Abstract
Aims
To determine 3-year event rates in outpatients with vascular disease enrolled in the REduction of Atherothrombosis for Continued Health (REACH) Registry.
Methods and results
REACH enrolled 67 888 outpatients with atherothrombosis [established coronary artery disease (CAD), cerebrovascular disease, or peripheral arterial disease (PAD)], or with at least three atherothrombotic risk factors, from 44 countries. Among the 55 499 patients at baseline with symptomatic disease, 39 675 were eligible for 3-year follow-up, and 32 247 had data available (81% retention rate). Among the symptomatic patients at 3 years, 92% were taking an antithrombotic agent, 91% an antihypertensive, and 76% were on lipid-lowering therapy. For myocardial infarction (MI)/stroke/vascular death, 1- and 3-year event rates for all patients were 4.2 and 11.0%, respectively. Event rates (MI/stroke/vascular death) were significantly higher for patients with symptomatic disease vs. those with risk factors only at 1 year (4.7 vs. 2.3%, P < 0.001) and at 3 years (12.0 vs. 6.0%, P < 0.001). One and 3-year rates of MI/stroke/vascular death/rehospitalization were 14.4 and 28.4%, respectively, for patients with symptomatic disease. Rehospitalization for a vascular event other than MI/stroke/vascular death was common at 3 years (19.0% overall; 33.6% for PAD; 23.0% for CAD). For patients with symptomatic vascular disease in one vascular bed vs. multiple vascular beds, 3-year event rates for MI/stroke/vascular death/rehospitalization were 25.5 vs. 40.5% (P < 0.001).
Conclusion
Despite contemporary therapy, outpatients with symptomatic atherothrombotic vascular disease experience high rates of recurrent vascular events and rehospitalizations.
Keywords: Atherothrombosis, Risk factors, Coronary artery disease, Cerebrovascular disease, Peripheral arterial disease
Introduction
Vascular disease is a leading global cause of death and disability. Coronary artery disease (CAD) and stroke are projected to be the first and second leading causes of death in the world,1,2 and most of these events occur in underdeveloped or developing nations.3,4 Steps to better treat vascular disease and prevent these important events will have significant public health implications as well as reduce the financial impact for patients, healthcare systems, and governments.
Although lifestyle changes and targeted therapies reduce vascular disease and vascular events, such interventions are often either underutilized or are not applied appropriately for some at-risk populations.5–7 A better understanding of how such therapies are used and their impact on various populations could significantly influence medical practices, with the goal of preventing vascular events.
The REduction of Atherothrombosis for Continued Health (REACH) Registry is a contemporary international outpatient study of patients either with vascular disease or at high risk for developing vascular disease (Appendix).8 Patients are treated according to the best judgement and practices of their primary care physicians and followed on a longitudinal outpatient basis. No particular medications or interventions are tested as part of the REACH Registry. The treatment setting is non-academic outpatient physician practices. We used this unique registry to determine the 3-year outcomes of a diverse international patient population, with a focus on vascular events, risk factors, and therapies. Our hypotheses were that despite widely available therapies: (i) many patients receive suboptimal treatment and (ii) this group of patients has a high rate of subsequent major vascular events.
Methods
The design of the REACH Registry has been published previously.9 Briefly, REACH is an outpatient, non-specialist registry of patients with either stable symptomatic vascular disease [CAD, stroke, transient ischaemic attack (TIA), peripheral artery disease (PAD)] or with multiple risk factors for vascular disease (such as hypertension, diabetes mellitus, hyperlipidaemia, carotid stenosis, smoking, etc.). Detailed data about medication use are recorded. The treatments for each patient are determined by their medical practitioners, who are typically their primary care physician. The REACH Registry primarily enrolled patients who were not at a typical academic medical centre or university hospital. Rather, the focus was on outpatients cared for by non-specialists. Patients could not be enrolled in REACH if they were participating in another clinical research study or if they were currently hospitalized.
Patient enrolment began in December 2003 and ended in December 2004. A total of 67 888 patients were enrolled from 5587 different physician practices in 44 countries. Each nation had their own site-selection process designed to ensure diverse patient selection in different settings (urban and rural) and to account for the national patient and physician profiles and overall healthcare environment. Local institutional review boards reviewed and approved the protocol, and each enrolled patient was required to provide informed consent.
Follow-up
Patients were followed annually (every 12 ± 3 months) for key outcome events and medication use in most countries, except in the USA, where they were followed up every 6 months. Key outcome events included myocardial infarction (MI), stroke, vascular death, and rehospitalization for another vascular event or vascular procedure [i.e. congestive heart failure, unstable angina, vascular surgery, percutaneous coronary intervention (PCI)]. Further details of how these events were defined have been published previously.9 Patients were not censored after they had an MI, stroke, or vascular death. Data were also collected on serious bleeding events, which were defined as rehospitalization for bleeding and receipt of a blood transfusion, or rehospitalization for any type of cerebral haemorrhage. Patients were not censored if they had a serious bleeding event. Outcome events were typically not adjudicated, although ischaemic stroke and TIA had to be documented by a neurologist or recorded in hospital records.
For this report, we focused mainly on follow-up events for patients with symptomatic vascular disease at enrolment, since this group makes up the majority of the REACH population, has much higher event rates, and is often the target for most medical and procedural interventions in routine clinical practice. This group of patients is also the largest user of medical resources and healthcare finances.
Central data collection was used with a standardized international case report form. Demographic information, risk factors, medication use, employment status, smoking, and weight were recorded at each visit, along with key outcome events as noted earlier.
Statistical analysis
Continuous variables are expressed as mean ± SD; categorical variables are expressed as frequencies and percentages. Comparisons of baseline characteristics and concomitant medications between the numbers of symptomatic disease locations were made using χ2 tests. When comparing event rates across different disease bed types, patients were classified according to whether they had CAD, cerebrovascular disease (CVD), or PAD. Patients with polyvascular disease could have been included in more than one category.
The study retention rate was calculated by comparing the number of patients who completed the 3-year follow-up visit (or who died before 2-year follow-up) with the number of patients who were enrolled at centres that agreed to participate in the 3-year follow-up phase of the REACH Registry.
Cumulative event rates per 100 patient-years have been calculated at 3 years for selected endpoints (vascular death, non-fatal MI, non-fatal stroke, rehospitalization for another vascular event), according to the number of symptomatic disease locations; the number of events occurring within each period were added, divided by the total duration of participation in the registry (measured in years) for the patients, and multiplied by 100. All event rates were calculated using the Cox proportional hazard model. Events rates are reported as annualized rates, adjusted for age and sex. This adjustment was accomplished through the corrected group prognosis method in the Cox proportional hazards model previously described.8 The difference in hazard rates for selected endpoints according to the number of symptomatic disease locations was tested using the Wald χ2 test within the Cox proportional hazard model. All tests were two-sided with a significance threshold of P = 0.05. Cumulative event curves were constructed using the Kaplan–Meier approach. Statistical analyses were performed using SAS v9.1 software (SAS Institute Inc., Cary, NC, USA).
Results
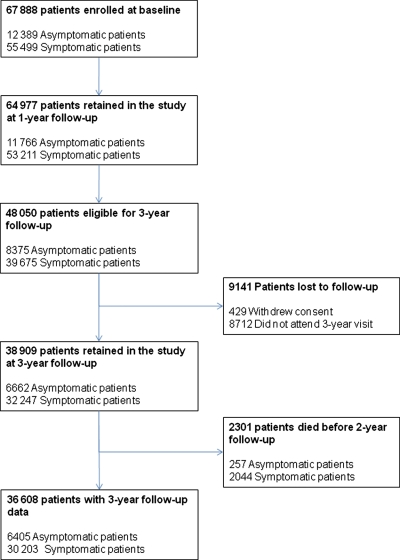
At baseline, the REACH Registry enrolled 67 888 patients, of which 55 499 had symptomatic disease, and 12 389 had asymptomatic disease (Figure 1). At 3 years, 48 050 patients were eligible for the 3-year follow-up analysis. Most of the attrition was related to certain study sites choosing not to participate in follow-up beyond 2 years, and 8712 patients did not attend the 3-year appointment (Figure 1). Of the 48 050 patients, 3-year follow-up data were available for 36 608. Patients without complete data at the 3-year follow-up included 8712 who did not return for the 3-year appointment, 2301 who were deceased, and 429 who withdrew consent. For this report, we focus mainly on patients with symptomatic vascular disease (n = 39 675), since they have higher event rates and are most likely to benefit from secondary prevention strategies. Among the 39 675 symptomatic patients potentially available for 3-year follow-up, data were available for 32 247, for an 81% retention rate.
Figure 1.
Patient flow, drop-out, and follow-up over 3 years in the REACH Registry.
Demographic data for symptomatic patients at baseline and 3 years are shown in Table 1. This population had high rates of several vascular risk factors, particularly hypertension (∼80%) and hypercholesterolaemia (∼70%). More than one in four patients (26%) were obese, and a significant minority (14%) of them were current smokers. Thus a significant portion of this population had risk factors that were modifiable or treatable.
Table 1.
Baseline demographic and risk factor data for REACH patients with symptomatic disease
| Variable | Symptomatic patients in REACH at baseline | Symptomatic patients at 3-year follow-up |
|---|---|---|
| n | 55 499 | 39 675 |
| Age, years (mean ± SD) | 68.5 ± 10.1 | 68.5 ± 10.0 |
| Men (%) | 66.8 | 67.8 |
| Hypertension (%) | 80 | 79.3 |
| Diabetes (%) | 37.5 | 36.0 |
| Elevated total cholesterol (%) | 70.2 | 68.7 |
| Obesity ≥30 kg/m2 (%) | 27.4 | 25.9 |
| Current smoker (%) | 14.4 | 14.3 |
| Heart failure (%) | 15.9 | 15.0 |
| Atrial fibrillation (%) | 11.7 | 11.4 |
Medication use was relatively high, yet not universal (Table 2). More than 90% of the symptomatic patients were taking an antihypertensive agent at baseline and 91.1% at 3 years. The majority of patients were also receiving some type of antithrombotic therapy as well as a lipid-lowering drug. The use of ‘at least one antithrombotic’ remained consistent at 92.4% at baseline and 92.1% at year 3; the use of oral anticoagulation was also stable (12.9% at baseline and 13.5% at 3 years); and the use of ‘at least one lipid-lowering drug’ was fairly stable, at 72.9% for baseline and 75.9% at 3 years. Many patients were taking other cardiovascular agents as well as antidiabetic medications. Although we do not have data about adherence to specific medications, the overall rate of medication use appears to be quite high.
Table 2.
Medication use for symptomatic patients at baseline and at 3-year follow-up
| Medicationa (%) | At baseline | At 3 years |
|---|---|---|
| At least one antihypertensive drug | 90.9 | 91.1 |
| At least one antithrombotic drug | 92.4 | 92.1 |
| Aspirin alone | 56.6 | 56.9 |
| Aspirin+another antiplatelet drug | 14.5 | 12.8 |
| Other antiplatelet drug alone | 13.6 | 14.2 |
| Oral anticoagulant drug | 12.9 | 13.5 |
| At least one lipid-lowering drug | 72.9 | 75.9 |
| Statin | 68.3 | 71.9 |
| Diabetic patients with at least one antidiabetic drugb | 87.3 | 84.6 |
aDenominators may vary due to missing data.
bPercentage calculated from 14 282 diabetic patients at baseline and 10 628 diabetic patients at 3 years.
There were differences in medication use among symptomatic patients based on gender, both at baseline and at 3 years. For example, the use of any antiplatelet agent was higher in men vs. women at baseline (93.5 vs. 90.1%, P < 0.0001) and at 3 years (93.3 vs. 89.6%, P < 0.0001). This was not explained by higher rates of anticoagulation use among women, since men had slightly higher rates of oral anticoagulation use at baseline (13.3 vs. 12.2%, P = 0.0014) and at 3 years (13.8 vs. 12.8%, P = 0.021). Similar differences were seen for the use of any lipid-lowering drug (73.6% in men vs. 71.4% in women at baseline, P < 0.0001; 76.9 vs. 73.8% at 3 years, P < 0.0001), and the use of any statin (69.2 vs. 66.4% at baseline, P < 0.0001; 73.0 vs. 69.5% at 3 years, P < 0.0001).
The gender differences in the use of antithrombotics could not be fully explained by differences in risk factors, since all of these patients had symptomatic ischaemic vascular disease and likely qualified for some type of antithrombotic therapy based on existing guidelines. For the use of lipid-lowering drugs, the percentage of men and women with elevated total cholesterol at baseline was similar (70.2% in men vs. 69.4% in women), indicating that other factors may be affecting medication usage in men vs. women.
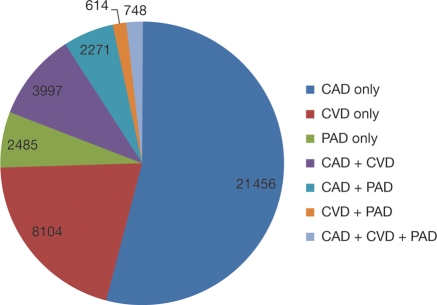
Among patients with symptomatic disease who were eligible for 3-year follow-up, 21 456 (54.1%) had only CAD, 8104 (20.4%) had only CVD, and 2485 (6.3%) had only PAD at baseline. We also found that 7630 had disease in more than one vascular bed, which included 3997 with both CAD and CVD, 2271 with both CAD and PAD, and 748 with the triad of CAD, CVD, and PAD (Figure 2).
Figure 2.
Type and distribution of monovascular and polyvascular disease at baseline in patients eligible for 3-year follow-up. Data labels report the number of patients with each disease type.
Event rates at 1 and 3 years
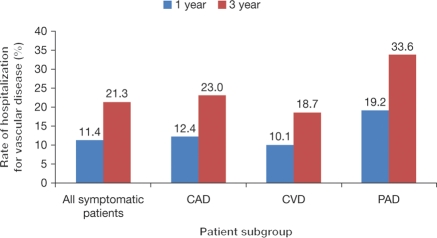
The rates of MI, stroke, vascular death, and rehospitalization for a vascular event at 1 and 3 years for symptomatic patients are shown in Table 3. For the composite endpoint of non-fatal MI, non-fatal stroke, or vascular death (hence defined as MI/stroke/vascular death), the 1-year and 3-year event rates were 4.7 and 12.0%, respectively (for patients enrolled with risk factors only, the event rates for MI/stroke/vascular death were 2.3 and 6.0%, at 1 year and 3 years, respectively). When rehospitalization for a vascular event is added to the composite endpoint, more than one in four patients at 3 years will experience one of these events. For MI/stroke/vascular death/rehospitalization, the 1- and 3-year event rates were 14.4 and 28.4%, respectively. Patients with PAD had the highest 3-year event rate of MI/stroke/death/rehospitalization, at 40.4%. These high rates are explained in part by the fact that rehospitalization for a vascular event other than MI/stroke/vascular death was a common occurrence at 3 years, with rates of 21.3% for the entire symptomatic cohort, 33.6% for patients with PAD, 23.0% for those with CAD, and 18.7% for patients with CVD (Figure 3). In fact, rehospitalization for a vascular event other than MI, stroke, or vascular death was more common than the composite endpoint of MI, stroke, and vascular death at both 1 and 3 years (Figure 3).
Table 3.
Outcome events (age- and sex-adjusted) after 1 and 3 years of follow-up in REACH patients with symptomatic disease
| Outcome | Total symptomatic population | Subgroup |
||
|---|---|---|---|---|
| Any CAD | Any CVD | Any PAD | ||
| At 1 year | n = 53 211 | n = 38 602 | n = 18 013 | n = 7911 |
| MI/stroke/vascular death | 4.7 | 4.5 | 6.5 | 5.4 |
| MI/stroke/vascular death/rehospitalizationa | 14.4 | 15.2 | 14.5 | 21.1 |
| Rehospitalizationa | 11.4 | 12.4 | 10.1 | 19.2 |
| At 3 years | n = 39 675 | n = 28 472 | n = 13 463 | n = 6118 |
| MI/stroke/vascular death | 12.0 | 11.6 | 15.4 | 14.8 |
| MI/stroke/vascular death/rehospitalizationa | 28.4 | 29.7 | 28.1 | 40.4 |
| Rehospitalizationa | 21.3 | 23.0 | 18.7 | 33.6 |
CAD, coronary artery disease; CVD, cerebrovascular disease; MI, myocardial infarction; PAD, peripheral arterial disease.
aRehospitalization for a vascular event other than MI, stroke, vascular death.
Figure 3.
Rates of rehospitalization (excluding rehospitalization for the primary endpoint of myocardial infarction/stroke/vascular death) for symptomatic patients in REACH at 1- and 3-year follow-up. Coronary artery disease, cerebrovascular disease, and peripheral arterial disease groups include patients with single bed as well as polyvascular disease.
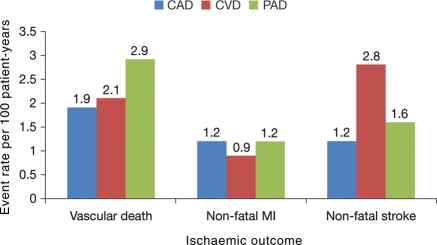
Analysis of event rates per 100 patient-years of follow-up showed differences in subsequent events based on the vascular bed initially involved (Figure 4). Patients with CVD were most likely to have a subsequent stroke (2.8 events per 100 patient-years), and those with PAD were most likely to have vascular death (2.9 events per 100 patient-years). Non-fatal MI was equally common among patients with either CAD or PAD (both had rates of 1.2 events per 100 patient-years).
Figure 4.
Event rates per 100 patient-years at 3 years for important vascular outcomes by the type of symptomatic disease present at study entry. Coronary artery disease, cerebrovascular disease, and peripheral arterial disease groups include patients with single bed as well as polyvascular disease.
We found important gender differences in 3-year outcomes in symptomatic patients. For the endpoint of MI/stroke/vascular death, the 3-year event rate was 12.5% for men and 10.9% for women (P = 0.0007 when age adjusted). Much of this difference was explained by the vascular death rate, which was 6.1% for men and 4.6% for women (P < 0.0001). For the composite endpoint of MI/stroke/vascular death/rehospitalization, there was a 2% higher rate in men compared with women (29.1 vs. 27.0%, P = 0.0004) at 3 years.
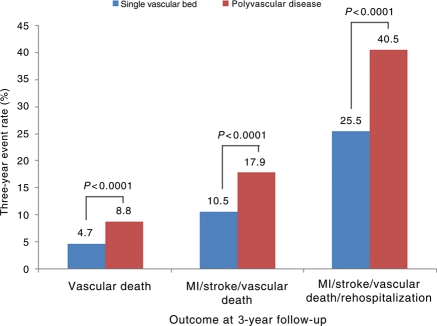
Patients with polyvascular disease had the highest event rates at 1 and 3 years. For patients with symptomatic vascular disease in one vascular bed compared with multiple vascular beds, the 3-year event rates for MI/stroke/vascular death/hospitalization were 25.5 vs. 40.5% (P < 0.001). When patients with polyvascular disease are compared with those with single-bed disease, the 3-year rates of vascular death are increased by 4%, the rate of MI/stroke/vascular death increased by 7%, and the rate of MI/stroke/vascular death/hospitalization increased by 15% (Figure 5).
Figure 5.
Three-year rates of myocardial infarction/stroke/vascular death, and myocardial infarction/stroke/vascular death/rehospitalization for symptomatic patients with monovascular or polyvascular disease.
Over the 3-year course of REACH, patients with PAD only at baseline had the highest risk of progressing to involvement of other vascular beds, which may explain some of the high event rates seen among this population. Almost 10% of the PAD patients progressed to polyvascular disease over 3 years, compared with ∼4% of patients with either CAD or CVD at baseline. The most common progression on a percentage basis was patients with PAD to develop CAD (6.1%), followed by patients with PAD to develop CVD (3.8%), and patients with CVD to develop CAD (3.7%). However, owing to the greater number of patients with CAD at baseline, this group had the largest number of patients who progressed to polyvascular disease. For the CAD group, they next developed CVD in years 1, 3, and 2 (in decreasing order of occurrence).
Event rates varied by geographical region (Table 4). Eastern Europe tended to have the highest overall event rates, whereas Australia and Japan had the lowest. North America and Western Europe also had above-average event rates despite the relatively high use of medications and vascular procedures in these regions. In general, the geographic differences in event rates tended to mirror the prevalence of vascular risk factors and the success in treating and controlling them.
Table 4.
Three-year event rates by geographic region
| North America | Latin America | Western Europe | Eastern Europe | Middle East | Asia | Australia | Japan | Total | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 11 604 | 1206 | 12 218 | 4326 | 392 | 3144 | 2551 | 4234 | 39 675 | |
| All-cause mortality | 9.80 | 11.51 | 9.25 | 11.68 | 7.87 | 9.85 | 6.86 | 5.21 | 9.06 | <0.0001 |
| 95% CI | 8.97–10.61 | 9.47–13.47 | 8.53–9.96 | 10.36–12.97 | 5.08–10.53 | 8.51–11.16 | 5.80–7.90 | 4.47–5.94 | 8.54–9.57 | |
| Vascular death | 6.02 | 7.61 | 5.79 | 9.03 | 5.61 | 6.64 | 2.70 | 2.42 | 5.57 | <0.0001 |
| 95% CI | 5.35–6.68 | 5.90–9.27 | 5.20–6.36 | 7.81–10.22 | 3.24–7.90 | 5.51–7.75 | 2.06–3.34 | 1.91–2.92 | 5.15–5.99 | |
| MI/stroke/vascular death | 11.99 | 13.65 | 11.85 | 17.99 | 13.25 | 13.13 | 7.57 | 8.63 | 11.96 | <0.0001 |
| 95% CI | 11.08–12.90 | 11.46–15.77 | 11.06–12.64 | 16.47–19.47 | 9.70–16.64 | 11.68–14.56 | 6.44–8.69 | 7.67–9.58 | 11.37–12.54 | |
| MI/stroke/vascular death/rehospitalization | 29.27 | 25.83 | 30.69 | 39.58 | 30.45 | 23.03 | 23.51 | 17.34 | 28.39 | <0.0001 |
| 95% CI | 28.09–30.43 | 23.24–28.32 | 29.62–31.75 | 37.90–41.21 | 25.85–34.77 | 21.38–24.64 | 21.68–25.29 | 16.10–18.56 | 27.62–29.16 |
CI, confidence interval; MI, myocardial infarction.
Major 3-year vascular outcome events and all-cause mortality for symptomatic patients in the REACH Registry. Some regions had higher than average rates of outcome events such as Eastern Europe and the Middle East; other regions such as Asia, Australia, and Japan had event rates that were lower than average. Event rates are age- and sex-adjusted. P-values indicate a statistically significant difference between at least one region and another.
Discussion
The data from this large, contemporary, international registry show that patients with symptomatic vascular disease have high rates of subsequent vascular events despite the high use of various standard medications and treatments. These results raise important questions about whether patients were actually treated according to guideline recommendations (as opposed to taking medications but not achieving therapeutic goals), about the need for more widespread use of medications, and the need for more potent and efficacious medications.8 This is particularly relevant for the REACH population, which had high rates of several common vascular risk factors.
Besides using medications and other approaches (i.e. surgery and endovascular procedures) for these patients, clearly more attention should be paid to adopting and achieving healthier lifestyles. Improving diet, increasing exercise, and smoking cessation are relatively easily available and inexpensive approaches to reducing the occurrence of vascular events.7,10 Nations and regions that have improved their lifestyles have achieved lower rates of coronary heart disease death and prevalence.7,10–12
The use of various medications such as antithrombotics, antihypertensives, and statins was fairly high in our study. We found small but significant differences in medication use by gender that were not explained by differences in underlying risk factors. Although the differences in the use of various medications by gender varied by only a few percentage points, when extrapolated across a global population affected by these treatable risk factors, such differences could mean that millions of women may not be receiving recommended therapies. Why women were less likely to receive certain medications is unclear. Although it could be argued that this did not translate into worse outcomes for women (men had higher event rates at 3 years), it is certainly possible that had the women been treated more aggressively, their events rates might have been even lower. This is clearly an area that requires further study using large international databases to determine whether there is a gender bias in terms of medication utilization. Prior studies have shown that women are less likely to undergo cardiac catheterization and coronary artery bypass surgery than men, further supporting gender bias in some care settings.13,14
We found high event rates at 3 years. More than one in four patients with symptomatic vascular disease will have an MI, stroke, vascular death, or be rehospitalized for another type of vascular event at 3 years. These outcomes are even more pronounced in those patients with PAD since 40% of these patients had a serious vascular event at 3 years. Clearly improved emphasis on the diagnosis and treatment of PAD is warranted.
On a global basis, these results represent tens of millions of vascular events occurring each year. In addition to the medical and personal impact of such events, the costs of recurrent events and hospitalization or rehospitalization are enormous. The annual cost of acute coronary syndromes in the USA is $150 billion, of which 60% is related to hospitalization.15 The annual cost for acute coronary syndromes is 3.3 billion € in Germany and 3.1 billion € in Italy.16 The cost for admission for stroke or TIA ranges from 3500 to 5000 € annually in Germany.17 Expenses for PAD range from 1800 to 6200 € each year, of which 45% are hospital related.18 On the basis of these figures, even small reductions in the number of these events could save national health care systems billions of dollars and Euros each year.
Patients with polyvascular disease represent a particular challenge. These patients were at much higher risk of subsequent events at both 1 year and 3 years. In addition, the progression from monovascular to polyvascular disease, particularly for the PAD group, was alarming, with almost 10% of patients showing such progression over 3 years. Such trends will clearly place additional healthcare burdens and costs on patients and healthcare systems. These high-risk groups should be targeted for aggressive identification and treatment of all vascular risk factors.19
There were significant geographic differences in outcome events. In general, Asian countries and Australia had lower event rates, whereas Eastern Europe and the Middle East had higher event rates. These differences were only partially explained by increased prevalence of risk factors in those regions. For example, at baseline, 85% of the symptomatic patients in Eastern Europe had hypertension and 48% were overweight. Among symptomatic patients from the Middle East, 81% had elevated cholesterol and 49% had diabetes.8 However, >97% of patients with hypertension in Eastern Europe were receiving at least one antihypertensive medication, and >85% of patients in the Middle East were receiving at least one lipid-lowering agent.8 This suggests that the high event rates seen in some regions are not simply a function of more risk factors or total lack of therapy. Baseline data from the REACH Registry showed that many patients are not treated to target goals for various risk factors such as hypertension and hyperlipidaemia.8 Questions about the effectiveness of some therapies, adherence to medications, and the adoption of healthier lifestyles should be addressed. Despite these issues, the aggressive treatment of modifiable risk factors has the potential to substantially reduce the risk of these vascular events.20
Study limitations
Any registry with selection criteria has the potential to be affected by ascertainment biases. We attempted to avoid such biases by having broad inclusion criteria with an obvious focus on patients with vascular disease and related risk factors. The diverse geographical distribution of enrolled patients and the wide variety of physician practices would also tend to mitigate any large ascertainment biases. Other limitations are that we could not document adherence to taking prescribed medications or over-the-counter medications, and most of the endpoints were not independently adjudicated. However, participating physicians and study coordinators were trained and instructed about measures to assess adherence to medication regimens and how to define and diagnose important endpoints.
To be enrolled in REACH, the participants had to be stable outpatients. Targeting stable outpatients may have actually resulted in an under-estimation of subsequent events, since it has been well documented that the period immediately following an acute coronary syndrome or ischaemic stroke is a time of high risk for a subsequent vascular event.21–23
Conclusions
The large multinational REACH Registry documented high rates of recurrent vascular events at 3 years in patients with symptomatic vascular disease. These events occurred in the setting of prevalent risk factors but also high use of various medications. These results point to the need for a multifaceted and aggressive approach to the prevention and treatment of vascular risk factors and vascular disease on a global basis. The high societal and medical costs of these common diseases mandate a comprehensive programme to reduce the occurrence of new and recurrent events.
Funding
The REACH Registry is sponsored by sanofi-aventis, Bristol-Myers Squibb, and the Waksman Foundation (Tokyo, Japan). Funding to pay the Open Access publication charges for this article was provided by sanofi-aventis and Bristol-Myers-Squibb.
Conflict of interest: All manuscripts in the REACH Registry are prepared by independent authors who are not governed by the funding sponsors and are reviewed by an academic publications committee before submission. The funding sponsors have the opportunity to review manuscript submissions but do not have authority to change any aspect of a manuscript.
M.J.A. has received research grants, honoraria, and consulting fees from sanofi-aventis, Bristol-Myers Squibb, AstraZeneca, Schering-Plough, Genentech, Boehringer Ingelheim; honoraria and consulting fees from The Medicines Company, Pfizer, PDL Biopharm Diadexus; and consulting fees from Eli Lilly, Merck, GlaxoSmithKline, Biosite, Cardax.
D.L.B. has received research grants from Bristol-Myers Squibb, Eisai, Ethicon, sanofi-aventis, and The Medicines Company; honoraria (donated to non-profits for >2 years)—AstraZeneca, Bristol-Myers Squibb, Centocor, Daiichi-Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Millennium, Paringenix, PDL, sanofi-aventis, Schering-Plough, The Medicines Company and tns Healthcare; speaker's bureau (>2 years ago)—Bristol-Myers Squibb, sanofi-aventis, and The Medicines Company; consultant/advisory board (any honoraria donated to non-profits)—Arena, AstraZeneca, Bristol-Myers Squibb, Cardax, Centocor, Cogentus, Daiichi-Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, McNeil, Medtronic, Millennium, Otsuka, Paringenix, PDL, Philips, Portola, sanofi-aventis, Schering-Plough, The Medicines Company, tns Healthcare, and Vertex; and expert testimony regarding clopidogrel (the compensation was donated to a non-profit organization).
J.-L.M. has received research grants, honoraria, and consulting fees from sanofi-aventis, Bristol-Myers Squibb, and Servier; honoraria and consulting fees from Boehringer Ingelheim.
E.M.O. has received grant support from Bristol-Myers Squibb, sanofi-aventis, Schering-Plough, Millennium Pharmaceuticals, Eli Lilly, and Berlex; consultancy fees from Inovise, Savacor, Liposcience, Response Biomedical, The Medicines Company, Datascope, and Abiomed; payment for speaker's bureau from CV Therapeutics and Schering-Plough within the last 3 years and is a shareholder of Inovise, Savacor, and Medtronic.
A.T.H. has received research grants from Bristol-Myers Squibb and sanofi-aventis, and honoraria from sanofi-aventis.
J.R. has received honoraria and consulting fees from sanofi-aventis, Bristol-Myers Squibb, Lundbeck, and Boehringer Ingelheim.
G.S. is an employee of sanofi-aventis.
S.G. has received honoraria and consulting fees from Eisai, sanofi-aventis, Daiichi-Sankyo, GlaxoSmithKline, Bristol-Myers Squibb, Otsuka, Bayer, Schering-Plough, Takeda, Astellas, AstraZeneca, Novartis, and Kowa; has received research grants from Pfizer, Ono, Eisai, Otsuka, Daiichi-Sankyo, sanofi-aventis, Takeda, and Astellas within the past 3 years.
S.S. has no disclosures.
C.-S.L. has no disclosures.
P.W.F.W. has received research grants from sanofi-aventis within the last 3 years.
Ph.G.S. has received research grant from sanofi-aventis (1999–2008); is on the speaker's bureau for Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Menarini, Medtronic, Nycomed, Pierre Fabre, sanofi-aventis, Servier, and The Medicines Company; is on the consulting/advisory boards for Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Endotis, GlaxoSmithKline, Medtronic, MSD, Nycomed, sanofi-aventis, Servier, and The Medicines Company; and is a stockholder for Aterovax.
Acknowledgements
The REACH Registry is endorsed by the World Heart Federation. A complete list of REACH investigators is accessible online at www.reachregistry.org. The REACH Registry enforces a no-ghostwriting policy. This manuscript was written and edited by the authors, who take full responsibility for its content. The first draft was written by Mark J. Alberts. We thank Sophie Rushton-Smith, PhD, for assistance with coordinating revisions and providing editorial help in preparing this manuscript, including editing, checking content and language, formatting, referencing, and preparing tables and figures.
Appendix
REACH Registry Executive Committee
Deepak L. Bhatt, MD, MPH, VA Boston Healthcare System and Brigham and Women's Hospital, Boston, MA, USA (chair); Ph. Gabriel Steg, MD, INSERM U-698, Université Paris 7, AP-HP, Paris, France (chair); E. Magnus Ohman, MD, Duke University Medical Center, Durham, NC, USA; Joachim Röther, MD, Johannes Wesling Klinikum Minden, Minden, Germany; Peter W.F. Wilson, MD, Emory University School of Medicine, Atlanta, GA, USA.
REACH Registry Global Publication Committee
Mark J. Alberts, MD, Northwestern University Medical School, Chicago, IL, USA; Deepak L. Bhatt, MD, MPH, VA Boston Healthcare System and Brigham and Women's Hospital, Boston, MA, USA (chair); Ralph D'Agostino, PhD, Boston University, Boston, MA, USA; Kim Eagle, MD, University of Michigan, Ann Arbor, MI, USA; Shinya Goto, MD, PhD, Tokai University School of Medicine, Isehara, Kanagawa, Japan; Alan T. Hirsch, MD, Minneapolis Heart Institute Foundation and University of Minnesota School of Public Health, Minneapolis, MN, USA; Chiau-Suong Liau, MD, PhD, Taiwan University Hospital and College of Medicine, Taipei, Taiwan; Jean-Louis Mas, MD, Centre Raymond Garcin, Paris, France; E. Magnus Ohman, MD, Duke University Medical Center, Durham, NC, USA; Joachim Röther, MD, Klinikum Minden, Minden, Germany; Sidney C. Smith, Jr, MD, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Ph. Gabriel Steg, MD, INSERM U-698, Université Paris 7, AP-HP, Paris, France (chair); Peter W.F. Wilson, MD, Emory University School of Medicine, Atlanta, GA, USA.
References
- 1.Rodgers A, Ezzati M, Vander Hoorn S, Lopez AD, Lin RB, Murray CJ. Distribution of major health risks: findings from the Global Burden of Disease study. PLoS Med. 2004;1:e27. doi: 10.1371/journal.pmed.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleck F. Cardiovascular disease—a global health time bomb. Bull World Health Organ. 2004;82:470–471. [PMC free article] [PubMed] [Google Scholar]
- 3.Levenson JW, Skerrett PJ, Gaziano JM. Reducing the global burden of cardiovascular disease: the role of risk factors. Prev Cardiol. 2002;5:188–199. doi: 10.1111/j.1520-037x.2002.00564.x. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 5.Dirks JH, Robinson SW, Alderman M, Couser WG, Grundy SM, Smith SC, Remuzzi G, Unwin N. Meeting report on the Bellagio Conference ‘prevention of vascular diseases in the emerging world: an approach to global health equity. Kidney Int. 2006;70:1397–1402. doi: 10.1038/sj.ki.5001781. [DOI] [PubMed] [Google Scholar]
- 6.Clement DL, Belch JJ. Vascular disease public education: the mandate is international. Int Angiol. 2004;23:1–4. [PubMed] [Google Scholar]
- 7.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104:2855–2864. doi: 10.1161/hc4701.099488. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PW. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295:180–189. doi: 10.1001/jama.295.2.180. [DOI] [PubMed] [Google Scholar]
- 9.Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau CS, Mas JL, Richard AJ, Rother J, Wilson PW. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J. 2006;151:786.e1–10. doi: 10.1016/j.ahj.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Yach D, Hawkes C, Epping-Jordan JE, Galbraith S. The World Health Organization's Framework Convention on Tobacco Control: implications for global epidemics of food-related deaths and disease. J Public Health Policy. 2003;24:274–290. [PubMed] [Google Scholar]
- 11.Khan S, Cleanthis M, Smout J, Flather M, Stansby G. Life-style modification in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2005;29:2–9. doi: 10.1016/j.ejvs.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Windler E, Zyriax BC. Life style changes for prevention of coronary heart disease. Herz. 2001;26:513–522. doi: 10.1007/pl00002056. [DOI] [PubMed] [Google Scholar]
- 13.Harrold LR, Esteban J, Lessard D, Yarzebski J, Gurwitz JH, Gore JM, Goldberg RJ. Narrowing gender differences in procedure use for acute myocardial infarction: insights from the Worcester Heart Attack Study. J Gen Intern Med. 2003;18:423–431. doi: 10.1046/j.1525-1497.2003.20929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weitzman S, Cooper L, Chambless L, Rosamond W, Clegg L, Marcucci G, Romm F, White A. Gender, racial, and geographic differences in the performance of cardiac diagnostic and therapeutic procedures for hospitalized acute myocardial infarction in four states. Am J Cardiol. 1997;79:722–726. doi: 10.1016/s0002-9149(96)00857-0. [DOI] [PubMed] [Google Scholar]
- 15.Kolansky DM. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care. 2009;15:S36–S41. [PubMed] [Google Scholar]
- 16.Taylor MJ, Scuffham PA, McCollam PL, Newby DE. Acute coronary syndromes in Europe: 1-year costs and outcomes. Curr Med Res Opin. 2007;23:495–503. doi: 10.1185/030079906X167462. [DOI] [PubMed] [Google Scholar]
- 17.Winter Y, Wolfram C, Schaeg M, Reese JP, Oertel WH, Dodel R, Back T. Evaluation of costs and outcome in cardioembolic stroke or TIA. J Neurol. 2009;256:954–963. doi: 10.1007/s00415-009-5053-2. [DOI] [PubMed] [Google Scholar]
- 18.Holler D, Claes C, von der Schulenburg JM. Treatment costs and quality of life of patients with peripheral arterial occlusive disease—the German perspective. VASA. 2004;33:145–153. doi: 10.1024/0301-1526.33.3.145. [DOI] [PubMed] [Google Scholar]
- 19.Carolei A, Chamorro A, Laloux P, Leys D, Rother J, Sander D, Stansby G, Weimar C. Identification and management of polyvascular disease in patients with noncardioembolic ischaemic stroke. Int J Stroke. 2008;3:237–248. doi: 10.1111/j.1747-4949.2008.00220.x. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 21.de Araujo Goncalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26:865–872. doi: 10.1093/eurheartj/ehi187. [DOI] [PubMed] [Google Scholar]
- 22.Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke. 2004;35:1925–1929. doi: 10.1161/01.STR.0000133129.58126.67. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto N, Kimura K, Yokota C, Yonemura K, Wada K, Uchino M, Minematsu K. Early neurological deterioration represents recurrent attack in acute small non-lacunar stroke. J Neurol Sci. 2004;217:151–155. doi: 10.1016/j.jns.2003.09.003. [DOI] [PubMed] [Google Scholar]