Abstract
Background
Patient mammogram reminders are effective but have not been fully implemented in practice to improve routine screening. The effectiveness of implementation and maintenance phases of a multimodal reminder program that incorporated automated calls capable of efficiently reaching large numbers of women was evaluated to improve repeat mammography screening.
Design
A quasi-experimental study was conducted in 2008 using electronic medical record data during three time periods: pre-reminder phase (2004), post-reminder implementation phase (2006), and post-reminder maintenance phase (January 1–July 1, 2007).
Setting/participants
Participants were female Kaiser Permanente Northwest HMO members aged 42 years or more who were 20 months past their last mammogram (index date) (N=35,104). The intervention program targeted women aged 50–69 years. Women aged 42–49 years (for whom clinical guidelines also recommend mammography) not targeted by the program constituted the primary comparison group (CG1).
Intervention
A “mammogram due soon” postcard was mailed to participants 20 months after their last mammogram, followed by up to two automated phone calls and one live call for nonresponders.
Main outcome measures
The outcome measure was the time until participants received a mammogram in the 10 months following the index date.
Results
Pre-reminder, 63.4% of targeted women completed a mammogram; this number increased to 75.4% in the post-reminder implementation phase; 80.6% completed a mammogram in the maintenance phases. After controlling for demographics and clinic visits, intervention women were 1.51 times more likely to complete a mammogram (CI=1.40, 1.62) post-reminder implementation, compared to CG1. The effect was maintained in 2007 (hazard ratio 1.81, CI=1.65, 1.99).
Conclusions
The study found that this multimodal reminder system could be effectively implemented and maintained in a large health system. If widely implemented, this intervention could substantially improve community mammography screening.
Introduction
Breast cancer is the most common type of cancer among women in the U.S.1 About one in eight women develop breast cancer during their lifetimes, and annually 46,000 women die from it.2 The early detection of breast cancer through mammography screening can reduce mortality from breast cancer in women aged 40 years or more, with greater absolute risk reduction in older women.3 The U.S. Preventive Services Task Force (USPSTF) recommends screening women every 1–2 years beginning at age 40 years. The strength of the evidence is greatest in women aged 50–69 years; women aged 40–49 years were added to the USPSTF recommendation in 2002, and mammograms are recommended in women aged 70 years or more if life expectancy is not compromised by comorbid disease.4 Many health plans targeted mammography screening improvement based on the National Center for Quality Assurance (NCQA) Health Plan Employer Data Information Set (HEDIS); this data set assessed mammography screening in women aged 50–69 years only, until 2007, at which point assessments for those aged 40–49 years were added.5
Current use of mammography is suboptimal, despite its proven effectiveness. Although the prevalence of screening mammography increased substantially between 1993 and 1998,6 this trend has flattened, and mammography rates are even declining in multiple geographic areas.7,8 As many as 30% of eligible women do not get regular screening exams,9 and improvements have remained sluggish for at least 10 years.7 Mammography will not achieve its potential to reduce mortality unless most women are screened regularly.4,10,11
There are multiple strategies known to be effective that healthcare organizations can use to increase mammography screening.11-16 Most studies have focused on encouraging mammography in the unscreened, and there has been a call for more research on methods of encouraging regular mammograms13,17-19 Patient reminders sent by mail, and telephone reminders utilizing live callers, have proven to be effective in improving screening rates,12,13,16 with live telephone calls being more effective than mailed reminders.16 Mailed reminders followed by a live telephone call appear to be the most cost-effective approach.20 This finding is not surprising given that practice-improvement literature has shown repeatedly that multimodal interventions to improve mammography screening rates are better than single-strategy interventions.13 However, these effective reminder programs have been slow to diffuse into practice and, even when they do so, are incompletely implemented and maintained.
The objective of the current study was to determine if a large-scale multimodal population-based screening-mammography reminder program, aimed at women who had previously had a mammogram, could increase the number of women receiving mammography screening. The reminder program began in January 2006.
Methods
The protocol for this study was approved by the IRB within the study HMO. The need for individual consent for data use was waived.
Study Setting and Data Sources
The study was conducted at Kaiser Permanente Northwest (KPNW), a nonprofit group-model HMO operating in southern Washington and northern Oregon, with 15 medical clinics and about 485,000 members. The demographic characteristics of the members are similar to those of the area population, with about 19% racial or ethnic minorities. Electronic databases provide data on patient membership, demographics, and healthcare utilization, including mammogram completion in radiology and procedures from inpatient admissions and outpatient visits, as well as from outside claims and referrals. These data capture over 95% of all medical care and pharmacy services members receive,21 and data are linked through each member’s health record number. Because screening mammography is a covered benefit, it is unlikely that members seek this exam outside of the plan, and the internal data are likely to be a nearly complete assessment of screening patterns. Kaiser Permanente mammography data, and similar data sets in other integrated care settings, have been used extensively in research.9
Study Design and Population
This retrospective quasi-experimental study evaluated the effectiveness of a multimodal reminder intervention that incorporated automated telephone calls to improve routine mammography screening. The study period included data from 2004–2007, and the study was conducted in 2008. Specifically, women were identified who were aged ≥42 years and whose most recent mammogram had been performed 20 months earlier. Patient cohorts were then developed for each of three time periods (Figure 1): the pre-reminder period (calendar year 2004); the first post-reminder year, in order to measure the effectiveness of the implementation of the reminder program (calendar year 2006); and the second post-reminder year, to measure the effectiveness of the maintenance of the reminder program (January 1–July 1, 2007). The date 20 months after each participant’s prior mammogram served as her index date. Each woman was required to have a minimum continuous membership from 24 months prior until 10 months after the index date (the follow-up period). The pre-reminder cohort (2004) included 14,831 women, the post-implementation (2006) cohort 17,361, and the maintenance (2007) cohort 7859, for a total of 35,104 unique individuals (patients who had been in the 2004 cohort could repeat in 2006 or 2007) across the three time periods.
Figure 1.
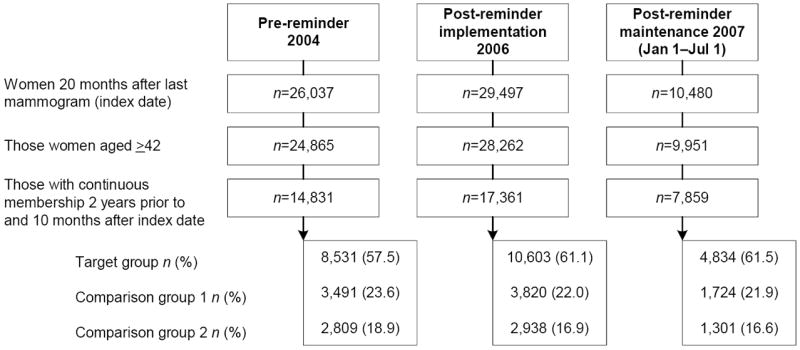
Study population flow for three cohorts: pre-reminder, post-reminder implementation, and post-reminder maintenance.
Target Group: mammogram recommended by USPSTF and patients targeted with reminders (age 50–69)
Comparison Group 1: mammogram recommended by USPSTF but patients not targeted (age 42–49)
Comparison Group 2: mammogram conditionally recommended by USPSTF (if life expectancy not compromised) but patients not targeted (age ≥70)
Comparison Groups
Each of the three time cohorts was further stratified into three groups. The target group consisted of women aged 50–69 years, the age group for whom mammograms were recommended by the USPSTF and who were the focus of the HEDIS mammography quality-improvement measure through the full study period; these women were targeted by reminders in 2006 and 2007. Two comparison groups of women were also developed, none of whom received the multimodal reminder program. Comparison Group 1 (CG1) consisted of women aged 42–49 years, the age group for whom mammograms were only recently recommended by guidelines and quality measures; the evidence base that mammography is helpful in this age group is weaker than it is for women aged 50–69 years.4,7 Comparison Group 2 (CG2) consisted of women aged ≥70 years, the group for which mammograms are conditionally recommended depending on life expectancy.4
Using these age-based comparison groups allowed adjustments to be made for background temporal trends occurring in mammography screening. The 10-month follow-up period was used to identify outcomes and clinic visits, and the 24-month pre-index period was used to ascertain prior mammograms and other explanatory variables.
Study Variables
The primary outcome was screening mammography completion. Patients’ ages were ascertained at the index date. Neighborhood SES was estimated from the census-tract block corresponding to each subject’s mailing address. Each individual’s racial category was obtained from electronic databases for 31,124 (77.7%) participants; the missing ones were geocoded. Patients were categorized into black, white, and other (Pacific Islander, Asian, and Native American). Information about years of college education, marital status, and whether the family income was <$40,000 per year was also obtained from geocoded data. In addition, as a measure intended to reflect disease burden,22,23 the mean number of unique generic drugs dispensed to each participant was determined. The patient’s assigned clinic was also determined, as well as whether she had visited her primary care provider or a gynecologist during the follow-up period.
The Mammography Patient Reminder Program
Starting in January 2006, women aged 50–69 years who were coming due for a mammogram and were 20 months from their prior mammogram were eligible for the KPNW reminder program (Figure 2). The goal was to encourage eligible women to have a mammogram at least every 24 months. Women who had had a bilateral mastectomy were excluded (approximately 0.2% per month). At 20 months after her prior mammogram, each patient was sent an informational postcard reminding her that she soon would be due for a mammogram and encouraging her to make an appointment. The mailing mentioned common barriers 9,24 to obtaining a mammogram and offered solutions.
Figure 2.

Mammogram reminder program to encourage routine mammograms
Those women who did not make an appointment for a mammogram by 21 months received an automated telephone reminder through Kaiser Voice Messaging (AVM). The automated telephone reminder, made on behalf of the patient’s primary care provider, reminded the patient that her mammogram was due soon, encouraged her to make an appointment, and provided instructions on how to do so. Contact information was provided to assist those who had questions. At 22 months, those who had not made an appointment received a second AVM call. These program elements were delivered by centralized HMO staff under a protocol after an HMO analyst extracted eligible patient lists monthly to deliver the appropriate intervention components. Staff was overseen by an internal medicine physician director. The quality-control program for the centralized program components included computer program updates, dual-analyst review, and mechanisms for patients and staff to provide feedback for data-and program-quality improvement.
At 23–24 months, the names and demographic information of women in the target group who had not yet made an appointment were given to local healthcare teams, so that follow-up live calls could be made (generally by radiology appointment clerks). A call script (available from the author) and training were provided by the centralized staff. Up to two messages were left requesting that the patient call staff back. The actual reminder was delivered only to the targeted individual, because of privacy concerns. Wait time for mammography varied from 0–30 days depending on the facility.
Usual Care
During the study period (2004–2007), in approximately the third quarter of each year, patients who were overdue for a mammogram (>24 months from prior mammogram or those who had never received one) were sent a reminder letter. In addition, the patient electronic medical record contained clinician information for health maintenance procedures due, including mammography.25,26 These procedures were active for the target and comparison groups in the pre-and post-reminder program periods.
Statistical Methods
Cox proportional regression was used for the primary analysis of time until completion of the mammogram, in months, during the 10-month follow-up period (30 months from the prior mammogram). The independent variables were group (target, CG1, and CG2), period (pre-reminder, post-reminder implementation, post-reminder maintenance), and their interaction. Group and period were dummy-coded, with CG1 and the pre-reminder period as the reference groups. The group-by-period interaction was included in the model to test whether, for the target group, change in time to completion of a mammogram, from the pre-implementation period to the implementation and maintenance periods, was greater than the change across periods for the two comparison groups. Several covariates that have been described as, or are suspected of, influencing mammogram completion were included: race, education, marital status, income, any visit to a primary care provider, any visit to an obstetrician/gynecologist, and number of medications.27-29 One model also included clinic site as a variable. The analyses accounted for the fact that some women were included in more than one time period through the use of clustering and robust SEs.
It was hypothesized that there would not be as great a change across the time periods in the comparison groups as in the target group. It was estimated that, with an analysis of 40,000 women who had an average mammography-completion rate of 55% at 6 months after their due date (30 months after their prior mammogram) without reminders, it would be possible to detect very small effects, such as hazard ratios (HRs) in the range of 1.035 to 1.036 (assuming that the covariates in the model explain 5%–10% of the variance in the outcome), with power of 0.80.
Results
Table 1 presents the baseline characteristics of patients in the three time cohorts. Mean age was 59 years across the three cohorts. Other demographic characteristics, as well as utilization of primary care provider and obstetrician/gynecologist visits during the follow-up period, remained similar across the time periods. Overall, 48.8% of women visited their primary care provider by 4 months, and 77.0% by 10 months, into the follow-up period (data not shown). The mean number of medications was higher in the maintenance cohort than in the pre-reminder and implementation cohorts (5.4 vs 3.9 and 3.8, respectively).
Table 1.
Characteristics of cohorts in three time periods,a n (%) unless otherwise indicated
| Variable | Pre-reminder 2004 | Post-implementation 2006 | Maintenance 2007 Jan 1–July 1 | p-valued |
|---|---|---|---|---|
| n | 14,831 | 17,361 | 7,859 | |
| Age (M±SD) | 59.4±11.4 | 59.3±10.8 | 59.1±10.9 | <0.001 |
| Neighborhood data | 0.046 | |||
| Raceb | 13,627 (92.3) | 15,989 (92.5) | 7,158 (91.7) | |
| White | 273 (1.9) | 275 (1.6) | 136 (1.7) | |
| Black | 859 (5.8) | 1,013 (5.9) | 516 (6.6) | |
| Other | ||||
| Some collegec | 6,063 (41.7) | 6,645 (38.9) | 3,319 (43.1) | <0.001 |
| Marriedc | 11,197 (77.1) | 13,476 (79.0) | 5,965 (77.5) | 0.038 |
| Income (family) <$40,000 per yearc | 2,159 (14.9) | 2,476 (14.5) | 1,050 (13.6) | 0.052 |
| PCP visite | 11,639 (78.5) | 13,392 (77.1) | 6,039 (76.8) | 0.002 |
| OB/GYN visite | 2,140 (14.4) | 2,500 (14.4) | 1,089 (13.9) | 0.440 |
| Number of medications (M±SD) | 3.9±4.3 | 3.8±4.3 | 5.4±5.0 | <0.001 |
For all women, 20 months had passed since their prior mammogram (index date). All variables were assessed during the baseline period, except for PCP and OB/GYN visits.
Race available at individual level in 31,124 (77.7%); the missing cases were geocoded
Based on geocoded information
Comparison across all three groups; p-values based on generalized estimating equation models
One or more visits to PCP or OB/GYN during 10 months of follow-up period OB/GYN, obstetrician/gynecologist; PCP, primary care provider
Table 2 presents the proportions of participants completing a mammogram by 10 months into the follow-up period for the three groups in the three periods. The proportion of the target group that completed a mammogram was 5412/8531 (63.4%) in the pre-reminder period. This increased to 75.4% (7995/10,606) in the first year post-implementation, and to 80.6% (3897/4834) in the second year of reminders. The proportion of women completing mammograms in CG1 and CG2 remained relatively flat through the study period, with slightly higher proportions observed in the younger comparison group (CG1: 46.4 % in pre-period, 48.2% in Year 1, and 47.0% in Year 2) versus the older comparison group (CG2: 41.9%, 43.1%, and 40.7% respectively.)
Table 2.
Mammography rates at 10 months of follow-up period by cohort (time period) and group, n (%)
| Group | Pre-reminder 2004 n=14,831 | Post-reminder implementation 2006 n=17,361 | Post-reminder maintenance 2007 Jan 1–July 1 n=7859 |
|---|---|---|---|
| Target groupa | 5412/8531 (63.4) | 7995/10,606 (75.4) | 3897/4834 (80.6) |
| Comparison Group 1b | 1619/3491 (46.4) | 1842 /3820 (48.2) | 810/1724 (47.0) |
| Comparison Group 2c | 1178/2809 (41.9) | 1266 /2938 (43.1) | 530/1301 (40.7) |
Mammogram recommended by USPSTF and patients targeted with reminders in post-implementation (2006) and maintenance (2007) phases (aged 50–69 years)
Mammogram recommended by USPSTF but patients not targeted (aged 42–49 years)
Mammogram conditionally recommended by USPSTF (if life expectancy not compromised) but patients not targeted (aged ≥70 years)
USPSTF, U.S. Preventive Services Task Force
Figure 3 graphically displays the proportion of women, by month, who completed mammograms over the 10 months of the follow-up period, by group and by period. The target group demonstrates improved time to mammogram completion in the first year post-implementation, and maintenance of the effect in the maintenance period (second year of reminders). The majority of the improvement is seen within the first 4 months of the follow-up period, during the time the reminder program was implemented. In Year 1, at 1 month after each program step, 9.9% of women in the target group had completed a mammogram after the postcard, 24% after automated call 1, 36.6% after automated call 2, and 46.6% after the live call. In the pre-period, 24.9% of women in the target group had completed a mammogram by 24 months, as compared to 46.6% in Year 1 and 52.8 % in Year 2. This finding is in contrast to the two comparison groups, which do not appear to have improved their mammogram completion rates in the post-reminder periods.
Figure 3.
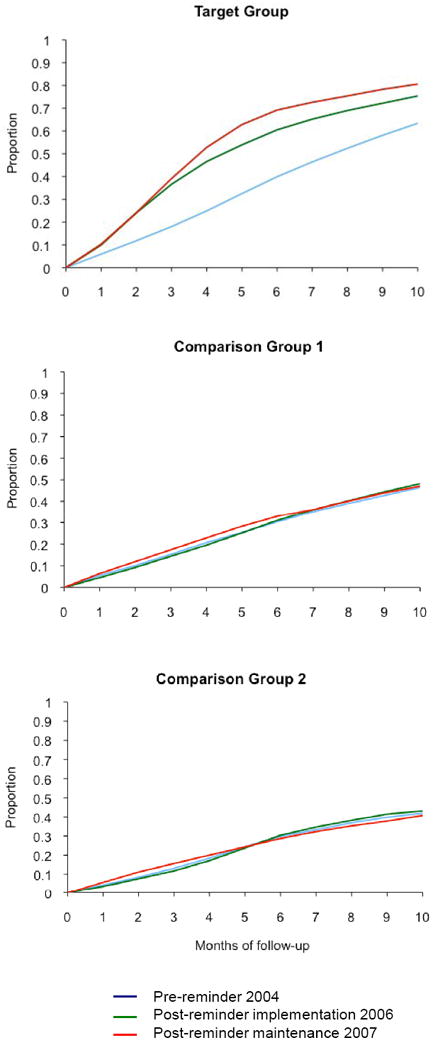
Proportion of women who have had a mammogram during 10 months of follow-up period
Figure 3: Proportion of women who have had a mammogram during 10 months of follow-up.
Target Group: mammogram recommended by USPSTF and patients targeted with reminders (age 50–69)
Comparison Group 1: mammogram recommended by USPSTF but patients not targeted (age 42–49)
Comparison Group 2: mammogram conditionally recommended by USPSTF (if life expectancy not compromised) but patients not targeted (age ≥70)
Table 3 presents the multivariate Cox proportional hazards model predicting time until mammogram. After adjusting for race, education, marital status, income, primary care provider and obstetrician/gynecologist visits, and number of medications, and including interactions of group by period, the target group was 1.51 times more likely (CI=1.40, 1.62; p<0.001) in the post-reminder implementation period, and 1.81 times more likely (CI=1.65, 1.99; p<0.001) in the post-reminder maintenance period, to have a mammogram compared to CG1 women. Women whose race was categorized as other were less likely to complete a mammogram (HR 0.93, CI=0.88, 0.99), as were those taking a greater number of medications (HR 0.99, CI=0.98, 0.99). Women with a college education (HR 1.08, CI=1.05, 1.11), who were married (HR 1.08, CI=1.04, 1.12), or who had visited a primary care provider (HR 1.90, CI=1.84, 1.97) or an obstetrician/gynecologist (HR 1.71, CI=1.65, 1.77) clinician during the follow-up period were more likely to complete a mammogram. A model that adjusted for clinic site did not materially alter the results.
Table 3.
Multivariate Cox proportional hazards model predicting time until mammograma
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Group | ||
| Target groupb | 1.59 (1.50, 1.68) | <0.001 |
| CG1c | 1.00 | |
| CG2d | 0.91 (0.84, 0.98) | 0.013 |
| Cohort (time period)e | ||
| Post-reminder implementation | 1.05 (0.98, 1.12) | 0.144 |
| Post-reminder maintenance | 1.06 (0.98, 1.16) | 0.156 |
| Pre-reminder | 1.00 | |
| Interactions (group by period) | ||
| Target group × post-reminder implementation | 1.51 (1.40, 1.62) | <0.001 |
| CG2 × post-reminder implementation | 0.97 (0.88, 1.08) | 0.602 |
| Target group × post-reminder maintenance | 1.81 (1.65, 1.99) | <0.001 |
| CG2 × post-reminder maintenance | 0.95 (0.83, 1.08) | 0.436 |
| Racef | ||
| Black | 1.01 (0.90, 1.12) | 0.919 |
| Otherg | 0.93 (0.88, 0.99) | 0.014 |
| Some collegeh | 1.08 (1.05, 1.11) | <0.001 |
| Marriedh | 1.08 (1.04, 1.12) | <0.001 |
| Income <$40,000 per yearh | 0.99 (0.94, 1.03) | 0.483 |
| PCP visits (any) | 1.90 (1.84, 1.97) | <0.001 |
| OB/GYN visits (any) | 1.71 (1.65, 1.77) | <0.001 |
| Number of medications | 0.99 (0.98, 0.99) | <0.001 |
During follow-up 10 months after index date; model includes 39,302 women with complete data (749 missing complete address for geocoding)
Mammogram recommended by USPSTF and patients targeted with reminders in 2006 and 2007 (aged 50–69 years)
Reference group; mammogram recommended by USPSTF but patients not targeted (aged 42–49 years)
Mammogram conditionally recommended by USPSTF (if life expectancy not compromised) but patients not targeted (aged ≥70 years)
Reference group: pre-reminder cohort
Reference group: white; race available at individual level in 31,124 (77.7%); those missing were geocoded
Includes Pacific Islander, Asian, and Native American
Based on geocoded information
CG1, Comparison Group 1; CG2, Comparison Group 2; HR, hazard ratio; OB/GYN, obstetrician/gynecologist; PCP, primary care provider; USPSTF, U.S. Preventive Services Task Force
Discussion
The current study found that a multimodal reminder program that incorporated automated telephone calls improved repeat mammography screening. Unadjusted results among targeted women revealed a 21.7% absolute improvement in the number of women screened at 24 months and a 12% improvement at 30 months in Year 1 compared to the number of women screened in the pre-reminder period. After controlling for confounders, in the first year of implementation, the likelihood of the target group completing their next mammogram by 10 months into the follow-up period improved 50% compared to a younger but untargeted comparison cohort. The positive effect was maintained in the second year of the program. Although this intervention did not target all women who were eligible for mammograms, the percentage of women this age with up-to-date mammograms (defined as having a mammogram in that year or the prior year) did improve with the reminder program (79.9% of 45,554 in 2005, 83.0% of 48,421 in 2006, and 84.1% of 49,532 in 2007 were up to date).
The intervention effect observed in the current study is consistent with effects from RCTs of patient reminder programs. Patient-directed reminders and invitations have been shown to increase the number of women receiving mammograms.11,30 In multiple studies, the combined effect for increased mammography utilization for patient-targeted interventions was estimated to be 13%,17 which is similar to the effect found here. However, most previous reminder trials have been conducted among the unscreened,17,30 in contrast to the previously screened population targeted in the current study. The prior patient-reminder trials that largely targeted previously screened women reported smaller intervention effects. One study31 used reminder letters only, two32,33 compared telephone counseling to mailed reminders, one combined34 and another compared35 patient and physician reminders, and two others36,37 included only low-income women.
Of particular note, the effect reported here was observed after implementation of a program that incorporated automated calls, a modality that, although it has been commonly advocated,11 has not been the focus of much mammography-reminder research. One prior nonrandomized study found that an interactive voice response system improved the odds of previously unscreened women completing a mammogram by 26%.38 Although costs were not specifically evaluated here, the current results indicate that automated calls may be a cost-effective approach to implementing a large-scale mammography-reminder program.
The findings reported here help inform the implementation of mammography-reminder systems in real-world practices. Although a large number of studies have addressed the efficacy of mammography-reminder systems, there has been little research addressing the implementation and maintenance of these programs.13,18,39 Prior trials have generally included fewer than 1000 patients,17,30 or they present data from only short-term follow-up periods.40 There is growing recognition that advances in cancer-control research are limited by a failure to translate research findings into practice.30 As long as efficacy and effectiveness trials are considered complete without adjunct research addressing what it will take to translate the results of such trials into real-world practices, the public-health potential of the original investments will not be realized.39
Other subgroup results from the current study shed further light on important factors relating to implementation of mammography-reminder programs. Consistent with other studies, it was found here that mammography completion is facilitated by contact with primary care providers28 and obstetricians/gynecologists.29 The current findings also suggest that insured patients who are sicker, or who are of Asian, Pacific Island, or Native American descent may need more support for completion of regular mammography screening. The latter finding needs to be interpreted with caution because racial data missing at the individual level were geocoded.
This study has other limitations. It was not an RCT, which would have provided the strongest evidence of effectiveness. Also, the comparison groups are clearly different from the targeted group. Although it would have been preferable to use a comparison group of similar age to the target group, this was not possible. However, this study had a strong quasi-experimental design that supports the findings and conclusions. Also, RCTs tend to play a limited role in informing the implementation gap in real-world settings13,19 because randomized effectiveness trials require a great deal of control over the environment on the part of researchers, thus threatening the external validity of the findings.41
It is not possible, however, to completely eliminate the possibility that concomitant unmeasured factors may explain some of the observed changes in mammography screening rates. This phenomenon is unlikely to explain our results because these unmeasured factors likely would have affected the control cohorts also. The reminder program was discussed with clinical managers, who said that clinician prompts at the point-of-care reminding patients to get mammograms were active throughout the study period, and that other co-interventions were unlikely. Managers also said that the live outreach calls (the fourth step in the reminder program) did not have the same quality-oversight as the centralized program components and that there was variation in their delivery.
Because the reminder program did not target women who were overdue for mammograms, or those who choose to screen every year, it is not possible to say how this program might affect those groups. In addition, the current study was conducted at a single HMO in two states, so findings may not be generalizable to other settings, although the direct-to-patient reminder system can certainly be implemented in most practice settings. Finally, because the data were pulled from electronic records and not from in-person interviews, individual race data were not complete, and other individually reported measures of SES were not available. Low income was not found to diminish the likelihood of mammography among this insured population, a potentially important finding given that mail and phone reminders have been thought to be ineffective among this demographic.42 However, the low-income-but-insured group in the current study may not be as low in income as low-income participants from other reported studies.
To our knowledge, this study is the first to find that a large population-based mammography-reminder program that integrated automated telephone calls could be effectively implemented and maintained, with the result that repeat mammography screening improved. Given that gaps in mammography screening are common, this intervention, if widely implemented, could substantially improve regular community mammography. Future studies should address practice-based factors that assist patients in completing mammograms within the context of a reminder program, as well as other factors that affect the reach and cost effectiveness of delivering the intervention to diverse patient groups in multiple settings.
Acknowledgments
This study was supported by Award R21CA124395 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the NIH.
We acknowledge the outstanding efforts of other members of the mammography reminder implementation team (Dr. Richard Bills, Michael Lassi, Ariel Hill) and the participating patients and staff at the study site. We also acknowledge Leslie Bienen for editorial support, Gail Morgan for project management, and Dixie Sweo for secretarial support.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Overmoyer B. Breast cancer screening. Med Clin North Am. 1999;83(6):1443–1557. doi: 10.1016/s0025-7125(05)70174-7. [DOI] [PubMed] [Google Scholar]
- 2.George SA. Barriers to breast cancer screening: an integrative review. Health Care Women Int. 2000;21(1):53–65. doi: 10.1080/073993300245401. [DOI] [PubMed] [Google Scholar]
- 3.Paquette D, Snider J, Bouchard F, et al. Performance of screening mammography in organized programs in Canada in 1996. The Database Management Subcommittee to the National Committee for the Canadian Breast Cancer Screening Initiative. CMAJ. 2000;163(9):1133–8. [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Preventive Services Task Force. Screening for breast cancer. www.ahrq.gov/clinic/uspstf/uspsbrca.htm.
- 5.National Committee for Quality Assurance. Osteoporosis management in women numerator. www.ncqa.org/programs/hedis/osteoporosis management in women numerator final 2004.xls.
- 6.Randolph WM, Mahnken JD, Goodwin JS, Freeman JL. Using Medicare data to estimate the prevalence of breast cancer screening in older women: comparison of different methods to identify screening mammograms. Health Serv Res. 2002;37(6):1643–57. doi: 10.1111/1475-6773.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Quality Assurance. The state of health care quality 2004: industry trends and analysis. Washington DC: NCQA; 2004. [Google Scholar]
- 8.Tampa Bay Business Journal. FMQAI: mammography rates decline in Florida. Tampa Bay Business Journal; 2004. Jun 14, [Google Scholar]
- 9.Valanis BG, Glasgow RE, Mullooly J, et al. Screening HMO women overdue for both mammograms and pap tests. Prev Med. 2002;34(1):40–50. doi: 10.1006/pmed.2001.0949. [DOI] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 42, April 2003. Breast cancer screening. Obstet Gynecol. 2003;101(4):821–31. doi: 10.1016/s0029-7844(03)00253-9. [DOI] [PubMed] [Google Scholar]
- 11.Baron RC, Rimer BK, Coates RJ, et al. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35(1S):S56–S66. doi: 10.1016/j.amepre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Bowie JV, Curbow BA, Garza MA, Dreyling EK, Benz Scott LA, McDonnell KA. A review of breast, cervical, and colorectal cancer screening interventions in older women. Cancer Control. 2005;12(2S):58–69. doi: 10.1177/1073274805012004S09. [DOI] [PubMed] [Google Scholar]
- 13.Rimer BK, Meissner H, Breen N, Legler J, Coyne CA. Social and behavioral interventions to increase breast cancer screening. In: Schneiderman N, Speers M, Silva J, Tomes H, Gentry J, editors. Integrating behavioral and social sciences with public health. Washington DC: American Psychological Association; 2001. p. 177. [Google Scholar]
- 14.Legler J, Meissner HI, Coyne C, Breen N, Chollette V, Rimer BK. The effectiveness of interventions to promote mammography among women with historically lower rates of screening. Cancer Epidemiol Biomarkers Prev. 2002;11(1):59–71. [PubMed] [Google Scholar]
- 15.Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136(9):641–51. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- 16.Bonfill X, Marzo M, Pladevall M, Marti J, Emparanza JI. Strategies for increasing women participation in community breast cancer screening. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabroff KR, Mandelblatt JS. Interventions targeted toward patients to increase mammography use. Cancer Epidemiol Biomarkers Prev. 1999;8(9):749–57. [PubMed] [Google Scholar]
- 18.Bero LA, Grilli R, Grimshaw JM, et al. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. BMJ. 1998;317(7156):465–8. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasick RJ, Hiatt RA, Paskett ED. Lessons learned from community-based cancer screening intervention research. Cancer. 2004;101(5S):1146–64. doi: 10.1002/cncr.20508. [DOI] [PubMed] [Google Scholar]
- 20.Vogt TM, Glass A, Glasgow RE, La Chance PA, Lichtenstein E. The safety net: a cost-effective approach to improving breast and cervical cancer screening. J Womens Health (Larchmt) 2003;12(8):789–98. doi: 10.1089/154099903322447756. [DOI] [PubMed] [Google Scholar]
- 21.Freeborn DK, Pope C. Promise and performance in managed care: the prepaid group practice model. Baltimore MD: Johns Hopkins University Press; 1994. [Google Scholar]
- 22.Schneeweiss S, Wang PS, Avorn J, Maclure M, Levin R, Glynn RJ. Consistency of performance ranking of comorbidity adjustment scores in Canadian and U.S. utilization data. J Gen Intern Med. 2004;19(5 Pt 1):444–50. doi: 10.1111/j.1525-1497.2004.30109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneeweiss S, Walker AM, Glynn RJ, Maclure M, Dormuth C, Soumerai SB. Outcomes of reference pricing for angiotensin-converting-enzyme inhibitors. N Engl J Med. 2002;346(11):822–9. doi: 10.1056/NEJMsa003087. [DOI] [PubMed] [Google Scholar]
- 24.Glasgow RE, Whitlock EP, Valanis BG, Vogt TM. Barriers to mammography and pap smear screening among women who recently had neither, one or both types of screening. Ann Behav Med. 2000;22(3):223–8. doi: 10.1007/BF02895117. [DOI] [PubMed] [Google Scholar]
- 25.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12(1):4–13. [PubMed] [Google Scholar]
- 26.Goins KV, Zapka JG, Geiger AM, et al. Implementation of systems strategies for breast and cervical cancer screening services in health maintenance organizations. Am J Manag Care. 2003;9(11):745–55. [PubMed] [Google Scholar]
- 27.Vernon SW, Vogel VG, Halabi S, Jackson GL, Lundy RO, Peters GN. Breast cancer screening behaviors and attitudes in three racial/ethnic groups. Cancer. 1992;69(1):165–74. doi: 10.1002/1097-0142(19920101)69:1<165::aid-cncr2820690128>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Miller AM, Champion VL. Mammography in older women: one-time and three-year adherence to guidelines. Nurs Res. 1996;45(4):239–45. doi: 10.1097/00006199-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Van Harrison R, Janz NK, Wolfe RA, et al. Characteristics of primary care physicians and their practices associated with mammography rates for older women. Cancer. 2003;98(9):1811–21. doi: 10.1002/cncr.11744. [DOI] [PubMed] [Google Scholar]
- 30.Ellis P, Robinson P, Ciliska D, et al. A systematic review of studies evaluating diffusion and dissemination of selected cancer control interventions. Health Psychol. 2005;24(5):488–500. doi: 10.1037/0278-6133.24.5.488. [DOI] [PubMed] [Google Scholar]
- 31.Kendall C, Hailey BJ. The relative effectiveness of three reminder letters on making and keeping mammogram appointments. Behav Med. 1993;19(1):29–34. doi: 10.1080/08964289.1993.9937562. [DOI] [PubMed] [Google Scholar]
- 32.Luckmann R, Savageau JA, Clemow L, Stoddard AM, Costanza ME. A randomized trial of telephone counseling to promote screening mammography in two HMOs. Cancer Detect Prev. 2003;27(6):442–50. doi: 10.1016/j.cdp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Taplin SH, Barlow WE, Ludman E, et al. Testing reminder and motivational telephone calls to increase screening mammography: a randomized study. J Natl Cancer Inst. 2000;92(3):233–42. doi: 10.1093/jnci/92.3.233. [DOI] [PubMed] [Google Scholar]
- 34.Burack RC, Gimotty PA, George J, Simon MS, Dews P, Moncrease A. The effect of patient and physician reminders on use of screening mammography in a health maintenance organization. Results of a randomized controlled trial. Cancer. 1996;78(8):1708–21. [PubMed] [Google Scholar]
- 35.Costanza ME, Stoddard AM, Luckmann R, White MJ, Spitz Avrunin J, Clemow L. Promoting mammography: results of a randomized trial of telephone counseling and a medical practice intervention. Am J Prev Med. 2000;19(1):39–46. doi: 10.1016/s0749-3797(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 36.Goel A, George J, Burack RC. Telephone reminders increase re-screening in a county breast screening program. J Health Care Poor Underserved. 2008;19(2):512–21. doi: 10.1353/hpu.0.0025. [DOI] [PubMed] [Google Scholar]
- 37.Partin MR, Slater JS, Caplan L. Randomized controlled trial of a repeat mammography intervention: effect of adherence definitions on results. Prev Med. 2005;41(3–4):734–40. doi: 10.1016/j.ypmed.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Crawford AG, Sikirica V, Goldfarb N, et al. Interactive voice response reminder effects on preventive service utilization. Am J Med Qual. 2005;20(6):329–36. doi: 10.1177/1062860605281176. [DOI] [PubMed] [Google Scholar]
- 39.Zapka JG, Lemon SC. Interventions for patients, providers, and health care organizations. Cancer. 2004;101(5S):1165–87. doi: 10.1002/cncr.20504. [DOI] [PubMed] [Google Scholar]
- 40.Jean S, Major D, Rochette L, Brisson J. Screening mammography participation and invitational strategy: the Quebec Breast Cancer Screening Program, 1998–2000. Chronic Dis Can. 2005;26(2–3):52–8. [PubMed] [Google Scholar]
- 41.Vernon SW, Briss PA, Tiro JA, Warnecke RB. Some methodologic lessons learned from cancer screening research. Cancer. 2004;101(5S):1131–45. doi: 10.1002/cncr.20513. [DOI] [PubMed] [Google Scholar]
- 42.Bailey TM, Delva J, Gretebeck K, Siefert K, Ismail A. A systematic review of mammography educational interventions for low-income women. Am J Health Promot. 2005;20(2):96–107. doi: 10.4278/0890-1171-20.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]


