Abstract
Schizophrenia (SZ) is primarily an adult psychiatric disorder in which disturbances elicited by susceptibility genes and environmental insults during early neurodevelopment initiate over a long time course neurophysiological changes that culminate in the onset of full-blown disease nearly two decades later. Aberrant postnatal brain maturation is an essential mechanism underlying the disease. Currently symptoms of SZ are treated with anti-psychotic medications that have variable efficacy and severe side effects. There has been much interest in the prodromal phase and the possibility of preventing SZ by interfering with the aberrant postnatal brain maturation associated with this disorder. It follows that it is crucial to understand the mechanisms that underlie the long-term progression to full disease manifestation to identify the best targets and approaches towards this goal. We believe that studies of certain SZ genetic susceptibility factors with neurodevelopmental implications will be key tools for this effort. Accumulating evidence suggests that Neuregulin-1 (NRG1) and DISC1 are likely to functionally converge and play key roles in brain development. We provide an update on the role of these emerging concepts in understanding the complex time-course of SZ from early neurodevelopmental disturbances to later onset, and suggest ways of testing them in the future.
Introduction
Schizophrenia (SZ) is a debilitating mental illness with a worldwide lifetime risk of about 1% characterized by positive symptoms (e.g., delusions and hallucinations), negative symptoms (e.g., affective flattening, apathy, and social withdrawal), and cognitive dysfunction. SZ is caused by a combination of genetic factors together with environmental insults, including prenatal infection, perinatal complication, and cannabis use. Recently, SZ has been described simply as a neurodevelopmental disorder [1, 2]. However, the onset of SZ occurs in young adulthood, in contrast to an earlier onset in childhood that occurs in many other neurodevelopmental disorders, such as autism. In the pathology of SZ, disturbances elicited by genetic susceptibility factors and environmental insults in prenatal and perinatal stages are likely to disturb postnatal brain maturation for many years, which results in the full-blown onset of the disease mainly after puberty [3].
Such pathological mechanisms underlying the long time-course of SZ have not yet been fully elucidated. One of the major reasons is the difficulty in designing longitudinal clinical studies for high-risk subjects many years before the disorder is manifested, although a small number of state-of-the-art brain imaging studies exist [4]. Lack of appropriate animal models to validate working hypotheses for the mechanisms also impedes progress. Although there are several interesting rodent models with specific brain lesions in early development that display phenotypic changes relevant to SZ only after puberty [5, 6], these models may not exactly replicate the etiologies of SZ.
Recent progress in psychiatric genetics has revealed several promising genetic susceptibility factors for SZ, including Neuregulin-1 (NRG1/Heregulin), the NRG1 receptor ErbB4 (HER4, a receptor tyrosine-protein kinase), and Disrupted-in-Schizophrenia-1 (DISC1) [7, 8]. The role of NRG1 as a risk factor for SZ has been supported by many association studies in more than one ethnic group [9]. Compelling genetic evidence for DISC1 was initially obtained from a large Scottish pedigree in which a majority of family members with disruption of DISC1 suffer from psychiatric illnesses, including SZ [10, 11]. Biological studies have revealed that both NRG1 and DISC1 are multifunctional in nature, with key roles during neurodevelopment [12–14]. Therefore, systematic studies with these factors from the time of the initial risks in early development to disease onset after puberty is likely to open a window on a mechanistic understanding of the ‘long-term’ neurodevelopmental processes in SZ.
Over the past three years, excellent review articles for individual risk factors for SZ, such as NRG1/ErbB4 and DISC1, have been published [9, 12–14]. Several reviews that discuss animal models for SZ are also available, but with an emphasis on behavioral assays in adult animals [15]. Nonetheless, as far as we are aware, few reports have addressed mechanistic approaches to long-term neurodevelopmental processes of SZ from the initial risk during pre- and perinatal stages, postnatal brain maturation, to the onset in young adulthood, especially by examining possible convergence of promising SZ genetic susceptibility factors at the functional levels in vivo. The extraordinary advances in the field over the past 1–2 years enable us to provide an overview of these issues, in particular focusing on the significance of “postnatal maturation” of the frontal cortex and associated circuitry, which are crucial for cognitive functions, such as working memory, and are frequently impaired in SZ patients now. It becomes also possible to discuss how such molecular approaches can suggest novel therapeutic strategies for this devastating disorder. In this review, we first outline long-term neurodevelopmental processes that might be disturbed in SZ (Fig. 1). Then, we describe roles of NRG1-ErbB4 and DISC1 in these processes (Fig. 2), suggesting convergence of these two cascades and finally ending with a discussion of relevant animal models.
Figure 1. Long-term neurodevelopmental processes, which are disturbed in schizophrenia (SZ).
The upper part depicts normal corticogenesis: radial migration of the neural progenitor cells from the subventricular zone towards the cortical plate to form the well defined cortical layers and elimination of connections in adolescence. The lower part shows details of the processes that might go wrong in SZ. SZ is primarily an adult psychiatric disorder in which disturbances elicited by susceptibility genes and environmental insults (risks/insults) during early development (indicated by three pink stars in the left side) disturb postnatal brain maturation. These factors, including genetic (e.g., Neuregulin-1/ErbB4 and DISC1) and environmental factors (e.g., birth hypoxia and congenital infection), are likely to impair some of the crucial processes in early development, including progenitor cell proliferation, neuronal migration, and dendritic arborization and outgrowth. Independent of such initial risks/insults, disease-associated intrinsic factors may also directly affect postnatal brain maturation (indicated by two pink central stars). The accumulation of such deleterious insults results in overall disturbance of proper postnatal brain maturation, which includes maturation of interneurons and dopaminergic projections, pruning of glutamate synapses, and myelination. Therefore, it is crucial to understand the mechanisms that underlie the long-term progression to the full disease manifestation in young adulthood to enable development of novel etiology-based therapeutic strategies. In this figure, interneuron maturation is plotted by an increased response of interneurons to dopamine D2 agonists in the prefrontal cortex [26], whereas mesocortical dopaminergic projection is based on the levels of tyrosine hydroxylase [34]. The relative amount/level of glutamatergic synapse density and myelination are depicted according to the previous publications [38, 47]. Molecular cascades involving NRG1/ErbB4 and DISC1 in each developmental stage (indicated by rectangles) are described in Figure 2. CP, cortical plate; SVZ, subventricular zone.
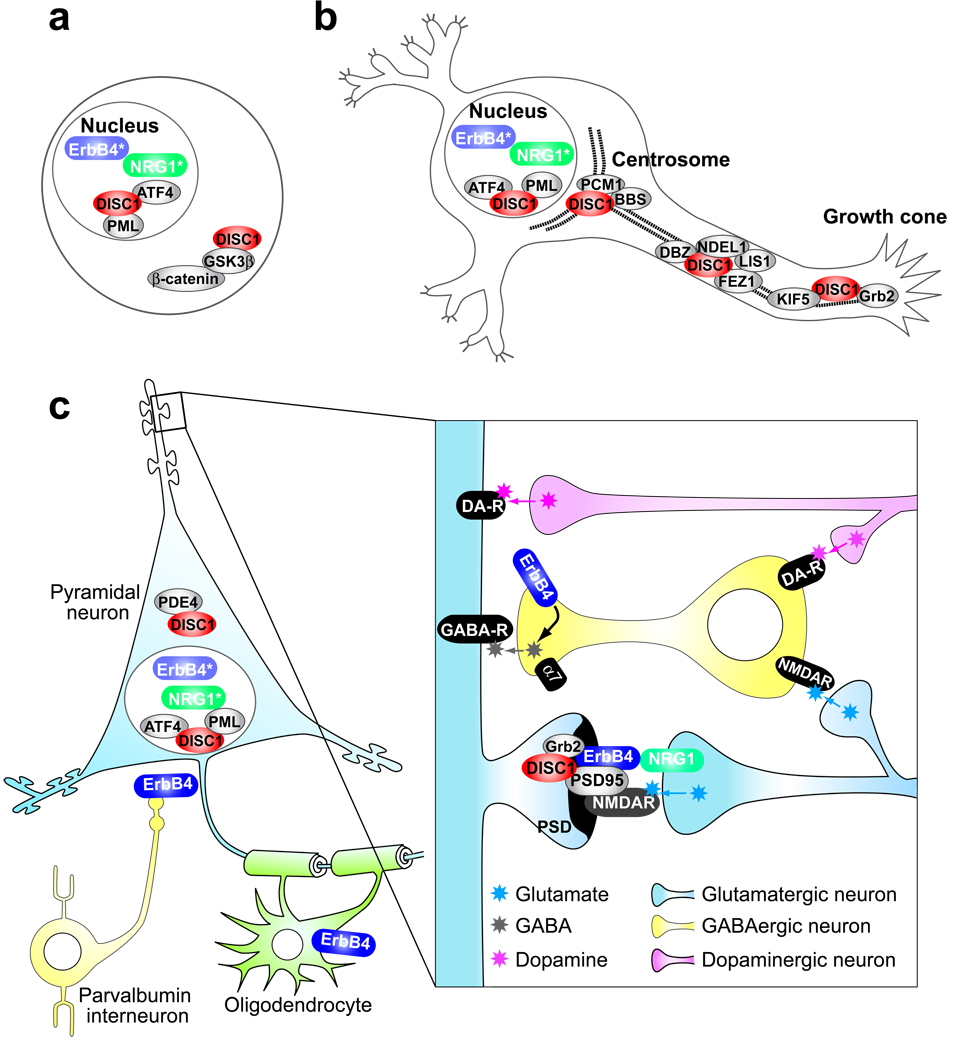
Figure 2. Convergence of two pleiotropic pathways: DISC1 and Neuregulin-1 (NRG1)–ErbB4, which are disturbed in schizophrenia.
(a) In neuronal progenitor cells, DISC1 plays an important role in regulating the Wnt-pathway by directly binding with GSK3β and modulating the stability of β-catenin. DISC1 is also localized in the nucleus where it potentially encounters intracellular domains of ErbB4 (ErbB4*) and neuregulin-1 (NRG1*). (b) In postmitotic neurons pre/perinatally DISC1 interacts with PCM1 (another risk gene for SZ) and BBS in the dynein motor complex in association with the centrosome and plays a key role in migration and arborization. DISC1 might contribute to outgrowth by interacting with many other cytoskeleton-associated proteins. Substantial levels of nuclear DISC1 are also observed. (c) In postnatal brains, neuronal connectivity among pyramidal neurons, interneurons, and dopaminergic projection from the ventral tegmental area underlies proper functions of the cortex. At the postsynaptic density (PSD) of the pyramidal neurons, in conjunction with the NMDA-type glutamate receptor, NRG1–ErbB4 and DISC1 cascades are likely to converge. These two cascades may also converge in the nucleus, mediating gene transcription. DISC1 also interacts with PDE4, regulating cAMP signaling. α7, alpha7 nicotinic acetylcholine receptor; ATF4, activating transcription factor 4; BBS, Bardet-Biedl syndrome; DBZ, DISC1-binding zinc-finger protein; DA-R, dopamine receptor; FEZ1, fasciculation and elongation protein zeta 1; GABAR, gamma-aminobutyric acid receptor; Grb2, growth factor receptor bound protein 2; GSK3β, glycogen synthase kinase 3β; KIF5, kinesin family member 5; LIS1, lissencephaly-1; NDEL1, nuclear distribution element-like 1; NMDAR, NMDA receptor; PCM1, pericentriolar material 1; PML, promyelocytic leukemia; PSD95, post-synaptic density protein 95.
Long-term neurodevelopmental processes which might be disturbed in SZ
Initial risks and insults during pre- and perinatal stages in SZ pathology
There is epidemiological support for the association of SZ with adverse events during prenatal and perinatal periods [3]. Among such events, birth complications, especially hypoxia, and viral infection in association with SZ provide some clues to the mechanisms underlying the initial risk for this disease [16, 17]. Minor physical anomalies, in particular in the craniofacial region and limbs, are seen in SZ patients, which are thought to be effected by events of the first and second trimester when progenitor cell proliferation and neural migration take place [18]. Minor cytoarchitectural abnormalities of neurons are observed without accompanying massive glial cell proliferation (gliosis) in autopsied brains from patients with SZ [19]. Existence of neuronal changes without gliosis supports the idea that this pathology is associated with damage during neurodevelopment. Dendritic changes and a smaller soma have been frequently reported in the brains of SZ patients [20, 21]. These changes may reflect direct disturbances of dendrites, but it is possible that these are compensatory outcomes in response to disconnectivity arising from earlier neurodevelopmental insults, such as defects of neuroprogenitor cell proliferation and migration. Taken together, in this prenatal/perinatal period critical for SZ , subtle disturbances in progenitor cell control, neuronal migration, dendritic growth and arborization, and combinations of these factors may contribute to the anatomical changes observed in SZ by neuropathology and brain imaging at a later time.
Possible disturbance of postnatal brain maturation in SZ pathology
The time lag between the major and initial disturbances during early neurodevelopment (prenatal/perinatal periods) and the late onset of SZ implies that these insults during early neurodevelopment may disturb postnatal brain maturation, which is required for the delayed onset after puberty. It is also conceivable that intrinsic factors (most likely genetic factors) contribute to the pathological processes in both prenatal/perinatal development and postnatal brain maturation, which are required for the onset of SZ [22]. Additional environmental factors may be crucial for the full manifestations of the genetic effects [23]. At least four elements may play a role in postnatal brain maturation associated with SZ: GABA interneuron maturation, pruning of glutamate synapses, maturation of dopaminergic projections (especially mesocortical dopaminergic projection), and oligodendrocyte differentiation/myelination, each of which we consider below.
i) GABA interneuron maturation
Characteristics of GABA-containing interneurons are dramatically changed during postnatal brain maturation, in particular the expression profiles of key molecules, such as GABA and dopamine receptors [24, 25]. Response to dopamine D2 agonists of fast-spiking interneurons in the prefrontal cortex becomes prominent after adolescence [26]. In the pathophysiology of SZ, interneuron deficits are thought to play an important role [27, 28]. Dysfunction of these fast-spiking interneurons can lead to dis-inhibition of pyramidal neurons in the cortex and hippocampus, as well as asynchrony of pyramidal neuron activation and cognitive impairment, all thought of as hallmarks of SZ pathophysiology [29]. Parvalbumin is a marker for a subclass of fast spiking interneurons. Post-mortem studies of SZ patient prefrontal cortex have detected changes in markers for specific sets of GABAergic neurons, such as a reduction in the number of parvalbumin-positive interneurons or in its expression level [30]. Therefore, understanding disturbances of postnatal interneuron maturation is currently believed to be very important to address the pathological mechanisms of SZ. It is unclear though whether such dysfunction occurs due to (1) intrinsic problems within the interneurons, (2) defects in connectivity with other cells, especially pyramidal neurons, or (3) both. Two important questions need to be addressed in association with initial risks and insults in pre- and perinatal brains; these include how initial disturbances of pyramidal neurons such as cell positioning by radial migration and dentritic arborization could affect postnatal interneuron maturation at a much later time, and how intrinsic maturation defects are triggered after possible disturbances in precursor cells of interneurons and tangential migration.
ii) Maturation of mesocortical dopaminergic projection
Dopamine plays an important role in the cerebral cortex by optimizing the signal-to-noise ratio of local cortical microcircuits in the prefrontal cortex [31]. Pharmacological and genetic studies, especially around the functional polymorphism of the dopamine-degrading enzyme catechol-O-methyltransferase (Val158Met), have suggested that cortical dopamine mediates proper information processing and working memory, which is impaired in SZ [32]. Dopaminergic projections from the ventral tegmental area (VTA) to the cortex display marked postnatal maturation [6, 33]. Until young adulthood, the concentration of dopamine and staining intensity of tyrosine hydroxylase (the rate-limiting enzyme in its synthesis) continue to increase in the prefrontal cortex [33, 34]. In some studies, decreases in tyrosine hydroxylase staining and dopamine levels are reported in the prefrontal cortex of SZ subjects [35, 36]. These observations may reflect defects of postnatal maturation of the mesocortical dopaminergic projection, but the mechanism underlying such disturbance remains elusive. Aberrant output of the pyramidal neurons, as a result of connectivity deficits between pyramidal neurons and interneurons occurring in early development, may potentially affect functions of the VTA where the dopaminergic neurons and other subcortical afferents originate. Alternatively, functional disconnection of the immature dopaminergic neurons to a disturbed cortical neuronal network caused by pre- and perinatal insults, may hamper proper maturation because of a lack of trophic support or other factors from cortical neurons to the distal side of dopaminergic neurons.
iii) Pruning of glutamate synapses
Glutamatergic synapses also undergo dynamic changes during postnatal brain maturation. Huttenlocher and colleagues [37] examined synaptic density in the middle frontal gyrus in autopsied brains from normal subjects, and found a dramatic reduction in number of synapses in late childhood and early adolescence. These eliminated synapses are asymmetric and mainly glutamatergic [38]. Because aberrant synaptic elimination in early adolescence could account for the timing of SZ onset and also certain neuropathological observations, the possibility of SZ as a disease of aberrant synaptic pruning has become a major working hypothesis [39]. The key question is simple: what causes such abnormal synaptic pruning? It is possible that abnormal synaptic connectivity arising from aberrant dendritic arborization that occurred in early neurodevelopment, in association with neuronal activity-dependent synaptic spine dynamics, underlies this deficit. It is also possible that neuro-immune interactions could be involved. For example, several key immune molecules, such as complement and MHC class I molecules, reportedly regulate synaptic function and elimination [40, 41].
iv) Myelination
The technological advance of diffusion tensor imaging by magnetic resonance techniques has permitted observation of white matter abnormalities in SZ patients [42]. In parallel, analyses of autopsied brains from SZ patients have established that expression profiles of oligodendrocyte-associated genes are changed in patients [43, 44]. Because myelination of the cortex occurs postnatally (in particular, completion of myelination in the frontal cortex occurs in young adulthood at the time of disease onset) [45–47], disturbances in myelination are thought to be very important in the pathophysiology of SZ. It is still uncertain how defects of early neurodevelopment influence myelination later. Disturbances of synaptic pruning may be linked to abnormal myelination. Alternatively, intrinsic disease-associated factors, such as genetic factors, may directly cause the disturbance of myelination just before the onset of the disease.
Functional disturbances in adult brains in SZ
Brain imaging studies have indicated there is further progression in the pathology of SZ brains for several years after the classic clinical diagnosis [48, 49]. Therefore, intrinsic mechanisms that underlie SZ, driven mainly by genetic factors, may influence functional deficits and progression in adult SZ brains also, in addition to their roles in pre- and perinatal brain development, as well as postnatal brain maturation. Deficits involving plasticity and associated intracellular signaling, such as cAMP signaling, may account for these problems.
Importance of models that reflect the etiologies and long-term neurodevelopmental processes of SZ
NMDA-type glutamate receptor antagonists, such as phencyclidine and ketamine, can elicit at certain doses SZ-like clinical manifestations in healthy humans [50]. Administration of these compounds to rodents elicits some pathological changes relevant to SZ. These drug-inducible models are useful as they may partially mimic the pathophysiology of SZ or functional disturbances observed in ‘adult’ brains in SZ. Their major drawback, however, is that they do not reflect the etiopathology and long-term neurodevelopmental processes of the human disease. The ability to treat SZ in its prodromal period successfully is one of, if not the ‘holy grail’, of SZ therapeutics. Such successful preventative treatment at prodromal stages would ameliorate the later emergence of the full repertoire of SZ symptoms [51]. To support such a goal, novel preclinical models that reflect the etiopathology and long-term neurodevelopmental processes of SZ are needed. Models such as conditional knockouts of NRG1/ErbB4 and DISC1 that cover the actual disease course are highly likely to enhance understanding of disease mechanisms, as well as prove critical for translational purposes.
Promising genetic susceptibility factors (NRG1, ErbB4 and DISC1): tools to address neurodevelopmental processes in SZ
The recent explosion of genome-wide association studies [52] and examination of copy number variations [53] are expected to disclose more SZ-associated genes. Identification of rare genetic mutants in other common brain diseases, such as Alzheimer's disease, has greatly aided the study of the pathogenesis of these diseases, including sporadic forms. Intriguingly, functional analyses of genetic susceptibility factors in cell models and of human brain imaging, have suggested that, instead of functioning independently from each other, these factors act in a synergistic manner in several common “pathways” that may contribute to the disease pathology [7]. From what is known about the growing list of risk factors today, NRG1, the NRG1 receptor ErbB4, and DISC1 may be the most useful in elucidating the questions discussed in this review. Both NRG1–ErbB4 and DISC1 cascades play key roles in many aspects of neurodevelopment, as described below. Nonetheless, until now these two cascades have been studied independently from one another. We will first summarize current knowledge of these two cascades during neurodevelopment (from pre- and perinatal periods to postnatal brain maturation), and second discuss their possible convergence, especially in appropriate animal models. Table 1 summarizes the current understanding of NRG1–ErbB4 and DISC1 cascades in each developmental stage. Recent advances in DISC1 biology [54–59], even in the short period since an extensive array of reviews was published [12, 14], have substantially changed the concept of signal convergence.
Table 1.
Physiological roles of DISC1 and NRG1/ErbB4 cascades at various stages of neurodevelopment, which seem to be disturbed in schizophrenia.
| Stage | Process | DISC1 | NRG1/ErbB4 | Major References |
|---|---|---|---|---|
| Pre/perinatal period (Cortex) |
Progenitor cell proliferation | +++ | ++ | [56, 85] |
| Radial migration | +++ | ++ | [66, 88] | |
| Tangential migration | ND | +++ | [89] | |
| Neurite outgrowth/ arborization | ++ | ++ | [75, 92] | |
| Astrogenesis | ND | +++ | [93] | |
| Postnatal maturation (Cortex) |
Interneuron maturation | ND | ++ | [99] |
| Dopaminergic neuron maturation |
ND | + | [96] | |
| Glutamatergic synapse maturation/pruning |
+ | ++ | [79, 94] | |
| Oligodendrocyte development/ Myelination |
+ | ++ | [59, 96, 98] | |
| Adulthood | Neurogenesis (Hippocampus) | +++ | ND | [56, 58] |
| Neuronal plasticity | ND | ++ | [94] | |
| cAMP signaling | ++ | ND | [60] | |
The roles of these two cascades in the pre/perinatal period, postnatal maturation, and adulthood are summarized. +++, well-established observation, including verification with in vivo models; ++, strongly indicated; +, suggested; ND, not addressed yet.
Roles of DISC1 in SZ pathology
Compelling genetic evidence for DISC1 was initially obtained from a large Scottish pedigree that included patients with SZ [60]. Since the time that this rare mutation of DISC1 was identified from this pedigree, many groups have conducted genetic association studies in several ethnic groups and now agree that DISC1 is a major risk factor for major mental illnesses, including both SZ and mood disorders [10, 14, 61, 62]. Studies of clinical subjects have reported that genetic variations of DISC1 influence brain function and anatomy [63–65]. In parallel with such genetic and clinical studies for DISC1, the cellular functions of DISC1 have been extensively studied. A current consensus is that DISC1 is a multifunctional anchoring molecule that regulates its interacting proteins in different subcellular compartments [12, 14]. In proliferating cells and immature neurons, DISC1 is found in association with the centrosome/microtubules and nucleus where it interacts with the dynein motor complex [66] and Activating transcription factor 4 (ATF4)/Promyelocytic Leukemia Protein (PML) transcriptional machinery [67], respectively. By contrast, in mature neurons, DISC1 is mainly located in the postsynaptic density (PSD) and the nucleus [68]. To date, cellular roles for DISC1 that have been characterized include neuronal progenitor cell proliferation, radial neuronal migration, dendritic arborization, and outgrowth, as well as regulation of cAMP metabolism and gene transcription.
i) Roles of DISC1 during pre- and perinatal stages (Fig. 2a, b)
DISC1 is highly expressed in the developing brain, especially the developing cortex [69]. DISC1 plays distinct roles in both neural progenitor cells and postmitotic neurons. Knockdown of DISC1 expression by in utero short-hairpin RNA (shRNA) injection at embryonic day 13 (E13) leads to decreased neural progenitor proliferation and premature neuronal differentiation in the developing cortex. Accordingly, the numbers of cells in the ventricular and subventricular zones in the cortex are decreased at E15 [57]. By contrast, knockdown of DISC1 in postmitotic neurons at E15, when radial neuronal migration becomes more prominent, leads to delayed neuronal migration [66]. This observation has independently been confirmed by a different laboratory, with an injection of DISC1 shRNA-expressing retroviruses at E14 and analysis at postnatal day 1 (P1) [58]. In both cases, DISC1 plays an important role in proper positioning of pyramidal neurons in the cortex. A possible role for DISC1 in tangential migration of interneurons remains to be established.
In neuronal progenitor cells, DISC1 interacts directly with GSK3β, which reduces phosphorylation of β-catenin and increases its stability[57]. Consequently, the Wnt-pathway is activated. By contrast, a role for DISC1 in radial neuronal migration may depend on its participation in the dynein motor complex, where DISC1 is required for proper anchoring of the proteins in the motor complex, which include Lissencephaly-1 (LIS1), Nuclear Distribution Element-Like-1 (NDEL1) and Bardet-Biedl-Syndrome (BBS) proteins [54]. Thus, independent knockdown of expression of these proteins per se also results in delayed neuronal migration in the developing cortex, phenocopying the effects of DISC1 knockdown. This motor complex also interacts with a centrosomal protein pericentriolar material-1 (PCM1), which is also a promising genetic risk factor for SZ [54, 70, 71].
Roles for DISC1 in neurite outgrowth have been studied mainly in cultured cells. Overexpression of a dominant-negative mutant DISC1 or knockdown of endogenous DISC1 leads to inhibition of neurite outgrowth in PC12 and primary neuron cultures [72–76]. More than one mechanism has been proposed, but validation has been limited to cell culture: for example, interactions of DISC1 with DISC1-binding zinc-finger protein (DBZ) in the presence of pituitary adenylate cyclase activating polypeptide (PACAP) [73], and DISC1 interactions with NDEL1 [72], kinesin-1 [76], or Grb2 [76] reportedly participate in this process. The influence of DISC1 on dendritic arborization in the cortex has been reported in a DISC1 genetically engineered model [55], as well as in mice with in utero injection of shRNA [66].
Substantial levels of DISC1 are also located in the nucleus in immature neurons. Nuclear DISC1 interacts with CREB family member transcription factors ATF4/5 and recruits nuclear co-repressor N-CoR to the transcriptional machinery [67, 77]. Because ATF4 is a key regulator of the stress response in neurons, nuclear DISC1 is likely to play a role in response to environmental factors relevant to SZ, such as birth hypoxia and congenital infection. In addition, nuclear DISC1 may also regulate progenitor cells via protein interaction with PML, which is known to regulate progenitor cell proliferation in the developing cortex [78].
ii) Direct influence of DISC1 on postnatal brain maturation (Fig. 2c)
Immuno-electron microscopy of normal human frontal and parietal cortex demonstrates prominent DISC1 staining in synapses, especially in PSD [68]. A protein-interactome study that identified the key protein–protein interaction networks around DISC1 indicates that DISC1 associates with many key proteins that regulate synaptic maturation and plasticity [79]. Thus, DISC1 may have a potential role in regulation of synaptic spines in association with neuronal activity, which could be critical for the pruning of glutamate synapses and, in turn, the connectivity of pyramidal neurons to interneurons.
A recent study with a zebrafish model reports that DISC1 controls development of oligodendrocytes [59]. Glial DISC1 is an area that should be studied extensively in the near future.
iii) Functional roles of DISC1 in adult brain (Fig. 2c)
In adult brain, DISC1 is highly expressed in the dentate gyrus of the hippocampus [69], where it regulates newborn neurons. Recently four convincing reports have provided a detailed characterization of these processes [55, 57, 58]. A single publication to date has provided preliminary but promising evidence indicating an impairment of adult hippocampal neurogenesis in SZ [80]. Until further replication and study, some caution is warranted, but it is an exciting entry point to begin to contemplate the manipulation of hippocampus neurogenesis therapeutically. In addition, as DISC1 is also a risk factor for mood disorders, the role of DISC1 in adult hippocampus might be more associated with mood disorders than with SZ.
A role for DISC1 protein in cAMP signaling was suggested by its interaction with phosphodiesterase-4 (PDE4), which degrades cAMP [81]. Because PDE4 inhibitors have been considered as potential therapeutic targets for psychiatric illnesses, this finding has clear therapeutic implications. Unfortunately owing to the unwanted side effects associated with most PDE4 inhibitors, this hypothesis might never be tested appropriately in man. In addition, there are other theoretical concerns as elevated cAMP levels as a result of changes in DISC1 regulation of PDE4, or PDE4 inhibition itself, would improve synaptic plasticity in the hippocampus but impair functioning of the prefrontal cortex complicating the potential therapeutic use of PDE4 inhibitors even further [82].
Roles of NRG1–ErbB4 in SZ pathology
For many years, NRG1 and its receptor ErbB4 have been studied in the areas of oncology and neuroscience. A landmark genetic association study with an isolated Icelandic population originally highlighted an important role for NRG1 in SZ [83]. Since that time, many studies have provided supportive evidence for a role of NRG-1–ErbB4 in SZ pathology. There are excellent review articles on NRG1–ErbB4 in association with SZ [9, 13, 84]. Therefore, we will summarize reports that are directly associated with long-term brain development and maturation, especially those associated with brain circuitry involving the frontal cortex and SZ pathology.
i) Roles of NRG1–ErbB4 during prenatal and perinatal stages (Fig. 2a, b)
Suggestive evidence for roles for NRG1 in proliferation of neuronal progenitor cells has been reported in cell culture, including studies with neuronal progenitors from embryonic neural stem cells [85]. Conditional ErbB4-null mice are resistant to NRG-induced cell proliferation in the subventricular zone in vivo [86]. These results support the idea that NRG1–ErbB4 signaling mediates progenitor cell proliferation, but details of the mechanism remain to be elucidated.
NRG1 plays roles in neuronal migration in various systems. In the cortex, NRG1 contributes to establishment of the radial glial scaffold [87], which aids radial migration of neurons along the scaffold to their final position in the cortex [88]. NRG1 is also important in tangential migration of interneurons to the cortex, which can influence the number of cortical GABAergic interneurons [89]. In addition, roles of NRG1 in radial migration in the cerebellum and tangential migration in the rostral migratory stream have been reported [90, 91]. Furthermore, relevant to SZ pathology, NRG1 expression is also required for proper axon guidance [92]. Finally, an excellent study reported that presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain [93].
ii) Direct influence of NRG1–ErbB4 on postnatal brain maturation (Fig. 2c)
Neuronal activity-dependent spine regulation participates in both spine formation and elimination of glutamate synapses. Activation and recruitment of ErbB4 into the synapse are required for this neuronal activity-dependent spine control [94]. Inside the postsynaptic scaffold, ErbB4 interacts with PSD protein 95 (PSD95), whose expression level, in turn, is controlled by NRG1 [95].
There are convincing data demonstrating the crucial roles of NRG1 in myelination in the peripheral nervous system [84]. Studies in cell culture and zebrafish also support the notion that NRG1 signaling plays a role in oligodendrocyte differentiation and myelination [59, 96, 97]. In transgenic mice expressing a dominant-negative ErbB4, fewer oligodendrocytes are observed in the cingulate cortex, a region that is thought to play a role in SZ [96]. The mice also display hypersensitivity to methamphetamine challenge, suggesting the influence of ErbB4 in function and maturation of dopaminergic neurons [96]. A recent study using NRG1-null mutants at various developmental stages cast doubt on this hypothesis, however, by showing normal myelination in these mice (but hypermyelination in NRG1-overexpressing mice) [98].
Finally, connectivity of interneurons and pyramidal neurons is important for function and maturation of interneurons. Modulation of activity-dependent GABA release in interneurons by presynaptic ErbB4 may mediate, at least in part, this process [99]. Conditional knockouts of NRG1-ERBB4 should help differentiate between the prenatal and postnatal effects.
iii) Functional roles of NRG1–ErbB4 in adult brain (Fig. 2c)
There are many reports supporting a concept that the NRG1–ErbB4 cascade modulates neuronal plasticity in adult brain [94, 99]. NRG1-induced expression of alpha7 nicotinic acetylcholine receptors (CHRNA7) on interneurons and its effects may contribute to the pathophysiology of SZ [100]. Given the increasing evidence suggesting a role of nicotinic acetylcholine signaling for cognitive functions and genetic association of CHRNA7 with SZ, the alpha7 receptor is a major target for many pharmaceutical companies as they look for agonists or positive allosteric modulators to treat the cognitive deficits associated with SZ [101].
In an excellent study with postmortem brains from patients with SZ, a marked increase in NRG1-induced activation of ErbB4 and suppression of NMDA-type glutamate receptor activation is observed, in comparison with normal controls. This supports the notion that the NRG1–ErbB4 cascade plays a role in adult brains from patients with SZ [102].
Possible convergence of NRG1–ErbB4 and DISC1 cascades (Fig. 2, Table 1)
As summarized above, both NRG1–ErbB4 and DISC1 cascades play roles in various aspects of pre- and perinatal periods, as well as in postnatal brain maturation. Because no single risk factor for SZ can cause the disease per se, additive or synergistic pathological effects seem likely.
At the molecular and cellular levels, there are at least two convergent sites of NRG1–ErbB4 and DISC1 action: first, all of these three molecules (NRG1 and ErbB4 via cleaved intracellular domains) directly mediate gene transcription in the nucleus [67, 93, 95]. Thus, testing whether these genes may synergistically regulate transcription of target genes is warranted. Second, both ErbB4 and DISC1 are located in the postsynaptic density of glutamate synapses [68, 103], where many other susceptibility factors for SZ, such as RGS4, CAPON, and neuronal nitric oxide synthase (nNOS), are also localized [104]. Growth factor receptor bound protein 2 (Grb2), an adaptor protein, interacts with both ErbB4 and DISC1 [76, 105] in the PSD. Neuronal activity-dependent synaptic pruning is likely to be mediated by these factors.
With respect to neuronal circuitry and function, both NRG1–ErbB4 and DISC1 cascades apparently mediate basic neuronal network formation in pre- and perinatal periods by regulating progenitor cell proliferation and migration. Nonetheless, more convincing data on in vivo roles for NRG1–ErbB4 and DISC1 in progenitor cell proliferation and tangential migration, respectively, are awaited. We believe that it is important to test how these two cascades contribute to the process, i.e. synergistically, complementarily, or both. If this question is clarified, a reasonable next step is to consider how other SZ genetic susceptibility factors, such as PCM1 and the nuclear distribution protein factor E homolog NDE1 [54, 70, 71, 106], and environmental factors such as hypoxia and viral infection [16, 17], might affect the process. Independent of their effects on early development, NRG1–ErbB4 and DISC1 cascades might also directly influence postnatal brain maturation. Previous reports clearly demonstrate that NRG1–ErbB4 is possibly involved in pruning of glutamate synapses [94], where other major susceptibility factors for SZ, such as nNOS and RGS4, may also be involved [104]. Roles for NRG1–ErbB4 in interneuron maturation and myelination are attractive hypotheses that warrant investigation. Analyses of how the DISC1 cascade may influence the four key elements of postnatal maturation, described above, are keenly awaited. In addition to direct effects of NRG1–ErbB4 and DISC1 cascades on early development and postnatal brain maturation, respectively, it is most important to tell how the initial risks/insults caused by these two cascades impair long-term postnatal brain maturation and how such accumulative effects result in the onset of SZ in young adulthood.
Animal models that can validate long-term and complicated neurodevelopmental processes of SZ
There are three major goals in generating animal models that can validate the long-term neurodevelopmental processes of SZ. First, as described just above, it is very important to clarify the influence of etiologically relevant risks/insults in pre- and perinatal periods on postnatal brain maturation, which, in turn, results in the full manifestation of endophenotypes and phenotypes relevant to SZ in young adulthood. It would be useful to distinguish effects of impairments on each developmental process, such as progenitor cell proliferation, neuronal migration, and arborization, to address the precise mechanisms. Second, the direct influence of etiological factors on postnatal brain maturation needs to be differentiated from their effect in early development. Third, as no single factor causes SZ per se, it is necessary to consider how each etiological factor functionally converges in a temporally and spatially regulated manner in vivo.
It will likely take a large system-biology-based approach (such as proteomic analysis) to tackle these problems, but we now have the technology and analytical capabilities to attempt this. State-of-the art genetic engineering of rodents, especially mice with conditional or inducible expression which enables gene deletion at different and limited time windows during pre- or post-natal development [107, 108], will be important tools. For example, induction of early postnatal expression of a C-terminal portion of DISC1 results in a cluster of SZ-related phenotypes, including reduced hippocampal dendritic complexity [109]. Genetic modulation in a specific lineage, such as selective targeting to interneurons or oligodendrocytes [96], in mice is also a promising approach. One limitation of the genetic engineering methods is the difficulty in modulating more than one gene at a time, as the etiology of SZ involves a combination of multiple genetic factors and environmental risks. Many groups have attempted to cross different SZ-related genetically engineered mice, but to date there are few data from such efforts. As a complementary method, in utero gene transfer to modulate expression of genes in the developing cerebral cortex may be useful because this method can modify expression of more than one gene at one time. As described for DISC1, in utero gene transfer can examine individual developmental processes and mechanisms by changing the timing of injection [57, 66]. Application of virus-mediated expression or RNAi constructs to genetically engineered mice can also address epistatic effects of disease risk factors.
There are several technical issues that critically influence data interpretation in studying NRG1–ErbB4 and DISC1 cascades. First, there is the molecular diversity of NRG1 and DISC1, which have many isoforms and variants. As shown in Supplementary Table, because each NRG1 isoform has different functions, the phenotypes of Nrg1-knockout models with deletion of different sets of isoforms are different from each other. Some mouse strains, including 129S6/SvEv, contain a critical deletion in the coding exon of the DISC1 gene [110]. Second, spatial and temporal elements should be considered in experimental design and data interpretation. For example, the dentate gyrus and developing cortex where NRG1–ErbB4 and DISC1 play key roles have distinct radial migration mechanisms, i.e. “outside-in” in dentate gyrus and “inside-out” in developing cortex. Therefore, it is understandable that DISC1 knockdown results in migration acceleration in the dentate gyrus but inhibition in the cortex [58, 66].
Concluding remarks
SZ is not a neurodevelopmental disorder in a simple sense, although a unique small portion of child-onset SZ exists and is extensively characterized [111]. SZ is primarily an adult psychiatric disorder in which initial risks/insults during early neurodevelopment at prenatal and perinatal stages are likely to disturb postnatal brain maturation for many years, resulting in the onset of the disease after puberty. Thus, to understand the mechanisms underlying SZ, it is essential to focus on the long-term disturbances of postnatal brain maturation and to consider how the initial insults in early development affect this process. Functional convergence of major etiological factors, such as NRG1/ErbB4 and DISC1 cascades, are likely to be useful tools to address this fundamental question (Box 1).
Key questions to be addressed towards better understanding of SZ pathology at the bench and promising translation to the bed.
- How do prenatal/perinatal brain insults lead to disturbances of postnatal maturation until the onset of SZ in young adulthood?
- Influences on postnatal interneuron maturation?
- Influences on postnatal maturation of the mesocortical dopaminergic projection?
- Impacts on synaptic pruning, especially in adolescence?
- Impacts on myelination?
- How can we address the time course of SZ pathology from initial brain insults in prenatal/perinatal periods to onset of the disease in young adulthood?
- Build and characterize animal models with genetic modulation of susceptibility factors for SZ, especially inducible and conditional mice.
- Focus on molecular pathways involving multiple genetic risk factors, such as NRG1- ErbB4 and DISC1, and their possible convergence at molecular and functional levels
- Examine possible functional convergence of risk factors, especially NRG1-ErbB4 and DISC1, in several key neurodevelopmental steps.
Current therapies for SZ and related disorders have limited efficacy and severe side effects, such as weight gain and diabetes. The origins of the majority of these compounds, which have efficacy driven by dopamine D2 antagonism , were from serendipitous observations in patients over 50 years ago [112]. Many efforts across both academia and industry are being started to hopefully fill the medicine cabinet of the future with new approaches that should attack SZ with a better appreciation of the disease and its underlying biology. Unfortunately, however, none of these approaches really derives from an understanding of the etiology-based molecular pathways of SZ. It is from this approach that we suggest novel therapeutic strategies to treat SZ, in a real disease-modifying sense, will emerge as is now touted for Alzheimer’s and other diseases. Furthermore, recent clinical studies have focused on subjects in the prodromal stages of SZ and have started to explore preventative medications that can block the progression to full-blown clinical diagnosis of SZ. Preventing disease onset in ‘at-risk’ patients would revolutionize treatment for psychiatric illnesses. To achieve this goal, understanding of disturbances in postnatal brain maturation in SZ is a crucial step.
Acknowledgments
We thank Drs Pamela Talalay, Amanda Law, and Eva Anton for critical reading of this manuscript. We acknowledge Ms Yukiko Lema for manuscript preparation. We apologize to authors who could not be cited due to space constrains. This work was supported by MH-084018 (A.S.), MH-069853 (A.S) as well as grants from Stanley (A.S.), CHDI (A.S.), HighQ (A.S.), S-R (A.S., A.K.), NARSAD (A.S., H.J-P; A.H-T., A.K.) and a fund from Brain Science Institute from JHU (A.S.).
Glossary
- Affective flattening
Diminished emotional expressiveness.
- Endophenotypes
Quantitative, heritable, trait-related deficits typically assessed by laboratory-based methods rather than clinical observation.
- Inside-out
When migrating neurons arrive in the cortical plate they bypass earlier-generated neurons to form the cortical layers in an inside-out sequence; deeper layers are the first to form, superficial layers the last.
- Prodrome
Early mild manifestations (in SZ: functional decline resulting from intrinsic abnormalties, possibly associated with cognitive and negative symptoms) appearing before the full-blown disease onset (in SZ: psychosis) and diagnosis.
- Radial migration
The main migration mode used by pyramidal neurons to reach from the subventricular zone to the cortical plate.
- Tangential migration
The migration mode used by interneurons to reach from medial ganglionic eminence to the cortex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 2.Rapoport JL, et al. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 3.Buka SL, Fan AP. Association of prenatal and perinatal complications with subsequent bipolar disorder and schizophrenia. Schizophr Res. 1999;39:113–119. doi: 10.1016/s0920-9964(99)00109-7. discussion 160-111. [DOI] [PubMed] [Google Scholar]
- 4.Pantelis C, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 5.Moore H, et al. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng KY, et al. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- 8.Owen MJ, et al. Schizophrenia: genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Blackwood DH, et al. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.St Clair D, et al. Association within a family of a balanced autosomal translocation with major mental illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- 12.Ishizuka K, et al. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 13.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chubb JE, et al. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- 15.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Clarke MC, et al. The role of obstetric events in schizophrenia. Schizophr Bull. 2006;32:3–8. doi: 10.1093/schbul/sbj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg SM, et al. Minor physical anomalies in schizophrenia: a metaanalysis. Schizophr Res. 2007;89:72–85. doi: 10.1016/j.schres.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 20.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 21.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 22.Cannon TD, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- 23.Henquet C, et al. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008;34:1111–1121. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung JP, Fritschy JM. Developmental profile of GABAA-receptors in the marmoset monkey: expression of distinct subtypes in pre- and postnatal brain. J Comp Neurol. 1996;367:413–430. doi: 10.1002/(SICI)1096-9861(19960408)367:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto T, et al. Protracted Developmental Trajectories of GABA(A) Receptor alpha1 and alpha2 Subunit Expression in Primate Prefrontal Cortex. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis DA, et al. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 29.Uhlhaas PJ, et al. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- 31.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 34.Lambe EK, et al. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akil M, et al. Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol Psychiatry. 2000;47:361–370. doi: 10.1016/s0006-3223(99)00282-6. [DOI] [PubMed] [Google Scholar]
- 36.Akil M, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 37.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 38.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keshavan MS, et al. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 40.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Huh GS, et al. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubicki M, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tkachev D, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 45.Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- 46.Sowell ER, et al. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 47.Giedd JN, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 48.Ho BC, et al. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 49.Mathalon DH, et al. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 50.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 51.White T, et al. The schizophrenia prodrome. Am J Psychiatry. 2006;163:376–380. doi: 10.1176/appi.ajp.163.3.376. [DOI] [PubMed] [Google Scholar]
- 52.O'Donovan MC, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- 53.Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 54.Kamiya A, et al. Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch Gen Psychiatry. 2008;65:996–1006. doi: 10.1001/archpsyc.65.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kvajo M, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen S, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao Y, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood JD, et al. Disrupted-in-schizophrenia 1 and neuregulin 1 are required for the specification of oligodendrocytes and neurones in the zebrafish brain. Hum Mol Genet. 2009;18:391–404. doi: 10.1093/hmg/ddn361. [DOI] [PubMed] [Google Scholar]
- 60.Millar JK, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 61.Hamshere ML, et al. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry. 2005;62:1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- 62.Hashimoto R, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- 63.Callicott JH, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cannon TD, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 65.Hennah W, et al. A haplotype within the DISC1 gene is associated with visual memory functions in families with a high density of schizophrenia. Mol Psychiatry. 2005;10:1097–1103. doi: 10.1038/sj.mp.4001731. [DOI] [PubMed] [Google Scholar]
- 66.Kamiya A, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 67.Sawamura N, et al. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatry. 2008;13:1138–1148. doi: 10.1038/mp.2008.101. 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirkpatrick B, et al. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 69.Schurov IL, et al. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry. 2004;9:1100–1110. doi: 10.1038/sj.mp.4001574. [DOI] [PubMed] [Google Scholar]
- 70.Gurling HM, et al. Genetic association and brain morphology studies and the chromosome 8p22 pericentriolar material 1 (PCM1) gene in susceptibility to schizophrenia. Arch Gen Psychiatry. 2006;63:844–854. doi: 10.1001/archpsyc.63.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Datta SR, et al. A threonine to isoleucine missense mutation in the pericentriolar material 1 gene is strongly associated with schizophrenia. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.128. [DOI] [PubMed] [Google Scholar]
- 72.Brandon NJ, et al. Disrupted in Schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. doi: 10.1016/j.mcn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Hattori T, et al. A novel DISC1-interacting partner DISC1-Binding Zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol Psychiatry. 2007;12:398–407. doi: 10.1038/sj.mp.4001945. [DOI] [PubMed] [Google Scholar]
- 74.Morris JA, et al. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- 75.Ozeki Y, et al. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shinoda T, et al. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007;27:4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sawamura N, et al. A form of DISC1 enriched in nucleus: altered subcellular distribution in orbitofrontal cortex in psychosis and substance/alcohol abuse. Proc Natl Acad Sci U S A. 2005;102:1187–1192. doi: 10.1073/pnas.0406543102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Regad T, et al. The tumor suppressor Pml regulates cell fate in the developing neocortex. Nat Neurosci. 2009;12:132–140. doi: 10.1038/nn.2251. [DOI] [PubMed] [Google Scholar]
- 79.Camargo LM, et al. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- 80.Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 81.Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 82.Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- 83.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corfas G, et al. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, et al. Neuregulin-1 increases the proliferation of neuronal progenitors from embryonic neural stem cells. Dev Biol. 2005;283:437–445. doi: 10.1016/j.ydbio.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 86.Ghashghaei HT, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci U S A. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmid RS, et al. Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc Natl Acad Sci U S A. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anton ES, et al. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 89.Flames N, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 90.Anton ES, et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 91.Rio C, et al. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 92.Lopez-Bendito G, et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sardi SP, et al. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 94.Li B, et al. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bao J, et al. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- 96.Roy K, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vartanian T, et al. A role for the acetylcholine receptor-inducing protein ARIA in oligodendrocyte development. Proc Natl Acad Sci U S A. 1994;91:11626–11630. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brinkmann BG, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woo RS, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, et al. Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olincy A, Stevens KE. Treating schizophrenia symptoms with an alpha7 nicotinic agonist, from mice to men. Biochem Pharmacol. 2007;74:1192–1201. doi: 10.1016/j.bcp.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hahn CG, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 103.Garcia RA, et al. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hashimoto R, et al. Postsynaptic density: a key convergent site for schizophrenia susceptibility factors and possible target for drug development. Drugs Today (Barc) 2007;43:645–654. doi: 10.1358/dot.2007.43.9.1088821. [DOI] [PubMed] [Google Scholar]
- 105.Ma L, et al. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23:3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burdick KE, et al. Elucidating the relationship between DISC1, NDEL1 and NDE1 and the risk for schizophrenia: evidence of epistasis and competitive binding. Hum Mol Genet. 2008;17:2462–2473. doi: 10.1093/hmg/ddn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li W, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pletnikov MV, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. 115. [DOI] [PubMed] [Google Scholar]
- 109.Li W, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clapcote SJ, Roder JC. Deletion polymorphism of Disc1 is common to all 129 mouse substrains: implications for gene-targeting studies of brain function. Genetics. 2006;173:2407–2410. doi: 10.1534/genetics.106.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46:1418–1428. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- 112.Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]