Abstract
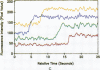
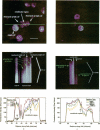
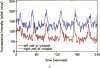
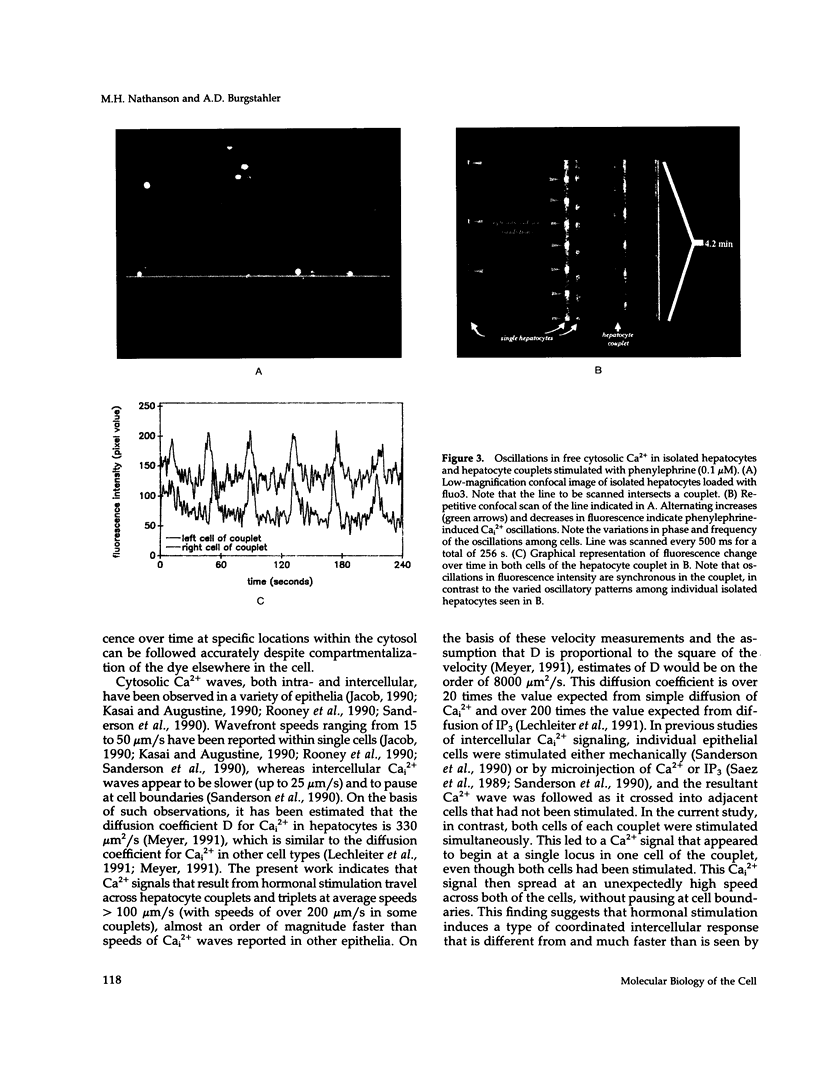
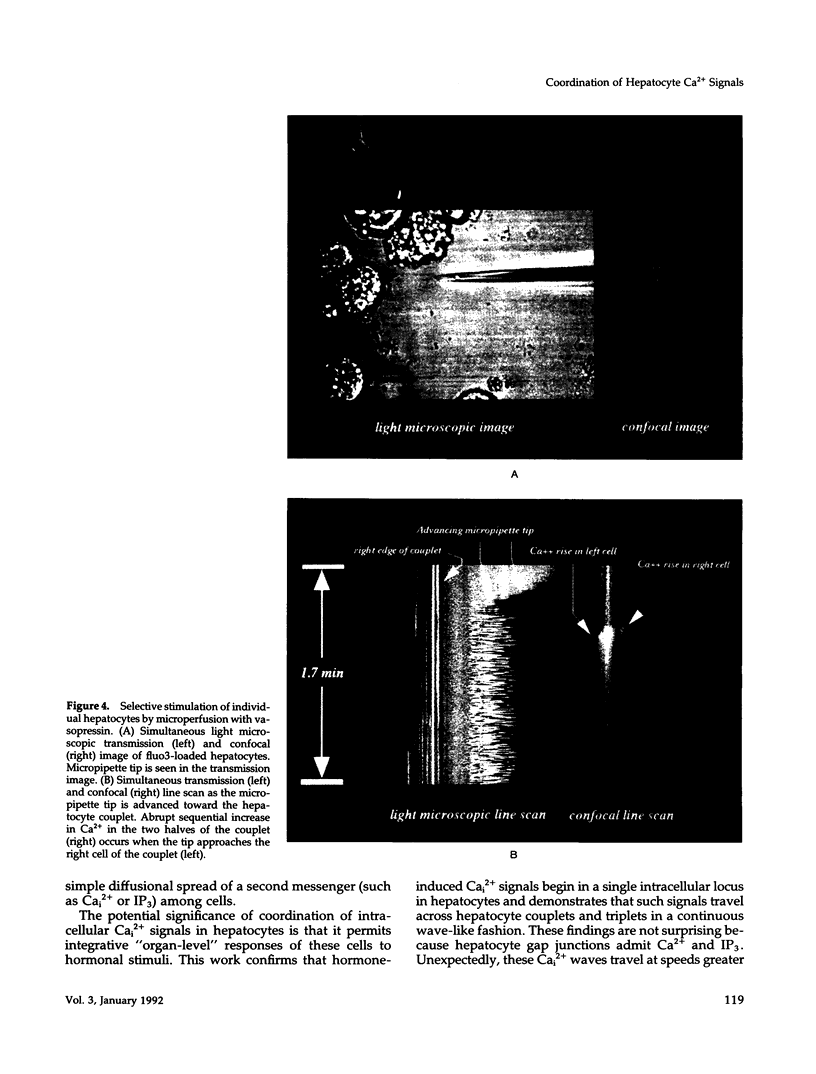
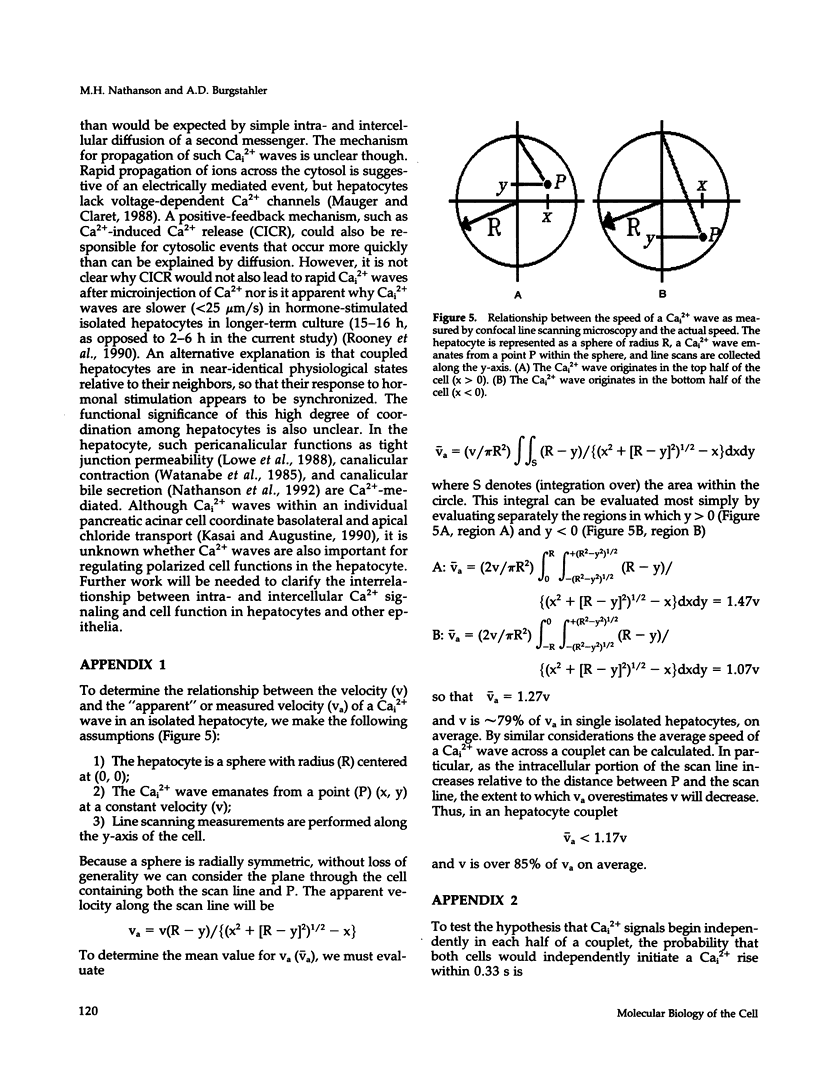
Excitable cells often display rapid coordination of hormone-induced intracellular calcium signals. Calcium elevations that begin in a single epithelial cell also may spread to adjacent cells, but coordination of hormone-induced signals among epithelial cells has not been described. We report the use of confocal microscopy to determine the inter- and intracellular distribution of cytosolic calcium in isolated rat hepatocyte couplets, an isolated epithelial cell system in which functional polarity is maintained. Both vasopressin and phenylephrine evoked sequential coordinated calcium signals in the couplets, even during cytosolic calcium oscillations. The coupling was abolished by closure of intercellular gap junction channels by treatment with octanol. These observations demonstrate that hormone-induced intracellular calcium signals are coordinated among hepatocytes and suggest that gap junction channels mediate this intercellular integration of tissue responsiveness.
Full text
PDF

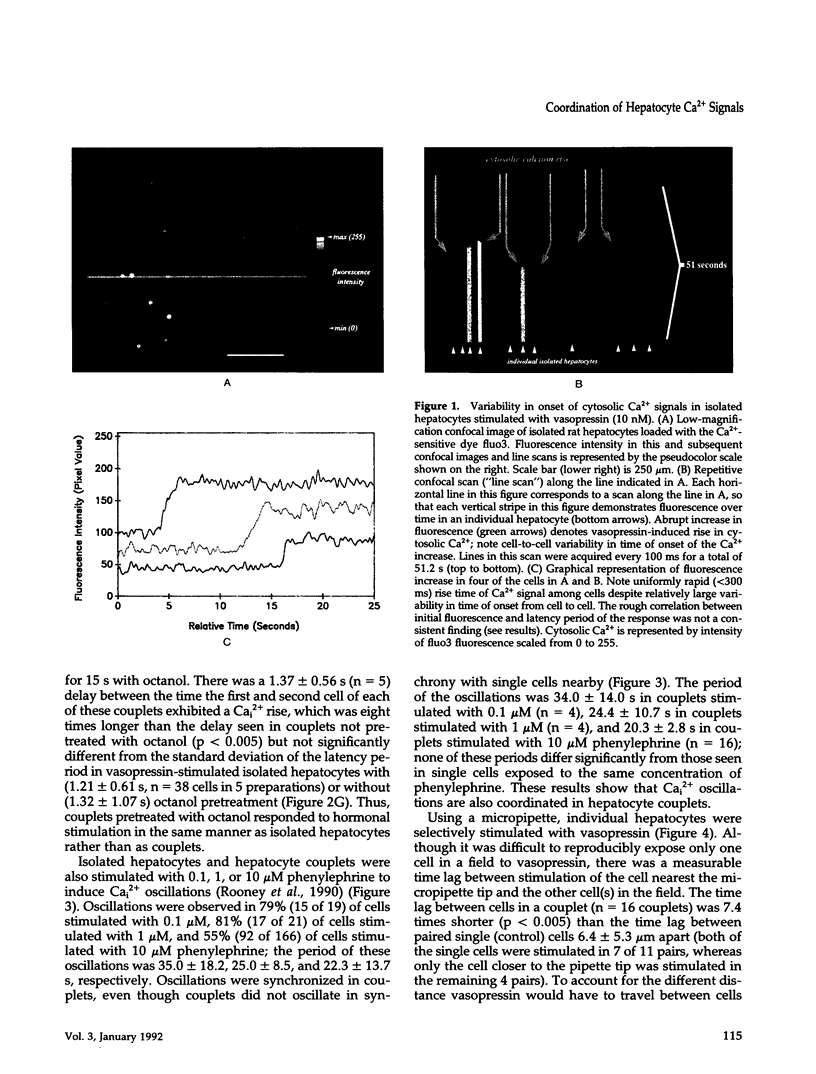
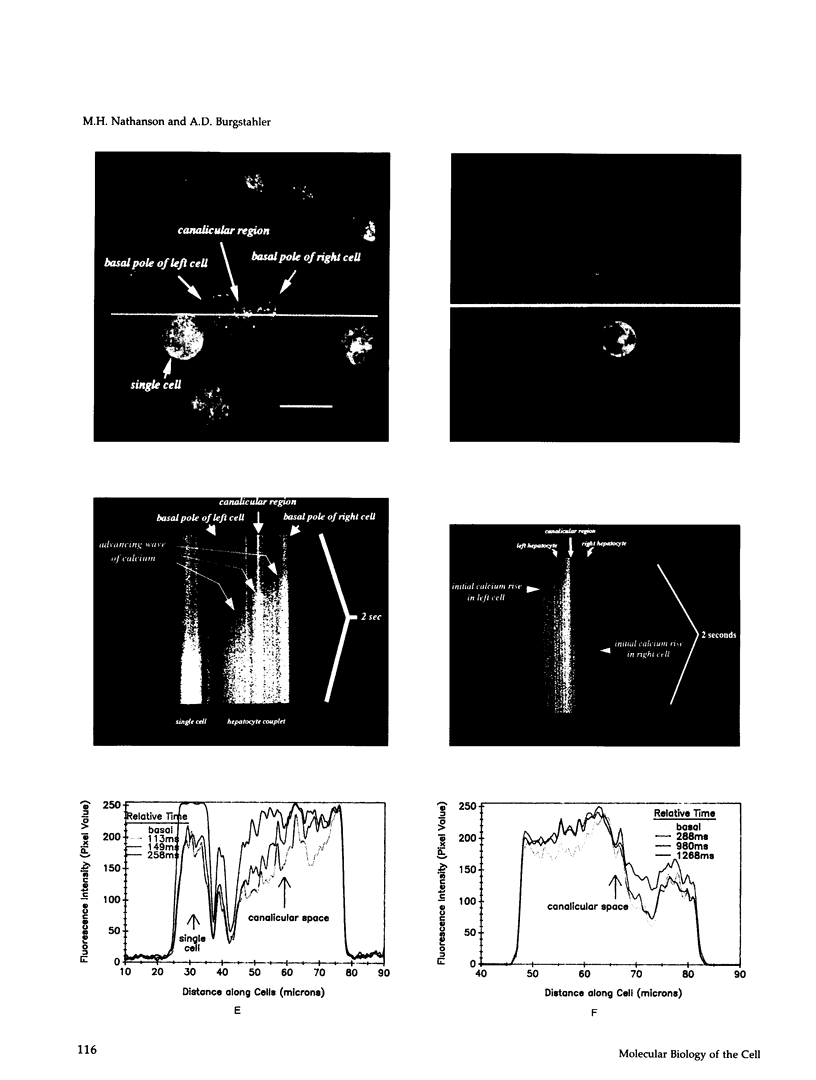
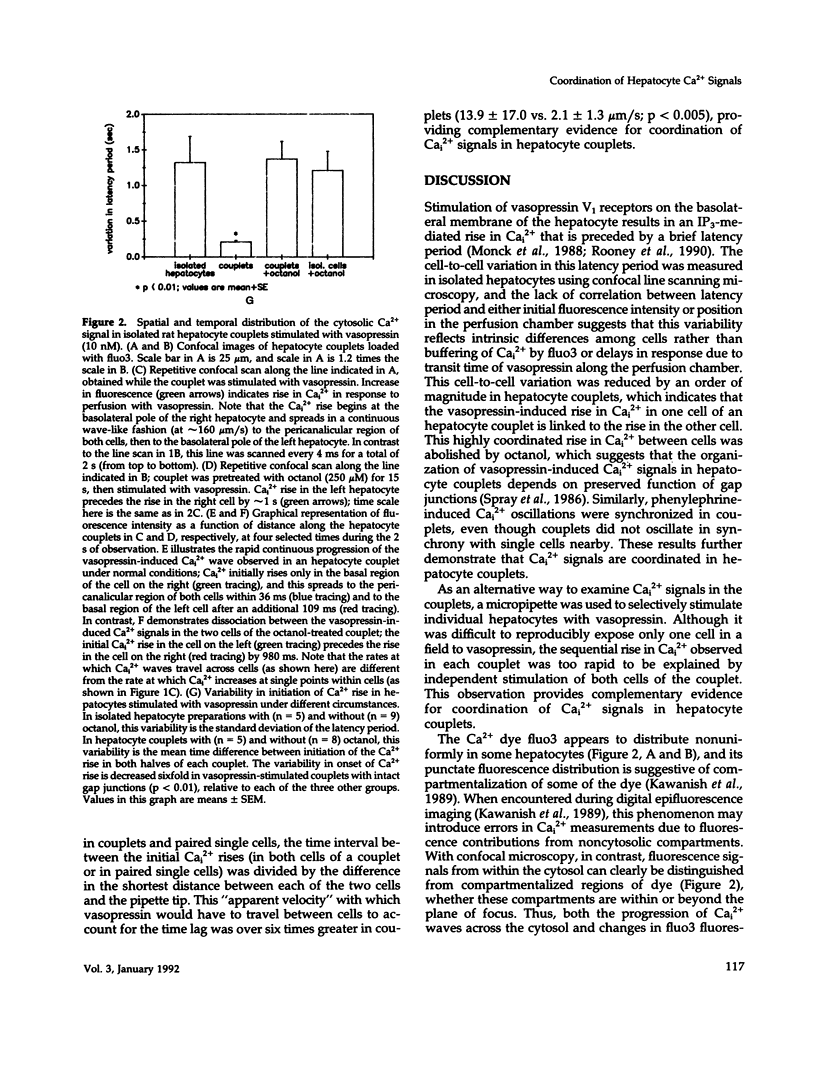

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. L., Gautam A., Graf J. Mechanisms of bile secretion: insights from the isolated rat hepatocyte couplet. Semin Liver Dis. 1988 Nov;8(4):308–316. doi: 10.1055/s-2008-1040552. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell A. H., Finkbeiner S. M., Cooper M. S., Smith S. J. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990 Jan 26;247(4941):470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Graf J., Boyer J. L. The use of isolated rat hepatocyte couplets in hepatobiliary physiology. J Hepatol. 1990 May;10(3):387–394. doi: 10.1016/0168-8278(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Jacob R. Imaging cytoplasmic free calcium in histamine stimulated endothelial cells and in fMet-Leu-Phe stimulated neutrophils. Cell Calcium. 1990 Feb-Mar;11(2-3):241–249. doi: 10.1016/0143-4160(90)90075-6. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Harootunian A. T., Tsien R. Y. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989 May 15;264(14):8179–8184. [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Kawanishi T., Blank L. M., Harootunian A. T., Smith M. T., Tsien R. Y. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989 Aug 5;264(22):12859–12866. [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lowe P. J., Miyai K., Steinbach J. H., Hardison W. G. Hormonal regulation of hepatocyte tight junctional permeability. Am J Physiol. 1988 Oct;255(4 Pt 1):G454–G461. doi: 10.1152/ajpgi.1988.255.4.G454. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Claret M. Calcium channels in hepatocytes. J Hepatol. 1988 Oct;7(2):278–282. doi: 10.1016/s0168-8278(88)80492-6. [DOI] [PubMed] [Google Scholar]
- Meda P., Bruzzone R., Chanson M., Bosco D., Orci L. Gap junctional coupling modulates secretion of exocrine pancreas. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4901–4904. doi: 10.1073/pnas.84.14.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. Cell signaling by second messenger waves. Cell. 1991 Feb 22;64(4):675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Reynolds E. E., Thomas A. P., Williamson J. R. Novel kinetics of single cell Ca2+ transients in stimulated hepatocytes and A10 cells measured using fura-2 and fluorescent videomicroscopy. J Biol Chem. 1988 Apr 5;263(10):4569–4575. [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990 Nov 16;250(4983):977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem. 1990 Jun 25;265(18):10792–10796. [PubMed] [Google Scholar]
- Sanderson M. J., Charles A. C., Dirksen E. R. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990 Jul;1(8):585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Burt J. M. Structure-activity relations of the cardiac gap junction channel. Am J Physiol. 1990 Feb;258(2 Pt 1):C195–C205. doi: 10.1152/ajpcell.1990.258.2.C195. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Ginzberg R. D., Morales E. A., Gatmaitan Z., Arias I. M. Electrophysiological properties of gap junctions between dissociated pairs of rat hepatocytes. J Cell Biol. 1986 Jul;103(1):135–144. doi: 10.1083/jcb.103.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Smith C. R., Phillips M. J. Coordination of the contractile activity of bile canaliculi. Evidence from calcium microinjection of triplet hepatocytes. Lab Invest. 1985 Sep;53(3):275–279. [PubMed] [Google Scholar]