Abstract
The effect of lengthening the distance in an adhesion molecule between the receptor binding site and the membrane anchor was studied by inserting four Ig-like domains into the two Ig domain lymphocyte function-associated antigen 3 (LFA-3) molecule. The extended molecule expressed in Chinese hamster ovary (CHO) cells bound to CD2 on T lymphocytes 4- to 20-fold more efficiently than the wild-type molecule at 4 degrees C. Treatment of the CHO clones with neuraminidase to remove sialic acid, or with deoxymannojirimycin to reduce the bulk of N-linked glycosylation, showed that adhesion to both the wild-type and the chimeric LFA-3 molecules was under the influence of cell-cell repulsive forces to a similar extent and that these treatments had less effect than lengthening LFA-3. At higher temperatures, such as 22 and 37 degrees C, the efficiency of binding to the wild-type LFA-3 increased to levels comparable with binding to extended LFA-3. Our results suggest that more distal locations of the adhesive binding site from the cell membrane anchor increase the efficiency of cell-cell adhesion by enhancing the frequency of receptor encounter with ligand and that more proximal locations of the adhesive binding site can provide efficient cell-cell adhesion at physiological temperatures.
Full text
PDF


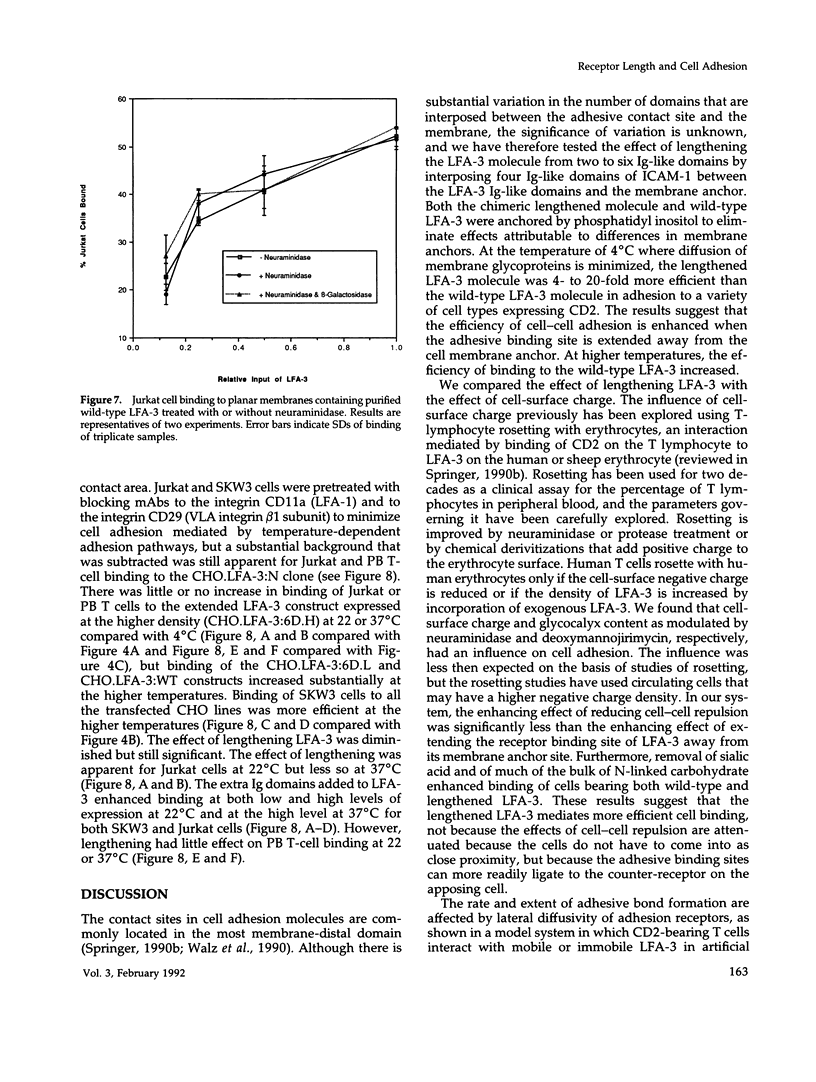
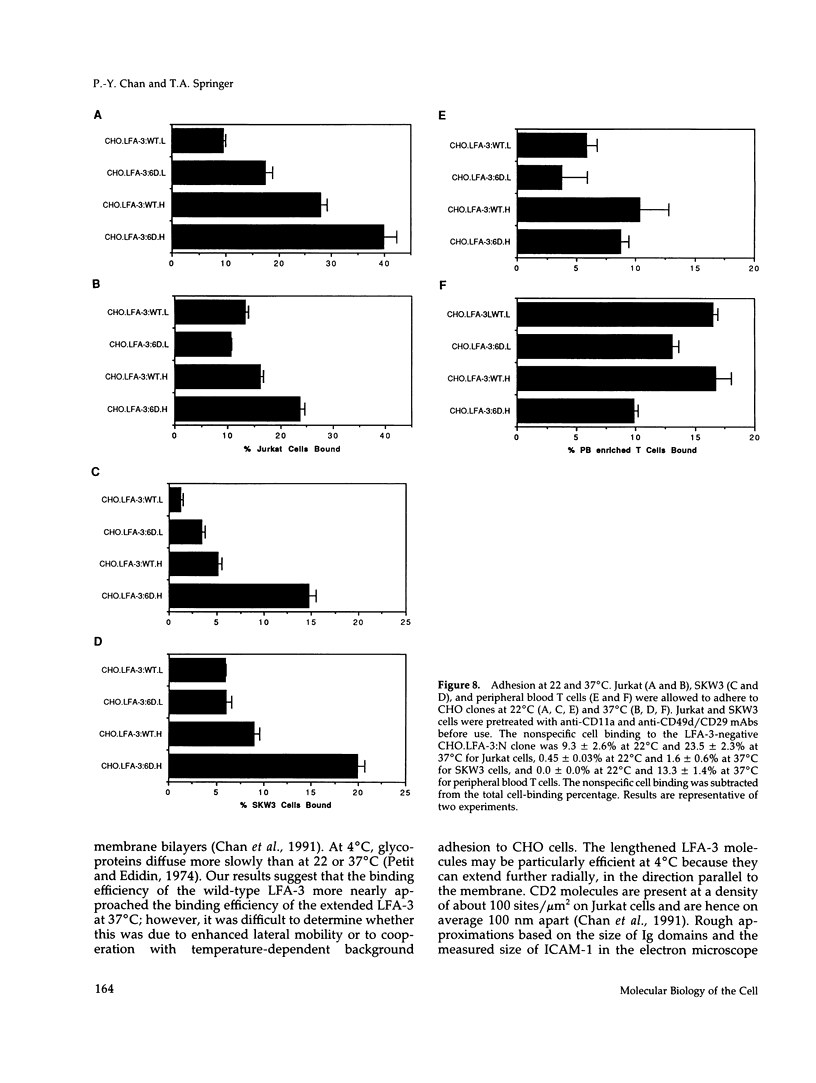


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Chan P. Y., Lawrence M. B., Dustin M. L., Ferguson L. M., Golan D. E., Springer T. A. Influence of receptor lateral mobility on adhesion strengthening between membranes containing LFA-3 and CD2. J Cell Biol. 1991 Oct;115(1):245–255. doi: 10.1083/jcb.115.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning S. M., Tuck D. T., Vollger L. W., Springer T. A., Singer K. H., Haynes B. F. Monoclonal antibodies to CD2 and lymphocyte function-associated antigen 3 inhibit human thymic epithelial cell-dependent mature thymocyte activation. J Immunol. 1987 Oct 15;139(8):2573–2578. [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., Marlin S. D., Springer T. A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991 Jun 14;65(6):961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Olive D., Springer T. A. Correlation of CD2 binding and functional properties of multimeric and monomeric lymphocyte function-associated antigen 3. J Exp Med. 1989 Feb 1;169(2):503–517. doi: 10.1084/jem.169.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Selvaraj P., Mattaliano R. J., Springer T. A. Anchoring mechanisms for LFA-3 cell adhesion glycoprotein at membrane surface. 1987 Oct 29-Nov 4Nature. 329(6142):846–848. doi: 10.1038/329846a0. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Guan J. L., Rose J. K. A membrane-anchored form but not the secretory form of human chorionic gonadotropin-alpha chain acquires polylactosaminoglycan. J Biol Chem. 1988 Apr 15;263(11):5314–5318. [PubMed] [Google Scholar]
- Hollander N., Selvaraj P., Springer T. A. Biosynthesis and function of LFA-3 in human mutant cells deficient in phosphatidylinositol-anchored proteins. J Immunol. 1988 Dec 15;141(12):4283–4290. [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Matsui M., Temponi M., Ferrone S. Characterization of a monoclonal antibody-defined human melanoma-associated antigen susceptible to induction by immune interferon. J Immunol. 1987 Sep 15;139(6):2088–2095. [PubMed] [Google Scholar]
- McClay D. R., Wessel G. M., Marchase R. B. Intercellular recognition: quantitation of initial binding events. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4975–4979. doi: 10.1073/pnas.78.8.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Mimms L. T., Zampighi G., Nozaki Y., Tanford C., Reynolds J. A. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Chang H. C., Wallner B. P., Stebbins C., Frey A. Z., Reinherz E. L. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature. 1989 May 25;339(6222):312–314. doi: 10.1038/339312a0. [DOI] [PubMed] [Google Scholar]
- Peterson A., Seed B. Monoclonal antibody and ligand binding sites of the T cell erythrocyte receptor (CD2). 1987 Oct 29-Nov 4Nature. 329(6142):842–846. doi: 10.1038/329842a0. [DOI] [PubMed] [Google Scholar]
- Petit V. A., Edidin M. Lateral phase separation of lipids in plasma membranes: effect of temperature on the mobility of membrane antigens. Science. 1974 Jun 14;184(4142):1183–1185. doi: 10.1126/science.184.4142.1183. [DOI] [PubMed] [Google Scholar]
- Plunkett M. L., Sanders M. E., Selvaraj P., Dustin M. L., Springer T. A. Rosetting of activated human T lymphocytes with autologous erythrocytes. Definition of the receptor and ligand molecules as CD2 and lymphocyte function-associated antigen 3 (LFA-3). J Exp Med. 1987 Mar 1;165(3):664–676. doi: 10.1084/jem.165.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Dustin M. L., Mitnacht R., Hünig T., Springer T. A., Plunkett M. L. Rosetting of human T lymphocytes with sheep and human erythrocytes. Comparison of human and sheep ligand binding using purified E receptor. J Immunol. 1987 Oct 15;139(8):2690–2695. [PubMed] [Google Scholar]
- Selvaraj P., Dustin M. L., Silber R., Low M. G., Springer T. A. Deficiency of lymphocyte function-associated antigen 3 (LFA-3) in paroxysmal nocturnal hemoglobinuria. Functional correlates and evidence for a phosphatidylinositol membrane anchor. J Exp Med. 1987 Oct 1;166(4):1011–1025. doi: 10.1084/jem.166.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. The sensation and regulation of interactions with the extracellular environment: the cell biology of lymphocyte adhesion receptors. Annu Rev Cell Biol. 1990;6:359–402. doi: 10.1146/annurev.cb.06.110190.002043. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Dustin M. L., Erickson H. P., Springer T. A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990 Apr 20;61(2):243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Kas E., Chasin L. A., Funanage V. L., Myoda T. T., Hamlin J. Effect of gamma rays at the dihydrofolate reductase locus: deletions and inversions. Somat Cell Mol Genet. 1986 Nov;12(6):555–566. doi: 10.1007/BF01671941. [DOI] [PubMed] [Google Scholar]
- Wallner B. P., Frey A. Z., Tizard R., Mattaliano R. J., Hession C., Sanders M. E., Dustin M. L., Springer T. A. Primary structure of lymphocyte function-associated antigen 3 (LFA-3). The ligand of the T lymphocyte CD2 glycoprotein. J Exp Med. 1987 Oct 1;166(4):923–932. doi: 10.1084/jem.166.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990 Nov 23;250(4984):1132–1135. doi: 10.1126/science.1701275. [DOI] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Y., Denning S. M., Mizuno S., Dupont B., Haynes B. F. A novel activation pathway for mature thymocytes. Costimulation of CD2 (T,p50) and CD28 (T,p44) induces autocrine interleukin 2/interleukin 2 receptor-mediated cell proliferation. J Exp Med. 1988 Oct 1;168(4):1457–1468. doi: 10.1084/jem.168.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]