlap.
Figure 4C plots alternation frequencies of nine individual observers during continuous viewing against their alternation frequencies during intermittent viewing. We do not observe a significant correlation between both variables ( p = 0.61). This suggests that alternations in both paradigms may draw on distinct neural mechanisms.
We were concerned that a potential correlation in Figure 4C might be masked by the two individual points in the top left of the graph. If we were to remove these two points we would find a positive correlation at the p < 0.05 level. Is there a reason to remove these points? The points correspond to two observers who combine a relatively high alternation frequency during continuous viewing with a low alternation frequency during intermittent viewing. We wondered whether their low number of alternations during intermittent viewing might be a spurious result, caused by a severe bias toward one of the two percepts. Such a bias could prevent alternations during intermittent viewing, while leaving continuous viewing relatively unaffected (Carter & Cavanagh, 2007). To control for this possibility we calculated each observer’s bias during intermittent viewing as the fraction of all presentations on which their preferred percept dominated. For these two observers the fractions were 0.6 and 0.62, respectively, compared to 0.59 averaged over the remaining observers. Hence, we conclude bias has had little influence on these data points. This provides no justification for removing these two data points from our analysis, supporting the conclusion that percept durations are not correlated between the two presentation paradigms.
Together, these results indicate that the alternation cycle observed during intermittent viewing has several features that distinguish it from the one during continuous viewing.
Computational model
Besides perceptual stabilization, intermittent presentation of an ambiguous stimulus can also give rise to a phenomenon that may be called perceptual destabilization. This occurs when the blank interval between presentations is relatively brief (between about 100 and 500 ms). Then, the percept that gains dominance at a given stimulus onset is generally the one that was suppressed on the previous presentation. In contrast to longer blank durations, which tend to give rise to percept repetitions, these brief blank durations thus cause perception to continually alternate from one presentation to the next (Klink, van Ee, Nijs et al., 2008; Kornmeier & Bach, 2004; Noest et al., 2007; Orbach et al., 1966).
Both perceptual stabilization and perceptual destabilization have been explained within the context of a single computational model (Brascamp et al., 2008; Noest et al., 2007). We will spend some time discussing this model, because our analyses indicate that the same model can also accommodate our present observations.
When alternations occur during intermittent viewing they usually do not occur during a single presentation. Instead, an alternation during intermittent viewing often comes about when the previously unperceived interpretation gains instant dominance at the next stimulus presentation. Thus, it was argued by Noest et al. (2007) that understanding perceptual stabilization and destabilization requires insight into the process that selects a percept at stimulus onset, rather than into the process that governs alternations during continuous presentation.
The model describes this selection process as a competition between mutually inhibitory neuron pools that each code a particular percept. To explain perceptual stabilization and destabilization the model relies on the idea that during dominance of a given percept the response characteristics of the neurons that code that percept are altered in a manner that does not immediately revert when dominance ends. Hence, these response characteristics carry a memory of prior dominance.
During perceptual stabilization this process allows neurons that code a previously dominant percept to respond more readily when the stimulus next appears. As a result, percept selection at stimulus appearance is biased toward the percept that dominated on prior presentations, and stabilization ensues.
During perceptual destabilization, in contrast, the changes in response characteristics are such that the neurons that code a previously dominant percept have a competitive disadvantage when the stimulus next appears. Thus, every stimulus appearance tends to prompt dominance of the previously suppressed percept, causing ongoing alternations from every presentation to the next.
Accounting for the existence of both stabilization and destabilization was a central objective of the model by Noest et al. (2007). How the model achieves this is explained in more detail in Appendix A. In short, the model posits that the trace that is left by perceptual dominance alters the response characteristics of the neurons that code the dominant percept in two distinct ways. One of these changes tends to benefit the previously dominant percept at the subsequent stimulus onset and thus causes perceptual stabilization, whereas the other tends to impede the re-emergence of that percept and, thus, can cause destabilization. Importantly, the relative functional strength of the two opponent effects depends on the level that the trace has reached. This is because the two effects grow at different rates as the trace accumulates. In general, a weak trace of past dominance primarily strengthens the associated representation and facilitates repeated dominance, whereas a strong trace tends to weaken the representation and prompt an alternation.
The existence of two counteracting effects of a trace of past dominance may seem particularly counterintuitive if one thinks of this trace in terms of persistent neural firing. The model, however, does not implement traces as persistent firing but as altered sensitivity of the neurons that represent a given percept. Many distinct effects of prior activity on neural sensitivity have been demonstrated. Such effects can either facilitate or impair neural response, and positive and negative effects often act simultaneously (e.g. Zucker & Regehr, 2002).
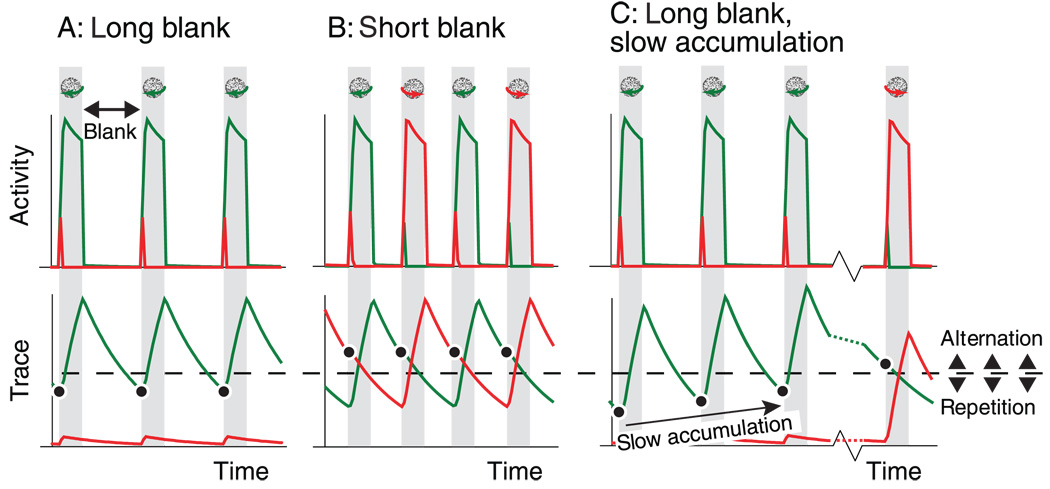
The properties of the model by Noest et al. (2007) are consistent with the psychophysical observation of perceptual stabilization and destabilization. A trace that has accumulated during dominance decays during stimulus absence. As a result, the longer a blank duration is, the lower the trace can fall before the next stimulus appearance. This explains why long blank durations tend to favor percept repetition (associated with a weak trace), whereas short blank durations tend to prompt alternations (due to a stronger trace). Figure 5A and 5B give a simplified illustration of this principle. The top panels show neural activity associated with perception of an ambiguous sphere that is presented intermittently. The bottom panels show the accumulation and decay of traces of past perception on the same time axis as the top panels. During dominance of a percept the associated trace builds up, whereas during suppression and during stimulus absence it decays. To schematically illustrate the difference between high and low levels of these traces, the dashed line separates levels that cause repetition from higher levels that cause alternation. In panel A the blank durations are long enough for a trace to fall below the dashed line before the moment a percept is selected at the next stimulus appearance (marked by a black dot), thus prompting repetitions. In panel B, in contrast, there is insufficient time for a trace to fall below the dashed line, and alternations ensue. A complete account of these principles is provided in Appendix A.
Figure 5.
Schematic illustration of the present model account of perception during intermittent viewing. Top panels: activity of the two percepts’ neural representations. Bottom panels: resulting traces that determine perception at the next stimulus appearance. The central idea is that weak traces strengthen a representation and cause repeated dominance, whereas strong traces weaken a representation and cause suppression. (A) Long blank durations allow traces to fall to a level that strengthens a representation (below dashed line) before the next stimulus onset, resulting in stabilization. (B) Brief blank durations do not allow sufficient decay between presentations, leading to perceptual alternations. (C) A trace that is composed of both fast and slow components can steadily grow from one presentation to the next. Then, blank durations that initially allow perceptual stabilization can, after many presentations, lead to an alternation when slow components lift the trace out of its stabilizing range.
Accommodating our findings with the model
Our experimental findings indicate that even at blank durations sufficiently long to promote stabilization, perception will eventually alternate. This can be explained within the framework sketched above. Details of this explanation are again given in Appendix A. It has previously been shown that traces of past perception accumulate on multiple timescales simultaneously (Brascamp et al., 2008). Not only do they build up and decay quickly with every presentation cycle; they also accumulate more slowly from one presentation to the next. This slow accumulation proceeds on timescales of minutes at least. Figure 5C schematically illustrates the consequences of this slow accumulation in the context of the present findings. In Figure 5C blank intervals are long enough to allow a trace of past perception to fall below the dashed line between presentations, and thus for perception to stabilize. However, slow components cause the trace of the stabilized percept to rise slightly higher with every presentation. This continues until the trace rises to a point where it is so strong that it prompts an alternation (rightmost black dot).
The account schematically illustrated in Figure 5 is consistent with the finding that alternations during intermittent presentation occur at regular intervals. The duration of the intervals that separate two consecutive perceptual alternations is determined by the number of presentations required for the accumulating trace to rise out of the ‘stabilizing’ range. If the trace starts accumulating from about the same level during every stabilization period, the resulting alternations will thus be approximately periodic. This requirement is met as long as the accumulating trace does not contain a major contribution of components that are even an order of magnitude slower than the slow ones we consider, and that would gradually accumulate from one stabilization period to the next. Such accumulation would result in progressive acceleration of the oscillation cycle, which, at least in our 30-minute sessions, is not evident.
Our finding that the rate of perceptual alternations increases with briefer blank intervals between presentations, is also consistent with the present account. First, at briefer blank durations the stimulus is present during a larger fraction of the time, so the slow components of the trace will take less time to accumulate. Second, a brief blank duration allows less time for the fast components to decay before the next stimulus onset. Thus, a smaller contribution of the slowly accumulating components is required before the combined trace causes an alternation.
In summary, this model account holds that during intermittent presentation of an ambiguous stimulus using relatively long blank durations perception initially stabilizes because perceptual dominance leaves a trace that helps the same percept gain dominance again when the stimulus reappears. Furthermore, as the trace builds further it reaches a point at which it takes on a suppressive quality, causing perception to alternate to the alternative interpretation. This alternative interpretation, in turn, will also accumulate a trace that initially keeps it stabilized but that eventually causes it to lose dominance, thus perpetuating the slow alternation cycle.
Experiment 3: Testing a model prediction
Prediction
We performed an additional experiment to test a prediction made by the above account. The prediction concerns the relation between blank duration and alternation probability. In the illustration of Figure 5, where the same blank duration (either short or long) was consistently applied throughout a session, perceptual alternations were scarce at long blank durations (panel A) and more common at short ones (panel B). The present prediction regards the situation where the blank duration is not fixed but, instead, varies randomly within a session. Under this condition, the model predicts that the relation between blank duration and alternation probability should not remain constant, but should slowly evolve within a session. For example, randomly drawing a short blank duration may promote an alternation at one moment during a session, but that same blank duration may actually prevent an alternation at another moment.
A full derivation of our prediction is provided in Appendix A, but some intuitive understanding can be gained without in depth analysis. The prediction relies on the interaction between slow and fast components of a trace of past perception. Imagine a moment during an experimental session at which one percept has been stabilized for several minutes. The trace of that stabilized percept has had sufficient time to slowly accumulate, even to a point where slow components bring the system close to an alternation (cf. Figure 5C). Besides this slow accumulation, there would still be a fast buildup and decay of the trace during every presentation cycle. Applying a brief blank duration at this moment would allow less time for these fast components to decay before the next stimulus onset. As a result the slow and fast components would both be relatively high at the next stimulus appearance, and jointly elevate the probability of a perceptual alternation. If, instead, a long blank duration were applied at this point, the resulting decay of the fast components would partly compensate for the highly accumulated slow components, thus reducing the likelihood of an alternation. In other words, toward the end of a stabilization period the probability of an alternation has a negative dependence on the duration of the blank period.
Now imagine that a long stabilization period of the first percept has just ended, and the second percept has just started to become stabilized. Because stabilization has just started, the trace that is keeping this second percept stabilized is largely composed of fast components. In contrast, any lingering trace of the first percept would contain slower components. If a long blank duration were applied at this moment, fast components could decay to a point where little of the stabilizing trace is left. As a result a perceptual alternation back to the first percept would become likely. Note that at the beginning of stabilization most alternations come about in this way: because the trace of the currently stabilized percept is too weak. The likelihood of an alternation occurring because the trace has become too strong, such as after prolonged stabilization, is small because there has been little opportunity for slow components to accumulate. We thus predict that at the beginning of a stabilization period the probability of an alternation has a positive dependence on the duration of the blank period.
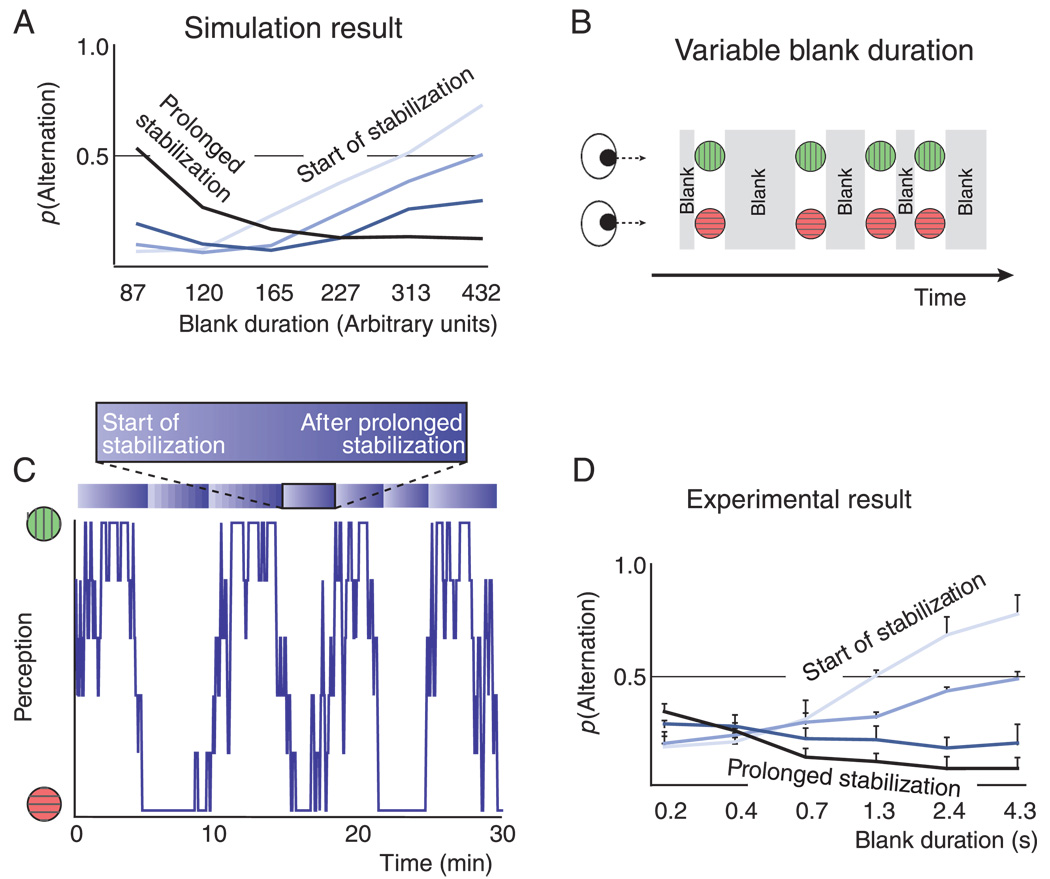
In sum, the model predicts that the relation between blank duration and alternation probability should reverse over the course of a period of stabilized perception. At the beginning of every stabilization period the probability that perception would alternate from one presentation to the next should rise with increasing blank duration, whereas toward the end of every stabilization period the probability should fall with increasing blank duration. Figure 6A illustrates this prediction by plotting simulation results produced by the model (see Appendix A). The figure displays alternation probability as a function of blank duration, as observed during a simulation run (see Methods). Data are separated into the period near the start of stabilization (light blue curve), the period after prolonged stabilization (dark blue curve), and two intermediate stages (intermediate colors).
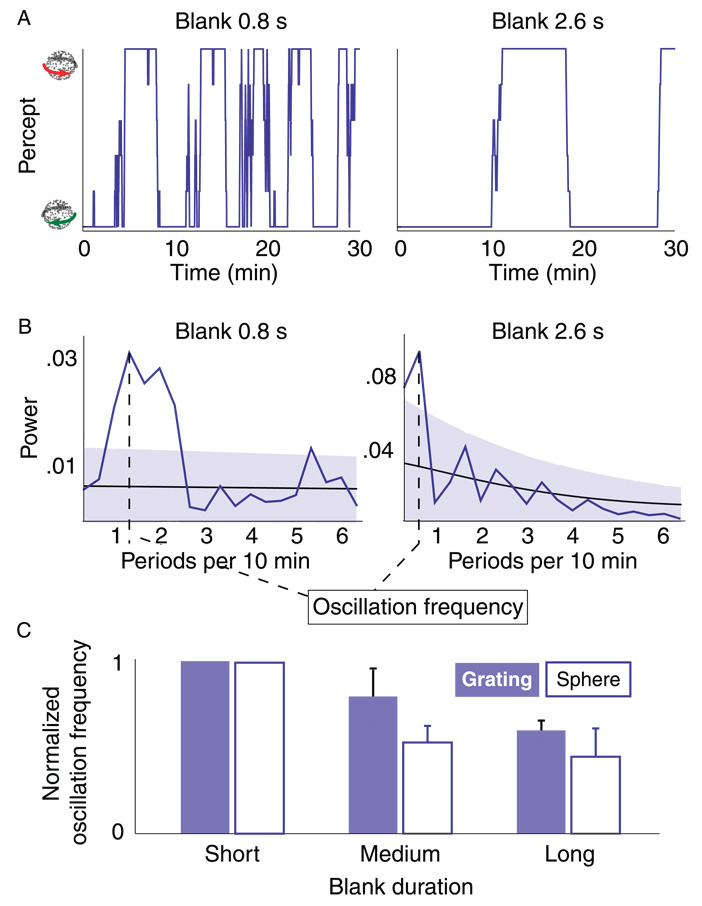
Figure 6.
Model prediction and empirical test. (A) Simulation result. The model predicts that the probability of an alternation should rise with blank duration at the beginning of a stabilization period (light curve), but fall with blank duration toward the end of a stabilization period (dark curve). During the periods in between these two extremes the relation between blank duration and alternation probability should be intermediate (intermediate colors). (B) Experimental timeline. A binocular rivalry stimulus was presented intermittently, with a variable blank duration between consecutive presentations. (C) Perception during a typical session (box car filtered with a window size of 5 presentations to enhance clarity). Perception alternated often between both interpretations, but there were underlying periods during which the same interpretation dominated on the vast majority of presentations. In our analysis we separated data into groups according to the position within such a period of relatively stable perception, ranging from ‘at the start of stabilization’ to ‘after prolonged stabilization’. This is schematically indicated above the plot. (D) Experimental result. Relation between blank duration (x-axis) and the probability of a perceptual alternation from a given presentation to the next (y-axis). At the beginning of stabilization (light curve) long blank durations promote alternations. After prolonged stabilization (dark curve) long blank durations promote stability. Intermediate shades correspond to intermediate stages of the stabilization sequence. These findings agree with the model prediction (Figure 6A). Error bars are standard errors of the mean (n = 4).
We performed an experiment to test the prediction under consideration, regarding the relationship between blank duration and alternation probability. In this experiment we presented a binocular rivalry stimulus intermittently, randomly varying the blank duration between each pair of presentations (Figure 6B). We then analyzed the relation between blank duration and alternations probability, separately for various periods within a slow alternation cycle. Would the relation between blank duration and alternation probability be different at the beginning of a period of stabilized perception than at the end, as in the model simulations of Figure 6A?
Methods
Four observers who viewed binocular rivalry in the previous experiments (three naive) performed an additional experiment using the same stimulus. Sessions lasted thirty minutes, during which the stimulus was presented intermittently and observers reported perception via key presses. The duration of every stimulus presentation was 0.6 s. Importantly, the blank duration separating consecutive presentations was varied randomly throughout a session (Figure 6B). Led by the model prediction described above, our intention was to investigate the effect of blank duration on alternation probability, for various periods within the slow perceptual alternation cycle separately.
Each blank duration within these sessions was drawn from a set of six durations ranging from 215 ms to 4.3 s, equally spaced on a log axis. Our choice of durations was based on a number of constraints suggested by our previous experiments. To adequately study the effect of blank duration we wished to sample as wide a range of blank durations as possible. However, Experiment 1 had shown that an excess of long blank durations might slow the alternation cycle (Figure 3) to a point where no full cycle occurs within a 30-minute session, which would preclude our intended comparison between the beginning and the end of a stabilization period. Conversely, too many short blank durations could cause perception to alternate so frequently that the slow and periodic alternation cycle would never develop (Figure 5B). This, too, would interfere with our intention to compare the beginning and the end of a stabilization period. As a compromise between these considerations we chose to sample a wide range of blank durations but we adjusted the relative probabilities of each of the six durations being drawn, so that intermediate durations occurred more often than short or long ones. The two central durations each had a probability of 23%, the outermost pair each had a probability of 10%, and the remaining two each had a probability of 17%. If our range of durations is plotted on a log axis, these probabilities correspond to a normal distribution centered on the middle of the range. The abundance of intermediate blank durations in this design ensured a robust slow alternation cycle. The long and short durations were scarce enough not to interfere with this cycle, yet they did provide the broad sampling range we aimed for.
In the model the predicted change in the relation between alternation probability and blank duration is brought about by the accumulation of slow perceptual traces. In the analysis of this experiment we thus needed a measure of the extent of accumulation of these slow traces throughout our experimental sessions. A slow trace of a percept is expected to accumulate when that percept is dominant and to decay when it is not. As a measure of the extent of accumulation of a slow trace we thus calculated a low-pass filtered version of the corresponding dominance periods during a session. For instance, the slow trace of percept 1, T1, was calculated by numerically integrating
| (1) |
over a time series of perceptual reports. On every integration step the value of K was set to 1 if the corresponding percept was dominant. It was set to 0 both if the corresponding percept was suppressed and during blank intervals. The time constant τ was set to 50 s. This calculation was then repeated for the trace of percept 2, T2.
For every time point within a session we calculated the difference between T1 and T2. The first 75 presentations of each session were considered a startup period in terms of slow accumulation, and were not included in this analysis. We separated all remaining stimulus presentations within a session into four groups according to the four 25% percentiles of the associated difference values. This produced four categories, ranging from ‘at the start of stabilization’ to ‘after prolonged stabilization’ (Figure 6C). The category labeled ‘at the start of stabilization’ is associated with a T value of the dominant representation that is much lower than that of the non-dominant representation; the one labeled ‘after prolonged stabilization’ corresponds to very high T value of the dominant representation compared to the non-dominant representation.
By assessing the relation between blank duration and alternation probability for each of these categories separately, we could investigate how this relation evolves throughout a period of stabilized perception. Using this procedure we could test our model prediction, which holds that the relation between blank duration and alternation probability should change systematically over repetitions of the same percept during stabilization, and thus should differ between the four categories.
Results: The relation between blank duration and alternations evolves as predicted
Figure 6C plots smoothed perception during a typical session. The course of the plot is slightly more erratic than that of the plots in Figure 2A and Figure 3A, probably because of perceptual instability brought about by the irregular blank duration. In spite of these alternations, prolonged periods can be identified during which the same percept is seen on the great majority of presentations.
To test our prediction that the effect of the blank duration should change over the course of a stabilization period, we separated the tracking data into subsets according to the location within such a stabilization period.
For each subset separately we calculated the probability that perception would alternate to the non-stabilized percept on a given stimulus appearance, as a function of the blank duration preceding that appearance. Results for all observers were similar. Observer-averaged results are shown in Figure 6D. Like in Figure 6B and 6C, the shades from light to dark correspond to the progression from the beginning of a stabilization period to the end of a stabilization period. The lightest shaded curve shows that at the beginning of a stabilization period the probability of an alternation increases with increasing off-duration. The darkest shaded curve shows the opposite effect: if a percept has already been stabilized for a long time, a long blank interval tends to keep it stabilized, whereas a brief interval will more likely yield an alternation. The intermediate shades have intermediate courses.
This experiment confirms that the relation between blank duration and alternation probability reverses over the course of a period of stabilized perception, as predicted by the model. At the beginning of a stabilization period an increased blank duration promotes alternations, whereas toward the end of a stabilization period it prevents alternations. This result provides support for the model account that is illustrated in Figure 5 and detailed in Appendix A.
Discussion
The model and its implications
Previously, versions of the model employed here have accounted for other findings regarding perception of intermittent ambiguous stimuli. These include perceptual stabilization and perceptual destabilization (Klink, van Ee, Nijs et al., 2008; Noest et al., 2007), the influence of both short-term and long-term perceptual history on subsequent perception (Brascamp et al., 2008) and the interaction between perceptual history and volitional control (Klink, van Ee, Nijs et al., 2008). In the present work we demonstrate further agreement between the model and empirical observations in experiments that capitalize on the occurrence of alternations during intermittent viewing. The explanatory power of this account indicates that it may accurately capture the essential interactions underlying perception of intermittently presented ambiguous stimuli, thus explaining both why consecutive presentations prompt percept repetition in some conditions and why they prompt alternations in others.
The model provides a good starting point for understanding the neural underpinnings of these phenomena. Among other things, the success of the model suggests that:
perception of intermittent ambiguous stimuli depends on a competition between alternative neural representations at the moment of stimulus onset;
memory in this paradigm is mediated by accumulating changes in neural sensitivity—not persistent activity—which drive perception by affecting this competition process;
these sensitivity changes occur on multiple time-scales simultaneously, the slowest ones spanning dozens of minutes;
positive and negative sensitivity changes go hand in hand and, depending on the circumstances, the net perceptual outcome of these changes can be either facilitation or suppression.
The present model was developed specifically with the intermittent presentation paradigm in mind, and its main merit lies in its ability to account for perception in that paradigm. It should be mentioned that, although the model also produces alternations during continuous viewing, it does not presently account for the full range of phenomena that may be observed in that latter paradigm. The model has not been rigorously tested for its predictions on the relation between stimulus intensity and percept durations during continuous viewing, as laid down in Levelt’s four propositions (Brascamp et al., 2006; Klink, van Ee, & van Wezel, 2008; Levelt, 1966). Furthermore, like many other models of bistable perception the present one is currently not equipped to deal with periods of mixed dominance, where observers experience a combination of the two alternative percepts. For instance, during binocular rivalry one may perceive parts of both eyes’ images at the same time (Brascamp, Knapen, Kanai, van Ee, & van den Berg, 2007; Yang, Rose, & Blake, 1992), and the ambiguous rotating sphere may give rise to the perception of two convex shells sliding past each other (Hol, Koene, & van Ee, 2003).
An alternative model for perceptual stabilization was proposed by Wilson (2007). A discussion of this model in the light of previous findings was provided elsewhere (Brascamp et al., 2008; Pearson & Brascamp, 2008). Regarding the present data set, we encountered several deviations between our observations and the behavior of this second model.
The model by Wilson (2007) does not currently contain elements with time constants of decay larger than about a second, which means there is no accumulating change over prolonged presentation sequences. Neither our basic observation of a slow periodic alternation cycle (Figure 2 and Figure 3), nor the finding that alternation behavior changes gradually over the course of a session (Figure 6), can therefore be reproduced, because both require slow changes on the order of minutes.
Furthermore, it is doubtful whether the existing model architecture of Wilson (2007) can simply be augmented with slower components to accommodate the present results. A main difficulty regards our experimental observation that intermittent presentations cause both percept repetitions and percept alternations within the same session. The mixed occurrence of alternations and repetitions is key to the data patterns of Figure 2, Figure 3, and Figure 6. It seems difficult to accommodate these findings using this model because the model does not transition smoothly from alternations to repetitions during intermittent viewing. On the contrary, once the model is made to produce alternations by applying brief blank durations, this tends to bring it into a state where it is unable to revert to percept repetitions within the same intermittent presentation sequence. In other words, perception is predicted to alternate at every stimulus appearance for the rest of the session, regardless of blank duration. This behavior is at variance with the present experimental findings. An example of this model behavior can be observed at the parameter settings used by Wilson (2007), his Figure 7) to demonstrate perceptual stabilization, by temporarily changing the blank duration from 4.5 s down to 1.5 s (causing an appropriate transition from repetitions to alternations) and then setting it back to 4.5 s (causing the alternations to continue in spite of the long blank durations, in disagreement with empirical observations).
Neural correlates
As yet few direct measurements are available of the neural correlates of perceptual stabilization and destabilization. Does our model account offer any suggestions that could guide the search for these correlates?
An essential characteristic of the present account is that it supposes that information on past perception is retained in the form of neural sensitivity, rather than as activity. In that sense the trace that stores prior perception is thus more akin to a latent capacity to generate a percept upon renewed stimulation, than to an active maintenance of the percept during stimulus absence. A similar distinction between sensitivity-based storage and activity-based storage has been noted in the context of working memory, where it was proposed that synaptic facilitation could mediate working memory in the absence of delay period spiking activity (Mongillo, Barak, & Tsodyks, 2008). An important implication of this idea in the present context is that sensitivity changes, contrary to persistent spiking activity, may not be easily identified in neural measurements taken during stimulus absence. They should, however, be visible in the response to an appearing stimulus. Existing work has measured activity during blank periods, and indeed demonstrated that potentially stabilization-related signals can be detected during those periods (Maier, Logothetis, & Leopold, 2002; Sterzer & Rees, 2008). However, present theory thus suggests that it may be even more informative to measure while the stimulus is present.
Another remark regards the intuitive assumption that perceptual events that immediately surround a neural measurement should be informative regarding the state of the neural memory trace. For instance, in search of a neural trace in binocular rivalry it would seem obvious to group measurements into those that immediately follow perception of the left eye’s image, and contrast those with measurements that follow perception of the right eye’s image (Maier et al., 2002; Sterzer & Rees, 2008). The present results indicate that perception of intermittently presented ambiguous stimuli depends in part on changes that occur over many minutes (see also Brascamp et al., 2008). Thus, slow and possibly large changes in the underlying neural circuitry take place as the same percept is seen over and over. Conversely, a relatively small final increment may make perception alternate. This implies that no single perceptual event, either directly preceding or directly following a neural measurement, may be very informative regarding the neural memory state, and that a longer term percept history should be considered instead.
In sum, present theory suggests that a search for neural concomitants of perceptual stabilization may benefit from approaches that do not center on sustained activity measured during stimulus absence and that do not use a limited number of perceptual events to categorize measured data. Instead, our account suggests it may be useful to take measurements in the presence of the stimulus, and to categorize data on the basis of a minute-scale history of past perception, using methods similar to the one used to form the four categories of Figure 6.
Traces accumulating on multiple timescales
It was previously shown that perception of intermittently presented ambiguous stimuli depends on perceptual traces that accumulate on various timescales; some on the order of seconds and others on the order of a minute (Brascamp et al., 2008). Our present results indicate that the slowest of these traces of past perception can accumulate over many minutes, in some cases even more than ten minutes. This is required to account for the observation that consecutive alternations are separated by up to fifteen minutes in some of our sessions. Is it plausible that neurons that code an interpretation of an ambiguous stimulus undergo such slow changes in sensitivity?
In general, stimulation of sensory neurons causes both very fast and very slow changes in the neurons’ response characteristics (Albrecht, Farrar, & Hamilton, 1984; Bonds, 1991; Kohn, 2007; Müller, Metha, Krauskopf, & Lennie, 1999; Ohzawa, Sclar, & Freeman, 1985; Ulanovsky, Las, Farkas, & Nelken, 2004). Also, changes in psycho-physically measured detection thresholds, which presumably reflect changes in sensory neurons’ response characteristics, are still in progress after more than ten minutes of stimulation (Rose & Evans, 1983; Rose & Lowe, 1982). A similar observation, of particular importance in the present context, is that the time course of the perceptual alternation cycle during continuous viewing of an ambiguous stimulus systematically changes during a session, pointing to progressive changes in the underlying neural structures (Suzuki & Grabowecky, 2002; Hupé & Rubin, 2003; Lehky, 1995; Suzuki & Grabowecky, 2007; van Ee et al., 2005). Some of these changes in the alternation cycle are still ongoing even after thirty minutes of continuous viewing (Hollins & Hudnell, 1980) and are thus indicative of neural changes on a timescale of dozens of minutes.
In sum, it is plausible that neurons involved in processing ambiguous stimuli undergo sensitivity changes on many timescales when they are activated, including timescales slow enough to account for our findings.
Dissociation of prolonged viewing and intermittent viewing
The overall lack of evidence in Figure 4 for a direct association between the perceptual oscillation cycle during intermittent viewing and the one observed during continuous viewing, is consistent with other empirical findings that distinguish the two. Carter and Cavanagh (2007) demonstrated that certain perceptual biases affect perception during intermittent but not continuous viewing. Also, when an intermittent viewing cycle is temporarily replaced by a continuous presentation episode, perception can alternate during continuous viewing without disrupting stabilization during intermittent viewing (Brascamp et al., 2008; Pastukhov & Braun, 2008). Finally, drawing attention away from an ambiguous stimulus slows alternations during continuous viewing (Paffen, Alais, & Verstraten, 2006) but promotes them during intermittent viewing (Kanai & Verstraten, 2006).
The view that the alternation cycle during intermittent viewing is not directly related to the one during continuous viewing, is further supported by current models (Brascamp et al., 2008; Noest et al., 2007; Wilson, 2007). These ascribe alternations in both paradigms to distinct neural events (discussed in Pearson & Brascamp, 2008). Note that this does not mean that these models suggest distinct brain areas for each type of presentation paradigm. On the contrary, our current model, for instance, uses the same network to account for perception during intermittent viewing as well as during continuous viewing, even though the events that cause model alternations are different in both paradigms (Noest et al., 2007, see also Appendix A).
Facilitation and suppression
A key property of our interpretation of the data is that it predicts a trace of past perception to have a facilitatory effect on percept selection when it has a moderate level, but a suppressive effect when it has a higher level. Thus, a percept tends to recur at stimulus onset if it has dominated sufficiently to accumulate a moderate trace, but it tends to become suppressed if the trace has accumulated higher (Figure 5). This idea is consistent with many psychophysical observations.
In the context of intermittently viewed ambiguous stimuli, this idea explains both our present findings and the transition from perceptual stabilization to perceptual destabilization at shorter blank intervals observed elsewhere (Klink, van Ee, Nijs et al., 2008; Noest et al., 2007; Orbach et al., 1966; see Figure 5).
The idea also accounts for several findings regarding the effect of unambiguous stimuli on subsequent perception of an ambiguous stimulus. When a disambiguated version of an ambiguous stimulus is viewed prior to the ambiguous stimulus itself, this can either cause the second stimulus to be perceived in the same way as the first one, or it can prompt dominance of the alternative interpretation. The distinction between the two effects lies in what may be called the energy of the unambiguous stimulus: brief or low contrast prior stimuli facilitate repeated dominance, whereas long or high contrast prior stimuli suppress the previewed interpretation (Brascamp et al., 2007; Kanai & Verstraten, 2005; Long, Toppino, & Mondin, 1992; Pearson, Clifford, & Tong, 2008). This is consistent with the idea of a transition from facilitation to suppression that occurs as a trace of past perception grows stronger. Indeed, the same model employed here can also account for the effects of unambiguous prior stimuli in certain conditions (Brascamp et al., 2007).
Previous work has shown that the effect of a blank interval inserted between two presentations of an ambiguous stimulus depends on the duration of the blank interval. As shown in Figure 5, brief blank intervals prompt alternations, whereas long blank intervals cause repetitions (Klink, van Ee, Nijs et al., 2008; Noest et al., 2007; Orbach et al., 1966). How can this be reconciled with the data in Figure 6, which show that the relation between blank duration and alternation probability depends on long-term perceptual history? The key difference between previous experiments and the present one is that here we varied the blank duration within sessions, whereas previous work used blocked conditions. During a session of consistently long blank intervals, traces of past perception remain sufficiently low to prompt percept repetition on the vast majority of presentations (Figure 5C). During sessions of consistently short blank intervals, on the other hand, traces have little time to decay during stimulus absence. As a result, traces associated with both percepts are relatively high throughout a session, resulting in many perceptual alternations. The main difference between our experiment and previous ones, therefore, is that we could dissociate the effect of a single blank duration from that of accumulation during previous presentations, whereas previous experiments, using a blocked design, measured both effects simultaneously.
Functional role of facilitation
The coexistence of facilitatory and suppressive effects of prior perception appears to be a common principle in experiments involving ambiguous stimuli. It seems to apply regardless of whether the prior stimulus itself is ambiguous or not (see above). Furthermore, it is evident in data from a wide variety of ambiguous test stimuli, including the binocular rivalry stimuli and structure-from-motion stimuli in this study, but also Necker cubes (Long et al., 1992; Orbach et al., 1966) and ambiguous apparent motion stimuli (Kanai & Verstraten, 2005). Thus, it appears that combined facilitation and suppression is a principle embodied in all neural circuits susceptible to fluctuations in response to invariant stimulation.
What is the functional use of these two opposing effects of past perception? Here we would like to speculate that facilitation and suppression correspond to two distinct requirements that the visual system has to meet. Suppression in our paradigm is the result of diminished neural sensitivity following prior activation. Diminished sensitivity is ubiquitous in neural systems (Albrecht et al., 1984; Bonds, 1991; Ohzawa et al., 1985). Various ways have been proposed in which such response attenuation can improve the efficiency and effectiveness of neural coding (reviewed by Kohn, 2007). Perceptual facilitation, on the other hand, corresponds to enhanced neural sensitivity in response to prior stimulation. We suggest that this enhanced sensitivity has the functional purpose of increasing the system’s readiness to respond to newly appearing stimuli that were of behavioral relevance in the past. Although this idea must remain speculative at this point, it is consistent with several experimental findings.
First, the idea that facilitation specifically aids the processing of appearing stimuli is suggested by psycho-physical data showing that past perceptual dominance can facilitate repeated dominance at stimulus reappearance, but invariably has the opposite effect of shortening dominance episodes during continuous viewing (Blake, Sobel, & Gilroy, 2003; Blake, Westendorf, & Fox, 1990; Fang & He, 2004). Similarly, unambiguous prior stimuli cause shortened dominance of the previewed interpretation during continuous viewing (Blake, Westendorf, & Overton, 1980; Fang & He, 2004; Long & Toppino, 1994; Long et al., 1992). Thus, facilitation seems to be restricted to stimulus onsets, whereas suppression is prominently observed during continuous stimulation as well. This is consistent with the idea that facilitation is specifically related to the processing of appearing stimuli, rather than sustained stimuli.
The second part of our conjecture is that facilitation enhances processing of stimuli specifically if they were of behavioral relevance in the past. One fairly automatized way in which the visual system may make a rough distinction between previously relevant stimuli and previously irrelevant stimuli is by observing the outcome of past visual selection processes, because stimuli that previously gained preferential processing had some asset that dissociated them from competing stimuli. This reasoning is similar to that employed to interpret instances of sequential priming in attentional selection (Kristjansson, 2006; Maljkovic & Nakayama, 2000). Thus, we propose that facilitation is particularly pronounced if the prior stimulus gained some form of preferential processing, whereas suppression occurs more indiscriminately. This idea provides some context for the observation that ambiguous prior stimuli have a stronger tendency to facilitate than unambiguous prior stimuli. Unambiguous stimuli do not involve clear preferential processing of the perceived interpretation, which is consistent with the fact that they leave only a modestly facilitatory trace.
In sum, we hypothesize that the history-driven facilitation that is evidenced in the perception of appearing ambiguous stimuli is functionally associated with processing of relevant stimuli when they reappear.
Conclusion
We demonstrate that intermittent presentation of an ambiguous stimulus can give rise to slow periodic alternations between both perceptual interpretations. The rate of these alternations depends on the duration of the blank period that separates consecutive presentations. These results demonstrate the presence of perceptual traces that can span dozens of minutes. These and other findings can be understood in terms of the dynamics of a slowly evolving neural network model. This model provides a plausible account of the computations underlying perception in this paradigm, and brings us closer to understanding its neural basis.
Figure A1.
Perception at stimulus appearance depends on the values of traces Ai and Aj. (A) General layout of the ‘percept choice landscape’. At moderate levels of traces Ai and Aj the population with the highest A value gains dominance (white region). This may be called a facilitatory effect of A. It is responsible for percept repetition. At higher levels of traces Ai and Aj the population with the lowest A value gains dominance (gray region). Thus, here A has a suppressive effect, which causes percept alternation. (B) Long blank durations lead to percept repetition. (C) Short blank durations cause perception to alternate on consecutive presentations.
Acknowledgments
The authors would like to thank Jeroen van Boxtel, Tomas Knapen, and André Noest for valuable discussion and comments on the manuscript. André Noest is specifically thanked for his suggestion of Equation A5. Support: IIE Fulbright Program and Vanderbilt International Grant Program (JB); NHMRC (Aus.) CJ Martin Fellowship 457146 (JP); NIH EY13358 (RB).
Appendix A
Model account of slow perceptual alternations
When an ambiguous stimulus is shown intermittently, perception tends to stabilize if the blank interval between presentations is on the order of seconds (Leopold et al., 2002; Orbach et al., 1963). If the blank interval lies below about half a second, however, dominance on every presentation tends to be opposite to dominance on the previous presentation (Klink, van Ee, Nijs et al., 2008; Noest et al., 2007; Orbach et al., 1966). Noest et al. (2007) accommodated these observations using a model that forms the backbone of our present account. It consists of two neural populations, each representing one perceptual interpretation. The two interact via cross inhibition, and their activities are modulated by a slow signal that depends on past activity. Each population is represented by two differential equations, one describing activity H, and another one for the slow history-dependent signal, called A. The two populations are indexed as i and j. We show only the equations for i; those for j are the same with indices i and j exchanged. The time derivative of activity H of population i is given by:
| (A1) |
Here Xi is i’s excitatory input, γ is a constant that determines the strength of cross inhibition and S[Hj] is a sigmoid function of Hj (H itself can be viewed as a population averaged membrane potential, and S[H] as a population averaged firing rate). The value of time constant τH is set to 1. The two terms that include the slow signal A in this equation are discussed below. Noest et al. (2007) used standard ‘leaky integration’ of spiking activity S[H] to model the slow signal A:
| (A2) |
Here τ > 1 is a time constant, chosen such that A rises and falls over a large fraction of its total range within a single stimulus presentation and following blank interval, respectively. α is another constant that modulates the maximum level of A.
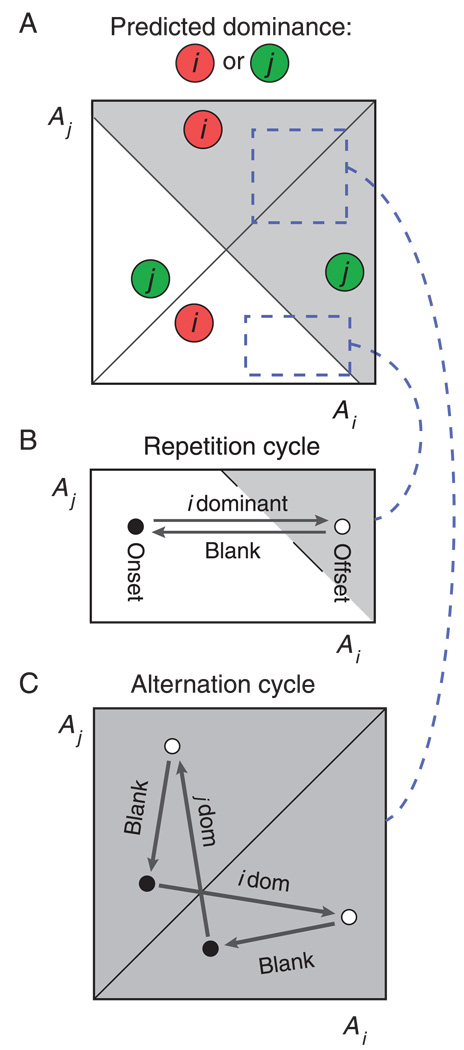
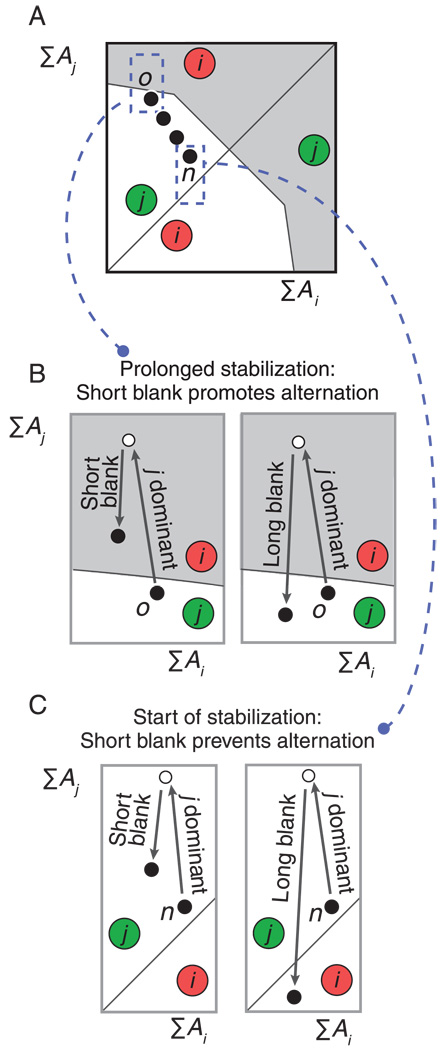
It can be seen in Equation A1 that the slow trace A affects activity H in two distinct ways. When A rises it reduces the response gain of this population via the term —(1 + Ai)Hi, whereas via the term +βAi it elevates the population’s resting activity. This combination of both a negative and a positive effect is responsible for the model’s capacity to generate both destabilization and stabilization of an intermittently presented ambiguous stimulus. When a stimulus reappears, the exact levels of Ai and Aj determine whether the positive effect or the negative effect of A will be decisive, and thus whether percept repetition or percept alternation will ensue. This is illustrated in Figure A1, panel A. Here the circled letters indicate which of the two perceptual interpretations will become dominant at stimulus appearance, as a function of Ai and Aj. The white area in panel A indicates regions where the percept associated with the highest A value becomes dominant. Because the recently dominant percept usually has the highest A value this corresponds to stabilization. The gray area indicates regions where the percept associated with the lowest A value becomes dominant, which corresponds to destabilization.
Figure A2.
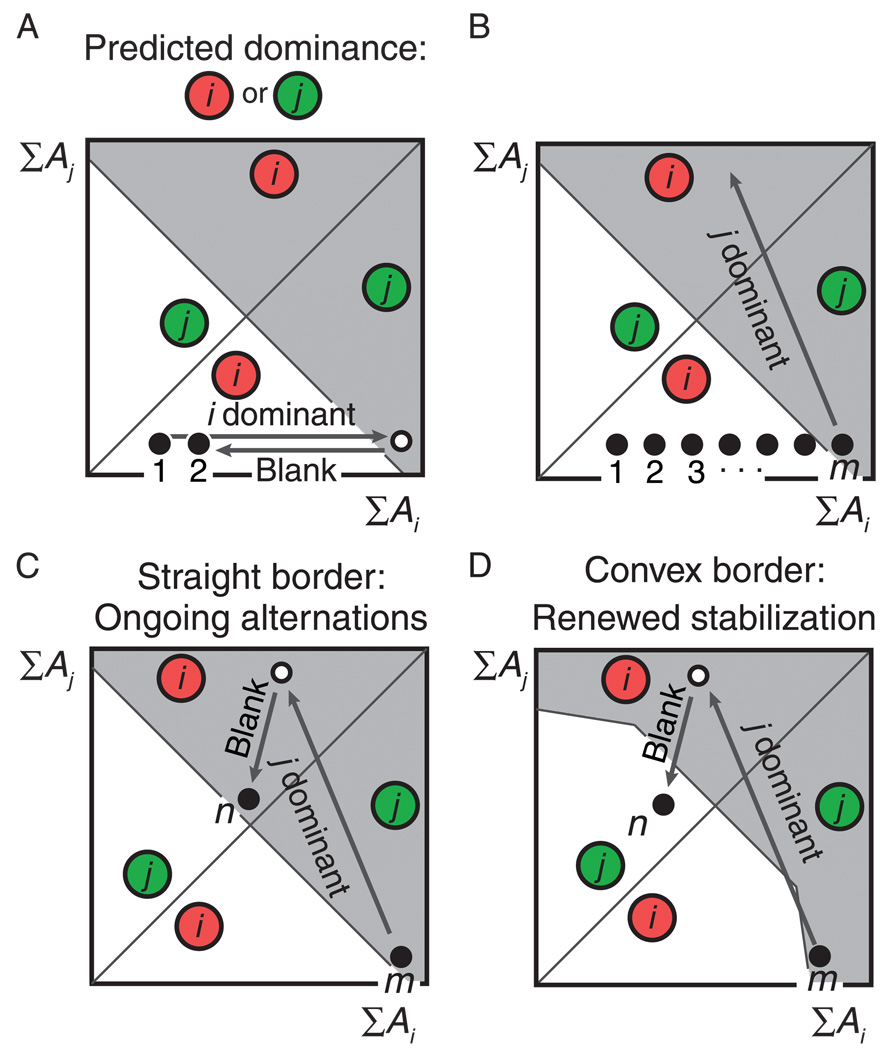
Expanding the model to accommodate the present findings. (A) and (B) The single-timescale trace (A) is replaced with a trace composed of both fast and slow components (∑A). (A) When slow components are added, the system slowly moves through the ‘percept choice landscape’ from one stimulus onset (black ball marked ‘1’) to the next (black ball marked ‘2’). (B) When this is repeated over many presentations, a perceptual alternation ensues at the first stimulus appearance at which the system is located in the gray region (black ball marked ‘m’). (C) Without further modification stimulus onset ‘m’ marks the start of ongoing perceptual alternations. This is not what is observed experimentally. (D) When the model is adjusted such that the border between the white and gray regions is convex rather than straight, stimulus onset ‘m’ marks the beginning of a stabilization period of the opposite percept j, in agreement with empirical results.
This approach assumes that percept selection is fully determined by the levels of Ai and Aj. This assumption is accurate, provided that both competing populations receive equal input (Xi = Xj), and that the blank duration is long enough for fast activities Hi and Hj themselves to fall to baseline before the stimulus reappears. This latter requirement can be expected to be met at all blank durations over about 100 ms.
Figure A1, panel B magnifies one section of panel A. It shows the course of the system in terms of this landscape during intermittent presentation with long blank intervals, prompting perceptual stabilization. The arrows indicate changes in A values during perceptual dominance of percept i (from the black ball to the white ball) and during stimulus absence (from the white ball back to the black one). Long periods of stimulus absence allow the system to sink into the white region before every stimulus onset (black ball). Thus, the same percept (i in this case) gains dominance on every presentation. Figure A1, panel C zooms in on another section of panel A. It demonstrates the situation when short blank intervals are applied instead of long ones. In this panel the system does not have time to fall into the white region before the stimulus reappears. Instead, the system remains in the gray region, resulting in opposite percepts on consecutive presentations: perceptual destabilization.
This basic model accounts for the existence of both perceptual stabilization and perceptual destabilization during intermittent viewing. However, it does not explain our present finding that the system slowly oscillates between periods of stable perception of one percept and periods of stable perception of the other. To account for that finding the model has to be expanded in two ways.
In the main text we argue that the infrequent and evenly spaced perceptual alternations in our paradigm are due to accumulation of A over the course of presentations. The period that separates consecutive alternations can be as long as ten minutes (barring the incidental perceptual alternation back and forth). This implies that A may accumulate over the course of hundreds of presentations: much slower than the time constant in Equation A2 allows. Thus, the model has to be expanded to include multiple A terms; faster ones responsible for up and down movements through the landscape during every single stimulus on–off cycle, and slower ones that cause the gradual accumulation of A over many presentations. We incorporate these requirements by replacing Equation A2 with three copies of the same equation, each for a different timescale.
| (A3a) |
| (A3b) |
| (A3c) |
Here τ1, τ2 and τ3 are three different time constants; one on the order of seconds, one on the order of minutes and one on the order of tens of minutes. In Equation A1 the A terms should now be replaced with the sum of A1, A2 and A3, written as ΣA.
| (A4) |
With this extension the model can account for the accumulation of slow changes over the course of multiple minutes, and it can explain how this accumulation can terminate a prolonged period of stable perception. This is illustrated in Figure A2, panels A and B. The two black dots in panel A represent two consecutive stimulus onsets, marked ‘1’ and ‘2.’ Percept i gains dominance at onset 1, and the resulting accumulation of ΣAi is reflected by the gray arrow marked ‘i dominant’. After the stimulus disappears (white dot) ΣAi decays as indicated by the arrow marked ‘blank’. These arrows reflect motions that are primarily due to the faster components of ΣAi, which respond relatively quickly to perceptual dominance and stimulus removal. However, some accumulation of the slower components also takes place. This slower accumulation ensures that at the moment of the subsequent stimulus onset (marked ‘2’) the system state has shifted to the right with respect to the previous onset (marked ‘1’). Panel B shows how this sequence is repeated over many presentations, until slow components have lifted ΣAi so far along the x-axis that, at a stimulus appearance marked ‘m,’ the system reaches the gray region. As a result percept j gains dominance, which constitutes a perceptual alternation. In other words, although an elevated level of ΣAi is initially responsible for the occurrence of stabilization, further accumulation of ΣAi over the course of a stabilization period causes perception to alternate. This means that perceptual stabilization is a process that eventually terminates itself. Below we will detail why the alternations produced by this model occur at regular intervals, as observed experimentally.
Figure A3.
Model prediction on the relation between randomly varied blank durations and alternation probability. (A) A period of stabilized perception of j continues from presentation ‘n’ through ‘o,’ and ends when further accumulation ∑Aj lifts the system across the region boundary on the top left of the landscape. (B) Outtake of panel A. Toward the end of the stabilization period alternations are promoted by applying short blank durations, whereas long blank durations strengthen perceptual stability. (C) Another outtake of panel A. At the beginning of the stabilization period stability can be strengthened by applying short blank durations (left), whereas long blank durations induce alternations to i. Simulation results using this model are presented in main text Figure 6A.
Note that independent empirical evidence has previously prompted the same conclusion that both fast and slow timescales of accumulation are required (Brascamp et al., 2008). That previous work indicated slow processes with time constants on the order of a minute; the extremely long stabilization periods in the present work show that even slower processes must be present as well.
The second extension implied by our findings is more intricate than the first. Its necessity is illustrated in panel C of Figure A2. This panel is essentially the continuation of panel B. It shows how the system would continue after having reached presentation m, if we do not modify the model any further. As soon interpretation j gains dominance over interpretation i, the system crosses to the opposite side of the landscape (arrow marked ‘j dominant’). This is because ΣAj accumulates while ΣAi decays. During the subsequent blank interval both ΣAi and ΣAj drop again (arrow marked ‘blank’). However, at the vast majority of parameter settings using this model, this drop does not move the system into the white region of the landscape. Instead, it stays in the gray region, and dominance would alternate back to interpretation i on the next presentation. Thus, once a slow trace has built up sufficiently to reach the gray region, it tends to stick in that region on subsequent presentations. As a result, the model without further modification predicts that a period of prolonged stabilized perception (Figure A2, panel B) will be followed by an indefinite period of constantly alternating perception! These alternations are very similar to what is observed at brief blank durations (Figure A1, panel C), but in this case they do not occur because brief blank durations allow insufficient time for fast components of ΣA to decay, but because slower components have been allowed to accumulate extensively.
In our experiments we do not observe stabilization giving way to ongoing perceptual alternations. Instead, after a period of stabilized perception of one interpretation has ended, we observe stabilized perception of the opposite interpretation. Geometrically speaking, this observation can be reconciled with our model, if we modify it such that the border between the white region and the gray region in the landscape is convex, not straight. Any type of convexity will tend to shift the model’s behavior in the direction indicated by the empirical data. This is illustrated in Figure A2, panel D. By definition a convex border runs lower near the x and y axis than it does near the diagonal ΣAi = ΣAj. Moreover, toward the end of a stabilized sequence the system is located close to one of the axes, because prolonged suppression has allowed the ΣA level of the non-stabilized interpretation to fall very low. Thus, the system crosses the border between the white region and the gray region at a location where that border runs relatively low. Panel D shows the first stimulus onset (marked ‘m’) that is located in the gray region, and also the subsequent course of events during dominance of interpretation j and the following blank interval. Due to the convexity, these events land the system in a region where the border between the two region runs relatively high. This allows the system to fall back into the white region (at the appearance marked ‘n’), in contrast to what happed with a straight border (panel C). This results in stabilized perception of the opposite percept, instead of continued perceptual alternations. In other words, if the border between the white region and the gray region is convex, a slow trace may build up sufficiently to terminate a sequence of perceptual stabilization, without automatically preventing stabilization of the opposite percept as well.
The alternations produced in this scenario are periodic if the slower components that comprise ΣA have the same values every time a stabilization period of a given percept begins, because then it takes them about the same number of presentations each time to accumulate to a level that causes an alternation. For instance, the appearance that initiates the first stabilization period of percept j (marked ‘n’ in Figure A2, panel D) should occur at about the same location in the landscape as the appearance that marks the beginning of the second stabilization period of that percept. There is good reason to expect this to be the case for all stabilization periods, with the exception of the very first stabilization period at the start of a session (Figure A2, panel B), which begins near the origin of the landscape. In the analysis of our empirical data we excluded the first 75 presentations of each session, thus avoiding this exceptional time period (see Methods). Regarding all remaining stabilization periods, the condition is met as long as no major accumulation takes place from one stabilization period to the next, so that each stabilization period of a given percept starts without much ‘memory’ of stabilization periods that preceded. It is possible that, in reality, A has components that are even slower than the slowest one considered here. In that case, true periodicity would give way to gradual acceleration of the alternation cycle over the course a session. This is not observed in our experiments, but it is conceivable that sessions longer than the 30 minutes we used would reveal such acceleration.
What does a convex border mean in terms of the architecture of our model? In fact, in the model the convexity can be achieved in a variety of ways, all involving the substitution of some linear term in Equation A4 with a nonlinear alternative. On the one hand, this means that convexity of the border is neurally plausible, because the assumption of linearity is often not met in neural systems. On the other hand it means that this particular finding is not a very rigorous model constraint and does not force any specific conclusion in terms of the underlying interactions. In our simulations we obtained a convex border by changing the way ΣA modulates the resting level of H or, in other words, by changing the +βΣA term:
| (A5) |
.
Here max[0, value] represents a threshold nonlinearity that returns value if value > 0, and 0 otherwise. β' is a constant. Thus, this equation implements a kind of cross interaction between trace ΣAi, and trace ΣAj. In the original model the resting level of Hi was modulated upward by its own memory trace ΣAi. In this expanded version it is both modulated upward by ΣAi and simultaneously modulated downward by the trace of the competing percept, ΣAj. Antagonistic interactions between oppositely tuned populations are common in neural systems (DeAngelis, Robson, Ohzawa, & Freeman, 1992; Snowden, Treue, Erickson, & Andersen, 1991).
Example parameter settings at which our model produces slow perceptual alternations similar to the ones observed experimentally are γ = 3.3, β = 0.21, β' = 0.04, α1 = 3, α2 = 0.5, α3 = 0.5, τ1 = 100, τ2 = 2000, τ3 = 4000 and X = 1. For the sigmoid function S[H] we use S[H] = . At these settings an alternation occurs about once every 175 presentations when using a stimulus presentation duration of 50 and a blank duration of 140 (arbitrary units).
It is worth commenting on the expansion from one timescale in Equation A2 to three timescales in Equation A3a–A3c. This expansion doubles the number of equations in the model, which appears to be a dramatic increase in the model’s free dimensions and thus a reduction of its parsimony. While this model is certainly less parsimonious than the original one (which was based on less data), several factors should be noted. First, the expansion merely copies Equation A2 to several timescales and thus changes little conceptually. Second, like many neural processes, the evolution of ΣA may well conform to a power law rather than to an exponential process (Anderson, 2001; Drew & Abbott, 2006). This casts a different light on the addition of timescales to our model, because capturing a power law function in differential equations necessitates an approximation using a series of exponential equations with different time constants.
In sum, we can account for our experimental findings using an expanded version of the model by Noest et al. (2007), which includes traces on multiple time scales, as well as a nonlinearity that causes a convex border between the white region and the gray region in the percept choice landscape.
Randomly varied blank durations
In the main text we predicted and confirmed that the relation between blank duration and alternation probability depends on the location within a long-term stabilization sequence. Figure A3 illustrates why our model implies this result. Panel A continues where Figure A2, panel D left off. A sequence of stable perception of interpretation i has just ended, and appearance ‘n’ marks the beginning of a sequence of j percepts. Each consecutive appearance, marked by the black balls starting at ‘n’ and ending at ‘o,’ is located slightly further to the top left. This is because during dominance of j ΣAj keeps accumulating, while the previously accumulated ΣAi gradually decays. The appearance marked ‘o’ is located toward the end of this stabilized sequence of j percepts. If ΣAj rises only slightly further from the location of appearance ‘o’, the system will land in the gray region where interpretation i is selected for dominance. When this happens, it will initiate a stabilized sequence of i percepts that is the mirror image of this panel, thus perpetuating the cycle of alternating periods of opposite dominance.
To see what this means in relation to the influence of the blank duration on perceptual alternations, we focus on two parts of the landscape in panel A. Panel B concentrates on the gray rectangle around appearance ‘o’. Starting from appearance ‘o’, dominance of interpretation j will cause further accumulation of ?Aj and decay of ΣAi. Whether the accumulation of ΣAj is sufficient to prompt a perceptual alternation, depends on the blank duration that follows. If the blank interval is relatively brief, the subsequent stimulus appearance is located in a region where interpretation i is selected and a perceptual alternation to interpretation i ensues (panel B, left image). If, on the other hand, the blank interval is longer, ΣAj has more time to decay (panel B, right image). As a result, the next stimulus appearance is located in a region where interpretation j is selected, resulting in percept repetition. Thus, once an interpretation has enjoyed a long period of stabilized perception, applying long blank durations should tend to prolong this stabilization even further, whereas short blank durations should prompt perceptual alternations.
It is worth commenting that all arrows in panel B of Figure A3 have a vertical extent that is much larger than their horizontal extent. The reason for this is that Figure A3 depicts a time period during which interpretation j repeatedly dominates over interpretation i, whereas interpretation i has enjoyed much dominance somewhat longer ago. Accordingly, the vertical extent of the arrows is mainly due to relatively fast components of ΣAj, which build up and decay during every presentation and subsequent blank, respectively. Interpretation i is not perceived in this time period. Therefore, its trace ΣAi is largely made up of residual slower components that have accumulated during the prior stabilization period of interpretation i (which was depicted in Figure A2, panel B). The gradual decay of these slow components is responsible for the modest horizontal extent of the arrows in these panels.
Panel C is a magnified view of the gray rectangle in panel A that is located around appearance ‘n’. Starting from appearance ‘n’ the system will move to the top left during stimulus presentation, as ΣAj accumulates and ΣAi decays. As in panel B, the vertical rise will be much larger than the horizontal decay, because ΣAj consists mainly of fast components and ΣAi mainly of slow ones. If the blank duration between presentations is sufficiently brief, the next stimulus appearance is located within a region where interpretation j is selected, causing percept repetition (panel C, left image). If, on the other hand, a longer blank interval is used, the system will sink into a region where interpretation i is selected, thus prompting a perceptual alternation to interpretation i (panel C, right image). This means that, if an interpretation has not yet enjoyed dominance on many consecutive presentations, applying short blank durations should help it gain dominance again, whereas long blank durations should cause perception to alternate to the opposite interpretation.
Bear in mind that the time period illustrated in Figure A3, panel C, is not only characterized by beginning stabilization of interpretation j, but also by recently terminated stabilization of interpretation i. As a result ΣAi is not zero, but still has significant slow components. If ΣAi were zero, applying long blank durations at this point would move the system toward the origin of the landscape, instead of across the main diagonal as sketched in panel C, right image. In that case, long blank durations would lead to a loss of all system memory, and thus a fifty-fifty chance of either percept becoming dominant at the next stimulus appearance (assuming no bias). Because in reality ΣAi is not zero, the probability of an alternation can actually rise above 50% as the blank duration is increased at this point in the stabilization sequence. This is observed both in our simulations and in our empirical data.
Figure 6A of the main text shows simulation results using our model. These simulations confirm that the model behaves as predicted in the qualitative analysis of Figure A3. The simulations were performed at the parameter settings provided above. Blank durations were drawn randomly from six values spaced equally along a log axis between 87 and 432 (arbitrary units). As in our psychophysical experiment, the two central durations were drawn most often (each constituted 25% of all blank intervals), the longest and shortest duration were drawn least often (8% each), and the intermediate durations fell in between at 17%. If our range of durations is plotted on a log axis, these probabilities correspond to a normal distribution centered on the middle of the range. The simulation was performed using a fourth order Runge-Kutta method with step size 0.1, with Poisson noise applied to S[H] on every step. This was implemented as
| (A6) |
S[H]old and S[H]new are the values before and after the addition of noise, respectively. Poisson(μ) is a random value from the Poisson distribution with mean μ and C is a constant that we set to 2000.
After performing the simulation we analyzed the simulated data using a procedure identical to the one we used to analyze the empirical data (see Methods). That is, we low-pass filtered our simulated perceptual data according to Equation 1 of the main text, using a time constant τ of 5000 (arbitrary units). This gave us one slow trace associated with past dominance of percept i and another one associated with past dominance of percept j. As described in the Methods section, the difference between these two traces can be used as an indication of the present stage within the alternation cycle. Thus, we separated all stimulus appearances into four categories according to the associated difference between the two traces. For each category we determined the probability of an alternation as a function of the preceding blank duration, leading to the plot depicted in Figure 6A of the main text.
Footnotes
Commercial relationships: none.
Contributor Information
J. W. Brascamp, Functional Neurobiology and Helmholtz Institute, Utrecht University, Utrecht, The Netherlands
J. Pearson, Department of Psychology, Vanderbilt University, Nashville, TN, USA
R. Blake, Department of Psychology, Vanderbilt University, Nashville, TN, USA
A. V. van den Berg, Functional Neurobiology and Helmholtz Institute, Utrecht University, Utrecht, The Netherlands
References
- Albrecht DG, Farrar SB, Hamilton DB. Spatial contrast adaptation characteristics of neurones recorded in the cat‘s visual cortex. The Journal of Physiology. 1984;347:713–739. doi: 10.1113/jphysiol.1984.sp015092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RB. The power law as an emergent property. Memory & Cognition. 2001;29:1061–1068. doi: 10.3758/bf03195767. [DOI] [PubMed] [Google Scholar]
- Blake R, Logothetis NK. Visual competition. Nature Reviews. Neuroscience. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Blake R, Sobel KV, Gilroy LA. Visual motion retards alternations between conflicting perceptual interpretations. Neuron. 2003;39:869–878. doi: 10.1016/s0896-6273(03)00495-1. [DOI] [PubMed] [Google Scholar]
- Blake R, Westendorf D, Fox R. Temporal perturbations of binocular rivalry. Perception & Psychophysics. 1990;48:593–602. doi: 10.3758/bf03211605. [DOI] [PubMed] [Google Scholar]
- Blake R, Westendorf DH, Overton R. What is suppressed during binocular rivalry? Perception. 1980;9:223–231. doi: 10.1068/p090223. [DOI] [PubMed] [Google Scholar]
- Bonds AB. Temporal dynamics of contrast gain in single cells of the cat striate cortex. Visual Neuroscience. 1991;6:239–255. doi: 10.1017/s0952523800006258. [DOI] [PubMed] [Google Scholar]
- Brascamp JW, Knapen TH, Kanai R, van Ee R, van den Berg AV. Flash suppression and flash facilitation in binocular rivalry. Journal of Vision. 2007;7:12. doi: 10.1167/7.12.12. 12 1–12, http://journalofvision.org/7/12/12/, doi:10.1167/7.12.12. [DOI] [PubMed] [Google Scholar]
- Brascamp JW, Knapen TH, Kanai R, Noest AJ, van Ee R, van den Berg AV. Multi-timescale perceptual history resolves visual ambiguity. PLoS ONE. 2008;3:e1497. doi: 10.1371/journal.pone.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brascamp JW, van Ee R, Noest AJ, Jacobs RH, van den Berg AV. The time course of binocular rivalry reveals a fundamental role of noise. Journal of Vision. 2006;6(11) doi: 10.1167/6.11.8. 8 1244–1256, http://journalofvision.org/ 6/11/8/, doi:10.1167/6.11.8. [DOI] [PubMed] [Google Scholar]
- Brouwer GJ, van Ee R. Endogenous influences on perceptual bistability depend on exogenous stimulus characteristics. Vision Research. 2006;46:3393–3402. doi: 10.1016/j.visres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Carter O, Cavanagh P. Onset rivalry: Brief presentation isolates an early independent phase of perceptual competition. PLoS ONE. 2007;2:e343. doi: 10.1371/journal.pone.0000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter OL, Pettigrew JD. A common oscillator for perceptual rivalries? Perception. 2003;32:295–305. doi: 10.1068/p3472. [DOI] [PubMed] [Google Scholar]
- Chen X, He S. Local factors determine the stabilization of monocular ambiguous and binocular rivalry stimuli. Current Biology. 2004;14:1013–1017. doi: 10.1016/j.cub.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Chong SC, Tadin D, Blake R. Endogenous attention prolongs dominance durations in binocular rivalry. Journal of Vision. 2005;5(11) doi: 10.1167/5.11.6. 6 1004–1012 http:// journalofvision.org/5/11/6/, doi:10.1167/5.11.6. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Robson JG, Ohzawa I, Freeman RD. Organization of suppression in receptive fields of neurons in cat visual cortex. 1992;68:144–163. doi: 10.1152/jn.1992.68.1.144. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Abbott LF. Models and properties of power-law adaptation in neural systems. Journal of Neurophysiology. 2006;96:826–833. doi: 10.1152/jn.00134.2006. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Stabilized structure-frommotion without disparity induces disparity adaptation. Current Biology. 2004;14:247–251. doi: 10.1016/j.cub.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Grossmann JK, Dobbins AC. Competition in bistable vision is attribute-specific. Vision Research. 2006;46:285–292. doi: 10.1016/j.visres.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hohwy J, Roepstorff A, Friston K. Predictive coding explains binocular rivalry: An epistemo-logical review. Cognition. 2008;108:687–701. doi: 10.1016/j.cognition.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Hol K, Koene A, van Ee R. Attention-biased multi-stable surface perception in three-dimensional structure-from-motion. Journal of Vision. 2003;3(7) doi: 10.1167/3.7.3. 3 486–498 http://journalofvision.org/3/7/3/, doi:10.1167/3.7.3. [DOI] [PubMed] [Google Scholar]
- Hollins M, Hudnell K. Adaptation of the binocular rivalry mechanism. Investigative Ophthalmology & Visual Science. 1980;19:1117–1120. [PubMed] [Google Scholar]
- Hupé JM, Rubin N. The dynamics of bistable alternation in ambiguous motion displays: A fresh look at plaids. Vision Research. 2003;43:531–548. doi: 10.1016/s0042-6989(02)00593-x. [DOI] [PubMed] [Google Scholar]
- Kanai R, Verstraten FA. Perceptual manifestations of fast neural plasticity: Motion priming, rapid motion aftereffect and perceptual sensitization. Vision Research. 2005;45:3109–3116. doi: 10.1016/j.visres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kanai R, Verstraten FA. Attentional modulation of perceptual stabilization. Proceedings of the Royal Society B: Biological Sciences. 2006;273:1217–1222. doi: 10.1098/rspb.2005.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Grabowecky M, Suzuki S. Stochastic resonance in binocular rivalry. Vision Research. 2006;46:392–406. doi: 10.1016/j.visres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Klink PC, van Ee R, van Wezel RJ. General validity of Levelt‘s propositions reveals common computational mechanisms for visual rivalry. PLoS ONE. 2008;3:e3473. doi: 10.1371/journal.pone.0003473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink PC, van Ee R, Nijs MM, Brouwer GJ, Noest AJ, van Wezel RJ. Early interactions between neuronal adaptation and voluntary control determine perceptual choices in bistable vision. Journal of Vision. 2008;8(5) doi: 10.1167/8.5.16. 16, 1–18 http://journalofvision. org/8/5/16/, doi:10.1167/8.5.16. [DOI] [PubMed] [Google Scholar]
- Kohn A. Visual adaptation: Physiology, mechanisms, and functional benefits. Journal of Neuro-physiology. 2007;97:3155–3164. doi: 10.1152/jn.00086.2007. [DOI] [PubMed] [Google Scholar]
- Kornmeier J, Bach M. Early neural activity in Necker-cube reversal: Evidence for low-level processing of a gestalt phenomenon. Psychophysiology. 2004;41:1–8. doi: 10.1046/j.1469-8986.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Kristjansson A. Rapid learning in attention shifts: A review. Visual Cognitive. 2006;13:324–362. [Google Scholar]
- Lehky SR. Binocular rivalry is not chaotic. Proceedings of the Royal Society B: Biological Sciences. 1995;259:71–76. doi: 10.1098/rspb.1995.0011. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Multistable phenomena: Changing views in perception. Trends in Cognitive Sciences. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Wilke M, Maier A, Logothetis NK. Stable perception of visually ambiguous patterns. Nature Neuroscience. 2002;5:605–609. doi: 10.1038/nn0602-851. [DOI] [PubMed] [Google Scholar]
- Levelt WJ. The alternation process in binocular rivalry. British Journal of Psychology. 1966;57:225–238. [Google Scholar]
- Levelt WJ. Note on the distribution of dominance times in binocular rivalry. British Journal of Psychology. 1967;58:143–145. doi: 10.1111/j.2044-8295.1967.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Long GM, Toppino TC. A new twist on the rotating-trapezoid illusion: Evidence for neural-adaptation effects. Perception. 1994;23:619–634. doi: 10.1068/p230619. [DOI] [PubMed] [Google Scholar]
- Long GM, Toppino TC, Mondin GW. Prime time: Fatigue and set effects in the perception of reversible figures. Perception & Psychophysics. 1992;52:609–616. doi: 10.3758/bf03211697. [DOI] [PubMed] [Google Scholar]
- Maier A, Logothetis NK, Leopold DA. Neural activity during stable perception of ambiguous displays in monkey visual cortex. Society for Neuro-science Abstracts. 2002 [Google Scholar]
- Maier A, Wilke M, Logothetis NK, Leopold DA. Perception of temporally interleaved ambiguous patterns. Current Biology. 2003;13:1076–1085. doi: 10.1016/s0960-9822(03)00414-7. [DOI] [PubMed] [Google Scholar]
- Maljkovic V, Nakayama K. Priming of pop-out: III. A short-term implicit memory system beneficial for rapid target selection. Visual Cognitive. 2000;7:571–595. [Google Scholar]
- Maloney LT, Dal Martello MF, Sahm C, Spillmann L. Past trials influence perception of ambiguous motion quartets through pattern completion. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3164–3169. doi: 10.1073/pnas.0407157102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M, Tong F. Can attention selectively bias bistable perception? Differences between binocular rivalry and ambiguous figures. Journal of Vision. 2004;4(7) doi: 10.1167/4.7.2. 2 539–551 http://journalofvision.org/4/7/2/, doi:10.1167/4.7.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- Moreno-Bote R, Rinzel J, Rubin N. Noise-induced alternations in an attractor network model of perceptual bistability. Journal of Neurophysiology. 2007;98:1125–1139. doi: 10.1152/jn.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science. 1999;285:1405–1408. doi: 10.1126/science.285.5432.1405. [DOI] [PubMed] [Google Scholar]
- Noest AJ, van Ee R, Nijs MM, van Wezel RJ. Percept-choice sequences driven by interrupted ambiguous stimuli: A low-level neural model. Journal of Vision. 2007;7(8) doi: 10.1167/7.8.10. 10, 1–14 http://journalofvision. org/7/8/10/, doi:10.1167/7.8.10. [DOI] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat‘s visual system. Journal of Neurophysiology. 1985;54:651–667. doi: 10.1152/jn.1985.54.3.651. [DOI] [PubMed] [Google Scholar]
- Orbach J, Ehrlich D, Heath HA. Reversibility of the Necker Cube. I. An examination of the concept of “Satiation of Orientation”. Perceptual and Motor Skills. 1963;17:439–458. doi: 10.2466/pms.1963.17.2.439. [DOI] [PubMed] [Google Scholar]
- Orbach J, Zucker E, Olson R. Reversibility of the Necker cube. VII. Reversal rate as a function of figure-on and figure-off durations. Perceptual and Motor Skills. 1966;22:615–618. [Google Scholar]
- Paffen CL, Alais D, Verstraten FA. Attention speeds binocular rivalry. Psychological Science. 2006;17:752–756. doi: 10.1111/j.1467-9280.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Pastukhov A, Braun J. Temporal characteristics of priming effects on the perception of ambiguous patterns [Abstract] Journal of Vision. 2007;7(9) 367a, http://journalofvision.org/7/9/367/, doi:10.1167/7.9.367. [Google Scholar]
- Pastukhov A, Braun J. A short-term memory of multi-stable perception. Journal of Vision. 2008;8(13) doi: 10.1167/8.13.7. 7,1–14 http://journalofvision.org/8/13/7/, doi:10.1167/ 8.13.7. [DOI] [PubMed] [Google Scholar]
- Pearson J, Brascamp J. Sensory memory for ambiguous vision. Trends in Cognitive Sciences. 2008;12:334–341. doi: 10.1016/j.tics.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Pearson J, Clifford CG. Determinants of visual awareness following interruptions during rivalry. Journal of Vision. 2004;4(3) doi: 10.1167/4.3.6. 6, 196–202 http:// journalofvision.org/4/3/6/, doi:10.1167/4.3.6. [DOI] [PubMed] [Google Scholar]
- Pearson J, Clifford CW. Mechanisms selectively engaged in rivalry: Normal vision habituates, rivalrous vision primes. Vision Research. 2005;45:707–714. doi: 10.1016/j.visres.2004.09.040. [DOI] [PubMed] [Google Scholar]
- Pearson J, Clifford CW, Tong F. The functional impact of mental imagery on conscious perception. Current Biology. 2008;18:982–986. doi: 10.1016/j.cub.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Tadin D, Blake R. The effects of transcranial magnetic stimulation on visual rivalry. Journal of Vision. 2007;7(7) doi: 10.1167/7.7.2. 2, 1–11 http://journalofvision. org/7/7/2/, doi:10.1167/7.7.2. [DOI] [PubMed] [Google Scholar]
- Pressnitzer D, Hupé JM. Temporal dynamics of auditory and visual bistability reveal common principles of perceptual organization. Current Biology. 2006;16:1351–1357. doi: 10.1016/j.cub.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Rose D, Evans R. Evidence against saturation of contrast adaptation in the human visual system. Perception & Psychophysics. 1983;34:158–160. doi: 10.3758/bf03211342. [DOI] [PubMed] [Google Scholar]
- Rose D, Lowe I. Dynamics of adaptation to contrast. Perception. 1982;11:505–528. doi: 10.1068/p110505. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Treue S, Erickson RG, Andersen RA. The response of area MT and V1 neurons to transparent motion. Journal of Neuro-science. 1991;11:2768–2785. doi: 10.1523/JNEUROSCI.11-09-02768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Rees G. A neural basis for percept stabilization in binocular rivalry. Journal of Cognitive Neuroscience. 2008;20:389–399. doi: 10.1162/jocn.2008.20039. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Grabowecky M. Evidence for perceptual “trapping” and adaptation in multistable binocular rivalry. Neuron. 2002;36:143–157. doi: 10.1016/s0896-6273(02)00934-0. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Grabowecky M. Long-term speeding in perceptual switches mediated by attention-dependent plasticity in cortical visual processing. Neuron. 2007;56:741–753. doi: 10.1016/j.neuron.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Farkas D, Nelken I. Multiple time scales of adaptation in auditory cortex neurons. Journal of Neuroscience. 2004;24:10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ee R, van Dam LC, Brouwer GJ. Voluntary control and the dynamics of perceptual bi-stability. Vision Research. 2005;45:41–55. doi: 10.1016/j.visres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Von Helmholtz H. vol. 9. Leipzig: Voss; 1867. Handbuch der physiologischen Optik. [Google Scholar]
- Wilson HR. Minimal physiological conditions for binocular rivalry and rivalry memory. Vision Research. 2007;47:2741–2750. doi: 10.1016/j.visres.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Rose D, Blake R. On the variety of percepts associated with dichoptic viewing of dissimilar monocular stimuli. Perception. 1992;21:47–62. doi: 10.1068/p210047. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annual Review of Physiology. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]