My dear colleagues and friends,
I am honored to have the privilege of delivering this oration before such an august gathering. I wish to pay my respects to the memory of the late Professor DLN Murthy Rao in whose name this oration was instituted. I thank my parents for their unwavering belief and support which nourished me during my formative years. I acknowledge with deep gratitude the influence of my teachers who, in more ways than just through education, were instrumental in shaping my growth. And, I place on record my indebtedness to the National Institute of Mental Health and Neurosciences, Bangalore, the institute which provided me with the opportunity to blossom in my career.
It is customary for the recipient of this award to showcase his research and to discuss its impact on the field. I am aware that I am best known for my work on electroconvulsive therapy (ECT) and psychopharmacology, especially in the context of animal models of investigation. However, listeners may be surprised and even amused to learn that, with the assistance of able students, colleagues, and collaborators at my institute and elsewhere, my fields of enquiry have been varied and wide. I am a curious person by nature and strongly believe that science knows no boundaries; if a question is worth asking, it is worth studying. As a result, in addition to ECT, I have worked and published in clinical psychopharmacology,[1–20] preclinical (basic science) psychopharmacology, [21–35] herbal medicine,[1,4,9,30,36–48] psychiatric phenomenology, [49–58] bipolar disorders,[8,11–13,56,59–65] statistics and research methodology, [66–76] family psychiatry[77–84] and fields such as environmental medicine, neuropsychology, education, and even snakebite.[85–92] My interest in these diverse areas persists to this very day. For those who may be curious, I have cited some representative references in this article; readers who would like a complete bibliography, or who desire reprints of specific articles, may write to me.
Without a shadow of doubt, an interest in ECT dominated my research during the past twenty-five years. Subjects that my colleagues and I addressed related to the history[93] and practice[94–96] of ECT; the efficacy[97–116] and adverse effects of the treatment in clinical and preclinical contexts;[117–126] methods and schedules of administration of ECT;[127–131] knowledge about, attitudes towards, and experiences with ECT;[132–142] and even literary issues.[143]
FIELDS OF SPECIAL INTEREST
Four areas seized our especial interest: the use of herbal medicines in the attenuation of the cognitive adverse effects of ECT,[38–47] the mechanism of action of ECT,[144–151] electrical aspects of ECT[97,98,127,151–165] and the safety and efficacy of unmodified ECT;[166–172] we addressed these areas as independent as well as interacting entities. I summarize our findings in the passages that follow. For clarity in communication, I will use the generic term ‘ECT’ to refer to both electroconvulsive therapy (in humans) and electroconvulsive shocks (in animal models).
Herbal medicines for the cognitive adverse effects of ECT
ECT induces anterograde and retrograde amnesia, as well as non-memory cognitive deficits. The amnestic deficits of ECT have been well characterized; whereas anterograde amnesia is mild and temporary, retrograde amnesia for autobiographical and nonautobiographical memories is frequent, and may be severe in a small proportion of patients; memories of lesser emotional salience are more vulnerable to loss. Although there is no evidence that ECT results in brain damage, and although there is evidence that ECT stimulates neuroplasticity and related changes in structures such as the hippocampus, amygdala, and prefrontal cortex, the use of electricity, the triggering of a seizure, and the cognitive adverse effects of the treatment are together responsible for the treatment being held in distrust.[134] To this extent, at least, the acceptability of ECT could improve should it prove feasible to contain the cognitive adverse effects without compromising the efficacy of the treatment.
Herbal medicines receive marketing approval in India without need for evidence of safety and efficacy.[46] We initiated a program to determine whether herbal treatments with putative neuroprotective effects could limit ECT-induced amnestic deficits. We used several different animal models of anterograde and retrograde amnesia in our experiments and obtained encouraging results with a complex herbal formulation,[38–42] with a simplified herbal formulation,[44–45] with the individual herbs Brahmi and Mandookaparni, separately and in combination,[47] but not with an aqueous extract of Shankapushpi.[43] Our findings have been summarized elsewhere along with a critical appraisal of the field, and a commentary on animal models and caveats concerning generalization from laboratory to clinical contexts.[46] Regrettably, as a result of a lack of funding, we were unable to do the translational research necessary to examine the safety and efficacy of herbal formulations in humans who are treated with ECT for psychiatric disorders; however, we did demonstrate that one formulation attenuates age-related cognitive decline in a community-based population.[1] The field remains open for further investigation.
The mechanism of action of ECT
Today, so much is known about the mechanism of action of ECT that it would take a book to review the field. Our contribution to this area has been small. Using animal models, we showed that ECT downregulates dopamine autoreceptors and alpha-2 adrenoceptors, and upregulates dopamine postsynaptic receptors, thereby facilitating both dopaminergic and noradrenergic neurotransmission.[144–149,151] Importantly, for the first time in literature, we showed that maintenance ECT maintains the alpha-adrenergic receptor change[149] and also that high but not low dose electrical stimuli elicit the dopamine postsynaptic receptor upregulation that is seen with ECT.[151]
Electrical aspects of ECT
Medications are dosed in terms of weight of the active ingredient. In ECT, the active ingredient is electricity. Curiously, most practitioners dose ECT in terms of the number of treatments administered; this is like asking patients to take a certain number of tablets without regard to the strength of the pill. Work by Sackeim and his colleagues, starting from the late 1980s, established that higher electrical doses with reference to the seizure threshold increase the likelihood of response in patients who receive unilateral ECT, and increase the speed of response whatever the electrode placement.[173–175] This encouraged us to probe deeper into electrical aspects of ECT.[97,98,127,151–165]
The stimulus in brief-pulse ECT is made up of four elements: pulse amplitude, pulse width, pulse frequency, and stimulus duration. These four elements can be combined in different ways to yield the same charge; that is, the same electrical dose. But, there is no assurance that differently constituted charges have identical biological effects.[155] In a tightly controlled study conducted in an animal model, we furnished unequivocal evidence that the seizure threshold is a variable point, and that, at constant electrical charge, it is lower when the electrical stimulus train duration is longer.[157] The importance of this study was underlined when the Association for Convulsive Therapy, USA, honored it with the ECT Investigator of the Year Award at its annual conference in San Francisco, during 2003.
In another study with far-reaching implications, we used power spectral electroencephalogram (EEG) analysis in an animal model to demonstrate how differently constituted electrical stimuli that were identical in charge could nevertheless differ in therapeutic efficacy and in adverse effect potential. We found that a narrow pulse width associated with high pulse frequency, especially in combination with a long stimulus train, were associated with three different EEG proxies of seizure efficacy: greater ictal power, greater postictal suppression, and greater interictal power, particularly in the lower frequency bands. In contrast, greater pulse width and lower pulse frequency showed no such associations. Stimulus-on time, number of pulses delivered, and rate of delivery of charge did not predict efficacy.[159] These findings could have much influence on the way ECT charges are constituted in clinical contexts. Clinical research on narrow pulse widths is now being pursued by several teams with encouraging results.
A chance observation during our work on the ECT seizure threshold led us to a dramatic new finding in neuroscience: that subconvulsive stimuli delivered to the brain lower the seizure threshold on subsequent days. In other words, the brain as a whole appears to respond more readily with a seizure discharge if previously primed with subconvulsive stimuli.[161] We confirmed this kindling effect in a controlled study.[162] Although we labeled the phenomenon ‘whole-brain kindling’, we later noted that what was actually kindled was probably only the brain territories involved in the triggering of the seizure.[163]
Unmodified ECT
In a 1991 survey of the Indian Psychiatric Society, we found that only 44% of respondents to a postal questionnaire invariably administered modified ECT.[95] Our initial response was one of shock and indignation.[95,166] However, in an observational study,[167] we found that, notwithstanding the 20-40% incidence of musculoskeletal complications with unmodified ECT in historical Western studies, only 1 of 50 patients who received a course of 6 unmodified treatments actually experienced a spinal complication (which turned out to be subclinical). We subsequently revised our stance to the cautious position that modified ECT is desirable, but in exceptional circumstances unmodified ECT may be better than no ECT. We also examined issues related to ethics, and the definition of what might constitute exceptional circumstances for a country such as India.[168–171] In our most recent study, we confirmed our finding that musculoskeletal complications with unmodified ECT are rare in Indian patients, and suggested that benzodiazepine-modification of the seizure (resulting in drug-induced muscle relaxation) may be one of several explanations for our unexpected findings.[172]
MECHANISMS UNDERLYING ECT-INDUCED AMNESTIC DEFICITS
Returning to the subject of our earlier research,[38–47] we reasoned that if we could understand what mechanisms explain ECT-induced amnestic deficits, we might be able to better develop treatments to diminish such deficits. Our lines of investigation followed two paths: we studied whether attenuating the systolic blood pressure surge during ECT results in decreased cognitive adverse effects[176–180] and we investigated the involvement of specific neurotransmitter mechanisms in ECT-induced amnestic deficits.[181–184] Curiously, two of the blood pressure surge studies themselves suggested neurotransmitter mechanisms.[177–178]
Hypertensive surge and cognitive impairment with ECT
It has long been known that there is a sharp surge in systolic blood pressure during the ECT seizure.[180] Hypothetically, this surge may breach the blood-brain barrier, causing hyperperfusion-related mild cerebral edema as well as a leak of proteins and other macromolecules into the CSF and interstitial spaces in the brain. These changes may disturb neuronal functioning, predisposing to cognitive impairment.[179] We reviewed the clinical literature on the subject and found some support for this hypothesis.[176] Accordingly, we used an animal model to study whether the calcium channel blockers verapamil and felodipine, administered shortly before ECT, can reduce the amnestic deficits of the treatment. We chose both verapamil and felodipine because both belong to the same category of drug; and because the latter has only peripheral action.
In this study,[176] we demonstrated that just two once-daily ECT sufficed to induce retrograde amnesia in rats trained in the Hebb-Williams maze; verapamil or felodipine, administered half an hour before each ECT, attenuated this amnesia. On the surface, these finding support our hypothesis. However, we realized that other mechanisms could also have been be involved: cerebral vasodilatation, enhancement of cholinergic tone, and inhibition of calcium-mediated excitotoxic impairment of neuronal functioning.
In order to further investigate our hypothesis, we studied the effects of a different antihypertensive agent: sodium nitroprusside[177] and we separately asked whether increasing blood pressure, using phenylephrine, worsens amnestic deficits with ECT.[178]
We found that three once-daily ECT successfully induced retrograde amnesia in a passive avoidance task, and that the pre-ECT administration of nitroprusside, despite increasing the seizure duration, reduced the severity of this amnesia. Unexpectedly, nitroprusside also improved recall in control rats which did not receive ECT.
What do we make of these results? Nitroprusside may have attenuated the amnesia by reducing the systolic blood pressure surge during ECT. But, nitroprusside may also have worked by other mechanisms, such as by improving cerebral perfusion through vasodilatation. And, nitroprusside is a nitric oxide donor, and nitric oxide mechanisms are known to facilitate learning and memory. Most importantly, the improved recall in control rats suggested that nitroprusside could have nonspecific effects on cognition that are independent of ECT and the blood-brain barrier breach mechanism. As a result, these findings[177] again left us without a firm conclusion.
So, we moved to the phenylephrine study.[178] Phenylephrine is a nonselective alpha receptor agonist which can be expected to raise blood pressure. We examined whether the administration of phenylephrine immediately before ECT would increase ECT-induced amnesia. As before, we found that three once-daily ECT induced retrograde amnesia in a passive avoidance task. Surprisingly, (and even more so because we found that, like nitroprusside, it increased seizure duration), phenylephrine attenuated the ECT-induced amnestic deficits. An additional unexpected finding was that phenylephrine also improved recall in rats which received sham ECT. This led us to conclude that the adrenergic effects of phenylephrine nonspecifically improved memory functioning; in this context, adrenergic mechanisms have long been known to facilitate memory consolidation and storage. Since phenylephrine increases blood pressure, our most recent findings weakened the hypothesis that ECT-induced cognitive impairment is a consequence of cerebral edema and blood-brain barrier breach resulting from the intra-ECT hypertensive surge. However, it remained possible that the adrenergic cognitive-facilitatory mechanisms of phenylephrine could have more than counterbalanced the hypertensive surge cognition-disturbing mechanisms of ECT as augmented by the drug.
A limitation of these experiments is that we did not actually measure blood pressure changes with the drugs that we administered. Nevertheless, the findings that nitroprusside, phenylephrine, and calcium channel blockers attenuate ECT-induced amnestic deficits are still positive results worthy of clinical examination. As far as the hypertensive surge hypothesis is concerned, the field remains open for further study.
Glucocorticoid mechanisms and ECT-induced amnesia
The hippocampus and the amygdala are both involved in learning and memory, and neurons in both structures richly express glucocorticoid receptors. Physiological activation of these receptors facilitates long-term potentiation (LTP) and neuroplasticity; that is, mechanisms which represent the cellular basis of learning and memory. However, excessive glucocorticoid stimulation (such as due to hypercortisolemia resulting from chronic stress, chronic depression, Cushing's syndrome, or the administration of exogenous steroids for therapeutic purposes) has been shown to induce a loss of dendritic spines and synapses, shrink the hippocampus, and impair cognition in both animal models and humans.[185]
Our review of literature[182] showed that ECT results in a hypercortisolemic surge that, though transient, lasts for hours after the seizure. Could this surge be responsible for the cognitive adverse effects of the treatment? Could blunting this surge attenuate ECT-induced cognitive impairments? We investigated the subject using the abortifacient drug mifepristone as an in vivo probe. In parentheses, mifepristone is used in gynecological practice because it is a competitive progesterone receptor antagonist; but mifepristone is also a highly potent glucocorticoid receptor antagonist. If ECT-induced hypercortisolemia stimulates hippocampal neurons physiologically, the transient hypercortisolemia could be a natural though incompletely effective defense against ECT-induced cognitive impairment; if so, mifepristone would worsen ECT-induced amnesia. But, if ECT-induced hypercortisolemia stimulates hippocampal neurons to a pathological degree, it would contribute to ECT-induced cognitive impairment; if so, mifepristone would reduce amnestic deficits with ECT.
As we had done earlier, we investigated the matter in an animal model. We administered mifepristone in two different doses (20 mg/kg/day and 40 mg/kg/day) one hour before each of five once-daily ECTs to rats that had been trained in a passive avoidance task. We tested recall a day after the last ECT. A part of our results is presented in Figure 1. ECT resulted in retrograde amnesia in the control but not in the mifepristone groups. The higher dose of mifepristone was associated with significantly better recall scores relative to the control group. Thus, mifepristone clearly protected against ECT-induced retrograde amnesia. This implicates the ECT-induced hypercortisolemic surge (with consequent overstimulation of glucocorticoid receptors) as one mechanism of ECT-induced amnestic deficits, and suggests that glucocorticoid mechanisms can be modulated to protect against the development of these deficits.
Figure 1.
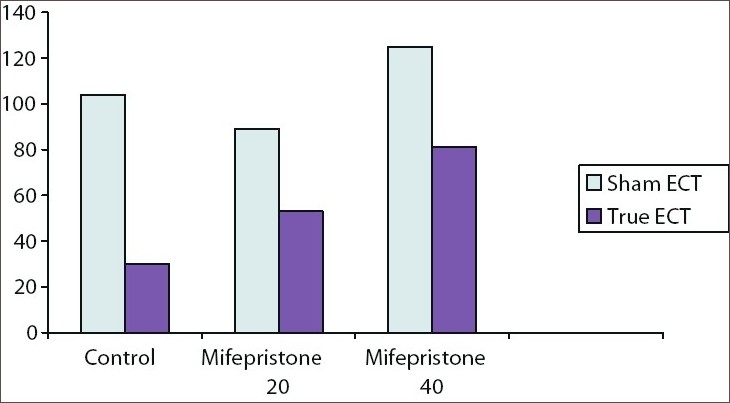
Mean recall scores in control and mifepristone (20mg/kg/day and 40 mg/kg/day) groups after five oncedaily ECT (lower scores indicate poorer recall; that is, greater retrograde amnesia). There was significant amnesia in the control but not mifepristone groups; recall scores were significantly higher in the mifepristone 40 mg/kg/day (but not 20 mg/kg/day) group relative to the control group
ECT and glutamateric excitotoxicity: A role for NMDA receptor, COX-2, and kynurenic acid modulation in ECT-induced amnesia
We next attempted something more ambitious: the study of the role of glutamatergic neurotransmission at the N-methyl D-aspartate (NMDA) receptor site, along with cyclooxygenase-2 (COX-2) and kynurenic acid lipid signalling mechanisms, in ECT-induced retrograde amnesia. Our work on this subject began with the well-known observation that chronic treatment with antiinflammatory drugs such as indomethacin in conditions such as rheumatoid arthritis is associated with a decreased risk of Alzheimer's disease; we therefore examined the neuroprotective effects of indomethacin and were pleasantly surprised to find that this drug reduced the severity of ECT-induced amnesia.[181] Indomethacin, however, is COX-nonselective and is, in fact, more effective against COX-1 than against COX-2; this is a negative for the drug because COX-2 is more important in learning mechanisms than COX-1.[183] Our review of literature[183] also showed that indomethacin increases kynurenic acid levels whereas COX-2 selective drugs such as celecoxib decrease kynurenic acid; as kynurenic acid dampens neurotransmission at all ionotropic glutamatergic receptor sites, and as glutamatergic neurotransmission is fundamental to LTP and neuroplasticity, celecoxib could be expected to demonstrate greater neuroprotective efficacy than indomethacin.
Normally, glutamate released during learning stimulates the NMDA receptor, launching a cascade of downstream events [Figure 2]. These events include activation of COX-2 and thence a conversion of membrane arachidonic acid to endogenous prostanoids, as well as a conversion of endogenous cannabinoids (which dampen NMDA signalling) into novel prostaglandins (which increase NMDA activity). These events also include the activation of platelet activation factor (PAF). The COX-2 and PAF activation result in feedback augmentation of the NMDA signal. Other reverberating circuits (including those that involve retrograde neurotransmission through arachidonic acid) that amplify the NMDA signal also develop.[183,184] The net effect is glutamate- and NMDA-dependent initiation of LTP in the hippocampus, and thence the hardwiring of learning and memory through neuroplasticity changes.
Figure 2.
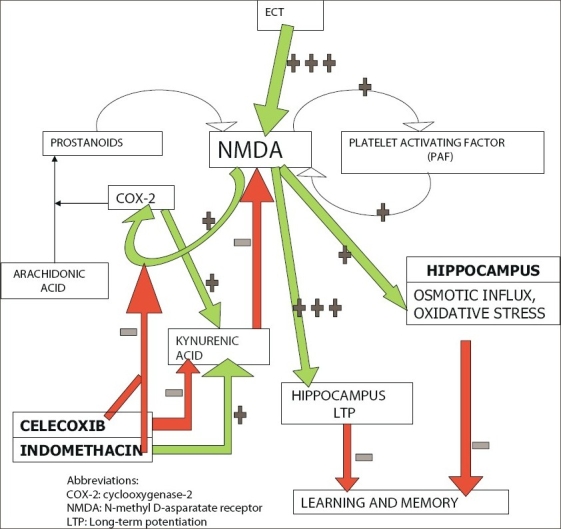
Glutamatergic mechanisms in learning, memory, and ECT-induced amnesia, and the europrotective mechanisms of indomethacin and celecoxib herein
Glutamatergic signaling is pathologically upregulated due to massive neuronal discharge during the ECT seizure. This may hypothetically result in a saturation of the cellular mechanisms that are responsible for hippocampal LTP and hence a decreased opportunity for the recruitment of these mechanisms for new learning. The consequence is ECT-induced amnesia.[183] Increased neuronal activity during the ECT seizure could additionally result in oxidative stress, and in an osmotic influx due to excessive (NMDA-mediated) entry of calcium into the cell; oxidative stress and osmotic load could both further contribute to ECT-induced cognitive impairment [Figure 2].
Under physiological conditions, COX-2 inhibitors inhibit learning and memory by dampening NMDA signaling. However, administration of COX-2 inhibitors pre-ECT could prevent glutamatergic excitotoxicity by blocking at least some of the reverberating circuits [Figure 2]; as a result, there could be less osmotic influx, less oxidative stress, and less saturation of LTP mechanisms. In consequence, ECT-induced amnesia may diminish. Based on the model that we constructed (simplified in Figure 2), we hypothesized that celecoxib would outperform indomethacin in this regard because, unlike indomethacin, celecoxib would decrease kynurenic acid, thereby facilitating normal NMDA signalling after the seizure.[183]
We again conducted our studies in an animal model. We administered indomethacin (4 mg/kg/day) or celecoxib (15 mg/kg/day) to rats that received five once-daily ECT. We tested recall of passive avoidance learning a day after the last ECT. A part of our results is summarized in Figure 3. ECT resulted in retrograde amnesia in the control but not in the indomethacin and celecoxib groups. Whereas indomethacin partly protected against ECT-induced amnestic deficits, retrograde amnesia was completely prevented in the animals which received celecoxib. These findings support the model that we constructed [Figure 2] and suggest that glutamatergic and COX-2 mechanisms can be modulated to protect against ECT-induced retrograde amnesia in clinical contexts. In parentheses, interested readers would do well to read our studies in the original for a more detailed exposition of the mechanisms involved.
Figure 3.
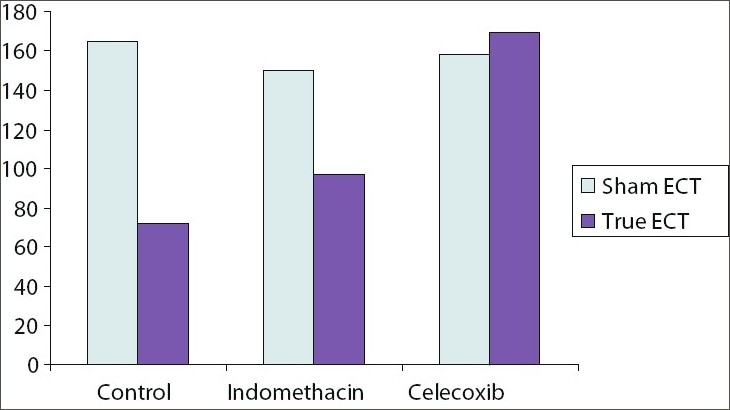
Mean recall scores in control, indomethacin (4 mg/kg/day), and celecoxib (15 mg/kg/day) groups after five once-daily ECT (lower scores indicate poorer recall; that is, greater retrograde amnesia). There was significant amnesia in the control but not indomethacin and celecoxib groups; recall scores were significantly higher with celecoxib (but not indomethacin) relative to controls.
WHERE DO WE GO FROM HERE?
As we described in an extensive review, we now know a great deal about the mechanisms underlying ECT-induced amnestic deficits[126] and there are extensive animal data to suggest pharmacological approaches towards the prevention of such deficits. Our own work suggests that certain herbal formulations, certain antihypertensive drugs (especially the calcium channel blockers), the glucocorticoid antagonist mifepristone, and certain antiinflammatory agents (especially the COX-2 inhibitors) justify further study in this regard. More specifically, translational research is now necessary to identify whether the findings from the laboratory can be extrapolated to the clinic.
Concluding notes
This has been a long journey, but there are still miles and miles to go. I am conscious that I cannot alone do all that is left to do. I am conscious that there is a large pool of talent in this country that is looking for direction in researching important questions in psychiatry and psychopharmacology. I now wish to devote a greater proportion of my time to the development of research talent so that ambitious students and academicians in this country can feature more prominently on the global map. From my experience during the past few years, I have come to realize that possibly the only model which will succeed in this goal is one that is based on the academic institute. It would give me much pleasure to provide interested institutes in this country with training in research methods and statistics; to provide research questions and methodologies for the study of these questions; and to provide backup support for data analysis and report writing. If at the end of these exercises Indian psychiatry grows stronger in research capacity, my efforts would not have been in vain.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
A paper presented for DLN Murthy Rao Oration, Indian Psychiatric Society, Kolkata, 2008
REFERENCES
- 1.Andrade C, Gowda S, Chaturvedi SK. Treatment of age-related cognitive decline with a herbal formulation: A double-blind study. Indian J Psychiatry. 1998;40:240–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade C. Risperidone worsens fluoxetine-treated OCD. J Clin Psychiatry. 1998;59:255–6. doi: 10.4088/jcp.v59n0509c. [DOI] [PubMed] [Google Scholar]
- 3.Andrade C. Obsessive-compulsive symptoms with risperidone. J Clin Psychiatry. 1999;60:261–2. doi: 10.4088/jcp.v60n0412b. [DOI] [PubMed] [Google Scholar]
- 4.Andrade C, Aswath A, Chaturvedi SK, Srinivasa M, Raguram R. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42:295–301. [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade C, Aswath A, Chaturvedi SK, Raguram R, Bhide A. A double-blind, controlled evaluation of the efficacy and adverse effect profile of sustained-release alprazolam. Indian J Psychiatry. 2000;42:302–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Andrade C, Srihari BS, Reddy KP, Chandramma L. Melatonin in medically ill patients with insomnia: A double-blind, placebo-controlled study. J Clin Psychiatry. 2001;62:41–5. doi: 10.4088/jcp.v62n0109. [DOI] [PubMed] [Google Scholar]
- 7.Kumar PN, Andrade C, Krishnankutty N, Nair SM. An open clinical trial with risperidone in chronic schizophrenia. Indian J Psychiatry. 2001;43:152–6. [Google Scholar]
- 8.Andrade C. Confusion and dysphoria with low-dose topiramate in a patient with bipolar disorder. Bipolar Disord. 2001;3:211–2. [PubMed] [Google Scholar]
- 9.Andrade C, Aswath A, Chaturvedi SK, Raguram R, Srinivasa M, Raguram R. GS-02 for dysthymic disorder: Results of a preliminary, open study. J Herbal Pharmacotherapy. 2002;2:49–55. [PubMed] [Google Scholar]
- 10.Patel V, Andrade C. Pharmacological treatment of severe psychiatric disorders in the developing world: Lessons from India. Curr Opin. 2003;17:1071–80. doi: 10.2165/00023210-200317150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Andrade C. Antidepressant-withdrawal mania: A critical review and synthesis of the literature. J Clin Psychiatry. 2004;65:987–93. [PubMed] [Google Scholar]
- 12.Andrade C. The risk of harm in mania and the very early time course of improvement: Important but neglected variables in treatment research. Bipolar Disord. 2004;6:446–7. doi: 10.1111/j.1399-5618.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 13.Berk M, Shin Shah I, Andrade C, Joyce P, Tiebang L, Ha KS. Australasian commentary on CANMAT guidelines for treatment of bipolar disorder. Bipolar Disord. 2005;7:83–6. doi: 10.1111/j.1399-5618.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 14.Andrade C, Bhakta SG, Singh NM. Controversy revisited: Selective serotonin reuptake inhibitors in pediatric depression. World J Biol Psychiatry. 2006;7:251–60. doi: 10.1080/15622970600702690. [DOI] [PubMed] [Google Scholar]
- 15.Kumar PNS, Andrade C, Bhakta SG, Singh NM. Melatonin in schizophrenic outpatients with insomnia: A double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:237–41. doi: 10.4088/jcp.v68n0208. [DOI] [PubMed] [Google Scholar]
- 16.Andrade C, Shah N, Chandra S. The new patent regime: Implications for patients in India. Indian J Psychiatry. 2007;49:56–9. doi: 10.4103/0019-5545.31520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade C, Kharawala S. First-vs second-generation antipsychotic drugs in schizophrenia. Arch Gen Psychiatry. 2007;64:978–9. doi: 10.1001/archpsyc.64.8.978-b. [DOI] [PubMed] [Google Scholar]
- 18.Andrade C. Rapid tranquilization in emergency psychiatry. BMJ. 2007;335:835–6. doi: 10.1136/bmj.39359.614387.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhakta SG, Andrade C. Melatonin treatment of fluoxetine-induced tardive dyskinesia. World J Biol Psychiatry. 2008 doi: 10.1080/15622970802650044. (in press) [DOI] [PubMed] [Google Scholar]
- 20.Kumar CN, Andrade C, Murthy P. A randomized, double-blind comparison of lorazepam and chlordiazepoxide in patients with uncomplicated alcohol withdrawal. J Stud Alcohol Drugs. 2008. (in press) [DOI] [PubMed]
- 21.Arunasmitha S, Andrade C, Pradhan N. Biphasic effect of apomorphine on rodent motility. Indian J Physiol Pharmacol. 1989;33:132–3. [PubMed] [Google Scholar]
- 22.Andrade C, Arunasmitha S, Pradhan N. Apomorphine-induced time-dependant potentiation of dopamine post-synaptic receptor response. NIMHANS J. 1990;8:53–5. [Google Scholar]
- 23.Andrade C, Pradhan N. Tardive dyskinesia: A potential new neurochemical animal model. Indian J Psychiatry. 1990;32:273–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Pradhan N, Harihar C, Das P, Andrade C. Heterogeneity in plasma homovanillic acid levels in schizophreniform disorder. Indian J Psychiatry. 1992;34:128–32. [PMC free article] [PubMed] [Google Scholar]
- 25.Naga Rani MA, Andrade C, David J. Effect of dothiepin on nociceptive response in diabetic rats. Indian J Physiol Pharmacol. 1992;36:93–6. [PubMed] [Google Scholar]
- 26.George J, Andrade C, Joseph T. Delayed effects of acute oral and chronic inhalational exposure to methylparathion on learning and memory in rats. Indian J Exp Biol. 1992;30:819–22. [PubMed] [Google Scholar]
- 27.George J, Andrade C, Joseph T. Alterations in immediate and recent memory following acute oral and chronic inhalational exposure to methylparathion in rats. Indian J Pharmacol. 1993;25:78–82. [PubMed] [Google Scholar]
- 28.Venkataraman BV, Naga Rani MA, Andrade C, Joseph T. Improved colorimetric method for cholinesterase activity. Indian J Physiol Pharmacol. 1993;37:82–4. [PubMed] [Google Scholar]
- 29.Jaiprakash R, Naga Rani MA, Venkataraman BV, Andrade C. Effect of felodipine on serum lipid profile in short-term streptozotocin-diabetes in rats. Indian J Exp Biol. 1993;31:283–4. [PubMed] [Google Scholar]
- 30.Andrade C, George J, Joseph T. BR-16A does not interfere with alpha-2 noradrenergic and dopamine postsynaptic receptor functioning. Indian J Psychiatry. 1993;35:179–80. [PMC free article] [PubMed] [Google Scholar]
- 31.Naga Rani MA, Venkataraman BV, Jaiprakash R, Andrade C. Effect of felodipine on cholinergic responses in isolated STZ diabetic rat. Indian J Physiol Pharmacol. 1993;37:80. [Google Scholar]
- 32.Naga Rani MA, Venkataraman BV, Jaiprakash R, Andrade C. Effect of felodipine on myocardial function and cholinergic responses in short-term streptozotocin diabetes in rats. Indian J Exptl Biol. 1994;32:629–32. [PubMed] [Google Scholar]
- 33.Jaiprakash R, Naga Rani MA, Venkataraman BV, Andrade C. Effect of felodipine on cholinergic responses of the colonic smooth muscle of streptozotocin-induced diabetic rats. Indian J Exp Biol. 1995;33:297–9. [PubMed] [Google Scholar]
- 34.Naga Rani MA, Chandran U, Andrade C, Venkataraman BV. Felodipine potentiates morphine analgesia in control and streptozotocin-diabetic female rats. Indian J Exptl Biol. 1996;34:663–6. [PubMed] [Google Scholar]
- 35.Andrade C, Alwarshetty M, Sudha S, Chandra JS. Effect of innate direction bias on T-maze learning in rats: Implications for research. J Neurosci Methods. 2001;110:31–5. doi: 10.1016/s0165-0270(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 36.Andrade C, Raj T, Udaya HB, Chandra JS. Effect of BR-16A on alpha-2 adrenergic, dopamine autoreceptor and dopamine postsynaptic receptor functioning. Indian J Pharmacol. 1994;26:292–5. [Google Scholar]
- 37.Andrade C. Herbal medical practice at the other side of the globe. Psychiatr Times. 2003;20:28–9. [Google Scholar]
- 38.Andrade C, Joseph J, Chandra JS, Venkataraman BV, Naga Rani MA. ECT-induced anterograde amnesia: Can the deficits be minimized? Convul Ther. 1994;10:59–64. [PubMed] [Google Scholar]
- 39.Joseph J, Venkataraman BV, Naga Rani MA, Andrade C. BR-16A protects against ECS-induced anterograde amnesia. Biol Psychiatry. 1994;36:478–81. doi: 10.1016/0006-3223(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 40.Andrade C, Udaya HB, Chandra JS. BR-16A restricts development of ECS-induced retrograde amnesia. Biol Psychiatry. 1995;37:820–2. doi: 10.1016/0006-3223(95)00039-J. [DOI] [PubMed] [Google Scholar]
- 41.Ramteke S, Andrade C, Faruqi S, Joseph J, Venkataraman BV, Naga Rani MA. BR-16A attenuates anterograde amnesia induced by electroconvulsive shocks in slow learning rats. Indian J Pharmacol. 1995;27:186–8. [Google Scholar]
- 42.Faruqi S, Andrade C, Ramteke S, Joseph J, Venkataraman BV, Naga Rani MA. Herbal pharmacotherapy for the attenuation of ECS-induced anterograde and retrograde amnestic deficits. Convulsive Ther. 1995;11:241–7. [PubMed] [Google Scholar]
- 43.Andrade C, Mathews M, George V, Anil M, George J, Naga Rani MA. Lack of effect of Shankapushpi in the attenuation of ECS-induced memory deficits. Indian J Psychiatry. 1996;38:11. [Google Scholar]
- 44.Vinekar AS, Andrade C, Sriprada VT, George J, Joseph T, Chandra JS. Attenuation of ECS-induced retrograde amnesia by using a herbal formulation. J ECT. 1998;14:83–8. [PubMed] [Google Scholar]
- 45.Andrade C, Anitha K, Moola B, Hegde R, Chandra JS. A simplified herbal formulation attenuates electroconvulsive shock-induced anterograde amnesia. J ECT. 1999;15:164–5. doi: 10.1097/00124509-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Andrade C, Sudha S, Venkataraman BV. Herbal treatments for ECS-induced memory deficits: A review of research and a discussion on animal models. J ECT. 2000;16:144–56. doi: 10.1097/00124509-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Andrade C, Chandra JS. Anti-amnestic properties of Brahmi and Mandookaparni in a rat model. Indian J Psychiatry. 2006;48:232–7. doi: 10.4103/0019-5545.31554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrade C. Indian herbal medicine and the treatment of addictive disorders: Focus on Ashwagandha (Withania somnifera) In: Ghodse H, editor. Herbal treatments for addictive disorders. London: St George's University of London; 2006. [Google Scholar]
- 49.Andrade C, Srinath S. True auditory hallucinations as a conversion symptom. Br J Psychiatry. 1986;148:100–2. doi: 10.1192/bjp.148.1.100. [DOI] [PubMed] [Google Scholar]
- 50.Andrade C, Gangadhar BN, Channabasavanna SM. Catatonic signs in schizophrenia. Br J Psychiatry. 1987;151:709. doi: 10.1192/s0007125000284390. [DOI] [PubMed] [Google Scholar]
- 51.Andrade C. Capgras syndrome: Delusion on illusion. J Clin Psychiatry. 1988;49:204. [PubMed] [Google Scholar]
- 52.Andrade C, Srinath S, Andrade AC. True hallucinations as a culturally sanctioned experience. Br J Psychiatry. 1988;152:838–9. doi: 10.1192/bjp.152.6.838. [DOI] [PubMed] [Google Scholar]
- 53.Andrade C, Srinath S, Andrade AC. True hallucinations in non-psychotic states. Can J Psychiatry. 1989;34:704–6. doi: 10.1177/070674378903400714. [DOI] [PubMed] [Google Scholar]
- 54.Andrade C, Rao NS. Musical obsessions. Indian J Psychiatry. 1997;39:178–80. [PMC free article] [PubMed] [Google Scholar]
- 55.Andrade C, Srihari BS. A preliminary survey of rhinotillexomania in an adolescent sample. J Clin Psychiatry. 2001;62:426–31. doi: 10.4088/jcp.v62n0605. [DOI] [PubMed] [Google Scholar]
- 56.Andrade C, Rao NS. Manic stupor. Indian J Psychiatry. 2001;43:285–7. [PMC free article] [PubMed] [Google Scholar]
- 57.Andrade C. A memorable patient: Straight from the patient's mouth. BMJ. 2002;325:1169. [Google Scholar]
- 58.Andrade C, Singh NM, Bhakta S. Simultaneous true seizures and pseudoseizures. J Clin Psychiatry. 2006;67:673. doi: 10.4088/jcp.v67n0420a. [DOI] [PubMed] [Google Scholar]
- 59.Desai NG, Andrade C, Gangadhar BN, Channabasavanna SM. Carbamazepine-lithium combination for lithium-resistant mania. Indian J Psychiatry. 1986;28:159–61. [PMC free article] [PubMed] [Google Scholar]
- 60.Andrade C, Gangadhar BN, Channabasavanna SM. Pathological neurotoxicity with lithium. Indian J Psychiatry. 1987;29:279–81. [PMC free article] [PubMed] [Google Scholar]
- 61.Andrade C, Pradhan N. Lithium therapy and prophylaxis. J Clin Psychiatry. 1990;51:37. [PubMed] [Google Scholar]
- 62.Suresh KP, Prasad KM, Mohan R, Andrade C, Ashok MV, Chaturvedi SK, et al. Once daily versus divided dosage lithium therapy in acute mania. Indian J Psychiatry. 1994;37:9–12. [PMC free article] [PubMed] [Google Scholar]
- 63.Mohan R, Suresh KP, Prasad KM, Ashok MV, Andrade C, Sreenivas KN, et al. Once daily lithium in the prophylaxis of mood disorders. Indian J Psychiatry. 1996;38:104–8. [PMC free article] [PubMed] [Google Scholar]
- 64.Andrade C. The pharmacological treatment of bipolar disorder: More questions than answers. In: Maj M, editor. Evidence and experience in psychiatry. Bipolar Disorder. World Psychiatric Association Series. Vol 5. London: John Wiley and Sons; 2002. pp. 252–5. [Google Scholar]
- 65.Mendhekar DN, Sunder KR, Andrade C. Aripiprazole use in a pregnant schizoaffective woman. Bipolar Disord. 2006;8:299–300. doi: 10.1111/j.1399-5618.2006.00316.x. [DOI] [PubMed] [Google Scholar]
- 66.Andrade C. Not significantly different is not the same as significantly similar (ltr) Biol Psychiatry. 1993;34:671. doi: 10.1016/0006-3223(93)90163-8. [DOI] [PubMed] [Google Scholar]
- 67.Andrade C, Rao NS. Naltrexone and alcohol dependance: Some methodological issues. Arch Gen Psychiatry. 1994;51:335–6. doi: 10.1001/archpsyc.1994.03950040079013. [DOI] [PubMed] [Google Scholar]
- 68.Andrade C, Choudhury P. Do Indian researchers read Indian research? Indian J Psychiatry. 1994;36:173–6. [PMC free article] [PubMed] [Google Scholar]
- 69.Andrade C. Yoga: Some unanswered questions. Indian J Psychiatry. 1995;37:189. [PMC free article] [PubMed] [Google Scholar]
- 70.Andrade AC, Andrade C, Lakshmi R, Reshma M. A scientific method for the study of quasi-scientific disciplines: Results of a preliminary investigation on astrological forecasts and behaviour. Indian J Psychol Med. 1996;19:32–8. [Google Scholar]
- 71.Andrade C. Analysis of repeated assessments data. Indian J Psychiatry. 1998;40:392–3. [PMC free article] [PubMed] [Google Scholar]
- 72.Andrade C, Kiran S, Rao NS, Choudhury P. Do Indian researchers read Indian research? A reappraisal, four years later. Indian J Psychiatry. 2000;42:203–8. [PMC free article] [PubMed] [Google Scholar]
- 73.Andrade C. Categorizing continuous variables. Can J Psychiatry. 2002;47:35. doi: 10.1177/070674370204700916. [DOI] [PubMed] [Google Scholar]
- 74.Andrade C. Yoga: Better treatment or better placebo? Indian J Psychiatry. 2002;44:83. [PMC free article] [PubMed] [Google Scholar]
- 75.Andrade C. Transcendental meditation and components of the metabolic syndrome: Methodological issues. Arch Intern Med. 2006;166:2553. doi: 10.1001/archinte.166.22.2553-a. [DOI] [PubMed] [Google Scholar]
- 76.Andrade C. Confounding. Indian J Psychiatry. 2007;49:129–31. doi: 10.4103/0019-5545.33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andrade C, Sarmah P, Channabasavanna SM. Psychological well-being and morbidity in parents of narcotic dependant males. Indian J Psychiatry. 1989;31:122–7. [PMC free article] [PubMed] [Google Scholar]
- 78.Sarmah P, Andrade C, Channabasavanna SM. Personality characteristics of parents of male narcotic dependant patients: A preliminary investigation of the narcotic family. Indian J Psychol Med. 1989;12:17–20. [Google Scholar]
- 79.Sarmah PL, Choudhury P, Andrade C, Channabasavanna SM. Dyadic adjustment in parents of narcotic dependant males. Indian J Psychol Med. 1994;17:35–42. [Google Scholar]
- 80.Andrade C, Choudhury P, Sarmah PL, Channabasavanna SM. Marital perceptions of parents of narcotic dependant males. Indian J Psychol Med. 1996;19:40–55. [Google Scholar]
- 81.Andrade C, Postma K, Abraham K. Influence of women's work status on the well-being of Indian couples. Int J Soc Psychiatry. 1999;45:65–75. doi: 10.1177/002076409904500108. [DOI] [PubMed] [Google Scholar]
- 82.Andrade C. Happier families: A self-help guide. Bombay: Pauline Sisters Bombay Society; 2000. [Google Scholar]
- 83.Andrade C. Basics of counselling. New Delhi: Indira Gandhi National Open University; 2001. [Google Scholar]
- 84.Andrade C. Existing trends in counselling services in India. In: Thomas G, editor. Counselling on HIV and family matters. New Delhi: Indira Gandhi National Open University; 2001. pp. 53–67. [Google Scholar]
- 85.Machado T, Andrade C. The psyche at high altitude. Indian J Clin Psychol. 1985;11:41–5. [Google Scholar]
- 86.Andrade AC, Fernandes C, Verghese L, Andrade C. Effect of negative ion atmospheric loading on cognitive functioning in human volunteers. Indian J Psychiatry. 1992;34:253–9. [PMC free article] [PubMed] [Google Scholar]
- 87.Andrade C, Tharaken J, Chari S. Detection of malingering through the use of Raven's standard progressive matrices. Indian J Psychiatry. 2001;42:36–40. [PMC free article] [PubMed] [Google Scholar]
- 88.Andrade C, Madhavan AP, Kishore ML. Testing logical memory using a complex passage: Development and standardization of a new test. Indian J Psychiatry. 2001;43:252–6. [PMC free article] [PubMed] [Google Scholar]
- 89.Andrade C. Multiple expansion disorder. Bipolar Disord. 2004;6:168–9. doi: 10.1111/j.1399-5618.2003.00095.x. [DOI] [PubMed] [Google Scholar]
- 90.Andrade C, Jamuna N. The Flynn effect: An alert to clinicians. Indian J Psychiatry. 2004;46:166–8. [PMC free article] [PubMed] [Google Scholar]
- 91.Andrade C. The national institute of open schooling. Indian J Psychiatry. 2008;50:227–8. doi: 10.4103/0019-5545.43618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simpson ID, Tanwar PD, Andrade C, Kochar DK, Norris RL. The Ebbinghaus retention curve: Training does not increase the ability to apply pressure immobilisation in simulated snake bite-implications for snake bite first aid in the developing world. Trans R Soc Trop Med Hyg. 2008;102:451–9. doi: 10.1016/j.trstmh.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 93.Ramanathan A, Andrade C. ECT in India: Historical snippets. Convulsive Ther. 1995;11:225–7. [Google Scholar]
- 94.Agarwal AK, Andrade C, Reddy MV. The practice of ECT in India: Issues relating to the administration of ECT. Indian J Psychiatry. 1992;34:285–97. [PMC free article] [PubMed] [Google Scholar]
- 95.Andrade C, Agarwal AK, Reddy MV. The practice of ECT in India. 2. The practical administration of ECT. Indian J Psychiatry. 1993;35:81–6. [PMC free article] [PubMed] [Google Scholar]
- 96.Andrade C. Electroconvulsive therapy in India. J ECT. 2006;22:231. doi: 10.1097/01.yct.0000235930.24032.5c. [DOI] [PubMed] [Google Scholar]
- 97.Andrade C, Gangadhar BN, Subbakrishna DK, Channabasavanna SM, Pradhan N. A double-blind comparison of sinusoidal wave and brief-pulse electroconvulsive therapy in endogenous depression. Convulsive Ther. 1988;4:297–305. [PubMed] [Google Scholar]
- 98.Andrade C, Gangadhar BN, Channabasavanna SM, Pradhan N. Does ECT stimulus waveform influence rate of recovery in endogenous depression? NIMHANS J. 1988;6:121–6. [Google Scholar]
- 99.Andrade C, Gangadhar BN, Swaminath G, Channabasavanna SM. Predicting the outcome of endogenous depression following electroconvulsive therapy. Convulsive Ther. 1988;4:169–74. [PubMed] [Google Scholar]
- 100.Andrade C, Gangadhar BN, Subbakrishna DK, Pradhan N, Channabasavanna SM. Clinical prediction of rate of response of endogenous depression to electroconvulsive therapy. Indian J Psychiatry. 1988;30:381–7. [PMC free article] [PubMed] [Google Scholar]
- 101.Andrade C, Gangadhar BN. When is an ECT responder an ECT responder? Convulsive Ther. 1989;5:190–1. [PubMed] [Google Scholar]
- 102.Andrade C, Gangadhar BN, Channabasavanna SM, Pradhan N. Clinical characteristics of endogenous depressives who respond to ECT. NIMHANS J. 1989;7:119–22. [Google Scholar]
- 103.Andrade C, Gangadhar BN, Vythilingam M, Channabasavanna SM, Pradhan N. Initial response to ECT as a predictor of outcome in endogenous depression. Indian J Psychiatry. 1989;31:293–5. [PMC free article] [PubMed] [Google Scholar]
- 104.Andrade C. Psychobiological frontiers of electroconvulsive therapy in depression: Evaluation of strategies for rational prescription and reduction in morbidity, Tilak Venkoba Rao Oration. Indian J Psychiatry. 1990;32:109–30. [PMC free article] [PubMed] [Google Scholar]
- 105.Andrade C. Seizure duration and related issues in ECT for endogenous depression. Indian J Psychiatry. 1993;35:43–7. [PMC free article] [PubMed] [Google Scholar]
- 106.Sarkar P, Andrade C, Kapur B, Das P, Sivaramakrishna Y, Harihar C, et al. An exploratory evaluation of ECT in haloperidol-treated DSM IIIR schizophreniform disorder. Convulsive Ther. 1994;10:271–278. [PubMed] [Google Scholar]
- 107.Andrade C. Assessment of the therapeutic adequacy of the ECT seizure: Current status. NIMHANS J. 1997;15:319–29. [Google Scholar]
- 108.Andrade C. Prediction of antidepressant efficacy from response to the first ECT. Indian J Psychiatry. 1998;40:394–5. [PMC free article] [PubMed] [Google Scholar]
- 109.Andrade C. Is dysthymia an indication for ECT? J ECT. 1999;15:280–1. doi: 10.1097/00124509-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Andrade C. Nortryptyline plus lithium increased time to relapse in unipolar depressed patients who responded to electroconvulsive therapy. Evidence Based Mental Health. 2001;4:117. [Google Scholar]
- 111.Andrade C, Kurinji S. Continuation and maintenance ECT: A review of recent research. J ECT. 2002;18:149–58. doi: 10.1097/00124509-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 112.Andrade C, Nelson A, Fink M. ECT in the management of major depression: Implications of recent research. World J Biol Psychiatry. 2003;4:139–40. doi: 10.1080/15622970310029909. [DOI] [PubMed] [Google Scholar]
- 113.Andrade C. Review: Electroconvulsive therapy reduces depressive symptoms. Evidence Based Mental Health. 2003;8:138. [Google Scholar]
- 114.Chanpattana W, Andrade C. ECT for treatment-resistant schizophrenia: A response from the far east to the UK NICE report. J ECT. 2006;22:4–12. doi: 10.1097/00124509-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 115.Thirthalli J, Bharadwaj B, Kulkarni S, Gangadhar BN, Kharawala S, Andrade C. Successful use of maintenance rTMS for 8 months in a patient with antipsychotic-refractory auditory hallucinations. Schizophr Res. 2008;100:351–2. doi: 10.1016/j.schres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Margoob MA, Ali Z, Andrade C. Efficacy of ECT in chronic, severe, antidepressant- and CBT-refractory PTSD: An open, prospective study (submitted) J ECT. 2009 doi: 10.1016/j.brs.2009.04.005. (submitted) [DOI] [PubMed] [Google Scholar]
- 117.Andrade C, Gangadhar BN, Channabasavanna SM. Mania associated with electroconvulsive therapy (ltr) J Clin Psychiatry. 1987;48:303–4. [PubMed] [Google Scholar]
- 118.Andrade C, Gangadhar BN, Swaminath G, Channabasavanna SM. Mania as a side effect of electroconvulsive therapy. Convulsive Ther. 1988;4:81–3. [PubMed] [Google Scholar]
- 119.Andrade C, Gangadhar BN, Channabasavanna SM. Further characterization of mania as a side effect of ECT. Convulsive Ther. 1990;6:318–9. [PubMed] [Google Scholar]
- 120.Krishnamurthy M, DeSouza C, Selvaraj V, Venkataraman BV, Naga Rani MA, Joseph T, et al. Effects of ECT-dothiepin combination on learning in rats. Indian J Exp Biol. 1993;31:831–3. [PubMed] [Google Scholar]
- 121.Andrade C. Need for homogeneity of diagnosis in studies on the cognitive effects of ECT. J ECT. 2003;19:122. doi: 10.1097/00124509-200306000-00015. [DOI] [PubMed] [Google Scholar]
- 122.Sonavane S, Borade S, Gajbhiye S, Shah N, Andrade C. Transient cortical blindness associated with electroconvulsive therapy. J ECT. 2006;22:155–7. doi: 10.1097/00124509-200606000-00017. [DOI] [PubMed] [Google Scholar]
- 123.Bhat T, Pande N, Shah N, Andrade C. Safety of repeated courses of ECT in a patient with Harrington rods. J ECT. 2007;23:106–8. doi: 10.1097/YCT.0b013e31805b7f10. [DOI] [PubMed] [Google Scholar]
- 124.Andrade C, Thirthalli J, Gangadhar BN. Unilateral nondominant electrode placement as a risk factor for recall of awareness under anesthesia during ECT. J ECT. 2007;23:201–3. doi: 10.1097/YCT.0b013e3180cab6a4. [DOI] [PubMed] [Google Scholar]
- 125.Andrade C, Bhakta S, Singh NM. Schizophrenia, osteoporosis, and an examination of the risk of fractures in patients receiving ECT. J ECT. 2007;23:219–20. doi: 10.1097/YCT.0b013e31814da9c2. [DOI] [PubMed] [Google Scholar]
- 126.Pigot M, Andrade C, Loo C. Pharmacological attenuation of ECT-induced cognitive deficits: Theoretical background and clinical findings. J ECT. 2008;24:57–67. doi: 10.1097/YCT.0b013e3181616c14. [DOI] [PubMed] [Google Scholar]
- 127.Andrade C. Double stimulation for eliciting an adequate treatment. Convulsive Ther. 1991;7:300–2. [PubMed] [Google Scholar]
- 128.Sanjay Kumar N, Andrade C, Sekhar RV, Venkataraman BV. Effect of different schedules of electroconvulsive shock on complex maze learning in rats. Indian J Psychiatry. 1991;33:232–5. [Google Scholar]
- 129.Andrade C, Rao NS, Sekhar RV, Naga Rani MA, Venkataraman BV. Relationship between rate of administration of electroconvulsive shocks and rate of learning in rats: Implications for the practice of ECT. Convulsive Ther. 1994;10:206–11. [PubMed] [Google Scholar]
- 130.Andrade C. Duration of liquid fast before ECT. J ECT. 1998;14:120–1. doi: 10.1097/00124509-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 131.Andrade C, Reddy KP, Srihari BS, Sudha S, Chandra JS. Effects of zopiclone and lorazepam on ECT seizure duration: Clinical implications of findings from an animal model. Indian J Psychiatry. 2000;42:308–11. [PMC free article] [PubMed] [Google Scholar]
- 132.Andrade C, Andrade AC, Limaye N, Reshma M, Ravishankar U. Knowledge about and attitudes towards ECT: A survey of urban general medical practitioners. Indian J Psychiatry. 1995;37:48–9. [Google Scholar]
- 133.Iyengar U, Andrade C, Andrade AC, Rao NS. Nonpsychiatric medical specialists' attitudes towards ECT. Indian J Psychiatry. 1995;37:48. [Google Scholar]
- 134.Andrade C, Rao NS. Medical students' attitudes towards electroconvulsive therapy. Convulsive Ther. 1996;12:86–90. [PubMed] [Google Scholar]
- 135.Andrade C. Electroconvulsive therapy: Formation of attitudes. Indian J Psychol Med. 1996;19:75–7. [Google Scholar]
- 136.Agarwal AK, Andrade C. Indian psychiatrists' attitudes towards ECT. Indian J Psychiatry. 1997;39:54–60. [PMC free article] [PubMed] [Google Scholar]
- 137.Andrade C, Mariadas B. Experiences of ECT in schizophrenic, manic and depressed patients. Indian J Psychiatry. 1998;40:69. [Google Scholar]
- 138.Andrade C, Mariadas B. Knowledge about and attitudes towards ECT in schizophrenic, manic and depressed patients. Indian J Psychiatry. 1998;40:69–70. [Google Scholar]
- 139.Andrade C. Why some psychiatrists may be unwilling to receive ECT. J ECT. 2005;21:198. doi: 10.1097/01.yct.0000172225.57298.b7. [DOI] [PubMed] [Google Scholar]
- 140.Andrade C. Knowledge about and attitudes towards ECT: Methodological issues. J ECT. 2005;21:255. doi: 10.1097/01.yct.0000194015.00185.08. [DOI] [PubMed] [Google Scholar]
- 141.Andrade C, Thyagarajan S. The influence of name on the acceptability of ECT: The importance of political correctness. J ECT. 2007;23:75–7. doi: 10.1097/yct.0b013e31806545a1. [DOI] [PubMed] [Google Scholar]
- 142.Andrade C. Patients and ECT: Knowledge or attitudes? Indian J Psychiatry. 2007;49:145. doi: 10.4103/0019-5545.33268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andrade C. ECT in poetry: In lighter vein. J ECT. 2002;18:167. doi: 10.1097/00124509-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 144.Gangadhar BN, Ramadevi G, Andrade C, Pradhan N. Dopaminergic effects of repeated electroconvulsive shocks. Convulsive Ther. 1989;5:157–61. [PubMed] [Google Scholar]
- 145.Andrade C, Pradhan N. Adrenergic receptor effects of a single electroconvulsive shock. Indian J Psychol Med. 1990;13:39–41. [Google Scholar]
- 146.Andrade C, Gangadhar BN, Meena M, Pradhan N. Dopamine post-synaptic receptor effects of restricted schedules of electroconvulsive shock. Indian J Psychiatry. 1990;32:297–301. [PMC free article] [PubMed] [Google Scholar]
- 147.Gangadhar BN, Ramadevi G, Andrade C, Pradhan N. Single electroconvulsive shock and dopamine autoreceptors. Indian J Psychiatry. 1990;32:302–4. [PMC free article] [PubMed] [Google Scholar]
- 148.Andrade C, Pradhan N. Alpha-2 adrenergic effects of repeated electroconvulsive shocks. Indian J Psychiatry. 1991;33:140–2. [Google Scholar]
- 149.Andrade C, Sudha S. Electroconvulsive therapy and the alpha-2 noradrenergic receptor: Implications of treatment schedule effects. J ECT. 2000;16:268–78. doi: 10.1097/00124509-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 150.Andrade C. Tumor necrosis factor-alpha, depression, and ECT: Towards a better understanding of the relationships. J ECT. 2004;20:197–8. doi: 10.1097/00124509-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 151.Andrade C, Srinivasamurthy GM, Vishwasenani A, Prakash GS, Srihari BS, Chandra JS. High but not low ECS stimulus intensity augments apomorphine-stimulated dopamine postsynaptic receptor functioning in rats. J ECT. 2002;18:81–3. doi: 10.1097/00124509-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 152.Gangadhar BN, Laxmanna G, Andrade C, Janakiramaiah N, Channabasavanna SM. The NIMHANS model ECT instrument: A technical report. Indian J Psychiatry. 1988;30:247–51. [PMC free article] [PubMed] [Google Scholar]
- 153.Gangadhar BN, Andrade C. Electrical aspects of electroconvulsive therapy: A review, I, Electrical issues. Indian J Psychol Med. 1989;12:53–60. [Google Scholar]
- 154.Gangadhar BN, Andrade C. Electrical aspects of electroconvulsive therapy: A review, II, Clinical and practical issues. Indian J Psychol Med. 1989;12:61–6. [Google Scholar]
- 155.Andrade C. Quantifying the ECT dose: The right unit remains elusive. J ECT. 2001;17:75. doi: 10.1097/00124509-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 156.Andrade C, Sudha S, Jyothsna K, Venkataraman BV. Effects of stimulus parameters on seizure duration and ECS-induced retrograde amnesia. J ECT. 2002;18:31–7. doi: 10.1097/00124509-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 157.Andrade C, Kurinji S, Sudha S, Chandra JS. Effects of pulse amplitude, pulse frequency, and stimulus duration on seizure threshold: A laboratory investigation. J ECT. 2002;18:144–8. doi: 10.1097/00124509-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 158.Andrade C, Thyagarajan S, Vinod PS, Srikanth SN, Rao NS, Chandra JS. Effect of stimulus intensity and number of treatments on ECS-related seizure duration and retrograde amnesia in rats. J ECT. 2002;18:197–202. doi: 10.1097/00124509-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 159.Sudha S, Andrade C, Mukundan CR, Chandra JS. Spectral EEG effects of electroconvulsive shock stimulus parameters: The development of a rationale for the optimization of the ECT stimulus. J ECT. 2003;19:197–210. doi: 10.1097/00124509-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Andrade C. Does absolute charge administered predict speed of response to right unilateral ECT? J ECT. 2003;19:121. doi: 10.1097/00124509-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 161.Kurinji S, Andrade C. ECS seizure threshold: Normal variations, and kindling effects of subconvulsive stimuli. J ECT. 2003;19:31–7. doi: 10.1097/00124509-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 162.Andrade C, Akki A, Nandakumar N, Chandra JS. Repeated subconvulsive electroconvulsive shocks lower the seizure threshold: A controlled study of whole-brain kindling. J ECT. 2003;19:81–3. doi: 10.1097/00124509-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 163.Andrade C. In vigorous defence of whole brain kindling and a reconsideration on naming the phenomenon. J ECT. 2004;20:275–6. doi: 10.1097/00124509-200412000-00022. [DOI] [PubMed] [Google Scholar]
- 164.Andrade C, Kurinji S, Laxmanna G. Fidelity of ECT devices: An alert to clinicians Indian J Psychiatry. Corrigendum, Indian J Psychiatry. 2003;45:239–43. 2004;46:278. [PMC free article] [PubMed] [Google Scholar]
- 165.Andrade C, Gangadhar BN. ECT devices in India. J ECT. 2005;21:253. doi: 10.1097/01.yct.0000183897.09302.54. [DOI] [PubMed] [Google Scholar]
- 166.Andrade C. Unmodified ECT: A note of caution. Indian J Psychiatry. 1995;37:99–100. [PMC free article] [PubMed] [Google Scholar]
- 167.Andrade C, Rele K, Sutharshan R, Shah N. Musculoskeletal morbidity with unmodified ECT may be less than earlier believed. Indian J Psychiatry. 2000;42:156–62. [PMC free article] [PubMed] [Google Scholar]
- 168.Andrade C, Shah N, Tharyan P. The dilemma of unmodified ECT. J Clin Psychiatry. 2003;64:1147–52. doi: 10.4088/jcp.v64n1002. [DOI] [PubMed] [Google Scholar]
- 169.Andrade C. Unmodified ECT: Ethical issues. Issues Med Ethics. 2003;11:9–10. [PubMed] [Google Scholar]
- 170.Andrade C. ECT: A measured defence. Issues Med Ethics. 2003;11:44–5. [PubMed] [Google Scholar]
- 171.Andrade C. What is the patient's perspective? Issues Med Ethics. 2003;11:101. [Google Scholar]
- 172.Shah N, Mahadeshwar S, Bhakta S, Bhirud M, Fernandes P, Andrade C. The safety and efficacy of benzodiazepine-modified ECT (submitted) J ECT. 2009 doi: 10.1097/YCT.0b013e3181d2711f. (submitted) [DOI] [PubMed] [Google Scholar]
- 173.Sackeim HA, Decina P, Kanzler M, Kerr B, Malitz S. Effects of electrode placement on the efficacy of titrated low-dose ECT. Am J Psychiatry. 1987;144:1449–55. doi: 10.1176/ajp.144.11.1449. [DOI] [PubMed] [Google Scholar]
- 174.Sackeim HA, Prudic J, Devanand DP, Kiersky JE, Fitzsimons L, Moody BJ, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328:839–46. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 175.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–34. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 176.Kamath S, Andrade C, Faruqi S, Venkataraman BV, Naga Rani MA, Candade VS. Evaluation of pre-ECS antihypertensive drug administration in the attenuation of ECS-induced amnesia in rats. Convulsive Ther. 1997;13:185–95. [PubMed] [Google Scholar]
- 177.Sudha S, Andrade C, Anand A, Guido S, Venkataraman BV. Nitroprusside and ECS-induced retrograde amnesia. J ECT. 2001;17:41–4. doi: 10.1097/00124509-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 178.Anand A, Andrade C, Sudha C, Guido S, Venkataraman BV. Phenylephrine and ECS-induced retrograde amnesia. J ECT. 2001;17:166–9. doi: 10.1097/00124509-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 179.Andrade C. ECT, hypertensive mechanisms and cognitive dysfunction. Indian J Psychol Med. 2000;23:40–1. [Google Scholar]
- 180.Andrade C. ECT and cardiovascular disorders. Arch Indian Psychiatry. 1995;2:105–9. [Google Scholar]
- 181.Rao NS, Andrade C, Reddy KP, Madappa KN, Thyagarajan S, Chandra JS. Memory protective effect of indomethacin against electroconvulsive shock-induced retrograde amnesia in rats. Biol Psychiatry. 2002;51:770–3. doi: 10.1016/s0006-3223(01)01219-7. [DOI] [PubMed] [Google Scholar]
- 182.Nagaraja N, Andrade C, Sudha S, Singh NM, Chandra JS, Venkataraman BV. Glucocorticoid mechanisms may contribute to ECT-induced retrograde amnesia. Psychopharmacology (Berl) 2007;190:83–90. doi: 10.1007/s00213-006-0593-y. [DOI] [PubMed] [Google Scholar]
- 183.Andrade C, Singh NM, Thyagarajan S, Nagaraja N, Rao NS, Chandra JS. Possible glutamatergic and lipid signalling mechanisms in ECT-induced retrograde amnesia: Experimental evidence for involvement of COX-2 and review of literature. J Psychiatr Res. 2008;42:837–50. doi: 10.1016/j.jpsychires.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 184.Andrade C, Thyagarajan S, Singh NM, Vinod PS, Rao NS, Chandra JS. Celecoxib as an in vivo probe of the mechanisms underlying retrograde amnesia in an animal model of ECT. J Neural Transm. 2008;115:1063–70. doi: 10.1007/s00702-008-0063-2. [DOI] [PubMed] [Google Scholar]
- 185.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]


