Abstract
Chemokines constitute a family of chemoattractant cytokines and are subdivided into four families on the basis of the number and spacing of the conserved cysteine residues in the N-terminus of the protein. Chemokines play a major role in selectively recruiting monocytes, neutrophils, and lymphocytes, as well as in inducing chemotaxis through the activation of G-protein-coupled receptors. Monocyte chemoattractant protein-1 (MCP-1/CCL2) is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages. Both CCL2 and its receptor CCR2 have been demonstrated to be induced and involved in various diseases. Migration of monocytes from the blood stream across the vascular endothelium is required for routine immunological surveillance of tissues, as well as in response to inflammation. This review will discuss these biological processes and the structure and function of CCL2.
Introduction
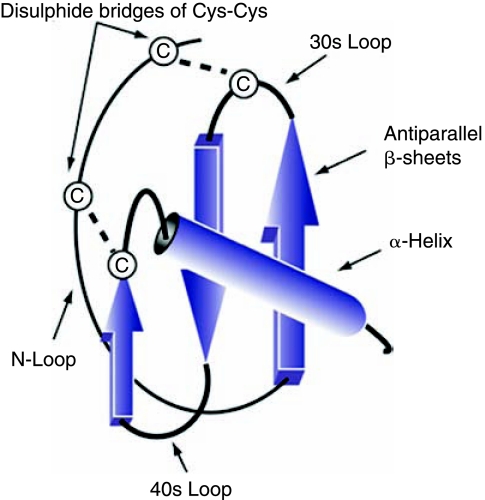
Chemokines (chemotactic cytokines) are small heparin-binding proteins that constitute a large family of peptides (60–100 amino acids) structurally related to cytokines, whose main function is to regulate cell trafficking. Chemokines were first identified in 1977 with the purification of the secreted platelet factor 4 (PF4/CXCL4) (Wu and others 1977). Since then, studies have identified more than 50 human chemokines and 20 chemokine receptors (Ruffini and others 2007). Chemokines can be classified into four subfamilies on the basis of the number and location of the cysteine residues at the N-terminus of the molecule and are named CXC, CC, CX3C, and C, in agreement with the systematic nomenclature (Rollins 1997) (Table 1). The genes for CXC chemokines are tightly and clustered mainly on chromosome 4, whereas the members of CC chemokines are encoded by genes that are located mainly on chromosome 17 (Table 1) (Naruse and other 1996). Chemokines are secreted in response to signals such as proinflammatory cytokines where they play an important role in selectively recruiting monocytes, neutrophils, and lymphocytes. Once induced, the directed migration of cells expressing the appropriate chemokine receptors occurs along a chemical ligand gradient known as the chemokine gradient. This allows cells to move toward high local concentrations of chemokines (Callewaere and others 2007). The structure of chemokines comprises three distinct domains: (1) a highly flexible N-terminal domain, which is constrained by disulfide bonding between the N-terminal cysteine(s); (2) a long loop that leads into three antiparallel β-pleated sheets; and (3) an α-helix that overlies the sheets (Baggiolini and Loetscher 2002) (Fig. 1). Structure-function studies have revealed that the N-terminal region is important for receptor binding and activation (Clark-Lewis and others 1991). Interestingly, all chemokine structures described to date more or less conform to this three-dimensional pattern, even though they may bear little homology at the primary amino acid level (Clore and Gronenborn 1995). The majority of the chemokine ligands have a molecular mass between 8 kDa and 12 kDa and contain 1–3 disulfide bonds. Their sequence homology is highly variable, ranging from <20% to >90%. Several chemokines function by forming dimers in solution or upon their interaction with GAG (e.g., CC or CXC chemokines) (Crown and others 2006). This dimerization is facilitated through the involvement of the antiparallel β-sheet with the cysteine residues near the N-terminal domain (CC chemokine) or the two α-helices (CXC chemokine).
Table 1.
Chemokine Receptors and Their Ligands
| Family | Systematic name | Human ligand (alternative name) | Chemokine receptor(s) | Chr. | Funct. |
|---|---|---|---|---|---|
| C Chemokine (γ chemokine) | XCL1 | Lymphotactin α, ATAC, SCM-1α | XCR1 | 1q24.2 | D |
| XCL2 | SCM-1β | XCR1 | 1q24.2 | D | |
| CCL1 | I-309 | CCR8 | 17q11.2 | I | |
| CCL2 | MCP-1, MCAF, TDCF | CCR2 | 17q11.2 | I | |
| CCL3 | MIP-1α, LD78α | CCR1, CCR5 | 17q12 | I | |
| CCL3L1 | LD78β | 17q12 | I | ||
| CCL3L3 | LD78β | 17q12 | I | ||
| CCL4 | MIP-1β | CCR5 | 17q12 | I | |
| CCL4L1 | AT744.2 | 17q12 | I | ||
| CCL4L2 | 17q12 | I | |||
| CCL5 | RANTES | CCR1, CCR3, CCR5 | 17q12 | I | |
| CCL6 | Unknown | Unknown | |||
| CCL7 | MCP-3 | CCR1, CCR2, CCR3 | 17q11.2 | I | |
| CCL8 | MCP-2 | CCR1, CCR2, CCR3, CCR5 | 17q11.2 | I | |
| CCL9/CCL10 | Unknown | CCR1 | |||
| CCL11 | Eotaxin | CCR3 | 17q11.2 | I | |
| CC Chemokine (β chemokine) | CCL12 | Unknown | CCR2 | ||
| CCL13 | MCP-4 | CCR1, CCR2, CCR3 | 17q11.12 | I | |
| CCL14 | HCC-1 | CCR1 | 17q12 | H | |
| CCL15 | HCC-2, Lkn-1, MIP-1δ | CCR1, CCR3 | 17q12 | H | |
| CCL16 | HCC-4, LCC-1, LEC | CCR1, CCR2, CCR5 | 17q12 | H | |
| CCL17 | TARC | CCR4 | 16q13 | D | |
| CCL18 | PARC, DC-CK1, AMAC-1 | Unknown | 17q12 | H | |
| CCL19 | ELC, Exodus-3, MIP-3β | CCR7 | 9p13.3 | H | |
| CCL20 | LARC, Exodus-1, MIP-3α | CCR6 | 2q36.3 | D | |
| CCL21 | SLC, 6Ckine, Exodus-2 | CCR7 | 9p13.3 | D | |
| CCL22 | MDC, STCP-1 | CCR4 | 16q13 | D | |
| CCL23 | MPIF-1, Ckβ8, MIP-3 | CCR1 | 17q12 | I | |
| CCL24 | Eotaxin-2, MPIF-2 | CCR3 | 7q11.23 | I | |
| CCL25 | TECK | CCR9 | 19p13.3 | H | |
| CCL26 | Eotaxin-3 | CCR3 | 7q11.23 | I | |
| CCL27 | CTACK, ILC | CCR10 | 9p13.3 | H | |
| CCL28 | MEC | CCR3, CCR10 | 5p12 | U | |
| CXCL1 | GROα, MGSA-α | CXCR2, CXCR1 | 4q13.3 | I | |
| CXCL2 | GROβ, MGSA-β | CXCR2 | 4q13.3 | I | |
| CXCL3 | GROγ, MGSA-γ | CXCR2 | 4q13.3 | I | |
| CXCL4 | PF-4 | CXCR3B | 4q13.3 | U | |
| CXCL4V1 | 4q13.3 | U | |||
| CXCL5 | ENA-78 | CXCR2 | 4q13.3 | I | |
| CXCL6 | GCP-2 | CXCR1, CXCR2 | 4q13.3 | I | |
| CXCL7 | NAP-2 | Unknown | 4q13.3 | I | |
| CXC Chemokine (α chemokine) | CXCL8 | IL-8 | CXCR1, CXCR2 | 4q13.3 | I |
| CXCL9 | MIG | CXCR3-A, CXCR3-B | 4q21.1 | I | |
| CXCL10 | IP-10 | CXCR3-A, CXCR3-B | 4q21.1 | I | |
| CXCL11 | I-TAC | CXCR3-A, CXCR3-B | 4q21.1 | I | |
| CXCL12 | SDF-1α/β | CXCR4, CXCR7 | 10q11.21 | H | |
| CXCL13 | BCA-1, BLC | CXCR5 | 4q21.1 | H | |
| CXCL14 | BRAK, Bolekine | Unknown | 5q31.1 | I | |
| CXCL15 | Unknown | Unknown | |||
| CXCL16 | CXCR6 | 17p13 | I | ||
| CXCL17 | DMC | Unknown | 19 | U | |
| CX3C Chemokine (δ chemokine) | CX3CL1 | Fractalkine | CX3CR1 | 16q13 | I |
Table 1 summarizes the chemokine receptors and their known ligands and illustrates the fact that many different ligands bind the same receptor and many ligands bind multiple receptors (Bacon and others 2002). Functions are as follows: I, inflammatory; H, homeostatic; D, dual (homeostatic and inflammatory); U, unknown as described previously (Zlotnik and others 2006). Abbreviations: Chr, chromosome location; Funct, functions.
FIG. 1.
Schematic representation of the three-dimensional structure of chemokines. Three-dimensional representation of a chemokine, where α and β sheet, as well as the cysteine residues and the 30s and 40s loops, are shown. Note that all chemokines share a typical Greek key structure that is stabilized by disulfide bonds between conserved cysteine residues. The term Greek key refers to a kind of secondary structure or motif of a protein sequence.
Chemokines induce chemotaxis through the activation of G-protein-coupled receptors (GPCRs), which also involves adhesion molecules and glycosaminoglycans (GAGs) (Hyduk and others 2007). Chemokines bind to specific cell surface transmembrane receptors coupled with heterotrimeric G proteins, whose activation leads to the activation of intracellular signaling cascades that prompt migration toward the chemokine source. Proteins within each family but not between families can competitively bind to the same receptor on target cells (Leonard and Yoshimura 1990). Although proteins within each subset share structural similarity, they have chemotactic potential for additional diverse types. For example, CCL2, RANTES, and MIP-1β are chemotactic for monocytes but function through two separate receptors (Sozzani and others 1993). In addition, both CCL2 and RANTES target memory T-cells (Carr and others 1994; Maghazachi and others 1994), whereas MIP-1β acts preferentially on naive T-cells (Adams and others 1994). Note that of these three chemokines, only RANTES causes chemotaxis and activation of eosinophils (Ebisawa and others 1994).
Chemokines are also grouped into two main functional subfamilies: inflammatory and homeostatic chemokines. Inflammatory chemokines control the recruitment of leukocytes in inflammation and tissue injury, whereas homeostatic chemokines fulfill housekeeping functions such as navigating leukocytes to and within secondary lymphoid organs as well as in the bone marrow and the thymus during hematopoiesis (Wagner and others 2007). In addition to this major biological function, accumulating evidence suggests critical roles of chemokines in development, hematopoiesis, lymphocyte trafficking and homing, angiogenesis, and malignancy (Xia and Frangogiannis 2007).
Finally, in addition to their roles in the immune system, chemokines and chemokine receptors are also involved in the pathology of a number of diseases (e.g., HIV-1/AIDS), autoimmune disorders (e.g., psoriasis, rheumatoid arthritis, and multiple sclerosis), pulmonary diseases (asthma and chronic obstructive pulmonary disease), transplant rejection, cancer, and vascular disease.
MCP-1/CCL2
The monocyte chemoattractant protein-1 (MCP-1/CCL2) is a member of the C-C chemokine family, and a potent chemotactic factor for monocytes. MCP-1 is believed to be identical to JE, a gene whose expression is induced in mouse fibroblasts by platelet-derived growth factor (Cochran and others 1983). However, the human homolog that has been best characterized as CCL2 was first purified from human cell lines on the basis of its monocyte chemoattractant properties.
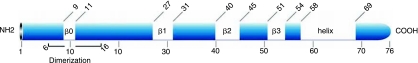
CCL2 is the first discovered human CC chemokine. Located on chromosome 17 (chr.17, q11.2), human MCP-1 is composed of 76 amino acids and is 13 kDa in size (Van Coillie and others 1999). MCP belongs to a family composed of at least four members (MCP-1, −2, −3, and −4). The domain structure of human MCPs is shown in Figure 2. The sequence homology between CCL2 and other family members is high and varies between 61% for CCL8 and CCL4, and 71% for CCL7 (Van Coillie and others 1999). The primary protein structures of human CCL2, CCL7, and CCL8 were initially determined using purified natural material, whereas human CCL13 protein sequence was deduced from isolated cDNAs. In addition to these proteins, different molecular mass forms of CCL2 have been purified, but these seem to be caused by O-glycosylation. Glycosylation of CCL2 has been shown to slightly reduce its chemotactic potency. Note that in this review, we will discuss the role of unglycosylated CCL2.
FIG. 2.
Amino-acid sequence alignment of human MCPs. Conserved cysteine residues are indicated by alignment and spacing. Consensus sequence residues are in dark grey, whereas conserved and mutated CCL2 residues are shown in black and light grey, respectively.
CCL2 is produced by a variety of cell types, either constitutively or after induction by oxidative stress, cytokines, or growth factors. Mutational analysis of CCL2 has resulted in the identification of two regions of the primary structure that are critical for biological activity (Beall and others 1996). The first region consists of the sequence from Thr-10 to Tyr-13, whereas the second region that appears to also be functionally important consists of residues 34 and 35. Mutation of either residue 10 or 13 causes a decrease in CCL2 activity (Ebisawa and others 1994). The importance of the second region was indicated by results with two mutations, one introducing a proline between Ser-34 and Lys-35, and the other a replacement of those two residues with the sequence Gly-Pro-His. Either of these mutations severely decreased CCL2 activity. In addition to these two regions, it has also been reported that cell-type specificity of CCL2 is affected by mutation of residues 28 and 30, but not of residue 30 alone (Beall and others 1996). Further, deletion of residues at the N-terminal domain results in loss of CCL2 activity (Gong and Clark-Lewis 1995) despite the fact that some of these N-terminus deletion mutants act as CCL2 antagonists (Gong and others 1997).
CCL2 is produced by many cell types, including endothelial, fibroblasts, epithelial, smooth muscle, mesangial, astrocytic, monocytic, and microglial cells (Cushing and others 1990; Standiford and others 1991; Brown and others 1992; Barna and others 1994). These cells are important for antiviral immune responses in the peripheral circulation and in tissues. However, monocyte/macrophages are found to be the major source of CCL2 (Yoshimura and others 1989a,b). CCL2 regulates the migration and infiltration of monocytes, memory T lymphocytes, and natural killer (NK) cells. Note that CCL2 is among the most studied member of the chemokine family, and has been shown to be a potential intervention point for the treatment of various diseases, including multiple sclerosis (Sorensen and others 2004), rheumatoid arthritis (Hayashida and others 2001), atherosclerosis (Kusano and others 2004), and insulin-resistant diabetes (Sartipy and others 2003). All the functions of CCL2 were first identified on the basis of an in vitro assay using purified protein, which were reproduced and confirmed later in vivo (Fuentes and others 1995; Gunn and others 1997). Further, the CCL2 pathway has been validated using animal models, in which CcL2 and its receptor CCR2 were knocked out (Kurihara and others 1997). It should be, however, noted that knockout mice for CcL2 and its receptor CCR2 are viable. However, abnormalities in monocyte recruitment and cytokine expression in CcL2−/− mice were observed (Lu and others 1998).
Using asymmetrically labeled CCL2 in NMR experiments, the solution structure of CCL2 dimer has been determined (Handel and others 1996). These studies indicated that the secondary structure of CCL2 consists of four regions of β-sheet. These include residues 9–11 (β0), 27–31 (β1), 40–45 (β2), and 51–54 (β3). In addition to the four strands of sheet, there are two helical regions. A long helix extends from approximately residue 58 to residue 69 (Fig. 3). Moreover, it was also found that residues 6–16 are involved in the dimerization interface of CCL2 (Zhang and Rollins 1995). The residues involved in the interface include Asn6, Ala7, Val9, Cys11, Tyr13, Asn 14, Phe15, and Thr16 near the N-terminus, and Glu 50, Ile51, and Cys 52. The overall secondary and quaternary structures of CCL2 monomers and dimers resemble RANTES and MIP-1β (Meunier and others 1997). The protein complex appears elongated with the two monomers oriented to give a fairly large pocket. Structures of monomeric and dimeric CCL2 in two crystal forms namely I and P forms, respectively, have also been determined (Lubkowski and others 1997). Recently, structures of CCL2 in complex using blocking antibody were also determined (Reid and others 2006). Note that it is possible to attenuate the function of CCL2 by allowing it to form a dimer with a nonfunctional mutated form of CCL2. For example, it has been reported that an N-terminal deletion mutant of CCL2 (7ND), which lacks the N-terminal amino acids 2–8, acts as a dominant-negative inhibitor of CCL2 and blocks the CCL2/CCR2 signal pathway in vivo (Kitamoto and others 2003). Further, it has been reported that this CCL2 mutant (7ND) and wild-type CCL2 form a heterodimer, which binds to the CCL2 receptor (CCR2) and completely inhibits CCL2-mediated monocyte chemotaxis in vitro (Zhang and Rollins 1995). Transgenic mice expressing the 7ND gene were found to block CcL2 pathway and, thus, prevent the formation of atherosclerotic lesions without having any effect on serum lipid concentrations (Ni and others 2001). Finally, as shown in Table 2, several cellular events lead to the induction or suppression of CCL2 expression.
FIG. 3.
Schematic representation of CCL2 structure. Note that all the domains are also indicated.
Table 2.
Conditions Affecting MCP-1/CCL2 Production: Summary of Cellular Events Leading to Increased Production Secretion of MCP-1 Protein
| Treatments | Effects | References |
|---|---|---|
| Secretory phospholipases A(2) | Phosphorylation/MAPK p38 and ERK1/2 | Granata and others 2006 |
| Leukotriene receptor antagonists (LTRAs) | Downregulates CCL2-induced chemotaxis | Hung and others 2006 |
| Trichostatin A (inhibitor/histone deacetylases) | Supresses LPS-induced CCL2 | Aung and others 2001 |
| Doxycycline | Reduces CCL2 production | Raza and others 2006 |
| Cytomix | Stimulates CCL2 production | |
| Lysophospholipids | Enhances IL-1-mediated CCL2 expression | Lin and others 2006 |
| Quercetin (3,3′,4′,5,7-pentahydroxyflavone) | Reduces TNF-α-induced CCL2 expression | Nanua and others 2006 |
| Tat-C3 exoenzyme, dominant-negative RhoA | Inhibits TGF-β1-mediated CCL2 expression | Kim and others 2006 |
| Calcium channel blockers (Amlodipine & Manidipine) | Decreases angiotensin-II-mediated CCL2 | Toba and others 2006 |
| Heme oxygenase-1 | Decreases CCL2 expression | Shokawa and others 2006 |
| FR 167653 (p38 MAPK inhibitor) | Reduces CCL2 and TGF-β expression | Takaishi and others 2003 |
| SB 203580 (p38 MAPK inhibitor), PD 98059 (ERK), AG 490 (JAK-2) | Suppresses IL-4 and IL-13-mediated CCL2 production | Kamei and others 2006 |
| Cell cycle proteins: | Suppresses: | Nonomura and others 2006 |
| p21 (Cip1) | IL-1 receptor (IL-1RI) | |
| p16 (INK4a) | MMP-3 and CCL2 | |
| p18 (INK4c) | MMP-3 and CCL2 | |
| Retinoblastoma gene product | MMP-3 and CCL2 | |
| Propentofyllin (antiallodynic agent) | Dampen CCL2 and MIP-2 released by astrocytes by improving glutamate transport | Tawfik and others 2006 |
| Anti-Fas antibody and TNF-α | Upregulation of MMP9, CCL2, and ICAM-1 in astrocytes | Ogier and others 2005 |
| Minocycline | Downregulation through inhibiting microglial activation | Carmen and others 2006 |
| vMIP-II | Chemokine receptor antagonist | Ghirnikar and others 2001 |
| SDF-α (CXCL12) | Upregulates CCL2 and IL-8 by P13-K and p38-dependent mechanisms | Calderon and others 2006 |
| Pyrrolidine dithiocarbamate (potent antioxidant and an inhibitor of NF-κB) | Prevents Tat-induced COX-2 expression Abrogates neuroparasite-mediated induction of CCL2 and IL-8 | Flora and others 2005 |
| NS-398 inhibitor of cyclooxygenase-2 (COX-2) | Attenuates Tat-mediated upregulation of CCL2, IL-1β, TNF-α, and iNOS | Nakao and others 2005 |
| Attenuates activation of microglial cells | ||
| Attenuates IL-1β-induced angiogenesis through inhibiting macrophage infiltration | ||
| Toll-like receptor 3 (TLR3) signaling | Involved in the induction of chemokine and cytokine genes in astrocytes in response to viral infections | Yang and others 2006 |
| Dominant-negative mutant of MCP-1 (7ND) | Blocks CCL2/CCR2 signaling pathway Blocks CCL2-mediated monocyte chemotaxis | Ni and others 2001 |
| Duffy antigen receptor for chemokines (DARC) (negative growth regulator) | Overexpression causes the inhibition of tumorigenesis and/or metastasis tumor angiogenesis and CCL2 expression | Wang and others 2006 |
| Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) | Induces IL-8 and CCL2 resulting in the invasion of adenocarcinoma cells but also increases distant tumor metastasis | Trauzold and others 2006 |
MCP receptors
Many of the CC chemokine receptors (CCRs) have been cloned based on well-conserved motifs of the earlier identified IL-8 receptors. Many of the genes encoding these CCR proteins of about 360 amino acids are closely linked on chromosome 3p21-22 (Wells and others 1996). All chemokine receptors identified are GPCRs, belonging to the rhodopsin or serpentine receptor family. Generally, these receptors are composed of a short extracellular N-terminus, seven hydrophobic transmembrane domains each connected by three extracellular and three intracellular loops, and a C-terminal intracellular region. Most of the CCRs that bind one or more MCPs are shared by other CC chemokines (as shown in Table 1). As MCPs have a broad cell spectrum, their receptors are expressed on various leukocyte types. In addition, all human MCPs are known to bind to at least two receptors (Table 1). Also, it should be noted that many receptors such as CCR2 respond to several different ligands. Therefore, understanding implications of these interactions (chemokines-receptors) for their in vivo functions are becoming more challenging. However, it seems that many regulatory mechanisms may come into picture to eliminate redundancy and give each chemokine a unique and specific function (Mantovani 1999).
CCL2 mediates its effects through its receptor CCR2, and, unlike CCL2, CCR2 expression is relatively restricted to certain types of cells. There are two alternatively spliced forms of CCR2, namely, CCR2A and CCR2B, which differ only in their C-terminal tails (Charo and others 1994). CCR2A is the major isoform expressed by mononuclear cells and vascular smooth muscle cells (Bartoli and others 2001), whereas monocytes and activated NK cells express predominantly the CCR2B isoform. It is possible that CCR2A and CCR2B may activate different signaling pathway and exert different actions. For example, CCL2 chemotaxis of CCR2A-positive cells occurs without Ca2+ mobilization, but Ca2+ flux is induced in the CCR2B-positive cells (Sanders and others 2000; Cho and others 2007). It has been reported that CCL2 is capable of increasing the expression of CCR2A but not CCR2B in synoviocytes obtained from patients with rheumatoid arthritis (Cho and others 2007). It is important to note that CCR2 has dual roles and has both proinflammatory and anti-inflammatory actions. The proinflammatory role of CCR2 is dependent on APCs and T cells, whereas the anti-inflammatory role of CCR2 is dependent on CCR2 expression in regulatory T cells. Further, as many as seven single nucleotide polymorphisms (SNPs) have been reported for CCR2. However, there is little evidence to suggest that any particular one of them affects clinical disease outcome in patients with acute idiopathic anterior uveitis (Yeo and others 2006). CCR2-deficient mice are resistant to the induction of sensory neuropathies (Thacker and others 2007). In addition, CCR2-null mice immunized with type II bovine collagen were found to be more susceptible to collagen-induced arthritis than the wild-type mice (Quinones and others 2004).
Mechanisms and pathways for monocyte recruitment
In a study performed by the Van Furth group, it was demonstrated that the half-life of circulating monocytes in humans is about three times longer than in mice, which is estimated to be around 340 million monocytes leaving the circulation daily (van Furth and Diesselhoff-den Dulk 1980). Further, in mice, under steady-state conditions, about half of the circulating monocytes are cleared from the blood stream daily (van Furth and Cohn 1968; van Furth and others 1973). A considerable fraction of circulating monocytes enters the tissues of the body, differentiating into macrophages. In contrast, immature dendritic cells within the tissue are able to leave via afferent lymphatic vessels to the draining lymph nodes, where they mature, present antigens to T cells, and die within a few days of arrival. Thus, a large fraction of monocytes can potentially be cleared as a by-product of immune surveillance.
CCL2 has been demonstrated to recruit monocytes into foci of active inflammation (Ajuebor and others 1998). However, it remains unclear whether monocytes use the same molecular signals to migrate into tissues as part of the constitutive or steady-state efflux from blood. In this regard, evidence has been provided describing the involvement of prostaglandin E2 in the attraction of monocytes to the site of inflammation and their maturity into macrophages (Kurth and others 2001). CCL2 secreted in or injected into skin arrives in the draining lymph nodes where it can be presented on the surface of high endothelial venules (HEVs) for recruitment of lymphocytes (Palframan and others 2001). Further, they found that CCL2 was the main chemokine responsible for recruiting monocytes. In these studies, the authors showed that only a fraction (∼2%) of the circulating monocyte pool is recruited to the lymph nodes (Palframan and others 2001). It is not clear whether these cells are representative of the majority of circulating monocytes, or they represent an important subset destined to reach draining lymph nodes. In addition to CCL2, several other chemokines were also shown to be involved in the recruitment of monocytes. In this regard, it has been demonstrated that the stimulation of RANTES leads to recruitment of monocytes/macrophages (Brown and other 1996; Haberstroh and others 1998).
Genetic variations of CCL2 and diseases
Genetic variations of CCL2 have been reported to influence the serum levels of CCL2 and the incidence of myocardial infarction (Tucci and others 2004). Two SNPs of CCL2, namely, G-927C and A-2578G, were found to be associated with carotid intima-media thickness, which reflects generalized atherosclerosis and is predictive of future vascular events (Brenner and others 2006). Further studies examined the distribution of SNPs in the CCL2 gene in tuberculosis (Flores-Villanueva and others 2005). The authors found that the probability of developing tuberculosis was 2.3- and 5.4-fold higher in carriers of CCL2 genotypes AG and GG, respectively, than in homozygous AA. These findings suggest that persons bearing the CCL2 genotype GG produce higher concentrations of CCL2, which inhibits the production of IL-12 p40 in response to Mycobacterium tuberculosis and increases the likelihood that M. tuberculosis infection will progress to active pulmonary tuberculosis. In addition, the influence of genetic variation in CCL2 on HIV-1 pathogenesis has been examined using large cohorts of HIV-1-infected adults and children. Results showed that in adults, homozygosity for the CCL2 −2578G (alternatively designated −2518) allele was associated with a 50% reduction in the risk of acquiring HIV-1. However, once HIV-1 infection was established, this same CCL2 genotype was associated with accelerated disease progression and a 4.5-fold increased risk of HIV-associated dementia (HAD) (Gonzalez and others 2002). Finally, HIV-patients with a mutated CCL2 allele have an undetectable viral load after treatment with protease inhibitor–based antiretroviral therapy (Coll and others 2006).
CCL2 and immune response
Apart from recruiting and directing leukocyte movement, several lines of evidence indicate that CCL2 might influence T-cell immunity. First, CCL2 expression is associated with the development of polarized Th2 responses (Chensue and others 1995; Handel and others 1996) and CCL2 enhances the secretion of IL-4 by T cells (Karpus and others 1997). Second, in Th2 immune-mediated diseases, such as asthma, CCL2 is expressed at high levels and its neutralization in animal models ameliorates disease (Gonzalo and others 1998). Finally, other chemokines and their receptors are linked to specific responses of T-helper cells (Sallusto and others 1998). In contrast to other chemokines of the C-C family, which trigger the Th1 phenotype upon their interaction with CCR5 on T-helper cells (Van Coillie and others 1999), CCL2 acts as a potent factor in the polarization of Th0 cells toward a Th2 phenotype (Gu and others 2000). The T-lymphocyte differentiation process is initiated by the ligation of the T-cell receptor (TCR). Cytokines present during the initiation of a T-cell response determine the development of the particular T-helper subset (Rogge and others 1997). Polarization of the T-cell subsets occurs in the secondary lymphoid organs to which Th0 cells preferentially migrate. Memory lymphocytes and effector precursor cells, in contrast, migrate to peripheral tissues (Picker and Butcher 1992). It is likely that, given their different effectors function, Th1 and Th2 cells are differentially recruited to peripheral sites of infection (Lichtman and Abbas 1997). For example, it has been shown that Th1 cells, but not Th2 cells, express a functional ligand for P- and E-selectin and therefore are selectively recruited to sites where Th1 immune responses occur (Austrup and others 1997). There may be a direct role for CCL2 in the development of Th2 cells. It appears that CCL2 can directly activate the IL-4 promoter, as IL-4 production is increased in cells that are given a primary TCR stimulus in the presence of CCL2. A higher level of CCL2 augments the Th2 response (Karpus and others 1997). These findings provide an important clue as to why there is a switch from Th1 to Th2 cytokine response in HIV-1 disease. The reciprocal inhibition between Th1 and Th2 cytokines, such as IL-4, is a major factor that governs Th2 differentiation and inhibits the development of IFN-γ-secreting cells (Brown and Hural 1997). This may be important for the effective regulation of the immune response to viruses. Moreover, Th1 and Th2 cells, because of their different chemokine receptor expression pattern induced at least in part by CCL2, are likely to have different susceptibility to HIV strains that use different fusion coreceptors.
Role of CCL2 in disease
Both CCL2 and its receptor CCR2 are induced and involved in various diseases (Table 3). This involvement has mainly been demonstrated using genetically deficient mice, antibody- or inhibitor-mediated neutralization in mice, as well as epidemiological studies in humans. In this section, we will briefly discuss the role of CCL2 in different diseases mainly focusing on AIDS.
Table 3.
Involvement of CCL2 in Different Diseases: List of Diseases that Are Affected by CCL2
| Effects | Mechanism | References |
|---|---|---|
| Thrombus formation | By generating tissue factor | Charo and Taubman 2004 |
| Tuberculosis | Lower levels of IL-12 p40 | Flores-Villanueva and others 2005 |
| Immunotolerance in endometriosis | Apoptosis of T-lymphocytes | Salem and others 2006 |
| Recurrent miscarriage | IL-1β-induced MCP-1 | Huang and others 2006 |
| Viral clearance from CNS | Increased infiltration of T lymphocytes | Carmen and others 2006 |
| Multiple sclerosis | Correlation between CCL2 and axonal damage CCL2 and IP-10 in hypertrophic astrocytes | Tanuma and others 2006 |
| Secondary progressive multiple sclerosis | ||
| Nociception (perception of pain) | CCL2-mediated depolarization of neurons | Sun and others 2006 |
| HIV-neurological complications | Tat-mediated upregulation of COX-2, MCP-1, IL-1β, TNF-α, and iNOS, and activation of microglial cells | Flora and others 2005 |
| Oxygen-induced injury (retinopathy) | Marked increase in CCL2 in microglia/macrophages | Davies and others 2006 |
| HIV-associated neurocognitive impairment | Correlation between plasma MCP-1 and tissue status TNF-α and anisotrphy measurements | Ragin and others 2006 |
| Tumor neovascularity | CCL2 influence by affecting macrophage infiltration | Wang and others 2006 |
| Brain development and heterologous desensitization, influence of neuronally active pharmacological agents such as opioids and cannabinoids | Chemokine-based intracellular communication interaction with neurotransmitter systems | Adler and others 2006 |
| Nephropathy | p38 MAPK phosphorylation | Granata and others 2006 |
| Inflammatory bowel disease | CCL2-mediated differentiation of intestinal macrophages | Spoettl and others 2006 |
| Allergic asthma | IL-4 and IL-13-induced release of CCL2 in bronchial epithelium | Ip and others 2006 |
| Rheumatoid arthritis | Increased CCL2 before onset | Rantapaa-Dahlqvist and others 2007 |
| Insulin resistance | Increased MCP-1, TNF-α, and IL-6 | Kamei and others 2006 |
| Ischemia-related neuronal death | Astrocytes expressing CCL2, MIP1-α, and cells expressing its receptors | Sakurai-Yamashita and others 2006 |
| Exitotoxic (NMDA) neuronal injury | CCL2 production by astrocytes | Katayama and others 2002 |
Role in HIV-1 pathogenesis
Owing to higher CCR2 expression, memory CD4+ T cells and monocytes are the main cells to be recruited by CCL2, which helps making them primary targets for HIV-1 infection (Matsushima and others 1989). Because monocytes/macrophages are found to be the major source of CCL2 in vitro and in vivo, this may explain why HIV-1-infected migrating monocytes are considered to be a potent contributor to spread the disease (Cinque and others 1998). Altered CCL2 expression may also contribute to HAD. Accordingly, CCL2 was markedly elevated in the cerebrospinal fluid (CSF) of HIV-infected patients with cytomegalovirus (CMV) encephalitis (Bernasconi and others 1996). High levels of CCL2 may underlie monocyte recruitment and tissue damage in CMV encephalitis, and may represent a rapid and useful tool in the diagnostic armamentarium for neurological disorders associated with HIV infection (Sozzani and others 1997). Note that the plasma levels of CCL2 correlates with virus load in HIV-1 infection (Weiss and others 1997; Chang and others 2004). HIV encephalitis was strongly associated with high CSF CCL2 levels, which also correlated with high HIV-1 RNA levels in the CSF, but not to plasma viremia (Ragin and others 2006). These findings support a model whereby HIV encephalitis is sustained by virus replication in monocytic cells, a process amplified by the recruitment of mononuclear cells via HIV-induced CCL2 (Cinque and others 1998). Further, SIV-infected macaques that developed moderate-to-severe encephalitis had significantly higher MCP-1 levels in CSF than in plasma as early as 28 days after inoculation, which suggests that the CSF:plasma MCP-1 ratio may be a valuable prognostic marker for the development of HIV-induced central nervous system (CNS) disease (Zink and others 2001). Furthermore, CCL2 levels have been shown to diminish in HIV-1 patients after indinavir (a viral protease inhibitor) treatment (Bisset and others 1997). Interestingly, CCL2 could be a potent suppressor of HIV-1 when HIV-1-infected peripheral blood lymphocytes are used as target cells (Frade and others 1997). In this regard, it has been shown that CCL2 may inhibit HIV-1 infection by blocking viral attachment to CCR2 and CCR5 coreceptors (Homan and others 2002). The mechanisms of induction of CCL2 in the brains of HIV-infected patients are not well characterized. However, it has been shown that the HIV-1 transactivator protein Tat significantly increases the expression and release of CCL2 by astrocytes (Conant and others 1998). Tat-induced CCL2 expression is mediated at the transcriptional level through a minimal promoter DNA region containing 213 nucleotides upstream of the translational start site (Lim and others 2000). Site-directed mutagenesis studies indicate that Sp1, AP1, and NF-κB binding sites are critical for both constitutive and Tat-enhanced expression of the CCL2 promoter. Further studies demonstrated that Tat might cooperate with Smad3 and C/EBPβ factors to induce CCL2 gene expression (Abraham and others 2003, 2005). In this regard, it is also important to note that the C-terminal domain of Smad3 (MH2) downregulates CCL2 production (Eldeen and others 2006). In addition, CCL2 was released from human lung microvascular endothelial cells (HMVEC-Ls) in a dose- and time-dependent manner following Tat treatment (Park and others 2001). Further, CCL2 was shown to be induced following treatment of the cells with morphine and HIV-1 Tat protein (El-Hage and others 2005). Treatment of astrocytes with the κ-opioid receptor (KOR) ligand trans-3,4-dichloro-N-methyl-N[2-(1-pyrolidinyl) cyclohexyl] benzeneacetamide methanesulfonate (U50,488) inhibited Tat-induced CCL2 production in a concentration-dependent manner (Sheng and others 2003). However, in another study with neurons, CCL2 was demonstrated to play a protective role against the toxic effects of glutamate and Tat (Eugenin and others 2003).
Role in cardiovascular disease
Several studies have linked CCL2 to cardiovascular disease. Using CCL2- or CCR2-deficient mice to examine atherosclerosis, it was demonstrated that, in the absence of CCL2 or its receptor, CCR2, there was a substantial reduction in arterial lipid deposition (Boring and others 1998). Further, amelioration from the disease was associated with diminished numbers of macrophages in the arterial wall consistent with a model in which CCL2 contributes to atherosclerosis by attracting monocytes into the subendothelium via CCR2 activation (Dawson and others 1999). Increased plasma levels of CCL2 following balloon angioplasty of coronary arteries predicts early restenosis, which may represent an accelerated form of atherosclerosis (Cipollone and others 2001). Finally, at a population level, a polymorphism in the CCL2 promoter has been demonstrated to be associated with an increased risk of an individual to suffer from coronary artery disease (Szalai and others 2001).
Role in cancer
Chemokines and their receptors have been detected in most tumors (Conti and Rollins 2004). However, to date no susceptibility gene in any cancer has been mapped on to a chemokine or chemokine receptor. Chemokines are involved in a broad array of normal host activities that impact cancer; therefore, it is possible that they will be found to have important effects on cancer pathogenesis. For this reason, chemokines might be expected to have either growth-promoting or growth-inhibiting influences on cancer cells depending on the particular setting in which they are expressed. Further, because of their ability to attract and activate lymphocytes, some chemokines might be expected to stimulate host antitumor responses. On the other hand, some of the chemokines are known to possess angiogenic activities, which could potentially contribute to tumor growth and progression. Some of the tumor-associated molecular alterations that increase macrophage infiltration and macrophage-mediated angiogenesis include increased expression of CCL2 and VEGF, both of which are highly expressed in breast cancer cells (Ohta and others 2003). CCL2 expression in tumor cells is significantly correlated with the extent of tumor-associated-macrophage (TAM) infiltration (Sato and others 1995), and in particular both CCL2 and VEGF expressions have been positively correlated with TAM infiltration, angiogenesis, and poor survival in breast cancer (Valkovic and others 2002).
Monocytes are critical for the initiation of tumor arteriogenesis because they adhere to and invade endothelium activated by the increased shear stress that results from large pressure differences between perfused areas (Scholz and others 2001). The involvement of monocytes in arteriogenesis was discovered in 1976 (Schaper and others 1976), shortly before their role in angiogenesis in 1977 (Polverini and others 1977). CCL2 is once again implicated in this process because it not only attracts monocytes, but also promotes their adhesion by inducing them to upregulate MAC-1, the receptor for intracellular adhesion molecule-1 (ICAM-1) that is expressed in activated endothelium (Scholz and others 2000). Finally, note that CCL2 has antitumor activity. This was demonstrated by its ability to augment cytostatic activity against tumor cells upon addition to macrophages in tissue culture (Zachariae and others 1990) and by its ability to induce FAS ligand protein expression in cultured endometrial stromal cells, thus driving cells to apoptosis.
Role of CCL2 in other diseases
Both CCL2 and its receptor CCR2 have been found to be elevated and to play a pivotal role in the development of atherosclerosis (Namiki and others 2002). Moreover, differentiation of intestinal macrophages was disturbed by CCL2, suggesting that CCL2 could play a role in the disturbed intestinal differentiation that occurs in the mucosa of patients suffering from inflammatory bowel disease (Spoettl and others 2006). Further studies linked the induction of CCL2 by IL-4 and IL-13 in human bronchial epithelial cells to its potential involvement in allergic asthma (Ip and others 2006). Further, CCL2 levels were significantly raised in individuals with rheumatoid arthritis (Rantapaa-Dahlqvist and others 2007). In addition, using a transgenic mouse model system, it has been shown that circulating CCL2 may contribute to insulin resistance in diabetic patients (Kamei and others 2006). Finally, CCL2 was also shown to be involved in neurological disorders such as ischemia-related neuronal death, where CCL2 levels were elevated in astrocytes leading to neuronal death (Sakurai-Yamashita and others 2006).
The importance of CCL2 and its receptor CCR2 is not limited to the manifestation of coronary artery disease but can be expected to play equally important roles in other inflammatory diseases. For example, similar results have been obtained in experimental allergic encephalitis, which is a rodent model for multiple sclerosis (Izikson and others 2000). In these studies, it was found that CcL2- or CCR2-deficient mice recruited many fewer monocytes into the CNS and the severity of disease was greatly reduced. Note that the mechanisms of recruitment of CCL2 in these diseases are not fully understood and in some cases remain to be identified.
Conclusions and Future Directions
One of the biggest challenges to define the role of chemokines and their receptors in inflammation-mediated diseases is that there are over 50 known chemokines and 20 chemokine receptors. Thus, it may not be practical to study all of them simultaneously in an experimental setting. It may be possible that mechanisms involved in causing ultimate damage may differ from one disorder to another with more than one chemokine/cytokine playing a significant role than the other. Similarly, upregulated expression of different chemokines and receptors may occur as a part of cytokine “cascade,” where the expression of one chemokine or its receptor is dependent on previous events. For this reason, future research should be directed toward identifying key early events that would be responsible for initiating the vicious cycle. Successful intervention of these early events would be very beneficial in avoiding downstream consequences that ultimately lead to symptomatic disease/disorder.
CCL2 remains one of the most studied of the chemokines. Valuable information regarding its pathway has come from structure-functional as well as transgenic mouse model. Although it has been associated with many pathological conditions such as cardiovascular disease, multiple sclerosis, rheumatoid arthritis, and so on, it plays an important role in routine immune surveillance and immune modulation, and in clearing acute viral infections. Any attempts to reduce CCL2 production or reduce CCR2 expression to achieve beneficial effects in some of the above-mentioned pathological conditions should be carefully weighed because of its role in the maintenance of health. Recruitment of monocytes/macrophages in a particular organ in response to inflammation is a vital response to eliminate invading pathogens through phagocytosis. Recently, recruitment of a large number of microglia from within the brain and monocytes from blood involved in clearing deposits of β-amyloid, a neurotoxic peptide that accumulates in the brain of individuals with Alzheimer's disease, has been demonstrated (Britschgi and others 2007; El Khoury and others 2007). In addition to clearing β-amyloid, microglia and blood-derived macrophages may enable neurons or astrocytes to limit β-amyloid accumulation and provide trophic support to neurons (White and others 2005). In this regard, it should be noted that there is no direct evidence linking DNA polymorphisms at the gene encoding CCL2 (−2518 A/G) and CCR2 (V64I) with the risk and/or the clinical outcome of Alzheimer's disease (Huerta and others 2004). However, a strong link was found between CCL2 polymorphism and other autoimmune diseases in specific populations. CCL2 (−2518 A/G) SNP was shown to be associated with the chronic stable angina pectoris within the Slovak population (Bucova and others 2008). Furthermore, a significant but not independent association was demonstrated between the (−2518G/A) polymorphism of the CCL2 gene and myocardial infarction in the Tunisian population (Jemaa and others 2008). This SNP was also observed in Czech patients with pulmonary sarcoidosis, which may be associated with Löfgren's syndrome (Navratilova and others 2007). Finally, CCL2 polymorphisms were described as associated with systemic lupus erythematosus in Mexican patients (Lima and others 2007).
Discovery of drugs, which block several upregulated chemokine receptors, may prove to be very effective if they are upstream of CCR2 expression. Proinflammatory cytokines, such as TNF-α, IL-1β, IL-6, and/or BDNF, could play a significant role in initiating the cascade. TNF-α, in particular, has been shown to upregulate CCL2 expression in sensory neurons (Milligan and others 2003; Twining and others 2004; Jung and others 2008). Blocking the actions of these proinflammatory cytokines on a chronic basis without causing major side effects would be a formidable challenge in future. If these drugs are to be targeted to the CNS, permeability issues also need to be taken into account. It is not clear whether a CCR2 receptor antagonist shown to be effective in crossing blood nerve barrier would be equally effective for the treatment of the CNS disorders (Hirakawa and others 2004). In fact, many CCR2 antagonists, such as INCB 3284 and INCB 8689 (Incyte), CCX915 (Chemo Centryx), and MLN 1202 (Millennium), are undergoing clinical trials for the treatment of various chronic inflammatory diseases. However, despite the growing evidence showing the ability of these antagonists to block autoimmune diseases in experimental animal models, clinical trials failed dramatically in humans, indicating a need to further elucidate the complex system of chemokine interactions (Kalinowska and Losy 2008). Furthermore, widespread use of these CCR2 antagonists may elicit harmful effects in the aging population, making them more vulnerable to Alzheimer's disease. Alternatively, CCL2 production itself may be targeted in tissues experiencing chronic inflammation. This could be achieved by using dominant-negative mutants of CCL2, by silencing CCL2 gene using RNAi technology. In any case, the vast knowledge being gathered on the functions of CCL2 may pave the way for developing safe strategies for controlling undesirable effects of chronic inflammation without compromising its beneficial effects.
Acknowledgments
We thank past and present members of the Center for Neurovirology and Department of Neuroscience for sharing ideas. We also thank Dr. Martyn K. White for editorial assistance. This work was supported by a Grant awarded by NIH to B.E.S.
References
- Abraham S. Sawaya BE. Safak M. Batuman O. Khalili K. Amini S. Regulation of MCP-1 gene transcription by Smads and HIV-1 Tat in human glial cells. Virology. 2003;309:196–202. doi: 10.1016/s0042-6822(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Abraham S. Sweet T. Sawaya BE. Rappaport J. Khalili K. Amini S. Cooperative interaction of C/EBP beta and Tat modulates MCP-1 gene transcription in astrocytes. J Neuroimmunol. 2005;160:219–227. doi: 10.1016/j.jneuroim.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Adams DH. Harvath L. Bottaro DP. lnterrante R. Catalano G. Tanaka Y. Strain A. Hubscher SG. Shaw S. Hepatocyte growth factor and macrophage inflammatory protein 1 beta: structurally distinct cytokines that induce rapid cytoskeletal changes and subset-preferential migration in T cells. Proc Natl Acad Sci USA. 1994;91:7144–7148. doi: 10.1073/pnas.91.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler MW. Geller EB. Chen X. Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2006;7:E865–870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajuebor MN. Flower RJ. Hannon R. Christie M. Bowers K. Verity A. Perretti M. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998;63:108–116. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- Aung H. McKenna SM. Ketoff NR. Jones L. Wu M. Hejal R. Rich EA. Toossi Z. Dysregulation of beta-chemokines in the lungs of HIV-1-infected patients. J Acquir Immune Defic Syndr. 2001;26:305–314. doi: 10.1097/00126334-200104010-00002. [DOI] [PubMed] [Google Scholar]
- Austrup F. Vestweber D. Borges E. Lohning M. Brauer R. Herz U. Renz H. Hallmann R. Scheffold A. Radbruch A. Hamann A. P- and E-selectin mediate recruitment of T-helper–1 but not T-helper–2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Bacon K. Baggiolini M. Broxmeyer H. Horuk R. Lindley I. Mantovani A. Maysushima K. Murphy P. Nomiyama H. Oppenheim J. Rot A. Schall T. Tsang M. Thorpe R. Van Damme J. Wadhwa M. Yoshie 0. Zlotnik A. Zoon K (IUIS/WHO Subcommittee on Chemokine Nomenclature) Chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002;22:1067–1068. doi: 10.1089/107999002760624305. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Loetscher P. Chemokines in inflammation and immunity. Immunol Today. 2000;21:418–420. doi: 10.1016/s0167-5699(00)01672-8. [DOI] [PubMed] [Google Scholar]
- Barna BP. Pettay J. Barnett GH. Zhou P. Iwasaki K. Estes ML. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J Neuroimmunol. 1994;50:101–107. doi: 10.1016/0165-5728(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Bartoli C. Civatte M. Pellissier JF. Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001;102:385–392. doi: 10.1007/s004010100394. [DOI] [PubMed] [Google Scholar]
- Beall CJ. Mahajan S. Kuhn DE. Kolattukudy PE. Site-directed mutagenesis of monocyte chemoattractant protein-1 identifies two regions of the polypeptide essential for biological activity. Biochem J. 1996;313:633–640. doi: 10.1042/bj3130633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi S. Cinque P. Peri G. Sozzani S. Crociati A. Torri W. Vicenzi E. Vago L. Lazzarin A. Poli G. Mantovani A. Selective elevation of monocyte chemotactic protein-1 in the cerebrospinal fluid of AIDS patients with cytomegalovirus encephalitis. J Infect Dis. 1996;174:1098–1101. doi: 10.1093/infdis/174.5.1098. [DOI] [PubMed] [Google Scholar]
- Bisset LR. Rothen M. Joller-Jemelka HI. Dubs RW. Grob PJ. Opravil M. Change in circulating levels of the chemokines macrophage inflammatory proteins 1 alpha and 11 beta, RANTES, monocyte chemotactic protein-1 and interleukin-16 following treatment of severely immunodeficient HIV-infected individuals with indinavir. AIDS. 1997;511:485–491. doi: 10.1097/00002030-199704000-00012. [DOI] [PubMed] [Google Scholar]
- Boring L. Gosling J. Cleary M. Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Brenner D. Labreuche J. Touboul PJ. Schmidt-Petersen K. Poirier O. Perret C. Schönfelder J. Combadière C. Lathrop M. Cambien F. Brand-Herrmann SM. Amarenco P GENIC Investigators. Cytokine poly- morphisms associated with carotid intima-media thickness in stroke patients. Stroke. 2006;37:1691–1696. doi: 10.1161/01.STR.0000226565.76113.6c. [DOI] [PubMed] [Google Scholar]
- Britschgi M. Wyss Coray T. Immune cells may fend off Alzheimer disease. Nature Med. 2007;13:408–409. doi: 10.1038/nm0407-408. [DOI] [PubMed] [Google Scholar]
- Brown MA. Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997;17:1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- Brown Z. Robson RL. Westwick J. Regulation and expression of chemokines: Potential role in gbomerulonephritis. J Leukocvte Biol. 1996;59:75–80. doi: 10.1002/jlb.59.1.75. [DOI] [PubMed] [Google Scholar]
- Brown Z. Strieter RM. Neild GH. Thompson RC. Kunkel SL. Westwick J. IL-1 receptor antagonist inhibits monocyte chemotactic peptide 1 generation by human mesangial cells. Kidney Int. 1992;42:95–101. doi: 10.1038/ki.1992.266. [DOI] [PubMed] [Google Scholar]
- Bucova M. Lietava J. Mrazek F. Petrkova J. Bernadic M. Buckingham T. Petrek M. Association of chronic stable angina pectoris with MCP-1–2518 A/G single nucleotide polymorphism in the Slovak population. Clin Chim Acta. 2008;392:71–72. doi: 10.1016/j.cca.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Calderon TM. Eugenin EA. Lopez L. Kumar SS. Hesselgesser J. Raine CS. Berman JW. A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol. 2006;177:27–39. doi: 10.1016/j.jneuroim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Callewaere C. Banisadr G. Rostene W. Parsadaniantz SM. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J Mol Endocrinol. 2007;38:355–363. doi: 10.1677/JME-06-0035. [DOI] [PubMed] [Google Scholar]
- Carmen J. Gowing G. Julien JP. Kerr D. Altered immune response to CNS viral infection in mice with a conditional knock-down of macrophage-lineage cells. Glia. 2006;54:71–80. doi: 10.1002/glia.20359. [DOI] [PubMed] [Google Scholar]
- Carr MW. Roth SJ. Luther E. Rose SS. Springer T. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. Ernst T. St Hillaire C. Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004;9:431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- Charo IF. Myers SJ. Herman A. Franci C. Connolly AJ. Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF. Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- Chensue SW. Warmington KS. Lukacs NW. Lincoln PM. Burdick MD. Strieter RM. Kunkel SL. Monocyte chemotactic protein expression during schistosome egg granuloma formation. Sequence of production, localization, contribution, and regulation. Am J Pathol. 1995;146:130–138. [PMC free article] [PubMed] [Google Scholar]
- Cho ML. Yoon BY. Ju JH. Jung YO. Jhun JY. Park MK. Cho CS. Kim HY. Expression of CCR2A, an isoform of MCP-1 receptor, is increased by MCP-1, CD40 ligand and TGF-beta in fibroblast like synoviocytes of patients with RA. Exp Mol Med. 2007;39:499–507. doi: 10.1038/emm.2007.55. [DOI] [PubMed] [Google Scholar]
- Cinque P. Vago L. Mengozzi M. Torri V. Ceresa D. Vicenzi E. Transidico P. Vagani A. Sozzani 5. Mantovani A. Lazzarin A. Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Cipollone F. Marini M. Fazia M. Pini B. lezzi A. Reale M. Paloscia L. Materazzo G. D'Annunzio E. Conti P. Chiarelli F. Cuccurullo F. Mezzetti A. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21:327–334. doi: 10.1161/01.atv.21.3.327. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I. Schumacher C. Baggiolini M. Moser B. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J Biol Chem. 1991;266:23128–23134. [PubMed] [Google Scholar]
- Clore GM. Gronenborn AM. Three-dimensional structures of alpha and beta chemokines. FASEB J. 1995;9:57–62. doi: 10.1096/fasebj.9.1.7821760. [DOI] [PubMed] [Google Scholar]
- Cochran BH. Reffel AC. Stiles CD. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Coll B. Alonso-Villaverde C. Parra S. Rabassa A. Martorell L. Joven J. Masana L. Influence of a monocyte chemoattractant protein 1 mutated allele on the response to protease inhibitor-based antiretroviral therapy. HIV Med. 2006;7:356–360. doi: 10.1111/j.1468-1293.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Conant K. Garzino-Demo A. Nath A. McArthur JC. Halliday W. Power C. Gallo RC. Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat- stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti I. Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14:149–154. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Crown SE. Yu Y. Sweeney MD. Leary JA. Handel TM. Heterodimerization of CCR2 chemokines and regulation by glycosaminoglycan binding. J Biol Chem. 2006;281:25438–25446. doi: 10.1074/jbc.M601518200. [DOI] [PubMed] [Google Scholar]
- Cushing SD. Berliner JA. Valente AJ. Territo MC. Navab M. Parhami F. Gerrity R. Schwartz CJ. Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MH. Eubanks JP. Powers MR. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis. 2006;12:467–477. [PubMed] [Google Scholar]
- Dawson TC. Kuziel WA. Osahar TA. Maeda N. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- Ebisawa M. Yamada T. Bickel C. Klunk D. Schleimer RP. Eosinophil transendothelial migration induced by cytokines. III. Effect of the chemokine RANTES. J Immunol. 1994;153:2153–2160. [PubMed] [Google Scholar]
- El Khoury J. Toft M. Hickman SE. Means TK. Terada K. Geula C. Luster AD. CCR2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nature Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Eldeen MB. Deshmane SL. Simbiri K. Khalili K. Amini S. Sawaya BE. MH2 domain of Smad3 reduces HIV-1 Tat-induction of cytokine secretion. J Neuroimmunol. 2006;176:174–180. doi: 10.1016/j.jneuroim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- El-Hage N. Gurwell JA. Singh IN. Knapp PE. Nath A. Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA. D'Aversa TG. Lopez L. Calderon TM. Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Flora G. Pu H. Lee YW. Ravikumar R. Nath A. Hennig B. Toborek M. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp Neurol. 2005;191:2–12. doi: 10.1016/j.expneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Flores-Villanueva PO. Ruiz-Morales JA. Song CH. Flores LM. Jo EK. Montano M. Barnes PF. Selman M Granados. A functional promoter polymorphism in monocyte chemoattractant ppotein-1 is associated with increased susceptibility to pulmonary tuberculosis. J Exp Med. 2005;202:1649–1658. doi: 10.1084/jem.20050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade JM. Llorente M. Mellado M. Alcami J. Gutierrez-Ramos JC. Zaballos A. Real G. Martínez-A C. The amino-terminal domain of the CCR2 chemokine receptor acts as co-receptor for HIV-1 infection. J Clin Invest. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes ME. Durham SK. Swerdel MR. Lewin AC. Barton DS. Megill JR. Bravo R. Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- Ghirnikar RS. Lee YL. Eng LF. Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat. J Neurosci Res. 2001;64:582–589. doi: 10.1002/jnr.1110. [DOI] [PubMed] [Google Scholar]
- Gong JH. Clark-Lewis I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J Exp Med. 1995;181:631–640. doi: 10.1084/jem.181.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JH. Ratkay LG. Waterfield JD. Clark-Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–137. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E. Rovin BH. Sen L. Cooke G. Dhanda R. Mummidi S. Kulkarni H. Bamshad MJ. Telles V. Anderson SA. Walter EA. Stephan KT. Deucher M. Mangano A. Bologna R. Ahuja SS. Dolan MJ. Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutan MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo JA. Lloyd CM. Wen D. Albar JP. Wells TN. Proudfoot A. Martinez-A C. Dorf M. Bjerke T. Coyle AJ. Gutierrez-Ramos JC. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and aiway hyper1-responsiveness. J Exp Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata F. Frattini A. Loffredo S. Del Prete A. Sozzani S. Marone G. Triggiani M. Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. Eur J Immunol. 2006;36:1938–1950. doi: 10.1002/eji.200535567. [DOI] [PubMed] [Google Scholar]
- Gu L. Tseng S. Horner RM. Tam C. Loda M. Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Gunn MD. Nelken NA. Liao X. Williams LT. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- Haberstroh U. Stilo K. Pocock J. Wolf G. Helmchen U. Wenzel U. Zahner G. Stahl RA. Thaiss F. L-arginine suppresses lipopolysaccharide-induced expression of RANTES in glomeruli. J Am Soc Nephrol. 1998;9:203–210. doi: 10.1681/ASN.V92203. [DOI] [PubMed] [Google Scholar]
- Handel TM. Domaille PJ. Heteronuclear (1H, 13C, 15N) NMR assignments and solution structure of the monocyte chemoattractant protein-1 (MCP-1) dimer. Biochemistry. 1996;35:6569–6584. doi: 10.1021/bi9602270. [DOI] [PubMed] [Google Scholar]
- Hayashida K. Nanki T. Girschick H. Yavuz S. Ochi T. Lipsky PE. Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 2001;3:118–126. doi: 10.1186/ar149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H. Okajima S. Nagaoka T. Kubo T. Takamatsu T. Oyamada M. Regional differences in blood-nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion. Neuroreport. 2004;15:405–408. doi: 10.1097/00001756-200403010-00004. [DOI] [PubMed] [Google Scholar]
- Homan JW. Steele AD. Martinand-Mari C. Rogers TJ. Henderson EE. Charubala R. Pfleiderer W. Reichenbach NL. Suhadolnik RJ. Inhibition of morphine-potentiated HIV-1 replication in peripheral blood mononuclear cells with the nuclease-resistant 2-5A agonist analog, 2-5A(N6B) J Acquir Immune Defic Syndr. 2002;30:9–20. doi: 10.1097/00042560-200205010-00002. [DOI] [PubMed] [Google Scholar]
- Huang SJ. Schatz F. Masch R. Rahman M. Buchwalder L. Niven-Fairchild T. Tang C. Abrahams VM. Krikun G. Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Huerta C. Alvarez V. Mata IF. Coto E. Ribacoba R. Martínez C. Blázquez M. Guisasola LM. Salvador C. Lahoz CH. Peña J. Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer's and Parkinson's disease. Neurosci Lett. 2004;370:151–154. doi: 10.1016/j.neulet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Hung CH. Li CY. Hua YM. Chen CJ. Yang KD. Jong YJ. Effects of leukotriene receptor antagonists on monocyte chemotaxis, p38 and cytoplasmic calcium. Pediatr Allergy Immunol. 2006;17:250–258. doi: 10.1111/j.1399-3038.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- Hyduk SJ. Chan JR. Duffy ST. Chen M. Peterson MD. Waddell TK. Digby GC. Szaszi K. Kapus A. Cybulsky MI. Phospholipase C, calcium, and calmodulin are critical for alpha4beta1 integrin affinity up-regulation and monocyte arrest triggered by chemoattractants. Blood. 2007;109:176–184. doi: 10.1182/blood-2006-01-029199. [DOI] [PubMed] [Google Scholar]
- Ip WK. Wong CK. Lam CW. Interleukin (IL)-4 and IL-13 upregulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin Exp Immunol. 2006;145:162–172. doi: 10.1111/j.1365-2249.2006.03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson L. Klein RS. Charo IF. Weiner HL. Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemaa R. Rojbani H. Kallel A. Ben Ali S. Feki M. Chabrak S. Elasmi M. Taieb SH. Sanhaji H. Souheil O. Mechmeche R. Kaabachi N. Association between the - 2518G/A polymorphism in the monocyte chemoattractant protein-1 (MCP-1) gene and myocardial infarction in Tunisian patients. Clin Chim Acta. 2008;390:122–125. doi: 10.1016/j.cca.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Jung H. Miller RJ. Activation of the nuclear factor of activated T-cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: A possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain. Mol Cell Neurosci. 2008;37:170–177. doi: 10.1016/j.mcn.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinowska A. Losy J. Investigational C-C chemokine receptor 2 antagonists for the treatment of autoimmune diseases. Expert Opin Investig Drugs. 2008;17:1267–1279. doi: 10.1517/13543784.17.9.1267. [DOI] [PubMed] [Google Scholar]
- Kamei N. Tobe K. Suzuki R. Ohsugi M. Watanabe T. Kubota N. Ohtsuka-Kowatari N. Kumagai K. Sakamoto K. Kobayashi M. Yamauchi T. Ueki K. Oishi Y. Nishimura S. Manabe I. Hashimoto H. Ohnishi Y. Ogata H. Tokuyama K. Tsunoda M. Ide T. Murakami K. Nagai R. Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- Karpus WJ. Lukacs NW. Kennedy KJ. Smith WH. Hurst SD. Barrett TA. Differential CC chemokine–induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- Katayama T. Minami M. Nakamura M. Ito M. Katsuki H. Akaike A. Satoh M. Excitotoxic injury induces production of monocyte chemoattractant protein-1 in rat cortico-striatal slice cultures. Neurosci Lett. 2002;328:277–280. doi: 10.1016/s0304-3940(02)00550-5. [DOI] [PubMed] [Google Scholar]
- Kim JS. Kim JG. Moon MY. Jeon CY. Won HY. Kim HJ. Jeon YJ. Seo JY. Kim JI. Kim J. Lee JY. Kim PH. Park JB. Transforming growth factor-beta1 regulates macrophage migration via RhoA. Blood. 2006;108:1821–1829. doi: 10.1182/blood-2005-10-009191. [DOI] [PubMed] [Google Scholar]
- Kitamoto S. Egashira K. Anti-monocyte chemoattractant protein-1 gene therapy for cardiovascular diseases. Expert Rev Cardiovasc Ther. 2003;1:393–400. doi: 10.1586/14779072.1.3.393. [DOI] [PubMed] [Google Scholar]
- Kurihara T. Warr G. Loy J. Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth I. Willimann K. Schaerli P. Hunziker T. Clark-Lewis I. Moser B. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J Exp Med. 2001;194:855–861. doi: 10.1084/jem.194.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano KF. Nakamura K. Kusano H. Nishii N. Banba M. Ikeda T. Hashimoto K. Yamamoto M. Fujio H. Miura A. Ohta K. Morita H. Saito H. Emori T. Nakamura Y. Kusano I. Ohe T. Significance of the level of monocyte chemoattractant protein-1 in human atherosclerosis. Circ J. 2004;68:671–676. doi: 10.1253/circj.68.671. [DOI] [PubMed] [Google Scholar]
- Leonard EJ. Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. [DOI] [PubMed] [Google Scholar]
- Lichtman AH. Abbas AK. T-cell subsets: recruiting the right kind of help. Curr Biol. 1997;7:242–244. doi: 10.1016/s0960-9822(06)00111-4. [DOI] [PubMed] [Google Scholar]
- Lim SP. Garzino-Demo A. The human immunodeficiency virus type 1 Tat protein up-regulates the promoter activity of the beta-chemokine monocyte chemoattractant protein 1 in the human astrocytoma cell line U-87 MG: role of SP-1, AP-1, and NF-kappaB consensus sites. J Virol. 2000;74:1632–1640. doi: 10.1128/jvi.74.4.1632-1640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima G. Soto-Vega E. Atisha-Fregoso Y. Sánchez-Guerrero J. Vallejo M. Vargas-Alarcón G. Llorente L. MCP-1, RANTES, and SDF-1 polymorphisms in Mexican patients with systemic lupus erythematosus. Hum Immunol. 2007;68:980–985. doi: 10.1016/j.humimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Lin CI. Chen CN. Chen JH. Lee H. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J Cell Biochem. 2006;99:1216–1232. doi: 10.1002/jcb.20963. [DOI] [PubMed] [Google Scholar]
- Lu B. Rutledge BJ. Gu L. Fiorillo J. Lukacs NW. Kunkel SL. North R. Gerard C. Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–618. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkowski J. Bujacz G. Boque L. Domaille PJ. Handel TM. Wlodawer A. The structure of MCP-1 in two crystal forms provides a rare example of variable quaternary interactions. Nat Struct Biol. 1997;4:64–69. doi: 10.1038/nsb0197-64. [DOI] [PubMed] [Google Scholar]
- Maghazachi AA. Al Aoukaty A. Schall TJ. C–C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J Immunol. 1994;153:4969–4977. [PubMed] [Google Scholar]
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- Matsushima K. Larsen CG. DuBois GC. Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S. Bernassau JM. Guillemot JC. Ferrara P. Darbon H. Determination of the three-dimensional structure of CC chemokine monocyte chemoattractant protein 3 by 1H two-dimensional NMR spectroscopy. Biochemistry. 1997;36:4412–4422. doi: 10.1021/bi9627929. [DOI] [PubMed] [Google Scholar]
- Milligan ED. Twining C. Chacur M. Biedenkapp J. O'Connor K. Poole S. Tracey K. Martin D. Maier SF. Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S. Kuwano T. Tsutsumi-Miyahara C. Ueda S. Kimura YN. Hamano S. Sonoda KH. Saijo Y. Nukiwa T. Strieter RM. Ishibashi T. Kuwano M. Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki M. Kawashima S. Yamashita T. Ozaki M. Hirase T. Ishida T. Inoue N. Hirata K. Matsukawa A. Morishita R. Kaneda Y. Yokoyama M. Local overexpression of monocyte chemoattractant protein-1 at vessel wall induces infiltration of macrophages and formation of atherosclerotic lesion: synergism with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22:115–120. doi: 10.1161/hq0102.102278. [DOI] [PubMed] [Google Scholar]
- Nanua S. Zick SM. Andrade JE. Sajjan US. Burgess JR. Lukacs NW. Hershenson MB. Quercetin blocks airway epithelial cell chemokine expression. Am J Respir Cell Mol Biol. 2006;35:602–610. doi: 10.1165/rcmb.2006-0149OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K. Ueno M. Satoh T. Nomiyama H. Tei H. Takeda M. Ledbetter DH. Coillie EV. Opdenakker G. Gunge N. Sakaki Y. Iio M. Miura R. A YAC contig of the human CC chemokine genes clustered on chromosome 17q11.2. Genomics. 1996;34:236–240. doi: 10.1006/geno.1996.0274. [DOI] [PubMed] [Google Scholar]
- Navratilova Z. Mrazek F. Kriegova E. Hutyrova B. Kolek V. du Bois RM. Petrek M. The MCP-1–2518 (A to G) single nucleotide polymorphism in Czech patients with pulmonary sarcoidosis: association with Löfgren's syndrome. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:33–38. [PubMed] [Google Scholar]
- Ni W. Egashira K. Kitamoto S. Kataoka C. Koyanagi M. Inoue S. Imaizumi K. Akiyama C. Nishida KI. Takeshita A. New anti-monocyte chemoattractant protein-1 gene therapy attenuates atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2001;103:2096–2101. doi: 10.1161/01.cir.103.16.2096. [DOI] [PubMed] [Google Scholar]
- Nonomura Y. Nagasaka K. Hagiyama H. Sekine C. Nanki T. Tamamori-Adachi M. Miyasaka N. Kohsaka H. Direct modulation of rheumatoid inflammatory mediator expression in retinoblastoma protein-dependent and -independent pathways by cyclin-dependent kinase 4/6. Arthritis Rheum. 2006;54:2074–2083. doi: 10.1002/art.21927. [DOI] [PubMed] [Google Scholar]
- Ogier C. Creidy R. Boucraut J. Soloway PD. Khrestchatisky M. Rivera S. Astrocyte reactivity to Fas activation is attenuated in TIMP-1 deficient mice, an in vitro study. BMC Neurosci. 2005;6:68. doi: 10.1186/1471-2202-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M. Kitadai Y. Tanaka S. Yoshihara M. Yasui W. Mukaida N. Haruma K. Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol. 2003;22:773–778. [PubMed] [Google Scholar]
- Palframan RT. Jung S. Cheng G. Weninger W. Luo Y. Dorf M. Littman DR. Rollins BJ. Zweerink H. Rot A. von Andrian UH. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IW. Wang JF. Groopman JE. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood. 2001;97:352–358. doi: 10.1182/blood.v97.2.352. [DOI] [PubMed] [Google Scholar]
- Picker LJ. Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- Polverini PJ. Cotran PS. Gimbrone MA., Jr Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Quinones MP. Ahuja SK. Jimenez F. Schaefer J. Garavito E. Rao A. Chenaux G. Reddick RL. Kuziel WA. Ahuja SS. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J Clin Invest. 2004;113:856–866. doi: 10.1172/JCI20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragin AB. Wu Y. Storey P. Cohen BA. Edelman RR. Epstein LG. Monocyte chemoattractant protein-1 correlates with subcortical brain injury in HIV infection. Neurology. 2006;66:1255–1257. doi: 10.1212/01.wnl.0000208433.34723.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantapaa-Dahlqvist S. Boman K. Tarkowski A. Hallmans G. Up regulation of monocyte chemoattractant protein-1 expression in anti-citrulline antibody and immunoglobulin M rheumatoid factor positive subjects precedes onset of inflammatory response and development of overt rheumatoid arthritis. Ann Rheum Dis. 2007;66:121–123. doi: 10.1136/ard.2006.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza M. Ballering JG. Hayden JM. Robbins RA. Hoyt JC. Doxycycline decreases monocyte chemoattractant protein-1 in human lung epithelial cells. Exp Lung Res. 2006;32:15–26. doi: 10.1080/01902140600691399. [DOI] [PubMed] [Google Scholar]
- Reid C. Rushe M. Jarpe M. van Vlijmen H. Dolinski B. Qian F. Cachero TG. Cuervo H. Yanachkova M. Nwankwo C. Wang X. Etienne N. Garber E. Bailly V. de Fougerolles A. Boriack-Sjodin PA. Structure activity relationships of monocyte chemoattractant proteins in complex with a blocking antibody. Protein Eng Des Sel. 2006;19:317–324. doi: 10.1093/protein/gzl015. [DOI] [PubMed] [Google Scholar]
- Rogge L. Barberis-Maino L. Biffi M. Passini N. Presky DH. Gubler U. Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Ruffini PA. Morandi P. Cabioglu N. Altundag K. Cristofanilli M. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392–2404. doi: 10.1002/cncr.22706. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yamashita Y. Shigematsu K. Yamashita K. Niwa M. Expression of MCP-1 in the hippocampus of SHRSP with ischemia-related delayed neuronal death. Cell Mol Neurobiol. 2006;26:823–831. doi: 10.1007/s10571-006-9077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem ML. El-Naggar SA. Kadima A. Gillanders WE. Cole DJ. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24:5119–5132. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Sallusto F. Lenig D. Mackay CR. Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SK. Crean SM. Boxer PA. Kellner D. LaRosa GJ. Hunt SW., 3rd Functional differences between monocyte chemotactic protein-1 receptor A and monocyte chemotactic protein-1 receptor B expressed in a Jurkat T cell. J Immunol. 2000;165:4877–4883. doi: 10.4049/jimmunol.165.9.4877. [DOI] [PubMed] [Google Scholar]
- Sartipy P. Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. Kuratsu J. Takeshima H. Yoshimura T. Ushio Y. Expression of monocyte chemoattractant protein-1 in meningioma. J Neurosurg. 1995;82:874–878. doi: 10.3171/jns.1995.82.5.0874. [DOI] [PubMed] [Google Scholar]
- Schaper J. Konig R. Franz D. Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. A combined SEM and TEM study. Virchows Arch A Pathol Anat Histol. 1976;370:193–205. doi: 10.1007/BF00427580. [DOI] [PubMed] [Google Scholar]
- Scholz D. Cai WJ. Schaper W. Arteriogenesis, a new concept of vascular adaptation in occlusive disease. Angiogenesis. 2001;4:247–257. doi: 10.1023/a:1016094004084. [DOI] [PubMed] [Google Scholar]
- Scholz D. Ito W. Fleming I. Deindl E. Sauer A. Wiesnet M. Busse R. Schaper J. Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- Sheng WS. Hu S. Lokensgard JR. Peterson PK. U50,488 inhibits HIV-1 Tat-induced monocyte chemoattractant protein-1 (CCL2) production by human astrocytes. Biochem Pharmacol. 2003;65:9–14. doi: 10.1016/s0006-2952(02)01480-6. [DOI] [PubMed] [Google Scholar]
- Shokawa T. Yoshizumi M. Yamamoto H. Omura S. Toyofuku M. Shimizu Y. Imazu M. Kohno N. Induction of heme oxygenase-1 inhibits monocyte chemoattractant protein-1 mRNA expression in U937 cells. J Pharmacol Sci. 2006;100:162–166. doi: 10.1254/jphs.sc0040188. [DOI] [PubMed] [Google Scholar]
- Sorensen TL. Ransohoff RM. Strieter RM. Sellebjerg F. Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur J Neurol. 2004;11:445–449. doi: 10.1111/j.1468-1331.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- Sozzani S. Introna M. Bernasconi S. Polentarutti N. Cinque P. Poli G. Sica A. Mantovani A. MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression. J Leukoc Biol. 1997;62:30–33. doi: 10.1002/jlb.62.1.30. [DOI] [PubMed] [Google Scholar]
- Sozzani S. Molino M. Locati M. Luini W. Cerletti C. Vecchi A. Mantovani A. Receptor-activated calcium influx in human monocytes exposed to monocyte chemotactic protein-1 and related cytokines. J Immunol. 1993;150:1544–1553. [PubMed] [Google Scholar]
- Spoettl T. Hausmann M. Herlyn M. Gunckel M. Dirmeier A. Falk W. Herfarth H. Schoelmerich J. Rogler G. Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes. Clin Exp Immunol. 2006;145:190–199. doi: 10.1111/j.1365-2249.2006.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford TJ. Kunkel SL. Phan SH. Rollins BJ. Strieter RM. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266:9912–9918. [PubMed] [Google Scholar]
- Sun JH. Yang B. Donnelly DF. Ma C. LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Szalai C. Duba J. Prohaszka Z. Kalina A. Szabo T. Nagy B. Horváth L. Császár A. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1 −2518 G/G genotype in CAD patients. Atherosclerosis. 2001;158:233–239. doi: 10.1016/s0021-9150(01)00423-3. [DOI] [PubMed] [Google Scholar]
- Takaishi H. Taniguchi T. Takahashi A. Ishikawa Y. Yokoyama M. High glucose accelerates MCP-1 production via p38 MAPK in vascular endothelial cells. Biochem Biophys Res Commun. 2003;305:122–128. doi: 10.1016/s0006-291x(03)00712-5. [DOI] [PubMed] [Google Scholar]
- Tanuma N. Sakuma H. Sasaki A. Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol (Berl) 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- Tawfik VL. Lacroix-Fralish ML. Bercury KK. Nutile-McMenemy N. Harris BT. Deleo JA. Induction of astrocyte differentiation by propentofylline increases glutamate transporter expression in vitro: heterogeneity of the quiescent phenotype. Glia. 2006;54:193–203. doi: 10.1002/glia.20365. [DOI] [PubMed] [Google Scholar]
- Thacker MA. Clark AK. Marchand F. McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- Toba H. Shimizu T. Miki S. Inoue R. Yoshimura A. Tsukamoto R. Sawai N. Kobara M. Nakata T. Calcium channel blockers reduce angiotensin II-induced superoxide generation and inhibit lectin-like oxidized low-density lipoprotein receptor-1 expression in endothelial cells. Hypertens Res. 2006;29:105–116. doi: 10.1291/hypres.29.105. [DOI] [PubMed] [Google Scholar]
- Trauzold A. Siegmund D. Schniewind B. Sipos B. Egberts J. Zorenkov D. Emme D. Röder C. Kalthoff H. Wajant H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- Tucci M. Barnes EV. Sobel ES. Croker BP. Segal MS. Reeves WH. Richards HB. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 2004;50:1842–1849. doi: 10.1002/art.20266. [DOI] [PubMed] [Google Scholar]
- Twining CM. Sloane EM. Milligan ED. Chacur M. Martin D. Poole S. Marsh H. Maier SF. Watkins LR. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain. 2004;110:299–309. doi: 10.1016/j.pain.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Valkovic T. Dobrila F. Melato M. Sasso F. Rizzardi C. Jonjic N. Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch. 2002;440:583–588. doi: 10.1007/s004280100458. [DOI] [PubMed] [Google Scholar]
- Van Coillie E. Van Damme J. Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- van Furth R. Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R. Diesselhoff-den Dulk MC. Mattie H. Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med. 1973;138:1314–1330. doi: 10.1084/jem.138.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R. Diesselhoff-den Dulk MM. Method to prove investigation of particles by macrophages with light microscopy. Scand J Immunol. 1980;12:265–269. doi: 10.1111/j.1365-3083.1980.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Wagner W. Roderburg C. Wein F. Diehlmann A. Frankhauser M. Schubert R. Eckstein V. Ho AD. Molecular and Secretory Profiles of Human Mesenchymal Stromal Cells and their Abilities to Maintain Primitive Hematopoietic Progenitors. Stem Cells. 2007;25:2638–2647. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- Wang J. Ou ZL. Hou YF. Luo JM. Shen ZZ. Ding J. Shao ZM. Enhanced expression of Duffy antigen receptor for chemokines by breast cancer cells attenuates growth and metastasis potential. Oncogene. 2006;25:7201–7211. doi: 10.1038/sj.onc.1209703. [DOI] [PubMed] [Google Scholar]
- Weiss L. Si-Mohamed A. Giral P. Castiel P. Ledur A. Blondin C. Kazatchkine MD. Haeffner-Cavaillon N. Plasma levels of monocyte chemoattractant protein-1 but not those of macrophage inhibitory protein-1alpha and RANTES correlate with virus load in human immunodeficiency virus infection. J Infect Dis. 1997;176:1621–1624. doi: 10.1086/517341. [DOI] [PubMed] [Google Scholar]
- Wells T. Power CA. Lusti-Narasimhan M. Hoogewerf AJ. Cooke RM. Chung CW. Peitsch MC. Proudfoot AE. Selectivity and antagonism of chemokine receptors. J Leukoc Biol. 1996;59:53–60. doi: 10.1002/jlb.59.1.53. [DOI] [PubMed] [Google Scholar]
- White FA. Sun J. Waters SM. Ma C. Ren D. Ripsch M. Steflik J. Codright DN. Lamotte RH. Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VY. Walz DA. McCoy LE. Purification and characterization of human and bovine platelet factor 4. Prep Biochem. 1977;7:479–493. doi: 10.1080/00327487708065515. [DOI] [PubMed] [Google Scholar]
- Xia Y. Frangogiannis NG. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm Allergy Drug Targets. 2007;6:101–107. doi: 10.2174/187152807780832265. [DOI] [PubMed] [Google Scholar]
- Yang X. Murthy V. Schultz K. Tatro JB. Fitzgerald KA. Beasley D. Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H2334–2343. doi: 10.1152/ajpheart.00252.2006. [DOI] [PubMed] [Google Scholar]
- Yeo TK. Ahad MA. Kuo NW. Spagnolo P. Menezo V. Lympany P. Lightman S. Chemokine gene polymorphisms in idiopathic anterior uveitis. Cytokine. 2006;35:29–35. doi: 10.1016/j.cyto.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Robinson EA. Tanaka S. Appella E. Leonard EJ. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989a;142:1956–1962. [PubMed] [Google Scholar]
- Yoshimura T. Yuhki N. Moore SK. Appella E. Lerman MI. Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen- stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989b;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- Zachariae CO. Anderson AO. Thompson HL. Appella E. Mantovani A. Oppenheim JJ. Matsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990;171:2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Rollins BJ. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Mol Cell Biol. 1995;15:4851–4855. doi: 10.1128/mcb.15.9.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC. Coleman GD. Mankowski JL. Adams RJ. Tarwater PM. Fox K. Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- Zlotnik A. Yoshie O. Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7:243. doi: 10.1186/gb-2006-7-12-243. [DOI] [PMC free article] [PubMed] [Google Scholar]