Abstract
Handedness refers to a consistent asymmetry in skill or preferential use between the hands and is related to lateralization within the brain of other functions such as language. Previous twin studies of handedness have yielded inconsistent results resulting from a general lack of statistical power to find significant effects. Here we present analyses from a large international collaborative study of handedness (assessed by writing/drawing or self report) in Australian and Dutch twins and their siblings (54,270 individuals from 25,732 families). Maximum likelihood analyses incorporating the effects of known covariates (sex, year of birth and birth weight) revealed no evidence of hormonal transfer, mirror imaging or twin specific effects. There were also no differences in prevalence between zygosity groups or between twins and their singleton siblings. Consistent with previous meta-analyses, additive genetic effects accounted for about a quarter (24.64%) of the variance (95% CI 20.17, 27.09%) with the remainder accounted for by non-shared environmental influences. The implications of these findings for handedness both as a primary phenotype and as a covariate in linkage and association analyses are discussed.
Keywords: laterality, behavioral genetics, left-handed, extended twin family design, asymmetry
Introduction
Handedness is first demonstrated between 9–10 weeks gestation, as embryos begin to exhibit single arm movements (Hepper, McCartney, & Shannon, 1998). The archaeological record of cultural and skeletal remains provides evidence of population level biases towards right-handedness in early humans (Steele, 2000; Toth, 1985). It has been hypothesized that lateralized behaviors either arose de novo in early homo sapiens (Annett, 2002; Corballis, 1997; McManus, 2002) or evolved from ancestral population level behavioral asymmetries (Vallortigara & Rogers, 2005). From a neuropsychological perspective, lateralization in the form of hand or foot preference remains the best behavioral predictor of cerebral lateralization. Left-hemisphere language dominance is reported in approximately 95% of right-handers and 70% of left-handers (Elias & Bryden, 1998; Pujol, Deus, Losilla, & Capdevila, 1999) and behavioral laterality has also been found to predict emotional lateralization (Elias, Bryden, & Bulman-Fleming, 1998).
Although there is evidence that behavioral laterality develops prenatally (Hepper, Wells, & Lynch, 2005), the extent to which this population level bias can be explained by genetic effects has been the topic of much debate. One method by which this may be explored is through the comparison of relatives who differ in the amount of genetic information they share. Twin studies, in which the similarity of identical (monozygotic; MZ) and non-identical (dizygotic; DZ) twin pairs are compared, provide estimates of the relative magnitude of genetic and environmental influences and have proved popular in studying behavioral laterality. Since the first study by Siemens (1924) there have been thirty-seven twin studies of handedness published (for reviews see McManus, 1980; Medland, Duffy, Wright, Geffen, & Martin, 2006; Sicotte, Woods, & Mazziotta, 1999).
Unfortunately, the results have been mixed and as handedness is typically analyzed as a binary trait (left or non-right vs right) the issue of sample size is nontrivial. For example, for a trait with a 10% prevalence (which is typical of left-handedness) where 30% of the variance is accounted for by an additive genetic effect, about 1000 pairs of twins would be required to reject a purely unique environmental model with 80% power (Neale, Eaves, & Kendler, 1994). Larger samples are required to distinguish between genetic and shared environmental influences (Neale, Eaves, & Kendler, 1994). However, the median sample size of the 35 studies reviewed by Medland et al (2006) was 189 pairs indicating a general lack of statistical power due to small sample sizes within the literature. Thus, with few exceptions (Basso et al., 2000; Medland et al., 2003; Neale, 1988; Orlebeke, Knol, Koopmans, Boomsma, & Bleker, 1996; Ross, Jaffe, Collins, Page, & Robinette, 1999), sample sizes have not been adequate to detect genetic or environmental effects that account for less than 50% of the total phenotypic variance with 80% power.
The aim of the present study was to characterize the heritability of hand preference (defined as writing/drawing hand or self reported preference) in a large genetically informative sample. To this end we used data from 54,270 twins and their non-twin siblings from 25,732 Australian and Dutch twin families. Previous twin studies of handedness have typically only compared the similarity of mono- and dizygotic twins. By using an extended twin and sibling design, the present study allowed tests of special twin effects both on the prevalence of left handedness and the covariation between siblings, thus also providing a test of the generalizability of these findings to the general population.
Behavioral laterality may be modified by cultural and environmental effects (Laland, Kumm, Van Horn, & Feldman, 1995) potentially masking genetic effects. Within western cultures, the prevalence of left-handedness (as defined by writing-hand) has gradually increased over the last century from around 2% in 1900 to between 10–15% in more recent samples (1990–2000) (Annett, 2002; McManus, 2002; Perelle & Ehrman, 1994). While cultural pressures have been hypothesized to decrease the prevalence of left-handedness, exposure to adverse environments and pathogenic insults has been hypothesized to increase the prevalence of left-handedness (Satz, Orsini, Saslow, & Henry, 1985). In addition, subtle neurological insults may also result in lasting changes in hand preference without deficits in other neuropsychological domains (Triggs, Tesar, & Yong, 1998). A wide range of pathogenic risk factors have been proposed, including, low birth weight, birth stress and ultrasound exposure (Bailey & McKeever, 2004; Bakan, Dibb, & Reid, 1973; Salvesen, 2002). Previous studies have typically found that lower birth weights were associated with higher rates of left-handedness (Hay & Howie, 1980; Orlebeke, Knol, Koopmans, Boomsma, & Bleker, 1996; Powls, Botting, Coooke, & Marlow, 1996). To account for these effects, birth cohort (year of birth) and birth weight were included as covariates in the current study.
Methods
Participants and Measures
The data were collected within a number of twin studies conducted in Australia and the Netherlands. The focus of these studies, the number of participants, the method of data collection and method of zygosity determination are summarized in Table 1. In the Netherlands twin registry Older twins study, self classification (left-handed, right handed or either) was used to determine handedness. In the Australian Twin ADHD Project and the Younger Netherlands twin study handedness was assessed by asking which hand is used for drawing. In all other studies, handedness was assessed as the hand used for writing. Following Annett (2002) and McManus (2002) reports of mixed handedness or ambidexterity, which were less than 1% of total reports, were classed as left handed. Previous studies have shown self report (left-handed, right handed or either) and hand used for writing (left vs. right) to be highly correlated .97 (data from Perelle & Ehrman, 1994). Similarly, drawing hand and writing hand are highly correlated .97 (estimated from parental report of drawing hand from the Australian Twin ADHD Project and self-reported writing hand from the Brisbane adolescent study as described below).
Table 1.
Sample Description (studies are listed in the order they joined the collaborative study). Note all samples were population based rather than samples selected on the trait of interest, for example the ADHD study collected data from all available participants regardless of ADHD status.
| Sample | Focus | Data collection | Zygosity type* | N Excluding overlaps | |
|---|---|---|---|---|---|
| Individuals | Families | ||||
| Brisbane Adolescent Twins - Memory Attention & problem solving Study (Wright et al., 2001) | Cognition and personality | Questionnaire completed during a data collection session | Confirmed by DNA | 2,515 (twins & sibs) | 1,033 |
| Australian Twin ADHD Project (Bennett et al., 2006) | Behavioral disorders of childhood (focus on ADHD) | Mailed Questionnaire completed by the parents | Questionnaires completed by the parents | 12,263 (twins & sibs) | 4,549 |
| Adult Australian twins -Sex Study (Kirk, Bailey, Dunne, & Martin, 2000) | Sexual orientation | Anonymous Mailed Questionnaire (Twins created a 10 digit ID to allow matching) | Pre- determined zygosity from questionnaires completed by the twins was pre-printed on the questionnaire | 4,791 (twins only) | 2,918 |
| Adult Australian twins - Asthma Study (Duffy, Mitchell, & Martin, 1998) | Asthma & Atopy | Mailed Questionnaire | Questionnaires completed by the twins, subsequently confirmed by DNA | 2,165 (twins only) | 1,265 |
| Younger Netherlands twin registry (Boomsma et al., 2002) | Longitudinal study of cognitive & physical development | Mailed Questionnaire completed by the parents | Questionnaires completed by the parents, subsequently confirmed by DNA for some twins | 16,836 (twins & sibs) | 8,425 |
| Adult Australian female MZ twins - Laterality Study (Medland et al., 2005) | Laterality - collecting phenotypes from genotyped individuals | Mailed Questionnaire | Confirmed by DNA | 286 (twins only) | 169 |
| South Australian twin study (Townsend, Richards, Hughes, Pinkerton, & Schwerdt, 2003) | Longitudinal study of dental development | Direct observation during a data collection session | Questionnaires completed by the parents | 1,131 (twins & sibs) | 567 |
| Older twins- Netherlands twin registry (Boomsma et al., 2002) | Broad longitudinal study assessing health, behavior and personality | Mailed Questionnaire | Questionnaires completed by the twins, subsequently confirmed by DNA for some twins | 10,235 (twins & sibs) | 4,229 |
| Twins Eye Study (Toh et al., 2005) | Corneal thickness & other ophthalmological traits | Direct observation during a data collection session | Confirmed by DNA | 392 (twins only) | 219 |
| Victorian Cancer Council - Young Adult Twin Study (White, Hopper, Wearing, & Hill, 2003) | Health and lifestyle cancer risk factors | Mailed Questionnaire | Questionnaires completed by the twins, subsequently confirmed by DNA for some twins | 1,818 (twins only) | 1,085 |
| Adult Australian twins - Male Pattern Baldness study (Nyholt, Gillespie, Heath, & Martin, 2003) | Laterality - collecting phenotypes from genotyped individuals | Mailed Questionnaire | Confirmed by DNA | 75 (twins only) | 44 |
| Adult Australian twins - Gambling study http://genepi.qimr.edu.au/studies/ga/?studycode=GA | Gambling behaviors and personality traits | Telephone Questionnaire | Questionnaires completed by the twins, subsequently confirmed by DNA for some twins | 1,421 (twins only) | 1,044 |
| Adult Australian twins - Osteoarthritis Study (Kirk et al., 2002) | Osteoarthritis | Mailed Questionnaire | Questionnaires completed by the twins, subsequently confirmed by DNA for some twins | 104 (twins only) | 57 |
| Adult Australian twins - Alcohol challenge study (Martin et al., 1985) | Metabolism of Alcohol | Direct observation during a data collection session | Questionnaires completed by the twins, subsequently confirmed by DNA for some twins | 238 (twins only) | 124 |
| Total | 54,270 (twins & sibs) | 25,723 | |||
Questionnaire methods of determining zygosity have previously demonstrated at least 95% agreement with DNA confirmation (Martin, 1975; Ooki, Yamada, Asaka, & Hayakawa, 1990)
As the majority of Australian studies recruited twins from the Australian Twin Registry which uses a centralized identification number system we were able to check for overlap between the Australian studies. When an individual had participated in more than one study or wave of data collection the most recent report was used (as described in the following paragraph we used the multiple reports of hand preference to assess the reliability of the measure). For one of the Australian studies (the Sex study) we were only able to identify the individuals who had returned a consent form, as the data in this survey were collected anonymously. To account for this we excluded the data from individuals who had returned a consent form for this study from any other data set (1215 individuals).
The large number of participants who contributed on multiple occasions within the Australian data set, and the longitudinal nature of the Netherlands adult twin study, afford an excellent opportunity to assess the test-retest reliability of hand-preference. Within the Australian data 1509 individuals reported their hand preference twice, while an additional 256 individuals reported their hand preference three times. The polychoric correlation between the multiple reports was .994 indicating the high reliability of self reported hand preference. Within the Netherlands adult twin study test-retest data were available for 6361 individuals (2948 reported twice, 1863 three times, 1206 four times, and 344 five times). As in the Australian data the polychoric correlation between the multiple reports of .993 supported the high reliability of this measure, which has been previously demonstrated to remain virtually unchanged in the absence of injury or insult (Liederman & Healey, 1986; Raczkowski, Kalat, & Nebes, 1974).
In addition, parent and self-reported handedness was available for 60 pairs of twins who had participated in both the Brisbane adolescent study and Australian Twin ADHD Project. The polychoric correlation between parent and self-reported handedness of .970 indicates the high reliability of parental report in these data (which may be expected as twins are allowed to help the parents complete the questionnaires). These results suggest that parental report is a valid method of data collection and comparable with self-report in terms of accuracy when the measures of handedness are salient. Based on these results it was decided that parental reports collected in the Australian Twin ADHD Project and younger Netherlands twin studies could be used to assess the handedness of their offspring.
Statistical Analyses
To model the binary hand preference data we employed the multifactorial threshold model which describes discrete traits as reflecting an underlying normal distribution of liability (or predisposition). Liability, which represents the sum of all the multifactorial effects, is assumed to reflect the combined effects of a large number of genes and environmental factors each of small effect and is characterized by phenotypic discontinuities that occur when the liability reaches a given threshold (Neale & Cardon, 1992). The distribution of hand preference assessed for multiple items is J-shaped. However, for self classification or writing hand the distribution is effectively binary. It is not difficult to conceptualize hand preference as reflecting the continuous and normally distributed measure of relative hand skill with a mean shifted towards the right as measured by a peg moving task (Annett, 1985).
All data analyses were conducted using maximum likelihood analyses of raw data within Mx (Neale, Boker, Xie, & Maes, 2006) which maximise the natural log of the following likelihood of the data:
with respect to Σi and μ, where k is the number of data observations for family i (which in this case is equal to the number of siblings for whom data is collected), Σi is the expected covariance matrix among the variables for family i, yi is a vector of observed scores obtained for the k variables for family i, μi is the vector of expected means for family i, and M is the number families. Corrections for known covariates, Sex, Year of Birth (both linear and quadratic effects), and birth weight were included with the threshold models in all data analyses. Year of birth ranged from 1906 to 2002 (median 1981) in the Australian data and from 1914 to 1998 (median 1989) in the Dutch data. To avoid computational difficulties year of birth was rescaled by subtracting 1950 and dividing by 10. Birth weight ranged from 454 to 5675g (mean 2647.76, sd 604) in the Australian data and from 580 to 5500g (mean 2594.60, sd 580) in the Dutch data. Birth weight was converted to a z-score before analysis.
As the majority of handedness studies have focused on prevalence effects, we undertook an extensive series of preliminary analyses to test these effects. In these tests we used a likelihood ratio chi-square test (LRT) to compare the fit (minus twice log-likelihood) of a model to that of a nested model in which constraints had been imposed (with the degrees of freedom equal to the change in the number of estimated parameters). We begin by testing for heterogeneity between studies and then between the different zygosity groups as described below and in tables 2 and 3.
Table 2.
Tests for heterogeneity between studies in the prevalence of left handedness and co-twin correlations after correcting for year of birth (linear and quadratic trends) and birth weight.
| Differences in Prevalence | Differences in Co-twin correlations | |||||
|---|---|---|---|---|---|---|
| Δχ2 | df* | p-value | Δχ2 | df* | p-value | |
| Australian Data | ||||||
| MZ Female | 21.385 | 20 | .375 | 13.068 | 10 | .220 |
| MZ Male | 16.855 | 20 | .662 | 1.229 | 10 | .999 |
| DZ Female | 21.512 | 18 | .254 | 12.922 | 9 | .166 |
| DZ Male | 23.279 | 20 | .275 | 12.285 | 10 | .266 |
| DZ Opposite-sex | 20.331 | 18 | .314 | 8.069 | 9 | .527 |
| Netherlands Data | ||||||
| MZ Female | 1.66 | 2 | .436 | .180 | 1 | .671 |
| MZ Male | 3.654 | 2 | .161 | .839 | 1 | .360 |
| DZ Female | 4.287 | 2 | .117 | 3.352 | 1 | .067 |
| DZ Male | 3.653 | 2 | .160 | .040 | 1 | .841 |
| DZ Opposite-sex | 5.562 | 2 | .062 | .980 | 1 | .322 |
The difference in df between zygosity groups among the Australian data reflects the fact that the Laterality study only collected data from female MZ twins and the male pattern baldness study collected data from MZ and DZ male twins
Table 3.
Tests for heterogeneity between zygosity groups in the prevalence of left handedness and co-twin correlations after correcting for year of birth (linear and quadratic trends) and birth weight.
| df | Australian Data | Netherlands Data | |||
|---|---|---|---|---|---|
| Δχ2 | p-value | Δχ2 | p-value | ||
| Testing of differences in the prevalence of left handedness between … | |||||
| First and second born twins (in same sex pairs) | 4 | 2.069 | .723 | 4.493 | .343 |
| MZ and DZ twins (in same sex pairs separately for males and females) | 2 | 1.533 | .465 | 1.691 | .429 |
| Same sex and opposite sex pairs (separately for males and females) | 2 | 5.90 | .052 | 1.819 | .403 |
| Twins and their non-twin siblings | 1 | 3.064 | .080 | 2.895 | .089 |
| Testing the effects of covariates on the prevalence of left handedness… | |||||
| Quadratic birth cohort effect | 1 | 10.557 | .001 | 2.216 | .137 |
| Quadratic and linear birth cohort effect | 2 | 31.165 | 1.71*10−7 | 4.869 | .088 |
| Sex effect | 1 | 22.833 | 1.77*10−6 | 32.319 | 1.31*10−8 |
| Birth weight effects (linear) | 1 | 20.788 | 5.13*10−6 | 14.539 | 1.37*10−4 |
| Testing of differences in the co-twin correlations between … | |||||
| Same sex pairs (separately for MZ and DZ twins) | 2 | 2.216 | .330 | 2.811 | .245 |
| Same sex and opposite sex DZ twins | 1 | .156 | .693 | 1.779 | .182 |
| DZ twins and twin-sibling/sibling-sibling correlations | 1 | 1.368 | .242 | .443 | .506 |
| MZ and DZ/sibling correlations | 1 | 6.650 | .010 | 19.908 | 8.13*10−6 |
| Testing for Familial aggregation (Setting all co-twin and sibling correlations to zero) | 1 | 95.836 | 1.25*10−22 | 57.421 | 3.52*10−14 |
Heritability estimates were obtained using variance component modeling of the twin and sibling data. In these analyses the total variance (σ2P) was partitioned in additive genetic (σ2A), non-shared or unique environmental effects (σ2E), and three shared environmental effects: familial environment effects (σ2F), a shared twin effect (σ2T), and a shared non-twin sibling effect (σ2S). The total variance (which was constrained to unity) was thus parameterized as:
While the covariance terms were parameterized as:
The addition of the σ2T and σ2S terms allow for two types of special twin effects. While the σ2F allows environmental influences shared equally by all siblings, the σ2T term allows for the covariance of the DZ twins to be higher than that of non-twin siblings; a significant σ2T might reflect more similar treatment of the twins or a developmental age effect. Conversely, the σ2S term allows for the presence of a special twin effect decreasing the covariance of the twins relative to their non-twin siblings; a significant σ2S effect might reflect an increased rate of phenocopies among the twins as compared to their non-twin siblings as a result of an increased rate of birth complications or a twin mirror imaging effect.
Results
Preliminary prevalence and covariance analyses
As both the Australian and Dutch data had been collected within multiple studies we started by checking for differences between studies within zygosity groups (male and female monozgyotic (MZ) and dizygotic (DZ) twins, and opposite sex pairs). Modeling the Australian and Dutch data separately, the fit of the model in which the prevalence and the co-twin correlations were allowed to vary for each sample was compared to the fit of a model in which the prevalence was constrained to be the same across samples, the fit of this model was then compared to the fit of a model in which the co-twin correlations were constrained to be equal across samples. There were no differences in the prevalence or co-twin correlations among the data sets collected from different studies within zygosity and nationality groups (results given in Table 2). As this first series of analyses revealed no differences between studies within groups, we combined the data across studies and proceeded to test for differences in the prevalence and co-twin correlations across zygosity groups within the Australian and Dutch data.
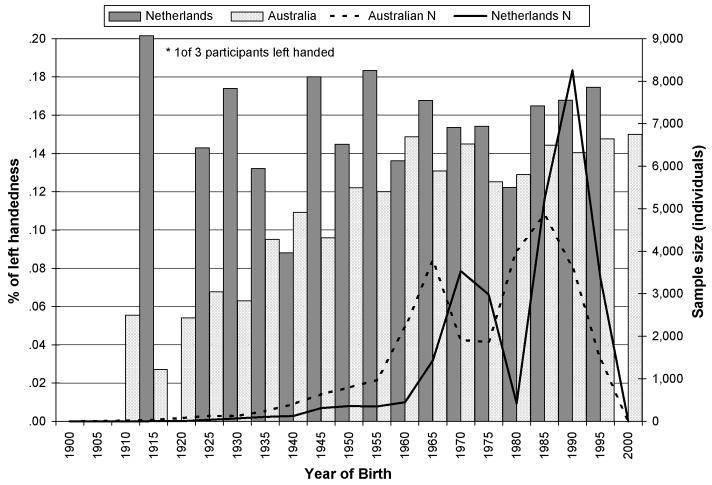
We then compared the fit of a series of increasing constrained models to test for differences in prevalence between zygosity groups, these was done separately for the Australian and Dutch data. This series of tests showed there were no differences in the prevalence of left handedness between: first and second born twins, MZ and DZ twins (by sex), twins born of same vs. opposite sex pairs (by sex) or between twins and their non-twin siblings (results given in Table 3). Thus, neither the Australian nor the Dutch data supported the presence of hormonal transfer effects (which would have led to differences between same and opposite sex twins; Elkadi, Nicholls, & Clode, 1999), or mirror imaging effects (which would have led to an increased prevalence of left handedness in MZ twins; Newman, 1928). The prevalence of left-handedness was greater in the Dutch than Australian sample and in both the Australian and Dutch data males were more likely to be left handed than females (raw prevalence of left handedness in females and males: Australian sample 12.5 and 14.6 %, Dutch sample 15.2 and 17.6%). To test for the effects of birth cohort were compared the fit of the model which included a correction for year of birth to a model in which this term was set to zero. As shown in Figure 1 the effects of birth cohort were highly significant in the Australian data but not in the Dutch sample which is predominantly younger.
Figure 1.
Prevalence of left handedness (shown by the bar graph) and sample size (number of individuals shown by the line graph) by year of birth for the Australian and Dutch samples. The prevalence of left handedness in the Dutch sample was truncated for the asterisked time period
Similarly, there were no sex differences in the co-twin correlations of same-sex pairs (within zygosity), nor between same and opposite sex DZ pairs, and the DZ co-twin correlations did not differ from the twin-sibling or sibling-sibling correlations (results given in Table 3). In both the Australian and the Dutch data the MZ and DZ correlations were significantly different indicating the presence of genetic effects. MZ co-twin correlations were low in both the Australian and Dutch data, .243 (95% confidence intervals .180–.304) and .241 (95%CI .182, .300) respectively, indicating a moderate familial contribution to hand preference. The magnitude of DZ co-twin correlations, .145 (95%CI .142, .188) and .070 (95%CI .019, .120) in the Australian and Dutch data, suggest genetic effects are the primary source of familial resemblance, or aggregation, for hand preference.
Heritability analyses
Variance components estimates were obtained from structural equation modeling of the raw hand preference data correcting for the effects of year of birth, birth weight and sex on the prevalence of left-handedness. The data from the Australian and Dutch samples were modeled separately allowing the prevalence and estimated variance components to differ between the two samples. We then fit a series of increasing constrained models, decreasing the number of freely estimated parameters in order to find the most parsimonious model that did not result in a significant loss of fit. As summarized in Table 4, the estimates of σ2A, σ2F, σ2T, σ2S, and σ2E could be equated between the two samples while allowing the prevalence of left-handedness to differ. Both twin (σ2T) and non-twin sibling (σ2S) effects could be dropped from the model without a significant loss of fit. In addition, the general shared family environment effect σ2F could also be dropped without a significant loss of fit. Conversely the additive genetic effect could not be dropped and an AE model in which all covariation between relatives was parameterized as being due to additive genetic effects provided a good fit to the data. As may be expected based on the co-twin correlations observed in this sample, the majority of variance 76.36% (95%CI 72.9, 79.8%) was accounted for by non-shared environmental influences with the remaining variance arising from additive genetic effects 24.64% (95%CI 20.2, 27.1%).
Table 4.
Results of the univariate genetic model fitting: model fitting summary and estimated variance components. Best fitting model is shaded. Abbreviations: A, additive genetic; F, familial genetic; T, twin genetic; S, sibling genetic, E, unique environmental. Fit of the saturated model in which separate estimates are estimated for the Australian (AUS) and Dutch (NL) data: -2LL 45200.745, df 54237.
| Model | Change in -2LL | Change in df | p-value | Variance components (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| A | F | T | S | E | ||||
| Separate estimates for the Australian (AUS) and Dutch (NL) data | AUS | .141 (.01,.29) | .049 (.04,.15) | .053 (.05,.07) | .069 (.06,.07) | .688 (.49,.82) | ||
| NL | .215 (.17,.26) | .000 (.00,.00) | .000 (.00,.00) | .000 (.00,.00) | .785 (.70,.83) | |||
| AFTSE | 4.124 | 5 | .532 | .236 (.15,.27) | .000 (.00,.00) | .000 (.00,.00) | .012 (.00,.02) | .7517 (.62,.80) |
| AFSE | 0 | 1 | 1.000 | .236 (.15,.27) | .000 (.00,.00) | - | .012 (.00,.02) | .7517 (.62,.80) |
| AFE | 0.038 | 1 | .303 | .237 (.15,27) | .000 (.00,.00) | - | - | .764 (.73,.80) |
| AE* | 0 | 1 | 1.000 | .237 (.20,27) | - | - | - | .764 (.73,.77) |
| FE* | 24.313 | 1 | .000 | - | .151 (.13,.18) | - | - | .849 (.72,.87) |
| E* | 178.549 | 2 | .000 | - | - | - | - | 1.00 |
These models are compared to the AFE model
Discussion
The heritability estimates from the current data are consistent with meta-analysis of data from 35 previous twin studies, which (when excluding the data used in the present study), also found an AE model to provide the best fit of the data and yielded estimates of 25.9% (95%CI 14.8, 29.9%) for the proportion of variance due to additive genetic effects and 74.2% (95%CI 70.1, 78.4%) for the unique environmental effects (Medland, Duffy, Wright, Geffen, & Martin, 2006). While the heritability of handedness in the current data is consistent with previous studies, the current analyses also yielded a number of important null results. There was no evidence of any special twin effects on either the prevalence of left handedness or the covariance between relatives. Thus, the present sample shows no evidence of hormonal transfer (the prevalence did not differ between twins born of same or opposite sex twin pairs) or mirror imaging (there were no differences in prevalence in MZ and DZ twins) and there was no evidence that twinning influenced handedness either through more similar environments or competition for resources. In addition, there was no evidence for sex differences in the covariance between relatives, nor significant family environment effects.
A number of competing ‘single gene’ models have been proposed within the literature (Annett, 1985; Crow, 2002; Klar, 1999; McManus, 1985). While we have not assessed the fit of these models, the polygenic model utilized here provided a good fit to the data. In addition, previous linkage analyses have identified a number of regions of interest including 2p12 q11, 17p11-q23 (Francks, DeLisi, Fisher et al., 2003; Francks et al., 2002), 10q26 (Van Agtmael, Forrest, & Williamson, 2002) and 12q21-23 (Warren, Stern, Duggirala, Dyer, & Almasy, 2006). Francks et al., (2003; 2007) have subsequently identified an imprinted gene, LRRTM1, within the 2p12-q11 region. The paternal copy of this gene is associated with both left-handedness and schizophrenia. An association has also been found between handedness and the X-linked Androgen receptor (Medland et al., 2005).
Significant birth cohort effects were identified in the Australian data and birth-weight effects were seen in both samples. However, the majority of variance was due to unique environmental influences even when correcting for these covariates. It is possible that there are other unidentified covariates that may potentially explain some of the high estimate of unique environmental influences. Another possible explanation lies with the assumption that there is no interaction between genetic and environmental effects. The standard univariate analyses described above assume homogeneity in the partitioning of variance within the sample. However, if interactions were present between the genetic and environmental effects, a single heritability estimate would be insufficient to describe the structure of the covariation within the data. Un-modeled interaction between genetic and non-shared environmental influences (GxE) would lead to over-estimates of the unique environmental effects (Eaves, Last, Martin, & Jinks, 1977). Future studies including pre and peri-natal risk variables such as birth stress, anoxia and ultra-sound exposure might help explain some of the unique environmental variance.
One of the advantages of these data for examining birth cohort effects was the wide range of year of birth which span almost a full century. The epoch related changes in prevalence of handedness in our sample are consistent with those reported elsewhere. For example, Levy (1974) found an increase in left-handedness from 2.2% in 1932 to 11.2% in 1972 within the United States following a parabolic curve. In Australia, Brackenridge (1981) found that the prevalence of left handedness increased from 2% in those born around 1890 to 10% in those born in 1930 and asymptotes to 13.2% in those born around 1970 following a sigmoid curve. Similarly, in the Netherlands, Beukelaar and Kroonenberg found the proportion of self-classified left-handers who wrote with their left hands increased from 0% in those born between 1900 and 1939 to 40% in those born between 1940 and 1944 and asymptotes to 100% in those born after 1965 (Beukelaar & Kroonenberg, 1986).
The main hypotheses proposed to account for these findings have focused on the cultural acceptance of left handedness and in particular the acceptance of left handed writing within class rooms. However, differential mortality of left- and right-handers (Halpern & Coren, 1988), adaptation to a right handed world (Porac & Coren, 1981) and changes in allele frequencies (McManus, 2002) have also been proposed.
The consistent finding of such systematic birth cohort effects across studies has important implications that should not be overlooked. Firstly, given that handedness itself is considered a covariate when analyzing both structural and functional brain asymmetries, and a wide range of neurological conditions including schizophrenia (Francks, DeLisi, Shaw et al., 2003; Orr, Cannon, Gilvarry, Jones, & Murray, 1999; Satz & Green, 1999; Shaw, Claridge, & Clark, 2001) and autism (Boucher & Birmingham University, 1977; Cornish & McManus, 1996; McManus, Murray, Doyle, & Baron-Cohen, 1992), these results suggest that the relationship between behavioral laterality (hand and foot preference) and cerebral laterality may be much more complicated than previously thought.
For example, many imaging studies select only right handed participants. However, given the high degree of cultural suppression within some cohorts it is possible that selection based on hand preference may lead to undesired heterogeneity within the sample. Conversely, it may be the case that left handedness in the presence of certain covariates, such as strong cultural suppression, may be more clinically meaningful than left handedness in general. Secondly, these results have important implications for linkage and association studies attempting to locate genes influencing hand preference or hand skill (which is usually measured as a continuous variable). The increase in power derived from the analysis of selected samples is based on the assumption of homogeneity of liability within the sample. However, given the results of the current analyses, linkage and association analyses of unselected samples would be recommended unless potential covariate effects can be well characterized within the data. In conclusion, we have analyzed the largest sample of twin and family data for hand preference collected to date (54,270 individuals from 25,732 families). Familial aggregation for hand preference was found to be consistent with additive genetic effects, which accounted for about a quarter of the variation in the trait.
Acknowledgments
The authors would like to thank the twins and their families for their valued participation and all those associated with data collection in the various studies.
In addition we would like to acknowledge the following funding sources: Queensland Institute of Medical Research data: NIH grants AA10249, AA07728, AA11998 from the U.S. National Institute on Alcohol Abuse and Alcoholism and DA12854 from the U.S. National Institute on Drug Abuse; Human Frontier Science Program (Grant Number RG0154/1998-b); the Australian National Health and Medical Research Council (NHMRC; Grant Numbers 921103, 941177); the Australian Research Council (Grant Numbers A79600334, A79906588, A79801419); the Government Employees’ Medical Research Fund (Australia), and a small Commonwealth AIDS Research Grant to N. G. M.
Netherlands Twin Registry data: This study was supported by The Netherlands Organization for Scientific Research (NWO-MW 904-61-193, NWO 575-25-006, NWO 904-61-090, NWO 985-10-002, NWO480-04-004), the Borderline Personality Disorder Research Foundation, NOW/SPINOZA 56-464-14192; NIMH, RO1 MH58799-03.
Adult Australian twins - Gambling study: National Institute of Mental Health grant MH66206
Australian Twin ADHD Project Data: NHMRC grants 11119 and 22905.
South Australian Twin Study Data: NHMRC Continuing Project Grant 980170
Victorian Cancer Council Data: NHMRC grant 262604 and funding from the Victorian Health Promotion Foundation (VicHealth).
Centre for Eye Research Data: NHMRC grant 350415 and the Ophthalmic Research Institute of Australia. DAM is the recipient of a Pfizer Australia research fellowship. In addition the authors would like to acknowledge the support of the Australian Twin Registry through an Enabling Grant from NHMRC.
SEM is supported by an Australian NHMRC Sidney Sax Fellowship (443036). DRN is supported by an NHMRC Fellowship (339462).
Statistical analyses were carried out using the Genetic Cluster Computer (http://www.geneticcluster.org) which is financially supported by the Netherlands Scientific Organization (NWO 480-05-003).
References
- Annett M. Left, Right, Hand and Brain: the Right Shift Theory. London: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- Annett M. Handedness and brain asymmetry: the right shift theory. Hove, East Sussex: Psychology Press; 2002. [Google Scholar]
- Bailey LM, McKeever WF. A large-scale study of handedness and pregnancy/birth risk events: Implications for genetic theories of handedness. Laterality: Asymmetries of Body, Brain & Cognition. 2004;9(2):175–188. doi: 10.1080/13576500342000013. [DOI] [PubMed] [Google Scholar]
- Bakan P, Dibb G, Reid P. Handedness and birth stress. Neuropsychologia. 1973;11:363–366. doi: 10.1016/0028-3932(73)90050-x. [DOI] [PubMed] [Google Scholar]
- Basso O, Olsen J, Holm NV, Skytthe A, Vaupel JW, Christensen K. Handedness and mortality: a follow-up study of Danish twins born between 1900 and 1910. Epidemiology. 2000;11(5):576–580. doi: 10.1097/00001648-200009000-00014. [DOI] [PubMed] [Google Scholar]
- Bennett KS, Hay D, Piek J, Pearsall-Jones J, Levy F, Martin N. The Australian Twin ADHD Project: Current status and future directions. Twin Research and Human Genetics. 2006;9:718–726. doi: 10.1375/183242706779462804. [DOI] [PubMed] [Google Scholar]
- Beukelaar LJ, Kroonenberg PM. Changes over time in the relationship between hand preference and writing hand among left-handers. Neuropsychologia. 1986;24(2):301–303. doi: 10.1016/0028-3932(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Vink JM, van Beijsterveldt TC, de Geus EJ, Beem AL, Mulder EJ, et al. Netherlands Twin Register: a focus on longitudinal research. Twin Research. 2002;5(5):401–406. doi: 10.1375/136905202320906174. [DOI] [PubMed] [Google Scholar]
- Boucher J Birmingham University E. Hand preference in autistic children and their parents. Journal of Autism and Childhood Schizophrenia. 1977;7(2):177–187. doi: 10.1007/BF01537728. [DOI] [PubMed] [Google Scholar]
- Brackenridge CJ. Secular variation in handedness over ninety years. Neuropsychologia. 1981;19(3):459–462. doi: 10.1016/0028-3932(81)90076-2. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The Genetics and Evolution of Handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295x.104.4.714. [DOI] [PubMed] [Google Scholar]
- Cornish KM, McManus IC. Hand preference and hand skill in children with autism. Journal of Autism and Developmental Disorders. 1996;26(6):597–609. doi: 10.1007/BF02172349. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Handedness, language lateralisation and anatomical asymmetry: relevance of protocadherin XY to hominid speciation and the aetiology of psychosis. Point of view. British Journal of Psychiatry. 2002;181:295–297. doi: 10.1192/bjp.181.4.295. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Mitchell CA, Martin NG. Genetic and environmental risk factor for asthma: a cotwin-control study. American Journal of Respiratory and Critical Care Medicine. 1998;157:840–845. doi: 10.1164/ajrccm.157.3.9702070. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Last K, Martin NG, Jinks JL. A progressive approach to non-additivity and genotype-environmental covariance in the analysis of human differences. British Journal of Mathematical and Statistical Psychology. 1977;30:1–42. [Google Scholar]
- Elias LJ, Bryden MP. Footedness is a better predictor of language lateralisation than handedness. Laterality. 1998;3(1):41–51. doi: 10.1080/713754287. [DOI] [PubMed] [Google Scholar]
- Elias LJ, Bryden MP, Bulman-Fleming M. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia. 1998;36(1):37–43. doi: 10.1016/s0028-3932(97)00107-3. [DOI] [PubMed] [Google Scholar]
- Elkadi S, Nicholls M, Clode D. Handedness in opposite and same-sex dizygotic twins: testing the testosterone hypothesis. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience. 1999;10:333–336. doi: 10.1097/00001756-199902050-00023. [DOI] [PubMed] [Google Scholar]
- Francks C, DeLisi LE, Fisher SE, Laval SH, Rue JE, Stein JF, et al. Confirmatory evidence for linkage of relative hand skill to 2p12-q11. American Journal of Human Genetics. 2003;72(2):499–502. doi: 10.1086/367548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francks C, DeLisi LE, Shaw SH, Fisher SE, Richardson AJ, Stein JF, et al. Parent-of-origin effects on handedness and schizophrenia susceptibility on chromosome 2p12-q11. Human Molecular Genetics. 2003;12(24):3225–3230. doi: 10.1093/hmg/ddg362. [DOI] [PubMed] [Google Scholar]
- Francks C, Fisher S, MacPhie L, Richardson A, Marlow A, Stein J, et al. A genomewide linkage screen for relative hand skill in sibling pairs. American Journal of Human Genetics. 2002;70:800–805. doi: 10.1086/339249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francks C, Maegawa S, Lauren J, Abrahams BS, Velayos-Baeza A, Medland SE, et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Molecular Psychiatry. 2007;12(12):1129–1139. 1057. doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF, Coren S. Do right-handers live longer? Nature. 1988;333(6170):213. doi: 10.1038/333213b0. [DOI] [PubMed] [Google Scholar]
- Hay DA, Howie PM. Handedness and differences in birthweight of twins. Perceptual and Motor Skill. 1980;51:666. doi: 10.2466/pms.1980.51.2.666. [DOI] [PubMed] [Google Scholar]
- Hepper PG, McCartney GR, Shannon EA. Lateralised behaviour in first trimester human foetuses. Neuropsychologia. 1998;36:521–534. doi: 10.1016/s0028-3932(97)00156-5. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Wells DL, Lynch C. Prenatal thumb sucking is related to postnatal handedness. Neuropsychologia. 2005;43(3):313–315. doi: 10.1016/j.neuropsychologia.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kirk KM, Bailey JM, Dunne MP, Martin NG. Measurement models for sexual orientation in a community twin sample. Behavior Genetics. 2000;30:345–356. doi: 10.1023/a:1026557719181. [DOI] [PubMed] [Google Scholar]
- Kirk KM, Bellamy N, O’Gorman LE, Kuhnert PM, Klestov A, Muirden K, et al. The validity and heritability of self-report osteoarthritis in an Australian older twin sample. Twin Research. 2002;5(2):98–106. doi: 10.1375/1369052022965. [DOI] [PubMed] [Google Scholar]
- Klar AJ. Genetic models for handedness, brain lateralization, schizophrenia, and manic-depression. Schizophrenia Research. 1999;39(3):207–218. doi: 10.1016/s0920-9964(99)00075-4. [DOI] [PubMed] [Google Scholar]
- Laland KN, Kumm J, Van Horn JD, Feldman MW. A gene-culture model of human handedness. Behavior Genetics. 1995;25:433–445. doi: 10.1007/BF02253372. [DOI] [PubMed] [Google Scholar]
- Levy J. Psychobiological implications of bilateral asymmetry. In: Dimond S, Beaumont J, editors. Hemisphere Function in the Human Brain. London: Elek; 1974. [Google Scholar]
- Liederman J, Healey JM. Independent dimensions of hand preference: reliability of the factor structure and the handedness inventory. Arch Clin Neuropsychol. 1986;1(4):371–386. [PubMed] [Google Scholar]
- Martin NG. The inheritance of scholastic abilities in a sample of twins. II. Genetical analysis of examinations results. Annals of Human Genetics. 1975;39:219–229. doi: 10.1111/j.1469-1809.1975.tb00125.x. [DOI] [PubMed] [Google Scholar]
- Martin NG, Perl J, Oakeshott JG, Gibson JB, Starmer GA, Wilks AV. A twin study of ethanol metabolism. Behavior Genetics. 1985;15(2):93–109. doi: 10.1007/BF01065891. [DOI] [PubMed] [Google Scholar]
- McManus IC. Handedness in Twins: A critical review. Neuropsychologia. 1980;18:347–355. doi: 10.1016/0028-3932(80)90130-x. [DOI] [PubMed] [Google Scholar]
- McManus IC. Handedness, language dominance and aphasia: A genetic model. Psychological Medicine Monographs Supplement. 1985;8:1–140. [PubMed] [Google Scholar]
- McManus IC. Right Hand, Left Hand: The origins of asymmetry in Brains, Bodies, Atoms and Cultures. London: Weidenfeld & Nicolson; 2002. [Google Scholar]
- McManus IC, Murray B, Doyle K, Baron-Cohen S. Handedness in childhood autism shows a dissociation of skill and preference. Cortex. 1992;28(3):373–381. doi: 10.1016/s0010-9452(13)80147-5. [DOI] [PubMed] [Google Scholar]
- Medland SE, Duffy DL, Spurdle AB, Wright MJ, Geffen GM, Montgomery GW, et al. Opposite Effects of Androgen Receptor CAG Repeat Length on Increased Risk of Left-Handedness in Males and Females. Behavior Genetics. 2005;35(6):735–744. doi: 10.1007/s10519-005-6187-3. [DOI] [PubMed] [Google Scholar]
- Medland SE, Duffy DL, Wright MJ, Geffen GM, Martin NG. Handedness in twins: joint analysis of data from 35 samples. Twin Res Hum Genet. 2006;9(1):46–53. doi: 10.1375/183242706776402885. [DOI] [PubMed] [Google Scholar]
- Medland SE, Wright MJ, Geffen GM, Hay DA, Levy F, Martin NG, et al. Special twin environments and their effects on the handedness of twins and their siblings. Twin Research. 2003;6(2):119–130. doi: 10.1375/136905203321536245. [DOI] [PubMed] [Google Scholar]
- Neale MC. Handedness in a sample of volunteer twins. Behavior Genetics. 1988;18:69–79. doi: 10.1007/BF01067076. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2006. http://www.vcu.edu/mx/: [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24(3):239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Newman HH. Studies of Human Twins II. Asymmetry reversal of mirrorimaging in identical twins. Biological Bulletin. 1928;55:298–315. [Google Scholar]
- Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic basis of male pattern baldness. Journal of Investigative Dermatology. 2003;121(6):1561–1564. doi: 10.1111/j.1523-1747.2003.12615.x. [DOI] [PubMed] [Google Scholar]
- Ooki S, Yamada K, Asaka A, Hayakawa K. Zygosity diagnosis by questionnaire. Acta Genetica Medica et Gemellologica. 1990;39:109–115. doi: 10.1017/s0001566000005626. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Koopmans JR, Boomsma DI, Bleker OP. Left-handedness in twins:genes or environment? Cortex. 1996;32:479–490. doi: 10.1016/s0010-9452(96)80005-0. [DOI] [PubMed] [Google Scholar]
- Orr KGD, Cannon M, Gilvarry CM, Jones PB, Murray RM. Schizophrenic patients and their first-degree relatives show an excess of mixed-handedness. Schizophrenia Research. 1999;39(3):167–176. doi: 10.1016/s0920-9964(99)00071-7. [DOI] [PubMed] [Google Scholar]
- Perelle IB, Ehrman L. An international study of human handedness: the data. Behavior Genetics. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- Porac C, Coren S. Lateral preferences and human behavior. New York: Springer-Verlag; 1981. [Google Scholar]
- Powls A, Botting N, Coooke RWI, Marlow N. Handedness in very-low-birthweight (VLBW) children at 12 years of age: Relation to perinatal and outcome variables. Developmental Medicine and Child Neurology. 1996;38:594–602. doi: 10.1111/j.1469-8749.1996.tb12124.x. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left handed people studies by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12(1):43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Ross DC, Jaffe J, Collins RL, Page W, Robinette D. Handedness in the NAS/NRC Twin Study. Laterality. 1999;4:257–264. doi: 10.1080/713754342. [DOI] [PubMed] [Google Scholar]
- Salvesen KA. Ultrasound and left-handedness: a sinister association? Ultrasound in Obstetrics and Gynecology. 2002;19(3):217–221. doi: 10.1046/j.1469-0705.2002.00659.x. [DOI] [PubMed] [Google Scholar]
- Satz P, Green MF. Atypical handedness in schizophrenia: some methodological and theoretical issues. Schizophrenia Bulletin. 1999;25:63–78. doi: 10.1093/oxfordjournals.schbul.a033367. [DOI] [PubMed] [Google Scholar]
- Satz P, Orsini DL, Saslow E, Henry R. The pathological left-handedness syndrome. Brain and Cognition. 1985;4:27–46. doi: 10.1016/0278-2626(85)90052-1. [DOI] [PubMed] [Google Scholar]
- Shaw J, Claridge G, Clark K. Schizotpy and the shift from dextrality: a study of handedness in a large non-clinical sample. Schizophrenia Research. 2001;50:181–189. doi: 10.1016/s0920-9964(00)00167-5. [DOI] [PubMed] [Google Scholar]
- Sicotte N, Woods R, Mazziotta J. Handedness in twins: a meta-analysis. Laterality. 1999;4:265–286. doi: 10.1080/713754339. [DOI] [PubMed] [Google Scholar]
- Siemens HW. Uber Linkshandigkeit [ as cited by Sicotte, Woods & Mazziotta, 1999] Virchow’s Archives. 1924;252:1–24. [Google Scholar]
- Steele J. Handedness in past human populations: skeletal markers. Laterality. 2000;5:193–220. doi: 10.1080/713754380. [DOI] [PubMed] [Google Scholar]
- Toh T, Liew SH, MacKinnon JR, Hewitt AW, Poulsen JL, Spector TD, et al. Central corneal thickness is highly heritable: the twin eye studies. Investigative Ophthalmology and Visual Science. 2005;46(10):3718–3722. doi: 10.1167/iovs.04-1497. [DOI] [PubMed] [Google Scholar]
- Toth N. Archaeological evidence for precranial right-handedness in the Lower and Middle Pleistocene, and its possible implications. Journal of Human Evolution. 1985;14:607–614. [Google Scholar]
- Townsend GC, Richards L, Hughes T, Pinkerton S, Schwerdt W. The value of twins in dental research. Australian Dental Journal. 2003;48(2):82–88. doi: 10.1111/j.1834-7819.2003.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Tesar DW, Yong M. Ipsilateral motor control in pathological left-handedness. Neurocase. 1998;4:65–69. [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28(4):575–589. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Van Agtmael T, Forrest SM, Williamson R. Parametric and non-parametric linkage analysis of several candidate regions for genes for human handedness. European Journal of Human Genetics. 2002;10:623–630. doi: 10.1038/sj.ejhg.5200851. [DOI] [PubMed] [Google Scholar]
- Warren DM, Stern M, Duggirala R, Dyer TD, Almasy L. Heritability and linkage analysis of hand, foot, and eye preference in Mexican Americans. Laterality. 2006;11(6):508–524. doi: 10.1080/13576500600761056. [DOI] [PubMed] [Google Scholar]
- White V, Hopper J, Wearing A, Hill D. The role of genes in tobacco smoking during adolescence and young adulthood: a multivariate behaviour genetic investigation. Addiction. 2003;98:1087–1100. doi: 10.1046/j.1360-0443.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- Wright MJ, De Geus E, Ando J, Luciano M, Posthuma D, Ono Y, et al. Genetics of Cognition: Outline of a Collaborative Twin Study. Twin Research. 2001;4:48–56. doi: 10.1375/1369052012146. [DOI] [PubMed] [Google Scholar]