Abstract
Content and function words have different roles in language and differ greatly in their semantic content. Although previous research has suggested that these different roles may be mediated by different neural substrates, the neuroimaging literature on this topic is particularly scant. Moreover, fMRI studies that have investigated differences between content and function words have utilized tasks that focus the subjects’ attention on the differences between these word types. It is possible, then, that task-related differences in attention, working memory, and decision-making contribute to the differential patterns of activation observed. Here, subjects were engaged in a continuous working memory cover task while single, task-irrelevant content and function words were infrequently and irregularly presented. Nonword letter strings were displayed in black font at a fast rate (2/sec). Subjects were required to either remember or retrieve occasional nonwords that were presented in colored fonts. Incidental and irrelevant to the memory task, content and function words were interspersed among nonwords at intervals of 12 to 15 sec. Both word types strongly activated temporal-parietal cortex, middle and anterior temporal cortex, inferior frontal gyrus, parahippocampal gyrus, and orbital frontal cortex. Activations were more extensive in the left hemisphere. Content words elicited greater activation than function words in middle and anterior temporal cortex, a sub-region of orbital frontal cortex, and the parahippocampal region. Words also evoked extensive deactivation, most notably in brain regions previously associated with working memory and attention.
Keywords: fMRI, language, semantic processing, content and function words
1. INTRODUCTION
Content and function words are two classes of words that follow the same rules of English orthography and phonology, but differ markedly in their role in language and in the degree to which they represent meaning. Content words are nouns, verbs, or adjectives that convey semantic information. They are often, but not always, associated with a physical object (e.g., house, table), have many associations to other words (e.g., dog, cat), and have meaning independent of context. The number of content words in a lexicon can always be increased as new objects or concepts are created or discovered. Thus, content words are often referred to as open-class words. Because of their semantic aspect, content words are likely to engage individuals in semantic activation, selection, and retrieval. Episodic memory processes may also be engaged, as individuals recall specific memories associated with a content word (e.g., school). In contrast, function words have linking and syntactic functions in context (e.g., although, while), but have few inherent associations to other words when presented outside of a sentence. The number of function words is generally fixed within a lexicon, and so they are also termed closed-class words. While content words retain their semantic properties independent of context, the degree to which function words engage syntactic processes outside of a sentential context is debated. Content words vary greatly in imageability, the ease with which an image can be generated for a given word (Paivio, 1971, 1983). For example, compare dog and truth. But function words are not imageable in and of themselves (e.g., of and the). In addition to these defining characteristics, collections of content and function words often differ along other confounding dimensions, such as word frequency and length.
As content and function words differ greatly in the semantic information that they convey, a comparison of brain regions activated by these two different word classes may provide important information about the representation of meaning in the brain. Content words, even when presented in isolation, can still convey a significant amount of semantic information and engage individuals in semantic processes. Function words provide an ideal comparison condition to help isolate semantic processes, as function words control for orthographic, phonological, and lexical processes. Moreover, syntactic processing will be limited by presenting all stimuli outside of a sentential context.
Previous research has suggested a different neurological organization for these two classes of words (Bradley & Garrett, 1983; Friederici, 1985; Friederici, Opitz, & von Cramon, 2000; Gordon & Caramazza, 1982; Nobre, Price, Turner, & Friston, 1997; Nobre & McCarthy, 1994; Segui, Mehler, Frauenfelder, & Morton, 1982). Much of this work has relied upon electrophysiological recordings made from scalp electrodes. A sequence of event-related potentials (ERPs) that discriminate content words, function words, and nonwords have been reported (e.g., Brown, Hagoort, & ter Keurs, 1999; King & Kutas, 1998; Kutas & Hillyard, 1983; Munte et al., 2001; Neville, Mills, & Lawson, 1992; Nobre & McCarthy, 1994; Osterhout, Bersick, & McKinnon, 1997). Neville and colleagues noted a negative-going electrophysiological deflection, located over left anterior frontal cortex (~280ms), that was elicited by function words, but not content words, which they attributed to grammatical processing (Neville, Mills, & Lawson, 1992). Others have also reported an early negativity, but attributed it to grammatical complexity (Brunelliere, Hoen, & Dominey, 2005) or grammatical category (Osterhout, Bersick, & McKinnon, 1997), rather than word class per se.
One characteristic in which content and function words differ markedly is word frequency. Investigations that have controlled for frequency, observed a negative ERP component for both word classes, in which latency was influenced by word frequency: the component had an earlier onset in words with higher frequencies (King & Kutas, 1998). A parametric investigation of frequency and word class also supported the N280’s sensitivity to frequency manipulations regardless of word class (Munte et al., 2001). Although processing of content and function words is almost certainly affected by context, this early electrophysiological negativity does not seem to be sensitive to context. It has been reported in word lists (King & Kutas, 1998; Munte et al., 2001; ter Keurs, Brown, & Hagoort, 2002), scrambled prose (Osterhout, Bersick, & McKinnon, 1997), and sentences (Brown, Hagoort, & ter Keurs, 1999; Munte et al., 2001; Osterhout, Bersick, & McKinnon, 1997; ter Keurs, Brown, Hagoort, & Stegeman, 1999).
Although scalp-recorded ERPs do not originate solely or directly from the brain regions that underlie the scalp topography, anterior inferior frontal brain regions have an important role in language. Patients with damage in or around left inferior frontal gyrus often develop Broca’s aphasia which is marked by limited speech production. Of particular relevance to the present experiment, the speech of these patients is often devoid of function words (Kearns, 2005). Electrophysiological investigations with Broca’s aphasics have shown that the N280 is absent in patients, in comparison to healthy adults (Brown, Hagoort, & ter Keurs, 1999; ter Keurs, Brown, & Hagoort, 2002; ter Keurs, Brown, Hagoort, & Stegeman, 1999). These findings suggest that the left inferior frontal regions are important for grammatical processing and may contribute to this early electrophysiological negativity.
However, scalp-recorded ERPs are limited in their ability to localize activation within the brain, and brain damage in patients is often diffuse or difficult to quantify precisely, so the results of these studies have not definitively established what neural structures differentiate content and function words. Only a few studies have investigated differing activations evoked by content and function words using functional neuroimaging. Nobre and colleagues (Nobre, Price, Turner, & Friston, 1997) used functional magnetic resonance imaging (fMRI) to demonstrate that content words activated a network of brain regions in the left hemisphere, including the anterior inferior frontal gyrus, the angular gyrus, medial and anterior temporal regions and posterior cingulate. These areas are consistent with previous reports of word activation (Binder et al., 1997; Fiez & Petersen, 1998; Price, 1998). In contrast, function words elicited largely left hemisphere activation in motor, premotor, posterior inferior frontal regions, middle temporal and supramarginal gyri. They suggested that because function words lack semantic properties, phonological and articulatory processes influenced the patterns of activation more for function than for content words.
Friederici and colleagues (Friederici, Opitz, & von Cramon, 2000) also used fMRI to investigate differential processing of content and function words during a semantic task (concrete/abstract judgment) and a syntactic task (noun/function word judgment). During the semantic task, inferior frontal regions and posterior portions of the superior and middle temporal gyri showed greater activation than during the syntactic task. During the syntactic task two separate left inferior frontal regions were activated. Moreover, an interaction between word class and concreteness was found during the syntactic task. This interaction was interpreted as an effect of prototypicality, in which atypical category members (i.e., abstract content words and concrete function words) elicited greater activation.
In both of these previous studies content and function words were embedded within different decision tasks that required the subject to make judgments about each word. This useful strategy provides some control over participants’ strategies. However, tasks can also engage additional cognitive processes – e.g., working memory, attention, decision-making, response planning and execution - that are not of direct relation to word processing. These ancillary cognitive processes may systematically vary across tasks along with the word processes of interest. An alternative approach is to provide a task that is unrelated to the presentation of the critical stimuli, but which otherwise imposes a constant engagement of working memory and other cognitive processes regardless of what word type is presented.
Here we embedded content and function words within a continually changing stream of nonwords (2/sec) in an adaptation design (Grill-Spector & Malach, 2001). Content and function words were presented within this stream every 12-15 seconds. The continual presentation of nonword letter strings served to maximally stimulate areas that responded only to perceptual or orthographic properties of the stimuli. Thus, any brain region that demonstrated activation above baseline, whether equally to both, or preferentially to one of the word classes must have responded on the basis of phonological, lexical, semantic or other high-level properties and not on the basis of perceptual and orthographic properties. In addition, during the rapid presentation of the word and nonword letter strings, participants performed a working memory task. This task was unrelated to the content and function words, and thus should not have differentially influenced the activations elicited by the critical stimuli. However, by engaging subjects in a demanding and concurrent task, we sought to limit whatever ancillary and task-irrelevant processes might be differentially engaged by these two word classes. We hypothesize that both content and function words will elicit activations in brain regions sensitive to phonological or lexical processes, such as angular gyrus and posterior middle temporal gyrus. Content words, but not function words, should elicit activations in brain regions sensitive to semantic processing, such as anterior middle temporal gyrus and anterior inferior temporal gyrus. Previous research has highlighted the importance of left inferior frontal gyrus in language processes. However, this region has been implicated in both articulatory processes, as well as semantic processes, such as semantic selection and semantic retrieval. It is possible that both content and function words could elicit activation in this region.
2. RESULTS
2.1 Behavioral Results
Subjects’ average accuracy for the matching task was 82.57% (SD=9.12%); the average response time (RT) was 1013.23ms (SD 166.19ms). Behavioral data from one subject was lost due to computer error. These data indicate that the match-to-sample task was demanding but that subjects were able to perform successfully.
2.2 fMRI Activation
Content and function words elicited activation in several brain regions: bilateral middle temporal gyrus (centroid MNI coordinates: L: -66, -16, -12; R: 66, -10, -16; R: 74, -26, -5; R: 66, -38, -12), Left inferior frontal gyrus (-47, 34, -14), Right orbital frontal gyri (34, 36, -20), left anterior middle temporal gyrus (-40, 12, -42), left angular gyrus (-54, -60, 36), left superior frontal gyrus (L: -11, 52, 23; L: -10, 36, 56) and right anterior parahippocampal gyrus (R: 24, -16, -24).
Figure 2 presents a left and right hemisphere surface view of the functional ROIs identified by the internal localizer analysis. The green overlays represent those functional ROIs defined by the FSL analysis of the initial data subset that comprised the internal localizer (see also Table 1). The red clusters within each functional ROI represent regions in which content words elicited greater activation than function words. These results are from the secondary voxel-based analysis performed on the data that was withheld from the functional localizer. See the Experimental Procedures section for more details on these analysis techniques. Where red and green regions overlap, voxels are shown in red. The ROI letter labels are used consistently across all figures in this manuscript.
Figure 2.
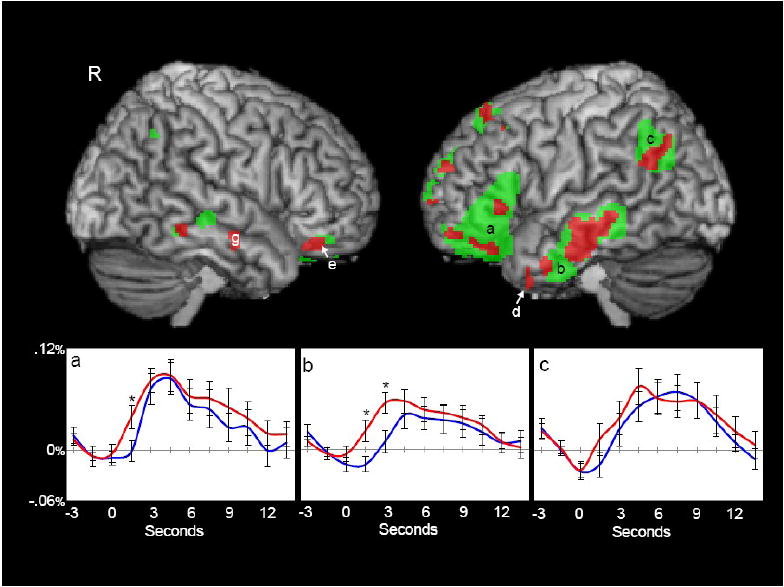
Activation elicited by content and function words. Regions in which either content or function words elicited a significant activation (green) and regions in which content words elicited significantly greater activation than function words (red) are shown on 3D volume renderings. Throughout the paper and figures, areas of activation are consistently labeled with letters (e.g., the area labeled b in Figure 2 is the same as that labeled b in Figure 3). Activations in left inferior frontal gyrus (a), left middle temporal gyrus (b, d), left angular gyrus (c), right orbital frontal gyrus (e), and right middle temporal gyrus (g) are shown. Hemodynamic time courses to content (red) and function words (blue) from left inferior frontal gyrus (a), left middle temporal gyrus (b) and left angular gyrus (c) are shown. Hemodynamic time courses represent the average time course (-3s - 13.5s) for the region. Time points with significant differences between content and function words are indicated with an asterisk.
Table 1.
Peak Coordinates and Z-values for regions in which either content or function words elicited significant activation (N=16)
| Location |
X | Y | Z | Max Z-value | # Voxels | |
|---|---|---|---|---|---|---|
| Area | Hemisphere | |||||
| Angular Gyrus (c) | Left | -54 | -60 | 36 | 3.0 | 701 |
| Middle Temporal Gyrus (b) | Left | -66 | -16 | -12 | 3.42 | 1117 |
| Middle Temporal Gyrus (g) | Right | 66 | -10 | -16 | 1.92 | 21 |
| Right | 74 | -26 | -5 | 2.07 | 19 | |
| Right | 66 | -38 | -12 | 1.85 | 16 | |
| Anterior Inferior Temporal Cortex (d) | Left | -40 | 12 | -42 | 1.79 | 13 |
| Inferior Frontal Gyrus (a) | Left | -47 | 34 | -14 | 3.99 | 2467 |
| Orbital Frontal Cortex (e) | Right | 34 | 36 | -20 | 2.24 | 90 |
| Parahippocampal Gyrus (h) | Right | 24 | -16 | -24 | 2.04 | 23 |
| Superior Frontal Gyrus | Left | -11 | 52 | 23 | 2.54 | 348 |
| Left | -10 | 36 | 56 | 2.27 | 159 | |
Coordinates represent the centroids of maximum activation in MNI space.
Activation was highly lateralized to the left hemisphere where three prominent ROIs are evident in inferior frontal gyrus (labeled ‘a’), the middle temporal gyrus (‘b’), and the temporo-parietal region near the angular gyrus (‘c’). The average waveforms derived from the data that was reserved from the initial FSL analyses that defined the ROIs are presented for these three regions (a, b, c). Significant differences in the time courses of activation were observed for the mean response from the middle temporal gyrus ROI, but not for the temporo-parietal or inferior frontal gyrus ROIs.
Smaller activations were also identified by the internal localizer in roughly homologous regions of the right temporal lobe (regions ‘e’ and ‘g’). The ROI in the right middle temporal gyrus (region ‘g’) differentiated the time courses from content and function words (hemodynamic responses not shown).
The left temporal gyrus activation also included a smaller more anterior ROI (‘d’) where all of the voxels identified in the internal localizer analysis also showed significant differences (content words > function words) in the subsequent voxel-based analysis. Figure 3 presents a series of sagittal cuts to illustrate the voxel-based analysis performed within these left lateral ROIs (labeled ‘b’ and ‘d’).
Figure 3.
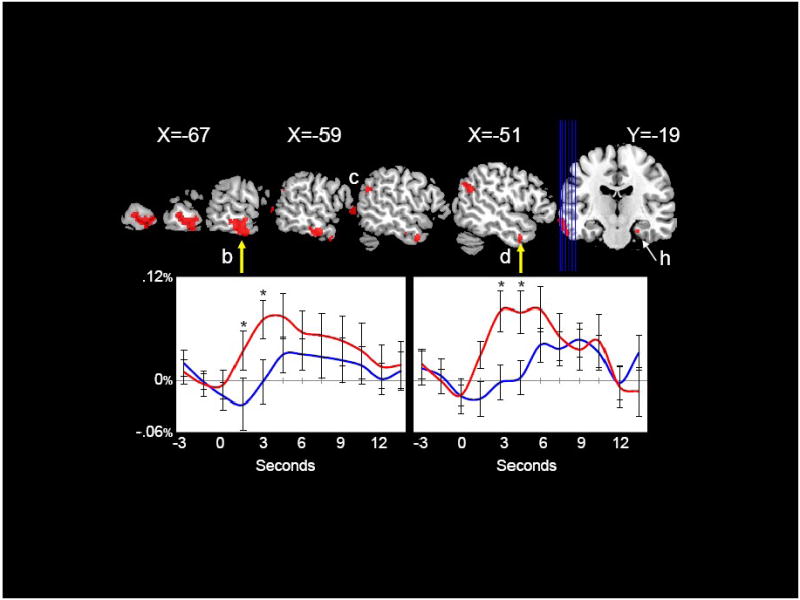
Left Middle Temporal Gyrus. Regions in which content words elicited significantly greater activation than function words (red) are displayed on sagittal slices through the left hemisphere. Two regions of activation in left middle temporal gyrus were found: a posterior region (b) and a more anterior, inferior region (d). Significant differences between content and function words in the parahippocampal gyrus can be seen in the coronal view. Hemodynamic time courses to content (red) and function (blue) words from the posterior region (left, b) and from the more anterior region (right, d) are shown. Time points with significant differences between content and function words are indicated with an asterisk. Coordinates are in MNI space.
Figure 4 shows two clusters (‘e’ and ‘f’) in bilateral regions of orbital frontal cortex (OFC) that strongly differentiated content and function words. These OFC clusters are also visible in the axial view presented in Figure 5. Figure 5 also shows the cluster of activity in the right anterior parahippocampal region (‘h’) where content words elicited greater activation than function words. This region is of particular interest because it has been previously found to differentiate content and function words in intracranial ERP recordings (McCarthy, Nobre, Bentin, & Spencer, 1995; Nobre, Allison, & McCarthy, 1994; Nobre & McCarthy, 1995).
Figure 4.
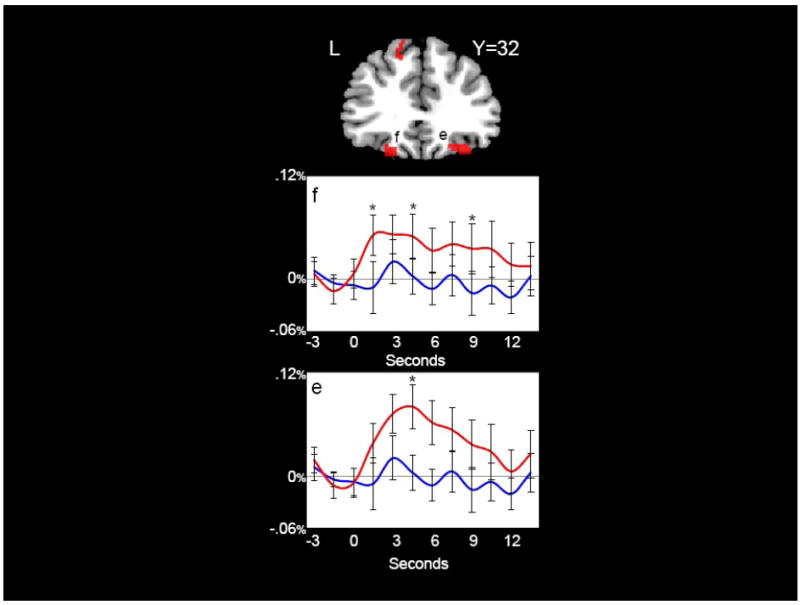
Orbital Frontal Gyri. Content words elicited significantly greater activation than function words (red) in right (e) and left (f) orbital frontal gyri. Hemodynamic time courses to content (red) and function (blue) words from right (e) and left (f) orbital frontal gyri are shown. Time points with significant differences between content and function words are indicated with an asterisk. Coordinates are in MNI space.
Figure 5.
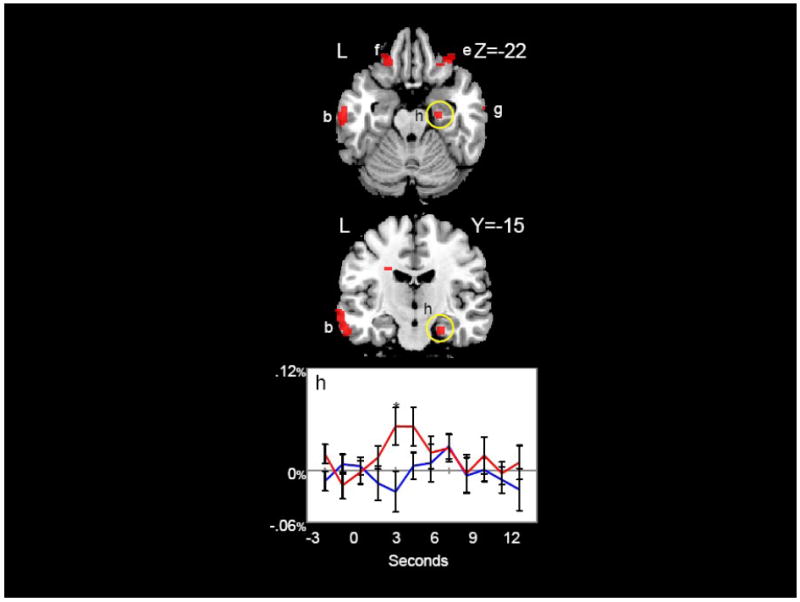
Temporal, Frontal, and Parahippocampal Regions. Regions in which content words elicited significantly greater activation than function words (red) are shown. Hemodynamic time courses to content (red) and function (blue) words in the right parahippocampal gyrus (h) are shown. Time points with significant differences between content and function words are indicated with an asterisk. Coordinates are in MNI space.
There were no regions in which function words elicited significantly greater activation than content words.
2.2.3 Areas of Deactivation
We also examined the data for areas in which either content or function words elicited significant deactivation. Figure 6 depicts areas of deactivation in blue in the left and right hemisphere surface views, and in a representative slice through the dorsolateral prefrontal cortex. For ease of comparison, the areas positively activated by words are presented in green.
Figure 6.
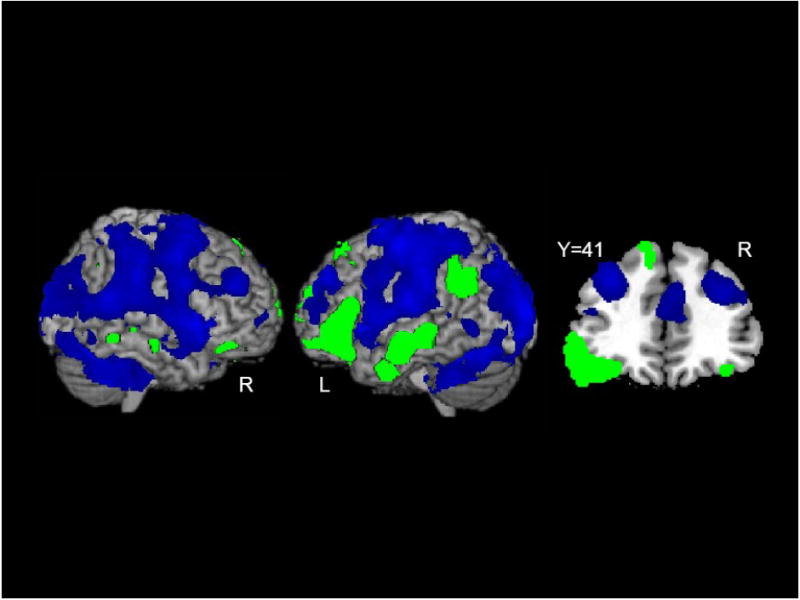
Areas of Deactivation. Regions in which either content or function words elicited a significant deactivation (blue) and regions in which either content or function words elicited a significant activation (green) are shown. A coronal slice through frontal gyri further illustrates the pattern of deactivations. Coordinates are in MNI space.
3. DISCUSSION
We used functional MRI to identify brain regions engaged while processing English words, and to identify brain regions that were differentially activated by the processing of content and function words. To emphasize automatic processing of single words, subjects were engaged in a demanding concurrent working memory task that was unrelated to either word class. The fMRI results revealed that words elicited activation in bilateral middle temporal gyrus, bilateral inferior and orbital frontal gyri, left anterior middle temporal gyrus, left angular gyrus, left superior frontal gyrus, and right anterior parahippocampal gyrus (Figures 2-5). Left hemisphere activations were larger in spatial extent compared to those in the right hemisphere.
Of greatest interest were several regions that were preferentially activated by content words, including bilateral anterior-inferior and middle temporal cortex, bilateral orbital frontal gyri, and right anterior parahippocampal gyrus.
The temporal lobe activations are largely consistent with previous reports of lexical and semantic processing (Binder et al., 1997; Fiez & Petersen, 1998; Price, 1998). Functional imaging experiments have demonstrated activity in this region when words were combined in meaningful grammatical sentences,(Humphries, Love, Swinney, & Hickok, 2005; Ikuta et al., 2006; Rossell, Price, & Nobre, 2003; Vandenberghe, Nobre, & Price, 2002), during both auditory and visual language processing (Lindenberg & Scheef, 2007), during semantic priming (Copland, de Zubicaray, McMahon, & Eastburn, 2007; Gold et al., 2006), and when word frequency was manipulated (Prabhakaran, Blumstein, Myers, Hutchison, & Britton, 2006). Previous research has indicated that damage to inferior and anterior temporal regions causes deficits in naming and identification of objects (Damasio, Grabowski, Tranel, Hichwa, & Damasio, 1996; Hodges & Patterson, 1996; Hodges, Patterson, Oxbury, & Funnell, 1992; Mummery et al., 1999; Snowden, Goulding, & Neary, 1989).
Activation that differentiated content and function words was also observed in the right anterior parahippocampal gyrus. Subdural intracranial recordings from the anterior medial temporal lobe (AMTL) have revealed a positive ERP at ~400 ms that is evoked by words, but not by nonword letter strings. The amplitude of this component is much smaller for function words compared to content words, and is attenuated when content words are primed by related words or sentence context (McCarthy, Nobre, Bentin, & Spencer, 1995; Nobre, Allison, & McCarthy, 1994; Nobre & McCarthy, 1995). While the current fMRI results reached significance only in the right hemisphere, the previously mentioned word-sensitive intracranial ERP recordings were observed in bilateral AMTL. The anterior parahippocampal gyrus has also been implicated in memory retrieval and familiarity based processes (for a review see, (Squire, 1991)). It is possible that the activation we observed here is also influenced by memory processes. Content words, in addition to engaging semantic processes, are likely to elicit episodic memories. However, it is unlikely that this activation is related to any task-related memory processes. The presentation of the content and function words was such that they never immediately preceded or followed task-related trials, limiting the influence of task-related activity on word trials. Moreover, if task-related memory was driving this activation, we would expect there to be equivalent activation across word types, rather than the differential activation, only for content words, that we observed.
One other factor that may have contributed to the observed differences between content and function words is orthographic neighborhood density. Orthographic neighborhood density is a measure of the number of words that can be created by recombining the letters of a word (Coltheart, Davelaar, Jonasson, & Besner, 1977). Lexical and semantic facilitation has been found for words with high density neighborhoods compared to those with low density neighborhoods (e.g., Forster & Shen, 1996; Sears et al., 1995, 1999; Andrews, 1997). For example, high density words were found to have a larger N400 compared to low density words; the authors attributed this to an increased amount of semantic information being generated for the high density words (Holcomb, Grainger & O’Rourke, 2002). Therefore, although this factor is defined on the basis of orthography, it is not necessarily orthography per se that is driving these effects. In a post-hoc analysis of orthographic density, we found that content words had significantly more dense neighborhoods compared to function words [t(148)=2.32, p=.021). Therefore, it is possible that this difference in neighborhood density contributed to the effects we found. However, as neighborhood density effects have been found in both lexical and semantic tasks, it is still possible that the greater activation for content words was influenced by the lexical neighbors’ semantic features.
The left angular gyrus was strongly activated by both content and function words compared to the nonword baseline condition, but did not strongly differentiate these two word types. The angular gyrus has been implicated in reading and is thought to have connections with occipital, temporal, and frontal regions (Mesulam, 1998). This area has been shown to be active during single word reading in normal readers, possibly responsible for orthographic-phonological translations or lexical retrieval (Horwitz, Rumsey, & Donohue, 1998; Pugh et al., 2000; Shaywitz et al., 1998; Warburton et al., 1996). In dyslexic readers, this area has been shown to be less active than in normal readers, especially during tasks that involve converting orthography to phonology (Horwitz, Rumsey, & Donohue, 1998; Pugh et al., 2000; Shaywitz et al., 1998). Rumsey and colleagues have found that increased blood flow in the angular gyrus is correlated with better reading skills in normal readers, but worse reading skills in dyslexic readers, suggesting both the importance of this area for reading and the dysfunction of this area in dyslexic readers (Rumsey et al., 1999).
The left inferior frontal gyrus also showed similar activation to content and function words. Many of the studies that have reported inferior frontal involvement in semantic processing have used tasks that require explicit semantic processes like semantic retrieval or selection (Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997; Wagner, Desmond, Demb, Glover, & Gabrieli, 1997; Wagner, Koutstaal, Maril, Schacter, & Buckner, 2000), although see (Petersen, Fox, Snyder, & Raichle, 1990). Our subjects did not have to perform any explicit semantic or lexical task. Moreover it seems unlikely that function words, which also elicited activation in this region, would engage subjects in semantically mediated processes.
Inferior frontal regions also have a well established role in working memory processes (Cohen et al., 1994; Cohen et al., 1997; McCarthy, Blamire, Puce, Nobre, Bloch et al., 1994; Petrides, Alivisatos, Meyer, & Evans, 1993). The overt task participants performed was a working memory task; it might be plausible to speculate whether this also contributed to this activation. We do not believe this is the case. Task performance and task-related trials did not occur in close temporal proximity to the content and function word trials. Because of this temporal disconnect between word and task trials, it is unlikely that activations time-locked to the word trials reflected task-related activity. Moreover participants were continually engaged in the overt task. Thus the task itself, like the presentation of the nonword trials, became part of the baseline activity. We cannot rule out the possibility that the content and function word trials themselves elicited some type of working memory engagement, distinct from the overt task itself, but again, this working memory processes would need to be equivalent for both content and function word trials, as both stimuli types elicited activation in this region.
It seems most parsimonious that participants were engaged in a linguistic process that both content and function word trials could easily elicit. Inferior frontal regions have also been implicated in phonological processing (Demonet et al., 1992; Demonet, Price, Wise, & Frackowiak, 1994; Fiez et al., 1995), and damage to this region produces language production deficits (Broca, 1861a; Broca, 1861b; Kearns, 2005). It is possible that although not instructed to do so, participants were sub-vocalizing the words and that this articulatory process contributed to the pattern of activation in this region.
We also found bilateral activation in the left and right orbital frontal gyri in which content words elicited greater activation than function words (Figure 5). A well established role for orbital frontal cortex has been established in motivation, reward, and emotional processing. Although, this was not an a priori region of interest, orbital frontal cortex has also been shown to be involved in attentional selection (Rushworth et al., 2005). In a PET study, Paradiso and colleagues found right orbital frontal cortex activation during the recall of a practiced word list and left orbital frontal cortex activation during the recall of a novel word list (Paradiso et al., 1997). In a related study, Andreasen and colleagues found bilateral anterior inferior frontal activation during the recall of novel word lists compared to recall of practiced word lists (Andreasen et al., 1995) and left orbital frontal cortex activation in response to conscious episodic memory recall (Andreasen et al., 1999). Others have indicated a role for fronto-polar regions, especially the right, in retrieval success (Rugg, Fletcher, Frith, Frackowiak, & Dolan, 1996). It is possible that when processing content words, participants recalled other words or memories associated with the content word. Although previous research has also implicated orbital frontal cortex in emotional related processes (Bechara, Damasio, & Damasio, 2000; Damasio, 1996; Davidson & Irwin, 1999), it is unlikely that emotional processes were driving this activation, as the content words presented in this experiment were neutral in valence.
We did not find any regions in which function words elicited significantly more activation than content words, which may reflect the fact that our experiment deemphasized syntactic processing. Function words and the syntactic processes associated with such words are largely context dependent. The connective utility of function words is limited when the context, crucial for making syntactic references, is absent.
One unexpected, but notable, finding was the extensive deactivation in both right and left hemispheres that occurred at the onset of both content and function words (Figure 6). Although deactivations were observed in several regions, the deactivation in the dorsolateral prefrontal cortex (dlPFC) observed here for words bears similarity to the deactivation of dlPFC in the delay interval of a match-to-sample task by task-irrelevant pictures (Dolcos & McCarthy, 2006).
The present study used fMRI to examine brain activation to content and function words in an experimental design that minimized task-related processing of words. All words elicited comparable activation in left angular gyrus and left inferior frontal gyrus. In no region did function words elicit greater activation than content words. Content words elicited greater activation than function words in bilateral middle temporal gyrus, left anterior-inferior temporal gyrus, bilateral orbital frontal cortex and right parahippocampal gyrus. This suggests a role for these regions in semantic processing that is independent of semantic task manipulations.
4. EXPERIMENTAL PROCEDURES
4.1 Participants
Sixteen right-handed, native English speaking healthy young adults (mean age 22.25; age range 18-34; 8 male) participated in this study. All participants had normal or corrected to normal vision, and none had a history of neurological or psychological disorders. Each subject provided informed consent and was paid for his or her participation. All experimental procedures were approved by the Duke University Medical Center Institutional Review Board.
4.2 Stimuli
Stimuli consisted of words and nonwords, each 3-8 characters in length. The words and nonword letter strings were padded on either side with pound signs such that each stimulus consisted of 12 characters with the word or nonword letter strings centered (e.g., ####door####). A fixed width font was used so that each stimulus subtended the same visual angle. Nonword letter strings (N= 4164) were created using a random letter generator that randomly selected letters of the alphabet. No valid English words were embedded in any portion of the nonwords, and nonwords were not constrained to follow English phonology. Word stimuli were selected from the MRC psycholinguistic database and consisted of 75 content words (concrete nouns) and 75 function words (Coltheart, 1981; Kucera & Francis, 1967; Wilson, 1988). All stimuli were equated for length (range: 3-8 letters, mean length content words: 4.85, mean length function words: 4.86, mean length nonwords: 4.86. Words were matched for frequency (mean frequency content words = 61.72, range: 0-274; mean frequency function words = 57.44, range 1-296; t(148)=0.36, p=.72) (Kucera & Francis, 1967). Stimuli were presented in four-minute runs. Fifteen volunteers completed 10 runs and one completed 8 runs (average = 148 critical trials) per subject. All stimuli were presented via LCD goggles (MRI Resonance Technologies, Los Angeles, CA, USA) using the CIGAL experimental control program (Voyvodic, 1999).
4.3 Experimental Task
Participants viewed a display in which nonwords were presented at a rate of 2/s and in which either a content or function word occurred every 12-15s. Word and nonwords were each presented for 500 ms duration (Figure 1) and the majority of stimuli were presented in black font. An occasional nonword stimulus was presented in blue font, which signaled the participant to remember that stimulus. After a variable 12-45s interval during which all stimuli were again presented in black font, a nonword stimulus was presented in red font. Participants responded with two buttons to indicate whether the red nonword stimulus was identical to the previous blue nonword stimulus, or not. Two fingers of the right hand were used to indicate this choice. After a short delay, another nonword stimulus, presented in blue font, appeared and the cycle was repeated. Thus, throughout the experiment, subjects were engaged in a variable interval match-to-sample task with colored nonword trials. Words were never relevant to this task and were not presented immediately prior to or following any task-related trials. Participants received a practice session to ensure they could competently perform this task.
Figure 1.
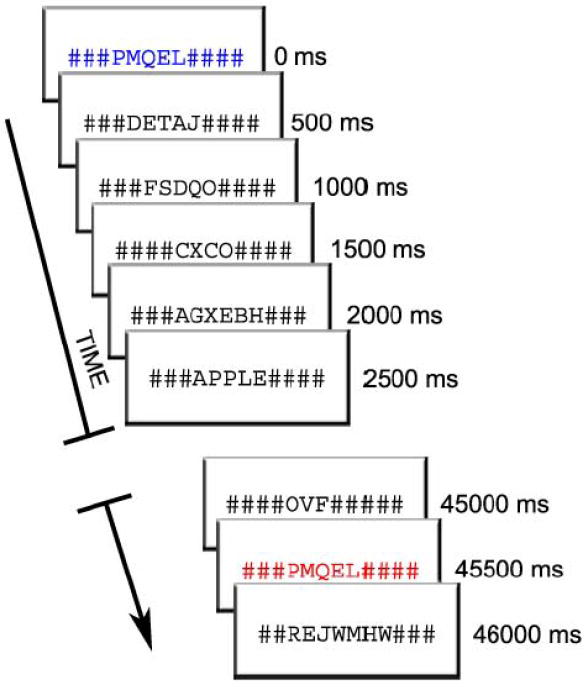
Experimental Design. Participants viewed a rapidly changing visual display of words and nonwords (duration=500ms). Most trials were nonwords and word trials occurred every 12 to 15 seconds. Participants performed a matching task in which they decided whether two colored nonword trials, separated by a varying time interval, were identical. The presentation of words was incidental to the task that participants performed.
4.4 Acquisition of MRI Data
MRI scanning was completed on a General Electric 4.0 Tesla LX Nvi MRI scanner equipped with a 41 mT/m gradient coil. A birdcage radio frequency (RF) head coil was used for RF transmission and reception (General Electric, Milwaukee Wisconsin, USA). Sagittal T-1 weighted localizer images were acquired and used to define a volume for high order shimming. The anterior and posterior commisures were identified for slice selection and shimming. A semi-automated high-order shimming program was used to ensure global field homogeneity. High-resolution structural images were acquired using a 3D fast SPGR pulse sequence (TR=2.2ms; TE=5.3 ms; FOV=24cm2; voxel size= .9375 × .9375 × 1.9mm). Functional images sensitive to blood oxygen level-dependent (BOLD) contrast were acquired using an inverse spiral pulse sequence (TR=1.5s; TE=35ms; FOV=24cm2; voxel size=3.75×3.75×3.8mm; 34 contiguous axial slices). Each of 10 runs consisted of the acquisition of a time series of 148 brain volumes. Four initial RF excitations were performed to achieve steady state equilibrium. These initial RF excitations were discarded.
4.5 Data Analysis
Our main analysis used the internal localizer approach described by Lerner and colleagues (Lerner, Hendler, & Malach, 2002) and also used in a recent study from our laboratory (Morris, Pelphrey, & McCarthy, 2006). In this localizer technique, data from three runs of the experiment, or approximately 30% of the total data, was used to identify functional regions of interest (ROIs) that were activated by all words. FSL was used to assess functional activations to all words as described below. The remaining data was used to measure differences between content and function words within those ROIs. Thus the definition of a functional ROI and the measurement of experimental effects at that ROI were done independently.
Preprocessing and first level analysis of each individual run for each subject (10 runs each for 15 subjects, and 8 runs for 1 subject) was performed using FSL version 3.3.5 [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, U.K.] (Smith et al., 2004). Functional image data were motion-corrected, high-pass filtered, and spatially smoothed using a Gaussian kernel (FWHM = 8 mm). No subject had movement greater than 3mm in the X, Y, or Z dimensions. Pre-whitening or voxel-wise temporal autocorrelation was estimated and corrected using FMRIB’s Improved Linear Model (FILM) (Woolrich, Ripley, Brady, & Smith, 2001). The skull and other coverings were stripped from the structural brain images using the FSL brain extraction tool (Smith, 2002). Functional images of each subject were co-registered to structural images in native space, and structural images were normalized to the MNI standard brain supplied with FSL. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. A double γ function was used to model the hemodynamic response for each word onset in each run.
The first level analyses from three experimental runs per subject were randomly selected and a second level analysis was performed for each subject. These 16 second level analyses were then combined into a group level analysis using the FMRIB Local Analysis of Mixed Effects (FLAME) (Beckman, Jenkinson, & Smith, 2003; Woolrich, Behrens, Beckman, Jenkinson, & Smith, 2004) to identify voxels that were activated by words compared to the nonword baseline condition, regardless of whether the words belonged to the function or content category. Because our interest was identifying functional ROIs for later testing, the resulting Z-maps were thresholded liberally at p <.05 (Z ≥1.9) with a minimum cluster size of 3 voxels. A custom Matlab program was used to label each functional ROI for later analysis.
The runs not used to identify functional ROIs (7 runs per each of 15 subjects, and 5 runs for 1 subject) were used to create separate peri-event averages for content and function words using the EventStats program (Gadde and McCarthy, ref note). The mean peri-event averages were calculated for each of the functional ROIs identified in the preceding step, and differences between the waveforms evoked by content and function words were calculated at each ROI.
In addition to the above, the single trial peri-event averages for each content and function word were measured, and a time-point by time-point t statistic was calculated at each voxel within the predefined functional ROIs for each subject (p<.01, t≥2.1). These t statistic peri-event waveforms were combined across subjects using a random effects analysis, and the difference in the mean amplitude of the hemodynamic response for each functional ROI was determined. Because some ROIs were spatially extensive, we also computed a voxel-based analysis for the voxels within each cluster as a secondary analysis to search for heterogeneous patterns of differential content – function word activation.
Percent signal change was determined by averaging the hemodynamic response elicited by content and function words for each functional region of interest and calculating the difference between baseline and peak points for each condition. Significance was thresholded at p<.01 (t≥2.1) in these subsequent analyses. Coordinates of the centroids of activation and their corresponding anatomical gyri were determined through the use of anatomical atlases. All reported coordinates are in Montreal Neurological Institute (MNI) space. Results are displayed overlaid on an individual subject’s brain normalized to MNI space.
Table 2.
Peak Coordinates and Z-values for regions in which content words elicited significantly greater activation than function words (N=16)
| Location |
X | Y | Z | Max Z-value | # Voxels | |
|---|---|---|---|---|---|---|
| Area | Hemisphere | |||||
| Middle Temporal Gyrus (b) | Left | -59 | -14 | -27 | 3.71 | 250 |
| Middle Temporal Gyrus (g) | Right | 66 | -10 | -21 | 3.84 | 21 |
| Anterior Inferior Temporal Cortex (d) | Left | -42 | 10 | -40 | 3.76 | 13 |
| Orbital Frontal Gyrus (f) | Left | -18 | 34 | -20 | 2.28 | 56 |
| Orbital Frontal Gyrus (e) | Right | 30 | 38 | -20 | 2.82 | 67 |
| Parahippocampal Gyrus (h) | Right | 24 | -14 | -27 | 2.57 | 20 |
Coordinates represent the centroids of maximum activation in MNI space.
Acknowledgments
This research was supported by NIMH grant MH-05286, and by a NSF graduate research fellowship to MTD. GM was also supported by a Department of Veteran’s Affairs Senior Research Career Scientist award. We thank Anuradha Ganapathy for help with experimental procedures and data analysis, and Brian Marion for help with data analysis and figure preparation.
Appendix 1
Content and Function Words
| CONTENT WORDS | ||
|---|---|---|
| WORD | LENGTH | FREQUENCY |
| AIRPORT | 7 | 19 |
| APPLE | 5 | 9 |
| ARM | 3 | 94 |
| BAT | 3 | 18 |
| BEAR | 4 | 57 |
| BEE | 3 | 11 |
| BOOK | 4 | 193 |
| BOY | 3 | 242 |
| BRIDE | 5 | 33 |
| BROTHER | 7 | 73 |
| CAR | 3 | 274 |
| CAT | 3 | 23 |
| CHIPS | 5 | 17 |
| CLOUD | 5 | 28 |
| COFFEE | 6 | 78 |
| COMB | 4 | 6 |
| COPS | 4 | 17 |
| CUP | 3 | 45 |
| DAGGER | 6 | 0 |
| DAUGHTER | 8 | 72 |
| DECK | 4 | 23 |
| DIRT | 4 | 43 |
| DRESS | 5 | 67 |
| EGGS | 4 | 12 |
| ENTRANCE | 8 | 57 |
| ENVELOPE | 8 | 21 |
| FARM | 4 | 125 |
| FISH | 4 | 35 |
| FLOWERS | 7 | 23 |
| FORK | 4 | 14 |
| FROG | 4 | 1 |
| GLASS | 5 | 99 |
| GLOVE | 5 | 9 |
| GRASS | 5 | 53 |
| GUN | 3 | 118 |
| HAIR | 4 | 148 |
| HAMMER | 6 | 9 |
| HAT | 3 | 56 |
| HORSE | 5 | 117 |
| ICE | 3 | 45 |
| INDIANS | 7 | 52 |
| KEY | 3 | 88 |
| KING | 4 | 88 |
| KNOB | 4 | 2 |
| LIBRARY | 7 | 62 |
| MOTHER | 6 | 215 |
| MOUNTAIN | 8 | 33 |
| MOUTH | 5 | 103 |
| NEEDLES | 7 | 15 |
| OCEAN | 5 | 34 |
| PANS | 4 | 16 |
| PEA | 3 | 0 |
| PEACHES | 7 | 3 |
| PEN | 3 | 18 |
| PEPPER | 6 | 13 |
| PIE | 3 | 14 |
| POOL | 4 | 111 |
| RIVER | 5 | 165 |
| SHADOW | 6 | 36 |
| SHEEP | 5 | 23 |
| SHIP | 4 | 83 |
| SILVER | 6 | 29 |
| SNAKE | 5 | 44 |
| SPIDER | 6 | 2 |
| STARS | 5 | 25 |
| STEEPLE | 7 | 9 |
| SUN | 3 | 112 |
| TABLE | 5 | 198 |
| THUNDER | 7 | 14 |
| UNCLE | 5 | 57 |
| WAGON | 5 | 55 |
| WALL | 4 | 160 |
| WHIP | 4 | 19 |
| WIFE | 4 | 228 |
| WINDOW | 6 | 119 |
|
FUNCTION WORDS | ||
| WORD | LENGTH | FREQUENCY |
| ABOVE | 5 | 296 |
| AFAR | 4 | 2 |
| AFOOT | 5 | 1 |
| AGO | 3 | 246 |
| ALAS | 4 | 10 |
| ALONE | 5 | 195 |
| ALREADY | 7 | 273 |
| AMID | 4 | 14 |
| AMONGST | 7 | 4 |
| ANEW | 4 | 6 |
| ANYONE | 6 | 140 |
| ASIDE | 5 | 67 |
| ASTRAY | 6 | 3 |
| AWHILE | 6 | 4 |
| AYE | 3 | 1 |
| BARRING | 7 | 3 |
| BESIDE | 6 | 78 |
| CIRCA | 5 | 1 |
| DULY | 4 | 10 |
| ELSE | 4 | 176 |
| ERE | 3 | 1 |
| ETC | 3 | 58 |
| FORE | 4 | 7 |
| FOREVER | 7 | 39 |
| FORTH | 5 | 71 |
| GRATIS | 6 | 1 |
| HENCE | 5 | 58 |
| HEREBY | 6 | 8 |
| HERS | 4 | 16 |
| HITHER | 6 | 2 |
| LEST | 4 | 17 |
| LIKEWISE | 8 | 18 |
| MAYBE | 5 | 134 |
| MINE | 4 | 59 |
| NAY | 3 | 2 |
| NEAR | 4 | 198 |
| NEITHER | 7 | 141 |
| NOBODY | 6 | 74 |
| NON | 3 | 10 |
| NONE | 4 | 108 |
| NOR | 3 | 195 |
| NOWADAYS | 8 | 12 |
| NOWHERE | 7 | 29 |
| OFT | 3 | 1 |
| ONESELF | 7 | 5 |
| ONTO | 4 | 60 |
| OURS | 4 | 27 |
| OVERALL | 7 | 12 |
| PRIOR | 5 | 47 |
| QUA | 3 | 2 |
| QUITE | 5 | 281 |
| SANS | 4 | 2 |
| SIC | 3 | 4 |
| SOON | 4 | 199 |
| SUPRA | 5 | 3 |
| THEE | 4 | 17 |
| THEIRS | 6 | 21 |
| THENCE | 6 | 6 |
| THEREBY | 7 | 33 |
| THINE | 5 | 1 |
| THOU | 4 | 14 |
| THY | 3 | 12 |
| TILL | 4 | 50 |
| UNDULY | 6 | 6 |
| UNLESS | 6 | 101 |
| UNTO | 4 | 16 |
| VERSUS | 6 | 9 |
| WHENCE | 6 | 3 |
| WHEREAS | 7 | 41 |
| WHOA | 4 | 1 |
| WHOM | 4 | 146 |
| WHOSE | 5 | 252 |
| YEA | 3 | 3 |
| YES | 3 | 144 |
| YON | 3 | 1 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, et al. PET studies of memory: Novel versus practiced free recall of word lists. Neuroimage. 1995;2:296–305. doi: 10.1006/nimg.1995.1037. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Paradiso S, Cizadlo T, Arndt S, Watkins GL, et al. The cerebellum plays a role in conscious episodic memory retrieval. Human Brain Mapping. 1999;8:226–234. doi: 10.1002/(SICI)1097-0193(1999)8:4<226::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S. The effect of orthographic similarity on lexical retrieval: Resolving neighborhood conflicts. Psychonomic Bulletin and Review. 1997;4:439–461. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beckman CF, Jenkinson M, Smith SM. General multi-level linear modelling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost R, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. The Journal of Neuroscience. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DC, Garrett MF. Hemispheric differences in the recognition of closed-and open-class words. Neuropsychologia. 1983;21:155–159. doi: 10.1016/0028-3932(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Broca P. Perte de la parole, remollissement chronique et destruction partielle du lobe anterieur gauche du cerveau. Bulletin de la Societe Anthropologie. 1861;2:235–238. [Google Scholar]
- Broca P. Remarques sur le siege de la faculte du language articule: suivies d’une observation d’aphemie. Bulletin de la Societe Anatomique de Paris. 1861;6:330–357. [Google Scholar]
- Brown CM, Hagoort P, ter Keurs M. Electrophysiological signatures of visual lexical processing: Open- and closed-class words. Journal of Cognitive Neuroscience. 1999;11(3):261–281. doi: 10.1162/089892999563382. [DOI] [PubMed] [Google Scholar]
- Brunelliere A, Hoen M, Dominey PF. ERP correlates of lexical analysis: N280 reflects processing complexity rather than category or frequency effects. Neuroreport. 2005;16(13):1435–1438. doi: 10.1097/01.wnr.0000177008.98860.69. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Forman SD, Braver TS, Casey BJ, Servan-Schreiber D, Noll DC. Activation of prefrontal cortex in a nonspatial working memory task with functional MRI. Human Brain Mapping. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1981;33A(4):497–505. [Google Scholar]
- Coltheart M, Davelaar E, Jonasson JT, Besner D. Access to the internal lexicon. In: Dornic S, editor. Attention and performance IV. Hillsdale, NJ: Erlbaum; 1977. pp. 535–555. [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Eastburn M. Neural correlates of semantic priming for ambiguous words: An event-related fMRI study. Brain Research. 2007;1131:163–172. doi: 10.1016/j.brainres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London B. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio A. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin R. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Price C, Wise R, Frackowiak RSJ. Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: a positron-emission tomography study in normal human subjects. Neuroscience Letters. 1994;182:25–28. doi: 10.1016/0304-3940(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Science. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Tallal P, Raichle ME, Miezin FM, Katz WF, Petersen SE. PET studies of auditory and phonological processing: effects of stimulus characteristics and task demands. Journal of Cognitive Neuroscience. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- Forster KI, Shen D. No enemies in the neighborhood: Absence of inhibitory neighborhood effects in lexical decision and semantic categorization. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22(3):696–713. doi: 10.1037//0278-7393.22.3.696. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Levels of processing and vocabulary types: Evidence from online comprehension in normals and agrammatics. Cognition. 1985;19:133–166. doi: 10.1016/0010-0277(85)90016-2. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, von Cramon DY. Segregating semantic and syntactic aspects of processing in the human brain: An fMRI investigation of different word types. Cerebral Cortex. 2000;10(7):698–705. doi: 10.1093/cercor/10.7.698. [DOI] [PubMed] [Google Scholar]
- Gadde S, McCarthy G. http://www.nbirn.net/tools/bxh_tools/index.shtm.
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of Automatic and Strategic Lexical-Semantics: Functional Magnetic Resonance Imaging Evidence for Differing Roles of Multiple Frontotemporal Regions. Journal of Neuroscience. 2006;26(24):6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B, Caramazza A. Lexical decision for open- and closed-class words: Failure to replicate differential frequency sensitivity. Brain and Language. 1982;15:143–160. doi: 10.1016/0093-934x(82)90053-0. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychologica. 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: A comparative neuropsychological study. Journal of the International Neuropsychological Society. 1996;2(6):511–524. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Grainger J, O’Rourke T. An electrophysiological study on the effects of orthographic neighborhood size on printed word perception. Journal of Cognitive Neuroscience. 2002;14:938–950. doi: 10.1162/089892902760191153. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Science. 1998;95(15):8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Love T, Swinney D, Hickok G. Response of Anterior Temporal Cortex to Syntactic and Prosodic Manipulations During Sentence Processing. Human Brain Mapping. 2005;26(2):128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta N, Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Iwata K, et al. Brain activation during the course of sentence comprehension. Brain and language. 2006;97(2):154–161. doi: 10.1016/j.bandl.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kearns KP. Broca’s Aphasia. In: Lapointe LL, editor. Aphasia and related neurogenic language disorders. 3. New York, NY: Thieme; 2005. pp. 117–141. [Google Scholar]
- King JW, Kutas M. Neural plasticity in the dynamics of human visual word recognition. Neuroscience Letters. 1998;244:61–64. doi: 10.1016/s0304-3940(98)00140-2. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis W. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Kutas M, Hillyard SA. Event-related brain potentials to grammatical errors and semantic anomalies. Memory & Cognition. 1983;11(5):539–550. doi: 10.3758/bf03196991. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Malach R. Object-completion Effects in the Human Lateral Occipital Complex. Cerebral Cortex. 2002;12:163–177. doi: 10.1093/cercor/12.2.163. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Scheef L. Supramodal language comprehension: Role of the left temporal lobe for listening and reading. Neuropsychologia. 2007;45(10):2407–2415. doi: 10.1016/j.neuropsychologia.2007.02.008. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Puce A, Nobre AC, Bloch G, Hyder F, et al. Functional magnetic resonance imaging of human prefrontal cortex during a spatial working memory task. Proceedings of the National Academy of Sciences. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe I: Intracranial distribution and neural generators. Journal of Neuroscience. 1995;15:1080–9. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Occipitotemporal activation evoked by the perception of human bodies is modulated by the presence or absence of the face. Neuropsychologia. 2006;44(10):1919–1927. doi: 10.1016/j.neuropsychologia.2006.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJS, Vandenbergh R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122(1):61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Munte TF, Wiering BM, Weyerts H, Szentkuti A, Matzke M, Johannes S. Differences in brain potentials to open and closed class words: class and frequency effects. Neuropsychologia. 2001;39:91–102. doi: 10.1016/s0028-3932(00)00095-6. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Mills DL, Lawson DS. Fractionating language: Different neural subsystems with different sensitive periods. Cerebral Cortex. 1992;2(3):244–258. doi: 10.1093/cercor/2.3.244. [DOI] [PubMed] [Google Scholar]
- Nobre A, Price C, Turner R, Friston K. Selective Processing of Nouns and Function Words in the Human Brain. Neuroimage. 1997;5(4):S53. [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–262. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related ERPs: Scalp distributions and modulation by word type and semantic priming. Journal of Cognitive Neuroscience. 1994;6:233–255. doi: 10.1162/jocn.1994.6.3.233. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe II: effects of word type and semantic priming. Journal of Neuroscience. 1995;15:1090–8. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout L, Bersick M, McKinnon R. Brain potentials elicited by words: word length and frequency predict the latency of an early negativity. biological psychology. 1997;46(2):143–168. doi: 10.1016/s0301-0511(97)05250-2. [DOI] [PubMed] [Google Scholar]
- Paivio A. Imagery and Verbal Processes. New York: Hold, Rinehart, & Winston; 1971. [Google Scholar]
- Paivio A. The empirical case for dual coding. In: Yuille JC, editor. Imagery, memory and cognition. Hillsdale, NJ: Erlbaum; 1983. pp. 307–322. [Google Scholar]
- Paradiso S, Facorro BC, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, et al. Brain activity assessed with PET during recall of word lists and narratives. Neuroreport. 1997;8(14):3091–3096. doi: 10.1097/00001756-199709290-00017. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME. Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science. 1990;249(4972):1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- Petrides ME, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proceedings of the National Academy of Sciences. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran R, Blumstein SE, Myers EB, Hutchison E, Britton B. An event-related fMRI investigation of phonological-lexical competition. Neuropsychologia. 2006;44(12):2209–2221. doi: 10.1016/j.neuropsychologia.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Price C. Functional Anatomy of Word Comprehension and Production. Trends in Cognitive Sciences. 1998;2(8):281–288. doi: 10.1016/s1364-6613(98)01201-7. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, et al. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychological Science. 2000;11(1):51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41(5):550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RSJ, Dolan RJ. Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain. 1996;119:2073–2083. doi: 10.1093/brain/119.6.2073. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace KL, Maisog JM, Andreason P. A functional lesion in developmental dyslexia: left angular gyral blood flow predicts severity. Brain and Language. 1999;70:187–204. doi: 10.1006/brln.1999.2158. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Gough PM, Alexander IH, Kyriazis D, McDonald KR, et al. Attentional Selection and Action Selection in the Ventral and Orbital Prefrontal Cortex. Journal of Neuroscience. 2005;25(50):11628–11636. doi: 10.1523/JNEUROSCI.2765-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears CR, Hino Y, Lupker SJ. Neighborhood frequency and neighborhood size effects in visual word recognition. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:876–900. [Google Scholar]
- Sears CR, Lupker SJ, Hino Y. Orthographic neighborhood effects in perceptual identification and semantic categorization tasks: A test of the multiple read-out model. Perception and Psychophysics. 1999;61:1537–1554. doi: 10.3758/bf03213116. [DOI] [PubMed] [Google Scholar]
- Segui J, Mehler J, Frauenfelder U, Morton J. The word frequency effect and lexical access. Neuropsychologia. 1982;20:615–627. doi: 10.1016/0028-3932(82)90061-6. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Science. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;3:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckman CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behavioral Neurology. 1989;2:167–182. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1991;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- ter Keurs M, Brown CM, Hagoort P. Lexical processing of vocabulary class in patients with Broca’s aphasia: an event-related brain potential study on agrammatic comprehension. Neuropsychologia. 2002;40:1547–1561. doi: 10.1016/s0028-3932(02)00025-8. [DOI] [PubMed] [Google Scholar]
- ter Keurs M, Brown CM, Hagoort P, Stegeman DF. Electrophysiological manifestation of open- and closed-class words in patients with Broca’s aphasia with agrammatic comprehension. An event-related brain-potential study. Brain. 1999;122(5):839–854. doi: 10.1093/brain/122.5.839. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Science. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre A, Price C. The Response of Left Temporal Cortex to Sentences. Journal of Cognitive Neuroscience. 2002;14(4):550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI integrating paradigm control, physiology, behavior and on-line statistical analysis. Neuroimage. 1999;10:91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JDE. Semantic repetition priming for verbal and pictorial knowledge: a functional MRI study of left inferior prefrontal cortex. Journal of Cognitive Neuroscience. 1997;9(6):714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner R. Task-specific repetition priming in left inferior prefrontal cortex. Cerebral Cortex. 2000;10(12):1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, et al. Noun and verb retrieval by normal subjects. Brain. 1996;119:159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Wilson MD. The MRC Psycholinguistic Database: Machine Readable Dictionary, Version 2. Behavioural Research Methods, Instruments and Computers. 1988;20(1):6–10. [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckman CF, Jenkinson M, Smith SM. Multi-level linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of FMRI data. Neuroimage. 2001;6:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]


