Abstract
Upon activation, naive CD4+ T cells differentiate into effector Th cell subsets. The stability and plasticity of effector Th cells have not been well understood. In this study we used an IL-17F-red fluorescent protein reporter mouse to analyze the plasticity of Th17 cells in vitro and in vivo. We found that in vitro generated Th17 cells poorly maintained their differentiation program in vitro and could be reprogrammed into other T cell lineages. Moreover, upon transfer into lymphopenic hosts, Th17 cells rapidly lost their IL-17 expression and were converted into Th1 cells independently of IL-7 signaling. However, Th17 cells maintained their phenotypes well in normal animals, even in the absence of Ag and inflammation. These results, although suggesting the plasticity of Th17 cells, also indicate active maintenance of their program in vivo.
Naive T cells, upon activation, differentiate into cytokine-expressing effector Th cells. In addition to Th1 and Th2, Th17 cells have been recently identified. Th17 cells were first marked by IL-17 production and subsequently were found to also secret IL-17F, IL-21, and IL-22 (1). Th17 cells have been show to play important roles in host defenses against various infections and in autoimmune diseases. Th17 differentiation is potently driven by TGF-β and IL-6 (2, 3) and is reinforced by IL-23 (4, 5). The Th17-specific master transcription factors retinoic acid-related orphan receptor (ROR)4γt and RORα, two orphan nuclear receptors, have been recently shown to regulate Th17 differentiation (6, 7).
It has been thought that effector differentiation is a “terminal” process for naive Th cells. However, recent results suggest the plasticity of Th subsets. For example, we and others have reported that Foxp3+ regulatory T cells could be reprogrammed to become Th17-like cells by IL-6 and other proinflammatory cytokines (8, 9).
In the current study, we use a Th17 reporter mouse to examine the plasticity of Th17 cells. We show that the in vitro generated Th17 cells, although not stable in vitro, maintain their cytokine expression programs in normal hosts in vivo, but they readily convert to Th1 cells in a lymphopenic environment.
Materials and Methods
Mice
Rag1-deficient and B6.SJL (CD45.1) mice were purchased from The Jackson Laboratory. The Il17frfp reporter mouse was generated by insertion of an IRES-mRFP-poly(A) cassette (where IRES is internal ribosome entry site, m is monomeric, and RFP is red fluorescent protein) into the exon 2 of the Il17f gene (10) and maintained on a 129 × B6 background or backcrossed six to seven generations onto B6 background. Il17frfp mice on B6 background were breed with OT-II transgenic mice. Mice were housed in the specific pathogen-free animal facility at M. D. Anderson Cancer Center, and the animal experiments were performed at the age of 6–10 wk with protocols approved by the Institutional Animal Care and Use Committee of the M. D. Anderson Cancer Center.
Th17 cell differentiation
CD4+CD25−CD62LhighCD44low cells from Il17frfp mice were FACS sorted and stimulated with plate bound anti-CD3 (2.5 μg/ml, BD Biosciences) and anti-CD28 (2.5 μg/ml, BD Biosciences) under Th17 conditions with 5 ng/ml TGF-β (Peprotech), 30 ng/ml IL-6 (Peprotech), 50 ng/ml IL-23 (R&D Systems), 10 μg/ml anti-IL-4 (clone 11B11), and 10 μg/ml anti-IFN-γ (clone XMG1.2). On day 4 after activation, RFP+ cells were FACS sorted and restimulated with plate bound anti-CD3/anti-CD28 in the presence or absence of IL-23 or under Th1 (10 ng/ml IL-12 (Peprotech) and 10 μg/ml anti-IL-4), Th2 (10 ng/ml IL-4 (Peprotech) and 10 μg/ml anti-IFN-γ), or inducible Treg (iTreg; 5 ng/ml TGF-β) conditions for 3 days. T cells were then washed and restimulated with PMA and ionomycin in the presence of BD GolgiStop (BD Biosciences) for 5 h, after which Foxp3-, IL-17-, IL-4-, and IFN-γ-producing cells were analyzed using intracellular staining. Intracellular staining for Foxp3 was performed by using a Foxp3 staining kit (eBioscience).
Quantitative real-time PCR
Total RNA was prepared from T cells using TRIzol reagent (Invitrogen). cDNA was synthesized using SuperScript reverse transcriptase and oligo(dT) primers (Invitrogen), and gene expression was examined with a Bio-Rad iCycler optical system using iQ SYBR Green real-time PCR kit (Bio-Rad Laboratories). The data were normalized to Actb reference. The primers for T-bet, GATA3, Foxp3, RORγt, T-bet, and Actb were previously described (4).
Analysis of Th17 cells in vivo
Naive Th cells from Il17frfp reporter mice were differentiated under Th17 conditions. Four days later, RFP+ cells were sorted by FACS and i.v. transferred into Rag1−/− mice. In some experiments, a total of 500 μg of control Ig (rat IgG) or anti-IL-7R Ab was i.p. injected at day 0, 1, 3, and 5. Six days later, splenocytes of recipients were stimulated with PMA/ionomycin for 4 h in the presence of BD GolgiPlug (BD Biosciences) and stained with FITC-conjugated anti-CD4 and PerCP-Cy5.5-conjugated anti-TCRβ followed by intracellular staining of various cytokines. In other experiments, RFP+ Th17 cells were transferred into B6.SJL congenic mice (CD45.1+) (5 × 106 cells/mouse). The recipient mice were left untreated or immunized subcutaneously with 50 μg of OVA peptide emulsified in IFA or CFA. Five days later, lymphoid cells from spleens were isolated and restimulated with OVA peptide for 24 h in the presence of GolgiStop for the last 5 h. Cells were stained with PerCP-Cy5.5-conjugated anti-CD45.2 together with allophycocyanin-conjugated anti-CD4 before they were analyzed by intracellular staining.
Results and Discussion
Plasticity of Th17 cells in vitro
Our recently developed Il17frfp reporter mice have allowed us to sensitively and faithfully mark Th17 cells (8). In the current study, we have also used these mice to analyze the plasticity of Th17 cells. Naive CD4+CD62LhighCD44low T cells from Il17frfp reporter mice were stimulated with plate-bound anti-CD3 and anti-CD28 together with TGF-β, IL-6, IL-23, and neutralizing Abs against IL-4 and IFN-γ. On day 4, RFP+ cells were sorted by FACS. Consistent with our previous report (8), ∼42–43% of sorted RFP+ cells could be stained by anti-IL-17 (Fig. 1A). In addition, RFP+ cells did not express IFN-γ or Foxp3 (Fig. 1A). Based on these results, we believe that RFP might be more sensitive in marking T cells competent in producing Th17 cytokines.
FIGURE 1.
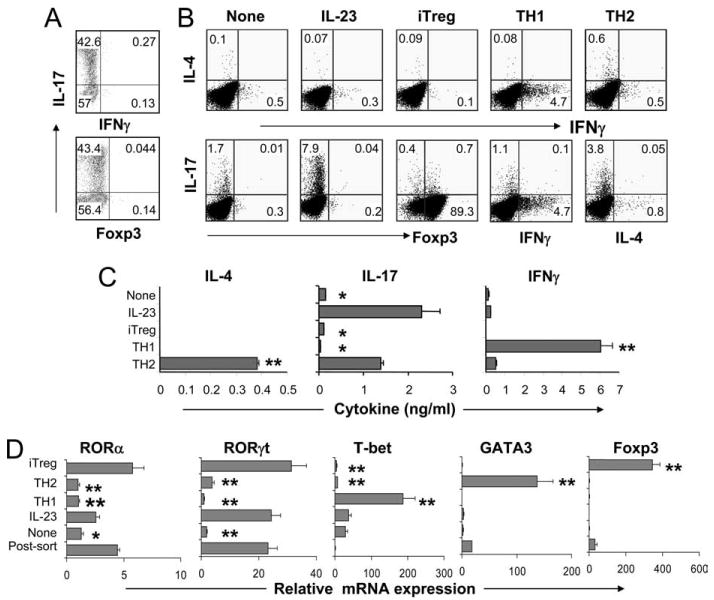
Plasticity of Th17 cells in vitro. A, Naive CD4+CD25−CD62LhighCD44low T cells from Il17frfp mice were polarized with plate-bound anti-CD3 and anti-CD28 under Th17 conditions (TGFβ, IL-7, anti-IL-4, anti-IFN-γ, and IL-23) for 4 days and RFP+ cells were sorted by FACS. After sorting, cells were assessed for IL-17, IFN-γ, and Foxp3 expression by intracellular staining. B–D, The sorted RFP+ cells were restimulated under the indicated conditions. IL-17-, IL-4-, IFN-γ-, and Foxp3-expressing cells were then analyzed by intracellular staining (B). CD4+ T cells were restimulated with anti-CD3 for 4 h for real-time PCR analysis (C) or for 24 h for cytokine measurement by ELISA (D). The experiment was performed twice with consistent results. p values were calculated with the t test; *, p < 0.05; **,p < 0.005.
After sorting, RFP+ cells were restimulated either in the presence or absence of IL-23 for 3 days. IL-17, IFN-γ, IL-4, and Foxp3 expressions were determined by flow cytometry. RFP+ cells quickly lost their IL-17 expression, although did not up-regulate IL-4, IFN-γ, or Foxp3 expression, which was associated with greatly reduced expression of RORα and RORγt (Fig. 1D). In the presence of IL-23, RFP+ cells maintained IL-17 as well as ROR expression (Fig. 1, B and D). These results support an important role of IL-23 in actively maintaining the Th17 program.
Stimulation of RFP+ cells under Th1, Th2, or iTreg polarizing conditions resulted in down-regulation of IL-17 expression and expressions of IFN-γ, IL-4, and Foxp3, respectively (Fig. 1B). Enhanced protein levels of IL-4 under Th2 conditions and IFN-γ under Th1 conditions were further confirmed by ELISA (Fig. 1C). Real-time RT-PCR analysis of Th1-, Th2-, and iTreg-specific genes revealed significantly enhanced expression of T-bet, GATA3, and Foxp3 following treatment with cytokines that favored the corresponding lineage differentiation (Fig. 1D). Reduced expression of IL-17 under Th1 and Th2 conditions correlated with decreased levels of RORγ and RORα. At this stage, we cannot rule out the possibility that RFP+ cells that were not stained with anti-IL-17 might be preferentially converted into Th1 cells. In contrast, the expression of RORγ and RORα mRNA did not change after the restimulation of Th17 cells under iTreg conditions (Fig. 1D). This result correlates with our earlier observation that Foxp3 overexpression under Th17 polarizing conditions inhibited IL-17, IL-17F, IL-21, and IL-22 cytokine expression without affecting RORα or RORγ mRNA levels (8). Others and we have shown that Foxp3 directly inhibited the ability RORγt and RORα to activate Th17-specific gene transcription (8, 11).
Thus, our results indicate that Th17 cells generated in vitro and enriched by a RFP marker are not stable in vitro. IL-23 regulates the maintenance of their cytokine expression programs, but cytokine environments that favor naive T cell differentiation into other lineages could reprogram these cells into Th1, Th2, and iTreg cells.
Conversion of Th17 cells to IFN-γ-producing cells in lymphopenic environments
The above results suggest that Th17 cells can be reprogrammed and redifferentiated into Th1, Th2, and iTreg cells. To understand whether such a regulation exists in vivo, naive cells from Il17frfp reporter mice were activated with anti-CD3 and anti-CD28 under two different Th17 conditions: IL-6, TGF-β, anti-IL-4, and anti-IFN-γ with or without IL-1 and IL-23. On day 4 after activation, RFP+ cells were sorted by FACS and i.v. injected into Rag1−/− recipient mice. The transferred RFP+ cells did not have any IFN-γ or IL-4 expression (Fig. 2A) and contained <1% Foxp3+ cells. Six days later, CD4+TCRb+ cells from spleen were analyzed for Foxp3, IL-17, IFN-γ, IL-4, and IL-10 expressions after restimulation with PMA plus ionomycin. Transferred cells demonstrated extremely reduced expression of IL-17, but in the meantime IFN-γ expression was greatly up-regulated (Fig. 2A). There was no increase in cells expressing IL-4, IL-10, or Foxp3. It has been reported that IL-23 and IL-1 enhance Th17 development/expansion in the presence of IL-6 and TGF-β (3–5). We thus added these cytokines in Th17 differentiation culture and found that the resulting RFP+ cells were still readily converted into IFN-γ-expressing Th1 cells in Rag1−/− mice. Therefore, in the lymphopenic environment, Th17 cells, during homeostatic proliferation, predominately became IFN-γ-positive cells. It remains possible that RFP+ cells that were not stained with anti-IL-17 might be preferentially converted into Th1 cells in vivo.
FIGURE 2.
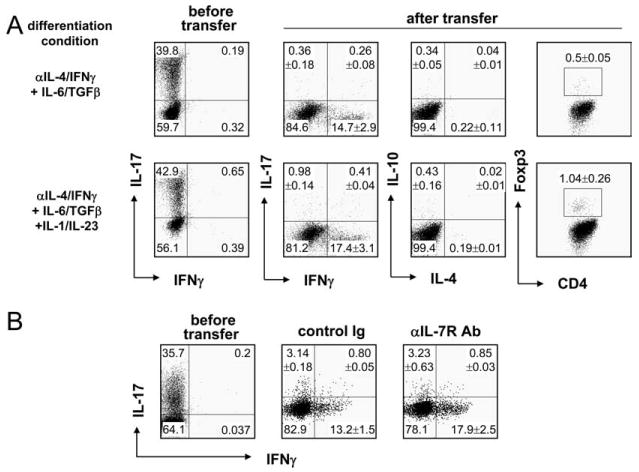
IL-17-producing T cells become IFN-γ-producing T cells in lymphopenic mice. Naive CD4+CD25−CD62LhighCD44low T cells were sorted from splenocytes and lymph node cells of Il17frfp mice and stimulated with plate-bound anti-CD3/CD28 Abs in the presence of the indicated cytokine combination (A) or IL-6 and TGF-β (B) with anti-IL-4/IFN-γ neutralization Abs. At day 4, RFP+ cells were further sorted and i.v. transferred into Rag1−/− mice. In B, a total of 500 μg of control Ig (rat IgG) or anti-IL-7RAb was i.p. injected on days 0, 1, 3, and 5. Six days later, splenocytes of recipients were stimulated with PMA/ionomycin for 4 h in the presence of GolgiPlug, stained with FITC-conjugated anti-CD4Ab and PerCP-Cy5.5-conjugated anti-TCRβ Ab followed by intracellular staining of indicated cytokines. CD4+TCRβ+ cells were gated and analyzed. Numbers in dot-plot quadrants represent the percentages. In A, the percentages (means ± SD) combining three independent experiments (n = 9 mice) are shown. In B, the percentages (means ± SD) combining two independent experiments (control Ig group, n = 5 mice; anti-IL-7R (αIL-7R) Ab group, n = 4 mice) are shown.
Multiple factors are associated with the lymphopenia-induced proliferation, among which IL-7 has been known to play an important role (12, 13). To examine whether the conversion of Th17 to Th1 cells in Rag1−/− host is IL-7 dependent, Rag1−/− mice receiving RFP+ cells were treated with a control (rat IgG) or IL-7R-blocking Abs. As expected, decreased TCRβ+ CD4+ cell populations were observed in the anti-IL-7R Ab-treated group when compared with control group (2.19 vs 1.33%, respectively), indicating that the IL-7R blockade reduced lymphopenia-induced proliferation. However, blocking of IL-7 signaling did not alter the fate of transferred Th17 cells, and the majority of the donor cells still produced IFN-γ. Thus, these results indicate that the conversion of Th17 cells into IFN-γ-producing cells in lymphopenic host is IL-7 independent.
Stability of Th17 cells in normal host
Next, we examined the plasticity of Th17 cells after their transfer into hosts with normal T cell compartments. Naive CD4+ T cells from Il17rfp OT-II mice were activated with OVA peptide and irradiated APC under Th17 conditions. On day 4 after activation, RFP+ cells sorted by FACS were transferred into B6.SJL congenic mice (CD45.1+). The recipient mice were either nonimmunized or immunized s.c. with OVA peptide emulsified in CFA. Five days later, CD45.1+ and CD45.2+CD4+ T cells were analyzed for Foxp3, IL-17, and IFN-γ expressions after restimulation with OVA peptide. As expected, enhanced IL-17 expression was detected in CD45.1+ host T cells after immunization, indicating that the immunization protocol was successful (Fig. 3A). Surprisingly, in CD45.2+ populations there was no reduction of IL-17-producing cells in the absence of immunization (Fig. 3A). In addition, enhanced IL-17 expression was detected in donor cells after immunization (Fig. 3A). Moreover, moderately enhanced IFN-γ single and IFN-γ/IL-17 dual expressers were also observed (Fig. 3A).
FIGURE 3.

Stability of TH17 cells in vivo. Naive CD4+CD25−CD62LhighCD44low T cells from Il17frfp OT-II mice were activated with OVA peptide and irradiated APC under Th17 conditions for 4 days, and RFP+ cells were sorted by FACS. Sorted cells (CD45.2+) were transferred i.v. into B6.SJL congenic mice (CD45.1+). A, The recipient mice were nonimmunized (Non/Immun.) or immunized (Immun.) s.c. with OVA peptide emulsified in CFA (three mice per group were analyzed individually,). Seven days later, spleen cells were restimulated with OVA peptide for 24 h and CD45.1+ and CD45.2+ CD4 T cells were assessed for IL-17, IFN-γ, and Foxp3 expression by intracellular staining. Numbers in dot-plot quadrants represent the percentages (means ± SD) combining two independent experiments (nonimmunized group, n = 6 mice; immunized group, n = 6 mice). B, The recipient mice were immunized s.c. with OVA peptide emulsified in CFA or IFA (three mice per group were analyzed individually). Seven day later, spleen cells were restimulated with OVA peptide or 24 h, and Ly5.2+ CD4 T cells were assessed for IL-17, IL-17F, IFN-γ, and Foxp3 expression by intracellular staining. Numbers in dot-plot quadrants represent the percentages (means ± SD) combining two independent experiments (IFA group, n = 6 mice; CFA group, n = 6 mice).
To further examine the role of inflammatory signals in maintaining phenotypes of Th17 cells, RFP+ cell-receiving B6.SJL congenic mice (CD45.1+) were immunized with OVA peptide emulsified in IFA or CFA. CD45.2+ donor cells in the noninflammatory conditions maintained their Th17 phenotype, similar to those in nonimmunized mice, whereas CFA further enhanced the percentages of IL-17-producing cells (Fig. 3B). This result indicates that in normal hosts, Th17 cells are stable and the addition of inflammatory signals will further enhance IL-17 expression or Th17 expansion.
In summary, we used a Th17 reporter to analyze the stability and plasticity of Th17 cells in vitro and in vivo. We found that in vitro, differentiated Th17 cells readily lose their polarized differentiation program, whereas IL-23 helps to maintain this program, possibly by maintaining ROR expression. In vivo, although some Th17 cells are converted to Th1 cells in lymphopenic environments, they maintain their cytokine expression in normal hosts. These results together suggest active environmental maintenance of a Th17 differentiation program in normal hosts, even in the absence of inflammation or Ag.
Acknowledgments
We thank E. Wieder for help on FACS sorting and analysis and the members of the Dong laboratory for their help.
Footnotes
The work is supported in part by National Institutes of Health Research Grant AR050772 (to C.D.) and a grant from the Leukemia and Lymphoma Society. R.N. is a recipient of a Scientist Development Grant from the American Heart Association. C.D. is a Leukemia and Lymphoma Society Scholar, a Trust Fellows of the M. D. Anderson Cancer Center, a Cancer Research Institute Investigator, and an American Lung Association Career Investigator.
Abbreviations used in this paper: ROR, retinoic acid-related orphan receptor; iTreg, inducible Treg; RFP, red fluorescent protein.
Disclosures: The authors have no financial conflict of interest.
References
- 1.Dong C. Th17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs Th-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 10.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. The J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-β-induced Foxp3 inhibits Th17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 13.Marleau AM, Sarvetnick N. T cell homeostasis in tolerance and immunity. J Leukocyte Biol. 2005;78:575–584. doi: 10.1189/jlb.0105050. [DOI] [PubMed] [Google Scholar]


