Abstract
Background and Purpose
Small-vessel knock is a recently reported Doppler ultrasound finding that has been identified in patients with cerebral ischemia. It has been hypothesized that knock-type signals are linked to the presence of either small-vessel occlusion or wall motion. The aim of this study was to investigate the origins of “knock-type” signals by reproducing occlusion of a peripheral artery model in vitro.
Methods
Synthetic bifurcations were fabricated from glass and latex and placed in a flow-rig mimicking physiological blood-flow conditions. The glass model permitted study of fluid flow in the absence of wall motion, whereas the latex model also produced wall motion effects. Vessels were artificially obstructed to examine Doppler signal characteristics associated with blood flow and wall motion.
Results
Complete obstruction of the peripheral branch of the glass model revealed discrete (<100 ms) knock-type signals caused by local fluid flow in the occluded branch. Imaging of the obstructed vessel using color Doppler revealed forward and reflected flow. The walls produced periodic bidirectional knock-type signals, which occurred during systole and were not related to the presence of an obstruction.
Conclusions
In our laboratory model, transcranial Doppler ultrasound was found to be capable of detecting knock signals produced by circulating fluid within an occluded branch. However, because similar signals are also generated by nonpathological wall motion, these results cannot be directly translated to a clinical setting. Clinicians should be careful to avoid casual overinterpretation of transcranial Doppler ultrasound data.
Keywords: acute stroke, cerebrovascular disease, Doppler, knock-type Doppler signals, small-vessel knock, transcranial Doppler
Transcranial Doppler ultrasound is becoming increasingly used as a “cerebral stethoscope” for monitoring patients with cerebral ischemia. Here we investigate a recent proposal that transcranial Doppler ultrasound also might be capable of localizing occluded branch vessels.1
Knock-type signals were first reported in the UK in 20041 and have since been independently confirmed by stroke research groups in Australia and the USA.2 The “knock” typically appears in the sonogram as a bright, short-duration, periodic, low-frequency signal, which may be bidirectional, unidirectional, or alternating.1-4 The signal can be distinguished from random artifacts by its persistent and regular appearance in the cardiac cycle. M-mode power Doppler5 reveals knock-type signals are localized to a specific depth within the head.
On the basis of transcranial Doppler ultrasound measurements recorded in patients with cerebral ischemia, Syme1,3,4 proposed that detection of knock signals may provide a diagnostic indicator of small-vessel occlusion. Despite a number of compelling case studies in which replacement of the knock with a small-vessel waveform was associated with dramatic neurological recovery,4 there is no broader consensus among the stroke community as to whether “knock-type” signals are clinically significant. A correspondence between the positions of ischemic lesions, neurological symptoms, and the location of knock signals has so far proved elusive.2 This suggests that knock signals may be nonpathological artifacts produced as a normal consequence of wall motion. Here we investigate the origins of knock signals by reproducing conditions for occlusion of a branch in vitro. Transcranial Doppler ultrasound signals from fluid flow and wall motion in a latex bifurcation were examined under controlled conditions using Doppler, B-mode, and color Doppler.
Materials and Methods
The in vitro flow circuit consisted of a fluid reservoir and C-flex tubing with 4.6-mm internal diameter and 0.8-mm wall thickness (Cole Parmer). Model arteries, with branches angled at ≈30° (Figure 1A), were submerged in a water tank and physiologically realistic pulsatile flow was generated using a programmable gear pump (Micropump model 120-000-1100). The 30-cm branch outlet was obstructed at several positions along its length using an adjustable clamp (Figure 1).
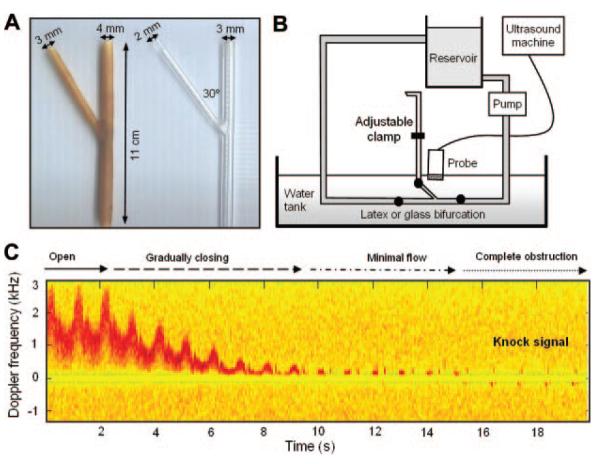
Figure 1.
A, Glass and latex models. B, Schematic of flow-rig design. C, Gradual obstruction of the branch vessel resulted in a knock-type Doppler signal (glass model).
Doppler signals were examined using a Duplex ultrasound system equipped with a 4- to 7-MHz linear array transducer (ATL; Ultramark 9). To permit observation of low-frequency signals, the wall filter was reduced to its minimum setting of 50 Hz. The sample length was 1 mm. The gain was set to 85% (before) and 45% (after) to optimize the signal from wall motion in the latex vessel when the circuit was filled with degassed water. Audio signals were recorded using a digital recording device (EXMI10 4-channel Direct Input Module, EX-UT10 Interface Unit, and EX-FA10 Analog output module; Sony) and displayed and analyzed off-line using Matlab (The Mathworks, Inc).
Glass Model
The glass bifurcation consisted of a piece of laboratory glassware with internal diameters of 3 mm (parent vessel) and 2 mm (branch). After flushing with water, ≈2 mL of blood-mimicking fluid7 was added to the circuit until the waveform was clearly defined and of similar intensity to that observed clinically. The center of the branch was then insonated during closure of the branch outlet using an adjustable clamp (Figure 1B).
Latex Model
The latex model was formed by coating a pair of detachable metal rods in liquid latex up to a wall thickness of ≈1 mm (≈7 coats; Figure 1A). Latex models with these dimensions have a similar compliance to real arteries and are a suitable substitute for wall-motion studies.6 After removal of the rods, the latex vessel had internal diameters of 4 mm (parent vessel) and 3 mm (branch). To avoid the contribution from wall motion being obscured by scattering from the fluid, only 0.5 mL of blood-mimicking fluid was initially added to the reservoir. Doppler signals from different positions within the lumen and at the artery wall were then characterized before and after clamping the side branch using the same gain settings. To further distinguish wall-motion scattering from fluid flow, the sample volume was then placed in the center of the obstructed branch vessel and the quantity of added blood-mimicking fluid was increased from 0.5 mL to 5 mL (in 0.5-mL drops). Finally, color Doppler images were obtained laterally and transversely along the length of the model.
Results
Glass Model
During closure of the vessel outlet, there was a gradual reduction in the velocity of the flow. This progressed to a “knock-type” signal when the vessel was completely obstructed (Figure 1C). The position of the clamp along the length of the outlet was varied between 5 and 30 cm and was not found to alter the characteristics of the knock signal.
Latex Model
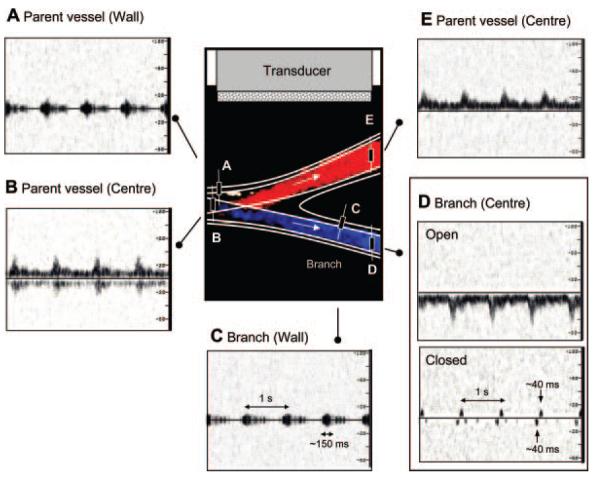
Motion of echogenic portions of the anterior wall lying at ≈90° to the beam produced a bidirectional periodic signal ≈100 to 300 ms in duration (Figure 2A, 2C). This signal was associated with a characteristic low-frequency “thump” sound resembling that observed clinically. The central Doppler frequency of the wall-motion signal corresponded to ≈350 Hz when the probe was oriented at ≈90° to the vessel. The temporal dependence of the wall-motion signal followed that of the principal waveform and was strongest during systole.
Figure 2.
Doppler signals observed in the latex model included bidirectional wall thump (A, C) and fluid flow in the main vessel (B, E). D, Obstruction of the branch gives rise to a knock-type signal from fluid-flow.
The knock-type signal from fluid flow at the center of the obstructed latex vessel was unidirectional and similar to that demonstrated in the glass model (Figure 2D). The appearance of the knock signal did not vary with the distance of the clamp from the bifurcation. At fixed gain, the knock signal increased in intensity with the addition of further blood-mimicking fluid, confirming that the signal was attributable to scatterers in the fluid rather than wall motion. The origins of the knock were also confirmed using color Doppler imaging. An example of longitudinal and transverse scans coinciding with the “knock” signal are shown during forward, reflected, and mixed flow in Figure 3.
Figure 3.
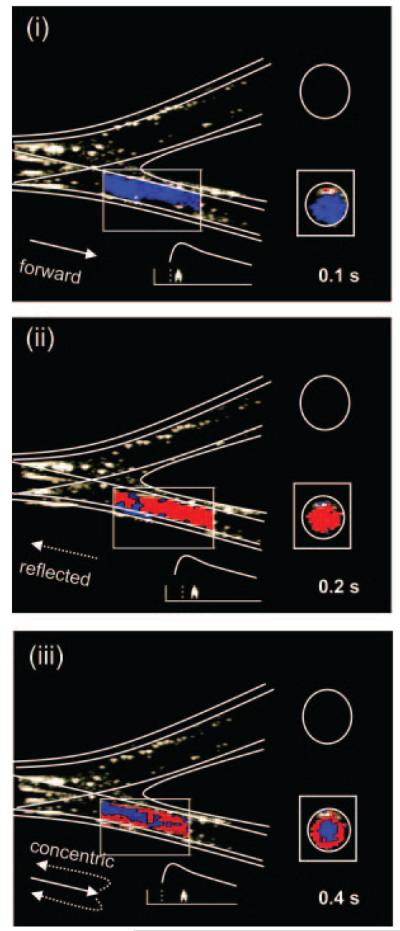
Color Doppler images of forward (I) and reverse (II) knock components, followed by concentric “shells” of mixed flow (III).
Discussion
Our study represents the first (to our knowledge) in vitro investigation of the origins of knock-type signals. We investigated a stiff glass vessel and compliant latex model and found knock signals to be reproducible in both systems.
The blood-mimicking fluid in our study was diluted to enable simultaneous observation of wall motion and fluid flow without altering the gain settings of the ultrasound machine. By controlling the concentration of scatterers in the fluid, we were able to distinguish wall motion from fluid flow. In color Doppler scans, the knock signal was seen to coincide with sudden “flashes” of forward and reflected flow, associated with redirection of fluid as it is recirculated to the parent vessel. In future work, these flow patterns could be investigated using 3-dimensional color Doppler and computational fluid dynamics simulations.
A limitation of our model is that the bifurcation dimensions were larger than expected clinically. Further work should investigate the effects of reducing the dimensions of the model. A more detailed investigation of backscatter intensity is also required.
It should also be noted that the wall motion signals produced by our latex model may not be representative of signals observed in clinical practice. In humans, there will be a broad range of vessel wall compliances and geometries. This has potential to produce a variety of different knock-type signals from wall motion. The durations and other characteristics of the signals observed in our model therefore may not be representative of those observed in patients.
The major finding of the current study is that knock signals are qualitatively consistent with, but not specific to, fluid flow within a fully obstructed branch vessel. Our results suggest that any branch that produces a Doppler signal could also produce a detectable knock signal when the branch is obstructed. Provided that abnormal flow signatures can be confidently distinguished from wall motion, transcranial Doppler ultrasound examination of patients' peripheral vessels may in principle provide a promising future means of detecting vascular occlusion.
Acknowledgments
We acknowledge the support of the University of Leicester, University Hospitals of Leicester NHS Trust and Austin Health. This work was performed as part of a British Medical Ultrasound Society (BMUS) and Australian Society for Ultrasound in Medicine (ASUM) exchange award. E.M.L.C. also acknowledges funding from the Wellcome Trust and British Heart Foundation. The authors also thank Paul Syme for his advice, and Werner Mess and Kirk Beach for helpful discussion.
Footnotes
Disclosures
None.
References
- 1.Syme PD. Detection of small vessel knock using transcranial Doppler ultrasonography. Advance Clin Neurosci Rehab. 2004;4:28–31. [Google Scholar]
- 2.Tsivgoulis G, Man BL, Lao AY, Sharma VK, Vadikolas K, Alexandrov AV. A spectrum of knock-type Doppler signals in the intracranial vessels. Stroke. 2009;40:644–647. doi: 10.1161/STROKEAHA.108.517797. [DOI] [PubMed] [Google Scholar]
- 3.Syme PD. The use of transcranial Doppler ultrasonography as a “cerebral stethoscope” for the assessment and treatment of acute stroke. J R Coll Physicians Edinburg. 2006;36:17–28. [Google Scholar]
- 4.Syme PD. Five further cases of spontaneous recanalisation during transcranial Doppler insonation—is this enhanced endogenous thrombolysis? J Cerebrovasc Dis. 2003;16(suppl4):47. [Google Scholar]
- 5.Moehring MA, Spencer MP. Power M-Mode Doppler (PMD) for Observing cerebral blood flow and tracking emboli. UltrasoundMed Biol. 2002;28:49–57. doi: 10.1016/s0301-5629(01)00486-0. [DOI] [PubMed] [Google Scholar]
- 6.Walker RD, Smith RE, Sherriff SB, Wood RFM. Latex vessels with customized compliance for use in arterial flow models. Physiol Meas. 1999;20:277–286. doi: 10.1088/0967-3334/20/3/305. [DOI] [PubMed] [Google Scholar]
- 7.Ramnarine KV, Nassiri DK, Hoskins PR, Lubbers J. Validation of a new blood mimicking fluid for use in Doppler flow test objects. Ultrasound Med Biol. 1998;24:451–459. doi: 10.1016/s0301-5629(97)00277-9. [DOI] [PubMed] [Google Scholar]