Abstract
Menstruation is the cyclic, orderly sloughing of the uterine lining on account of the interactions of hormones produced by the hypothalamus, pituitary, and ovaries. There is a tendency among parents and clinicians to view oligo-amenorrhea as a normal variant in the teen years. In fact, the 95th percentile for the time interval between cycles is 90 days. Thus, it is abnormal for an adolescent to be amenorrheic for greater than 3 months, even in the early gynecologic years. Identification of abnormal menstrual patterns throughout adolescence may permit early identification of potential health concerns for adulthood. Few problems in gynecologic endocrinology are as complex or challenging to the clinician as amenorrhea. However, thorough evaluation of menstrual cycle disorders in adolescence provides a window of opportunity for early diagnosis and treatment of conditions affecting the hypothalamic-pituitary-ovarian (HPO) axis. Here we discuss a systematic approach to the evaluation and treatment of amenorrhea in adolescents who do not have androgen excess. There is strong evidence that estrogen deficiency is a risk factor for later development of osteoporosis and hip fracture. Delay in the evaluation and treatment of disordered menses in some cases may contribute to reduced bone density. Both patients and clinicians need to view the ovary as an important endocrine organ that helps maintain health, especially bone health.
Keywords: menstrual cycle, amenorrhea, primary amenorrhea, secondary amenorrhea, hypothalamic-pituitary-ovarian axis, bone health, delay in diagnosis, hormone replacement therapy, estrogen deficiency
Background
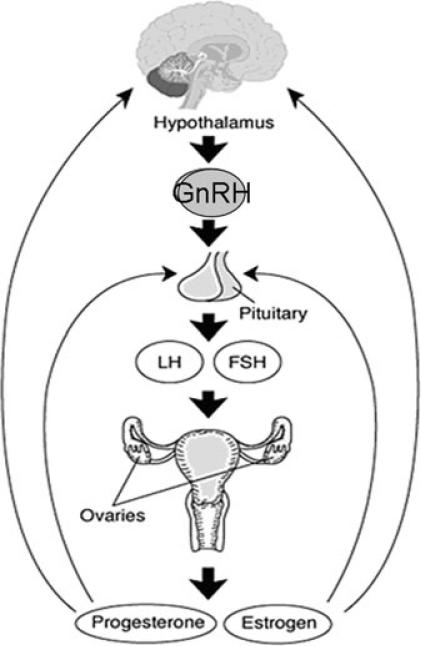
The hypothalamus, pituitary, and ovaries form an endocrine axis (known as the HPO axis) that functions via hormonal regulation and feedback loops (Fig. 1). This system governs the regulation of menstruation, which is the cyclic, orderly sloughing of the uterine lining. The hypothalamic central nervous system discharges GnRH in pulses, which are transported to the anterior pituitary, where they stimulate the gonadotrophs. In response to stimulation, these cells in turn synthesize, store, and secrete the gonadotropins FSH and LH. Furthermore, these trophic hormones stimulate the gonads to synthesize and secrete sex steroids. Hormone release in the HPO axis is regulated by a negative feedback on gonadotrophs in the anterior pituitary and by indirect inhibition at the level of the hypothalamus. Stimulation and negative inhibition complete the pathway between the hypothalamus, pituitary, and ovaries. Any disruption in this axis may result in amenorrhea or menstrual cycle disturbances.1
FIGURE 1.
The hypothalamic-pituitary-ovarian axis.
Why Should the Menstrual Cycle Be Considered a Vital Sign?
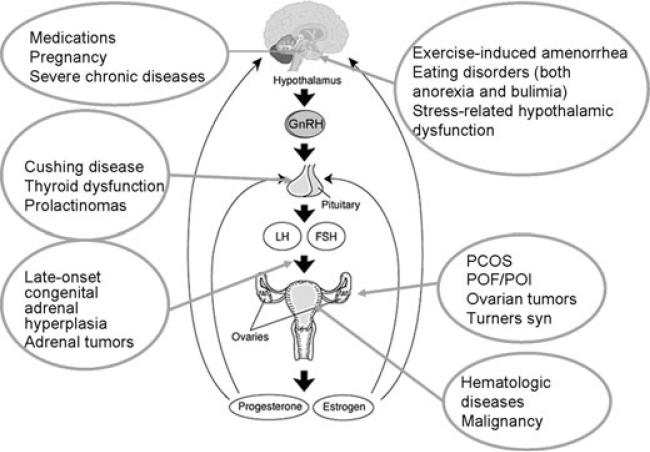
Evaluation of abnormal menstrual patterns throughout adolescence may permit early identification of potential health concerns for adulthood, just as abnormal blood pressure, heart rate, or respiratory rate may be key to the diagnosis of potentially serious health conditions.2 Figure 2 demonstrates the number of conditions that may result in irregularities or loss of the menstrual cycle. By including an evaluation of the menstrual cycle as an important vital sign, clinicians gain an additional tool in assessing overall health status for both patients and parents.
FIGURE 2.
Conditions associated with amenorrhea or disordered menses.
What Are the Implications of a Delay in Diagnosis?
In a busy clinical setting, it is tempting for clinicians to attribute loss of menstrual regularity to stress, an approach which may delay the diagnosis of an important underlying medical condition. In a study of 44 women with secondary amenorrhea due to primary ovarian insufficiency, more than half of the women visited a clinician's office three or more times before laboratory testing was performed to determine the diagnosis. In 25% of women, it took longer than 5 years for the diagnosis of primary ovarian insufficiency to be established.3 This delay was due both to the women themselves’ minimizing the importance of missing menses, as well as to their clinicians’ delaying laboratory testing to determine the diagnosis.
Delay in diagnosis contributes to reduced bone density by delaying proper therapy. In our unpublished data involving 442 women with primary ovarian insufficiency, approximately one-third of the women developed irregular menstruation at an age younger than 20 years. There was a mean delay in diagnosis of four and half years after onset of menstrual irregularity. Women with primary ovarian insufficiency who had the onset of menstrual irregularity before age 20 were more likely to have low bone density at the AP lumbar spine compared to women who had menstrual irregularity at age 20 or later. Also, women who had a delay in diagnosis of more than a year from the time of onset of menstrual irregularity were more likely to have reduced bone density than women diagnosed sooner.
Adolescence is a critical period for bone accretion, as more than half of peak bone mass is achieved during the teenage years.4 Estradiol is important in helping to build and maintain bone mass, but modifiable risk factors such as vitamin D deficiency, calcium intake, nutrition, exercise, and smoking are also important.5 Adolescents in whom bone mass is maximized would be expected have an advantage when their bone density declines with age, illness, or menopause.
What Should Prompt Evaluation of Menstrual Cycle Irregularity?
Few problems in gynecologic endocrinology are as challenging or taxing to the clinician as amenorrhea in a reproductive age woman. However, a thorough evaluation of menstrual cycle disorders in adolescence provides a window of opportunity to diagnose and treat conditions affecting the HPO axis in a timely manner.
Often, time constraints do not permit practitioners to obtain a thorough history and review of symptoms on the first visit. Scheduling a repeat visit to permit a more thorough evaluation may be necessary.
Another option is to use standardized history-taking instruments to collect this information in preparation for a return visit. In other cases, patients may be asked to keep a menstrual calendar and return in 3 months for reassessment. The importance of the ovary as an endocrine organ that helps maintain bone density should be stressed to the patient to help ensure her return.
Early menarche is associated with early onset of ovulatory cycles. When the age at menarche is younger than 12 years, 50% of cycles are ovulatory in the first gynecologic year (year after menarche). In contrast, it may take 8 to 12 years after menarche until females with later-onset menarche are fully ovulatory.6 Late menarche and menstrual irregularity are important risk factors for the development of osteoporosis and fractures later in life.4
Two large studies—one evaluating 275,947 cycles in 2,702 females and another reporting on 31,645 cycles in 656 females—support the observation that menstrual cycles in girls and adolescents typically range from 21 to approximately 45 days, even in the first gynecologic year.7,8
Normal ovulatory menstrual cycles occur at regular interval, 21–35 days, and last for <7 days. Any persistent deviance from this pattern such as oligomenorrhea, amenorrhea, polymenorrhea and menometrorrhagia deserve further evaluation (Table 1).
TABLE 1.
Menstrual conditions that may require evaluationa
| 1. | Menses have not started by 15 years of age |
| 2. | Menses occur 90 days apart, even for one cycle |
| 3. | Menses have not started within 3 years of thelarche |
| 4. | Menses have not started by 13 years of age, with no signs of pubertal development |
| 5. | Menses have not started by 14 years of age, with signs of hirsutism |
| 6. | Menses have not started by 14 years of age, with a history or examination suggestive of excessive exercise or eating disorder |
| 7. | Menses have not started by 14 years of age, with concerns about genital outflow tract obstruction or anomaly |
| 8. | Menses are regular, occurring monthly, and then become markedly irregular |
| 9. | Menses occur more frequently than every 21 days or less frequently than every 45 days |
| 10. | Menses last > 7 days |
From Diaz et al.2
What Are the Clinical Features Associated with Menstrual Irregularity?
There are a number of potential causes of amenorrhea (Table 2). Primary amenorrhea is defined as the failure of menses to occur by age 16 years, in the presence of normal growth and secondary sexual characteristics (Table 3). If by age 13, menses have not occurred and there is an absence of onset of puberty, such as breast development, an evaluation should be initiated.
TABLE 2.
Causes of amenorrhea without associated androgen excess11
| • Pregnancy |
| • Anatomic defects of outflow tract (normal FSH, normal E2) |
| ○ Intrauterine adhesions (Asherman's syndrome) |
| ○ Imperforate hymen |
| ○ Transverse vaginal septum |
| ○ Aplasia of the vagina, cervix, or uterus: Congenital absence of the uterus can be an isolated finding or it can occur in association with the complete androgen resistance syndrome, also known as testicular feminization. |
| • Ovarian causes (high FSH, low E2) |
| ○ Turner's syndrome |
| ○ Pure gonadal dysgenesis: The term “pure” here refers to the fact that the syndrome seems to have purely affected the gonad. No associated dysmorphic findings exist as are noted in Turner syndrome, which is often referred to as gonadal dysgenesis. Pure gonadal dysgenesis can occur with either a 46,XX or a 46,XY karyotype. |
| ○ Polycystic ovary syndrome |
| ○ Androgen-secreting ovarian tumor |
| ○ Struma ovarii |
| ○ Primary ovarian insufficiency or premature ovarian failure |
| ○ Autoimmune oophoritis |
| ○ 17,20-desmolase deficiency or 17-hydroxylase deficiency |
| ○ Radiation or chemotherapy |
| ○ Galactosemia |
| ○ FSH receptor mutation |
| • Pituitary causes (low FSH, low E2) |
| ○ Prolactinoma |
| ○ Other pituitary tumors (Cushing syndrome, acromegaly, thyrotropin) |
| ○ Autoimmune hypophysitis |
| ○ Pituitary radiation |
| ○ Sarcoidosis |
| ○ Hemachromatosis |
| ○ Hypothalamic causes |
| ■ Tumor such as craniopharyngioma or teratoma |
| ■ Infiltrative disorder such as sarcoidosis |
| ■ Kallmann syndrome |
| • Functional causes (low/normal FSH, low E2) |
| ○ Anorexia/bulimia |
| ○ Chronic disease |
| ○ Weight loss |
| ○ Malnutrition |
| ○ Depression or other psychiatric disorders |
| ○ Recreational drug abuse |
| ○ Psychotropic drug use |
| ○ Excessive exercise |
| ○ Idiopathy |
TABLE 3.
Causes of primary amenorrheaa
| Hypergonadotropic hypogonadism, 48.5%b |
| Abnormal sex chromosomes (Turner syndrome, 29.7%) |
| Normal sex chromosomes (46,XX, 15.4%; 46,XY, 3.4%) |
| Hypogonadotropic hypogonadism, 27.8% |
| Congenital abnormalities (isolated GnRH deficiency, 8.3%; forms of hypopituitarism, 2.3%; congenital CNS defects, 0.8%; constitutional delay, 6%) |
| Acquired lesions |
| Endocrine (CAH, 0.8%; Cushing syndrome, 0.4%; pseudohypo-parathyroidism, 0.4%; hyperprolactinemia, 1.9%) |
| Tumor (unclassified pituitary adenoma, 0.8%; craniopharyngioma, 1.1%; unclassified malignant tumor, 0.4%) |
| Systemic illness, 2.6% |
| Eating disorder, 2.3% |
| Eugonadism, 23.7% |
| Anatomic (CAUV, 16.2%; cervical atresia, 0.4%) |
| Intersex disorders (androgen insensitivity, 1.5%; 17-ketoreductase deficiency, 0.4%) |
| Inappropriate feedback, 5.3% |
Data from Reindollar et al.18
Percentages rounded to the nearest tenth.
Abbreviations: CAUV, congenital absence of the uterus and vagina; CNS, central nervous system; CAH, congenital adrenal hyperplasia.
Secondary amenorrhea is defined as the cessation of menses once they have spontaneously begun (Table 4). Oligomenorrhea is defined as menses occurring at intervals longer than 35 days. No consensus has been reached regarding the point at which oligomenorrhea becomes amenorrhea. Some authors suggest the absence of menses for 6 months constitutes amenorrhea, but the basis for this recommendation is unclear. Practically speaking, a woman who experiences loss of an established regular menstrual pattern for 3 months should have an evaluation to seek the cause.
TABLE 4.
Causes of secondary amenorrheaa
| Category | Approximate frequency (%) |
|---|---|
| Low or normal FSH | 66 |
| Weight loss/anorexia | |
| Nonspecific hypothalamic causes | |
| Chronic anovulation, including PCOS | |
| Hypothyroidism | |
| Cushing syndrome | |
| Pituitary tumor, empty sella, Sheehan syndrome | |
| Gonadal failure: high FSH | 12 |
| 46,XX | |
| Abnormal karyotype | |
| High prolactin level | 13 |
| Anatomic | 7 |
| Asherman's syndrome | |
| Hyperandrogenic states | 2 |
| Ovarian tumor | |
| Non-classic CAH | |
| Undiagnosed |
From the ASRM Practice Committee.19
The incidence of primary amenorrhea in the United States is less than 1%. The incidence of secondary amenorrhea (due to a cause other than pregnancy) is about 4% per year. No evidence indicates that the prevalence of amenorrhea varies according to national origin or ethnic group. However, local environmental factors related to nutrition and the prevalence of chronic disease undoubtedly have an effect. For instance, the age at first menses varies by geographic location, as demonstrated by a World Health Organization study comparing 11 countries, which reported a median age of menarche of 13–16 years across centers. No evidence suggests that the incidence of primary or secondary amenorrhea is related to race.
How Should Menstrual Cycle Irregularity Be Evaluated?
Evaluation of menstrual cycle irregularity depends on a careful assessment of the history, physical examination, and various laboratory and diagnostic tests. This should be done systematically according to the suspected origin of the dysfunction (i.e., outflow tract, ovary, or hypothalamus/pituitary).
History
Pregnancy is the most common cause of amenorrhea. Determining whether the patient is sexually active and whether she is using contraceptive methods is important. In some cases, the hormonal contraception itself may be the cause of the amenorrhea.
Inquiring about other aspects of growth and pubertal development is important. Breast development, pubertal growth spurt, and adrenarche are delayed or absent in persons with hypothalamic pituitary failure. A distinguishing factor in the case of isolated ovarian insufficiency or failure is that adrenarche occurs normally, while estrogen-dependent breast development and the pubertal growth spurt are absent or delayed.
Inquiry should be made regarding symptoms of androgen excess such as hirsutism, increased muscle mass, deepening of voice, or male pattern balding. The evaluation of patients with androgen excess is beyond the scope of this review.
Disorders of the Outflow Tract
A history of otherwise normal growth and pubertal development and cyclic pelvic pain in association with primary amenorrhea suggests the possibility of a congenital outflow tract abnormality such as imperforate hymen or agenesis of the vagina, cervix, or uterus. Prior history of a surgical procedure involving the endometrial cavity, especially if performed in the presence of infection, raises the possibility of uterine synechiae (Asherman's syndrome). History of minimal body hair, good breast development with blind vagina, and absent uterus is suggestive of androgen resistance syndrome.
Ovarian Disorders
Anomalies such as short stature, webbed neck, multiple ear infections, coarctation of the aorta, renal abnormalities, hypertension, pigmented nevi, short forth metacarpal and metatarsals, Hashimoto's thyroiditis, obesity, and osteoporosis suggest Turner syndrome. Symptoms of vaginal dryness, hot flashes, night sweats, or disordered sleep may be a sign of primary ovarian insufficiency or premature ovarian failure. The presence of these symptoms in young women demands timely further evaluation. Prior history of chemotherapy or radiation therapy may be associated with primary ovarian insufficiency.
Hypothalamic/Pituitary Disorders
Associated galactorrhea, headaches, or reduced peripheral vision could be a sign of intracranial tumor such as prolactinoma. These symptoms require immediate further evaluation. An impaired sense of smell in association with primary amenorrhea and failure of normal pubertal development may be related to isolated gonadotropin deficiency, as is observed in persons with Kallmann syndrome. Sarcoidosis can manifest insidiously, with development of mild fatigue, malaise, anorexia, weight loss, and fever. Because 90% of patients with sarcoidosis have pulmonary involvement at some stage of the disorder, cough and dyspnea may be present. Hemachromatosis may manifest as weakness, lassitude, weight loss, and a change in skin color.
Dieting with excessive restriction of energy intake, especially fat restriction, may lead to amenorrhea and associated bone loss. In extreme cases, the process may advance to anorexia nervosa, a potentially fatal condition. Associated symptoms are an intense fear of fatness and a body image that is heavier than observed. Eating disorders can be restrictive in nature or can be of a binge-eating/purging type. Major psychiatric disorders such as depression, obsessive–compulsive disorder, or schizophrenia may cause amenorrhea. Symptoms associated with these conditions may be detected upon review of systems. Autoimmune adrenal insufficiency, a potentially fatal condition, often manifests as vague and nonspecific symptoms. Amenorrhea may be the first clear symptom indicating a need for further evaluation to detect this condition. Amenorrhea may herald the onset of other autoimmune endocrine disorders such as hyperthyroidism, hypothyroidism, or autoimmune lymphocytic hypophysitis. The same is true for other endocrine disorders such as Cushing syndrome or pheochromocytoma. A careful review of symptoms may help uncover these disorders. Strenuous exercise related to a wide variety of athletic activities can be associated with the development of amenorrhea. Elicit a history regarding the type of exercise activity and its duration per week. Abuse of drugs such as cocaine and opioids have central nervous system effects that may disrupt the menstrual cycle. AIDS, HIV disease, or other types of immunodeficiency states may induce systemic infection, leading to chronic disease and amenorrhea. Occult malignancy with progressive weight loss and a catabolic state may lead to loss of menstrual regularity. A careful review of systems may help uncover such a disorder.
Physical Examination
Physical examination should begin with an overall assessment of nutritional status and general health. A clinician needs to be extra sensitive about pelvic exam in an adolescent who may be dealing with many unknowns about her health. In many instances, this could be her first pelvic exam. Measure height and weight and seek evidence for chronic disease or cachexia.
Hypothermia, bradycardia, hypotension, and reduced subcutaneous fat can be observed in persons with severe anorexia nervosa. In cases of frequent vomiting, look for possible dental erosion, reduced gag reflex, trauma to the palate, subconjunctival hemorrhage, and metacarpophalangeal calluses or bruises. Skin examination findings can also give clues to other endocrine disorders. Vitiligo or increased pigmentation of the palmar creases may herald primary adrenal insufficiency. Thin, parchment-like skin, striae, and evidence of easy bruising may be signs of Cushing syndrome. Warm, moist skin radiating excessive heat may be a sign of hyperthyroidism. The skin should be examined for evidence of androgen excess, such as hirsutism and acne. Acanthosis nigricans may be present in association with androgen excess related to insulin resistance.
Large pituitary tumors can cause visual-field cuts by impinging on the optic tract. In some cases, these visual-field cuts can be detected by simple confrontational testing.
The state of breast development and presence of galactorrhea should be assessed. In some cases, breast discharge can be expressed, yet the condition is not true galactorrhea. If the discharge is indeed milk, this can be confirmed by finding fat globules in the fluid using low-power microscopy. The presence of axillary and pubic hair should be evaluated. These are a marker of adrenal and ovarian androgen secretion. In cases of panhypopituitarism, sources of androgen are low, and pubic and axillary hair is sparse. In addition, some women develop the combination of autoimmune premature ovarian failure and autoimmune primary adrenal insufficiency. These women are also markedly androgen-deficient and have scant axillary and pubic hair. The same is true for persons with androgen insensitivity syndrome (testicular feminization), 17-hydroxylase deficiency, and 17,20-desmolase deficiency.
In cases of primary amenorrhea with otherwise normal pubertal development, pelvic examination may help detect imperforate hymen, a transverse vaginal septum, or cervical or uterine aplasia. Pelvic examination findings can provide physical evidence indicating the adequacy of estrogen production. Thin and pale vaginal mucosa with absent rugae is evidence of estrogen deficiency. Ovarian enlargement may be found upon pelvic examination in cases of autoimmune oophoritis, 17-hydroxylase deficiency, or 17, 20-desmolase deficiency. In these disorders, inadequate negative feedback supplied by the ovary permits excessive gonadotropin stimulation, which may cause ovarian enlargement with multiple follicular cysts. In some cases, these disorders manifest with an acute onset of pain related to ovarian torsion.
A general physical examination may undercover unexpected findings that are indirectly related to the loss of menstrual regularity (e.g., hepatosplenomegaly, which may lead to detection of a chronic systemic disease).
Laboratory Studies
In most cases, clinical variables alone are not adequate to define the pathophysiologic mechanism disrupting the menstrual cycle. The clinician must be concerned with an array of potential diseases. A pregnancy test should be the first step. Complete blood cell count, urinalysis, and serum chemistries may be indicated to help rule out systemic disease. Serum prolactin, FSH, and estradiol levels should be measured routinely in the initial evaluation of amenorrhea once pregnancy has been excluded.
Prolactin levels in excess of 200 ng/mL are generally observed only in the case of a prolactin-secreting pituitary adenoma (prolactinoma). In general, the serum prolactin level correlates with the size of the tumor. Psychotropic drugs, hypothyroidism, stress, and meals can also raise prolactin levels. Repeatedly elevated prolactin levels require further evaluation if the cause is not readily apparent.
Thyroid-stimulating hormone (TSH) and free thyroxine (T4) should be performed if symptoms or signs of hypothyroidism are present. An FSH level in the menopausal range is indicative of ovarian insufficiency. If a repeat value in 1 month confirms this finding, and amenorrhea persists, the diagnosis of premature ovarian failure or primary ovarian insufficiency is confirmed. Luteinizing hormone is elevated in cases of 17,20-lyase deficiency, 17-hydroxylase deficiency, and premature ovarian failure or primary ovarian insufficiency. Serum estradiol levels undergo wide fluctuations during the normal menstrual cycle. During the early follicular phase of the menstrual cycle, levels may be lower than 50 pg/mL. During the preovulatory estradiol surge, levels in the range of 400 pg/mL are not uncommon. In healthy menopausal women, estradiol levels are routinely lower than 20 pg/mL.
Imaging Studies
Magnetic resonance imaging (MRI) is indicated in the work-up of for pituitary or hypothalamic causes, associated headaches or visual-field cuts, profound estrogen deficiency with otherwise unexplained amenorrhea and hyperprolactinemia. Request imaging of the hypothalamic/pituitary area specifically, rather than a study of the entire brain. This achieves higher resolution.
Pelvic ultrasound and MRI may be helpful in delineating outflow tract abnormalities.
Other Tests
The development of accurate and reasonably priced hormonal assays has called into question the value of the progesterone withdrawal test. We do not recommend performing the test as part of the diagnostic algorithm for amenorrhea. Relying on the progesterone challenge test results can cause a delay in the diagnosis of potentially serious disorders. Prior to the development of readily available assays to measure serum levels of estradiol, the progesterone challenge test was used as a bioassay with which to demonstrate estrogen effect at the level of the endometrium. An intramuscular injection of 100 mg of progesterone in oil has been shown to predictably induce a withdrawal bleed if the circulating serum estradiol level is at least 50 pg/mL. However, the progesterone withdrawal test can provide inappropriately reassuring information that may delay the diagnosis of ovarian insufficiency and, possibly, other conditions. The progesterone withdrawal test is no substitute for evaluating ovarian health. Demonstrating the presence of normally functioning ovaries requires the concurrent measurement of serum estradiol and FSH.
Several validated instruments are available to measure dietary intake, mood disorder, and eating disorders, such as the Minnesota Nutrition Data Systems evaluation to assess dietary intake, Beck Depression Inventory to assess the mood, Modifiable Activity Questionnaire and Paffenbarger Questionnaire to assess the patient's level of physical activity, Multidimensional Eating Disorder inventory for anorexia and bulimia,9 and the bulimia test, revised, that is, the BULIT-R.10
Procedures
Hysterosalpingography and hysteroscopy are indicated in cases of possible Asherman's syndrome. Surgical repair is indicated in disorders of the outflow tract.
How Should Menstrual Cycle Irregularity Be Managed?
The treatment of primary amenorrhea will vary depending on the cause. Correcting the underlying disorder through surgical intervention when needed or treatments for specific disorders should be the first step. Medical care needs are defined by the cause of the amenorrhea and the desires of the patient. Ideally, treatment should be directed at correcting the underlying disease. In the case of outflow tract abnormalities, surgery may be indicated. In other cases, correcting the underlying pathology should restore normal ovarian endocrine function. Dopamine agonists are effective in treating hyperprolactinemia. In most cases, this treatment restores normal ovarian endocrine function and ovulation. Hormone replacement therapy is required to achieve and maintain peak bone density in patients whose underlying pathology cannot be reversed to restore normal endocrine function.
Some pituitary and hypothalamic tumors may require surgery and, in some cases, radiation therapy. The surgical procedure required for other outflow tract abnormalities depends on the specific clinical situation.
The causes of menstrual cycle disturbance leading to the development of amenorrhea are so diverse that in some complex cases, the situation is best addressed by a multidisciplinary team.11 For example, a patient with complete androgen resistance (testicular feminization) would benefit from the involvement of experts in endocrinology, human genetics, psychiatry, and reproductive surgery.
Geneticist: With hereditary causes of amenorrhea, such as the Kallmann syndrome, a geneticist's expertise can be helpful for the extended family and in counseling patients regarding the disorder.
Medical endocrinologist: In cases of pituitary/hypothalamic tumor, other endocrine disorders (e.g., central hypothyroidism, central adrenal insufficiency) may be involved. Generally, the expertise of a medical endocrinologist is required to assist in the treatment of patients who require neurosurgery to treat the underlying condition. In cases of hyperthyroidism or Cushing syndrome, the expertise of a medical endocrinologist is required to treat the underlying pathology.
Psychiatrist: Cases of major depression, anorexia nervosa, bulimia nervosa, or other major psychiatric disorders warrant consultation with a psychiatrist.
Reproductive surgeon: In some unusual cases, such as with vaginal agenesis, a reproductive surgeon with extensive experience in the specific disorder should be consulted.
Nutritionist: In many cases, exercise-induced amenorrhea is due to an imbalance in energy intake and expenditure. Nutritional counseling to increase energy intake without reducing exercise is a means of reversing the underlying pathology. Women who are underweight or who appear to have nutritional deficiencies should receive nutritional counseling and can be referred to a multidisciplinary team specializing in eating disorders.
General internal medicine specialist: In certain cases in which an underlying chronic disease process is present, the insights of an internist may be needed.
Hormone Replacement Therapy
Adolescents with Primary Amenorrhea
Girls with primary amenorrhea typically do not have symptoms of estrogen deficiency. However, with inadequate estrogen exposure over time, these patients are at increased risk for developing osteoporosis and possibly other health concerns. Young women in whom secondary sex characteristics have failed to develop fully should be exposed initially to very low doses of estrogen in an attempt to mimic the gradual pubertal maturation process. A typical regimen consists of an estrogen with a dosage equivalent to 25 mcg/day of transdermal estradiol (approximately 0.3 mg of conjugated equine estrogen) given unopposed (i.e., no progestogen) daily for 6 months with incremental dose increases at 6-month intervals until the required maintenance dose is achieved. Gradual dose escalation allows time to balance estrogen supplementation with need to grow in height and develop secondary sexual characters and often results in optimal breast development. It also allows time for the young woman to adjust psychologically to her physical maturation.12
Cyclic progestogen therapy, given 12 to 14 days per month, should be instituted once vaginal bleeding has occurred. Parenteral estrogen (transdermal or vaginal) is the preferred route of administration because it avoids first-pass liver metabolism and provides a more physiologic estradiol-to-estrone ratio. Moreover, it is less likely to increase sex hormone binding globulin (SHBG) and has little or no effect on circulating lipids, coagulation parameters, or C-reactive protein. The role of androgen replacement is unclear at this time and is the subject of ongoing investigation.13
Adolescents with Secondary Amenorrhea
Young women who experience estrogen deficiency may be candidates for hormone therapy. Results from studies about hormone therapy in postmenopausal women cannot be applied to girls and premenopausal women. Premenopausal women with estrogen deficiency sustain sex steroid–deficiency for more years than do naturally menopausal women, resulting in a significantly higher risk for osteoporosis14 and cardiovascular disease.15 The goal of therapy in young women with such conditions is to provide a hormone replacement regimen that maintains sex steroid status as effectively as the normal, functioning ovary. An estrogen dose equivalent to about 100 mcg transdermal estradiol (or 1.25 mg CEE) is needed to achieve adequate estrogen replacement in reproductively mature girls and young women. A progestogen such as medroxyprogesterone acetate or micronized progesterone should be given for 12 to 14 days per calendar month to prevent endometrial hyperplasia.12 Our preference is to use medroxyprogesterone acetate because there are data showing a protective effect on the endometrium at the dose of estrogen that we recommend.16 Other progestins have been evaluated with regard to endometrial effect at menopausal doses of estrogen (e.g., 0.625 mg CEE). The duration of HRT should in general be until approximately the physiological age of menopause (around 51–52 years).
In our experience, the Women's Health Initiative (WHI) study has discouraged many young women and their clinicians from starting hormone replacement therapy, although the results do not apply to this younger population. A secondary analysis of WHI randomized controlled trials of hormone therapy showed that there were no significant increases in risk due to hormone therapy for any outcome including CHD, stroke, and global index events at ages 50 to 59 years, and there was a reduction in total mortality in this age group (HR, 0.70; 95% CI, 0.51–0.96).17 In our opinion, benefits of hormone replacement therapy outweigh risks in young estrogen-deficient women.
Patient Education
By including an evaluation of the menstrual cycle as an additional vital sign, clinicians reinforce its importance in assessing overall health status for both patients and parents.
Physicians should make it clear to their patients with estrogen deficiency that their clinical situation differs from that of older women who have undergone normal menopause. They should take time to inform the patient of the potential benefits of HRT.
Summary and Conclusions
A variety of conditions affecting the hypothalamic-pituitary-ovarian (HPO) axis manifests as a final common symptom—irregular menstrual cycles. Thorough and systematic evaluation of menstrual cycle disorders in adolescence provides a window of opportunity for early diagnosis and treatment of disorders that may have long-term adverse health consequences. Adolescence is a critical period for bone accretion as more than half of peak bone mass is achieved during the teenage years. Delay in the evaluation and treatment of disordered menses in some cases may contribute to reduced bone density. The ovary is an important endocrine organ that helps maintain health, especially bone health.
Acknowledgments
This work was supported in part by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland. L.M.N. is a commissioned officer in the United States Public Health Service.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology. 7th ed. Lippincott Williams & Wilkins; Philadephia, PA: 2005. [Google Scholar]
- 2.Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–2250. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- 3.Alzubaidi NH, Chapin HL, Vanderhoof VH, et al. Meeting the needs of young women with secondary amenorrhea and spontaneous premature ovarian failure. Obstet. Gynecol. 2002;99:720–725. doi: 10.1016/s0029-7844(02)01962-2. [DOI] [PubMed] [Google Scholar]
- 4.Adams Hillard PJ, Nelson LM. Adolescent girls, the menstrual cycle, and bone health. J. Pediatr. Endocrinol. Metab. 2003;16(Suppl 3):673–681. [PubMed] [Google Scholar]
- 5.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J. Clin. Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J. Steroid. Biochem. 1984;20:231–236. doi: 10.1016/0022-4731(84)90209-7. [DOI] [PubMed] [Google Scholar]
- 7.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int. J. Fertil. 1967;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- 8.Vollman RF. The menstrual cycle. Major Probl. Obstet. Gynecol. 1977;7:1–193. [PubMed] [Google Scholar]
- 9.Garner DM, Olmsted MP. More on the eating disorder inventory. Am. J. Psychiatry. 1986;143:805–806. doi: 10.1176/ajp.143.6.aj1436805. [DOI] [PubMed] [Google Scholar]
- 10.Welch G, Thompson L, Hall A. The BULIT-R: Its reliability and clinical validity as a screening tool for DSM-III-R bulimia nervosa in a female tertiary education population. Int. J. Eat. Disord. 1993;14:95–105. doi: 10.1002/1098-108x(199307)14:1<95::aid-eat2260140113>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Nelson LM, Bakalov VK. Amenorrhea. [February 22, 2008];eMedicine Journal [serial online] 2005 May 17. Available at URL: www.emedicine.com/MED/topic117.htm.
- 12.DiPiro JT, Talbert RL, Yee GC. Pharmacotherapy: A Pathophysiological Approach. McGraw-Hill; New York: 2005. Hormone therapy in women. pp. 1493–513. [Google Scholar]
- 13.Braunstein GD. The Endocrine Society Clinical Practice Guideline and The North American Menopause Society position statement on androgen therapy in women: another one of Yogi's forks. J. Clin. Endocrinol. Metab. 2007;92:4091–4093. doi: 10.1210/jc.2007-1709. [DOI] [PubMed] [Google Scholar]
- 14.Anasti JN, Kalantaridou SN, Kimzey LM, et al. Bone loss in young women with karyotypically normal spontaneous premature ovarian failure. Obstet. Gynecol. 1998;91:12–15. doi: 10.1016/s0029-7844(97)00583-8. [DOI] [PubMed] [Google Scholar]
- 15.Kalantaridou SN, Naka KK, Papanikolaou E, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J. Clin. Endocrinol. Metab. 2004;89:3907–3913. doi: 10.1210/jc.2004-0015. [DOI] [PubMed] [Google Scholar]
- 16.Nelson LM, Covington SN, Rebar RW. An update: spontaneous premature ovarian failure is not an early menopause. Fertil. Steril. 2005;83:1327–1332. doi: 10.1016/j.fertnstert.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 17.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 18.Reindollar RH, Tho SPT, McDonough PG. Delayed puberty: an updated study of 326 patients. Trans. Gynecol. Obstet. Soc. 1989;8:146–162. [Google Scholar]
- 19.Practice Committee of the American Society for Reproductive Medicine Current evaluation of amenorrhea. Fertil. Steril. 2006;86:S148–S155. doi: 10.1016/j.fertnstert.2006.08.013. [DOI] [PubMed] [Google Scholar]