Abstract
The short reproductive cycle length observed in rodents, called the estrous cycle, makes them an ideal animal model for investigation of changes that occur during the reproductive cycle. Most of the data in the literature about the estrous cycle is obtained from rat because they are easily manipulated and exhibit a clear and well defined estrous cycle. However, the increased number of experiments using knockout mice requires identification of their estrous cycle as well, since (in)fertility issues may arise. In mice, like rats, the identification of the stage of estrous cycle is based on the proportion of cells types observed in the vaginal secretion. The aim of this unit is to provide guidelines for quickly and accurately determining estrous cycle phases in mice.
Key terms for indexing: Estrous cycle, mouse, vaginal smears, wet vaginal
In humans, the reproductive cycle, called the menstrual cycle, lasts approximately 28 days, in rodents this cycle, called the estrous cycle, lasts approximately 4-5 days. The success of reproduction in all mammals depends on the function of the hypothalamus-pituitary-gonads axis. In females, gonadotropin-releasing hormone (GnRH) neurons present in the septal area and hypothalamus send their axons to median eminence. GnRH released there reaches the anterior pituitary where the gonadotrophs are stimulated to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In the peripheral circulation, these hormones stimulate specific cells in the ovary leading to ovulation. Ovarian follicles secrete estrogen (E2) that reaches the hypothalamus. Most of the time, the plasma concentration of E2 is low. However, during the pre-ovulatory period E2 secretion reaches a peak, and GnRH neurons are stimulated (Sarkar et al., 1976; Freeman, 1994). E2 seems to regulate GnRH neurons indirectly, via interneurons. However, there are many questions needing to be answered in this field. Importantly, because E2 has a myriad of effects, questions are raised not only in the neuroendocrine field, but also in the neurosciences (e.g. synaptic plasticity, memory and learning and neurodegenerative diseases) as well as fields studying cardiological and/or renal function, sodium and water equilibrium, etc.
A short cycle length makes rodents an ideal animal model for investigating changes occurring during the reproductive cycle and historically rats have been the chosen model. Rats display, most of time, regular cycles; they are easy to manipulate; and the cycle is not disrupted easily even with the routine stress in the animal facility. However, as use of mice lines continues to increase, an understanding of the mouse estrous cycle is critical for investigators and in fact many knockout mice can exhibit puberty/fertility changes that can effect, simply, maintaining a knockout line. There are few published studies involving estrous cycle in mice. The stages of the estrous cycle are not as visually discernible as in rats, and handling mice requires more caution due to their aggressive behavior. Many studies involving reproduction and neuroendocrine systems have been carried out in knockout mice, and the controls often involve an ovariectomized and ovarian steroid replacement model. This model works well and may answer many questions, but in certain experiments, the intact animal may be the best option.
In studies involving the reproductive system and the influence of the estrous cycle on non-reproductive functions, vaginal smear cytology is used to determine the estrous cycle phases (Long & Evans, 1922). This method predicts the estrous cycle according to the proportion of three cells types observed in the vaginal smear: epithelial cells, cornified cells and leukocytes. Thus, the aim of this unit is to provide protocols for the rapid and accurate collection of vaginal smears and the determination of the estrous cycle phases in mice (Marcondez, 2002).
This unit includes a detailed description of each phase of estrous cycle in mice (see Description of stages of estrous cycle and hormonal considerations). Because experiments involving estrous cycle are made in post-pubertal animals, the procedures to determine the vaginal opening (VO) are included (see Basic Protocol 1) followed by a description for manipulating female mice, obtaining vaginal secretion, and identifying the stages of the estrous cycle (see Basic Protocol 2).
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Description of Stages of Estrous Cycle and Hormonal Considerations
The full estrous cycle in mice, as well in rat, occurs over 4 or 5 days and can be divided into four stages.
1. Proestrus
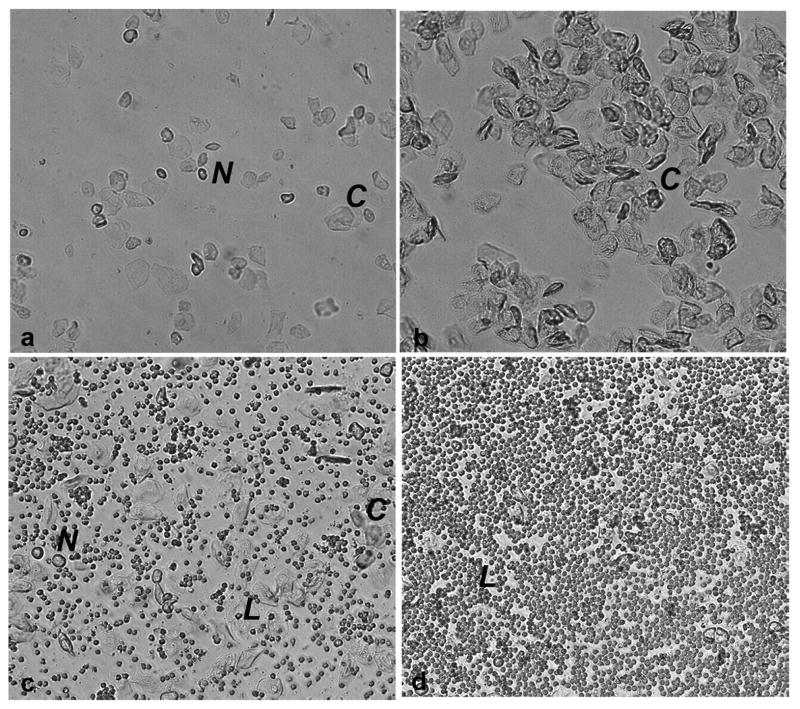
In this stage, there is a predominance of nucleated epithelial cells (Fig 1a). These cells may appear in clusters or individually. Occasionally, some cornified cells may appear in the sample. This stage corresponds to the pre-ovulatory day, when E2 increases (Walmer et al, 1992) and consequently, during the night, LH and FSH surge and ovulation occurs (Parkening et al., 1982).
Figure 1.
Photomicrographs of unstained vaginal secretion from mice at (A) pro-estrus, predominantly consisting of nucleated epithelial cells; (B) estrus, with anucleated cornified cells; (C) metestrus, consisting of the three types of cell, leukocytes, cornified, and nucleated epithelial cells; and (D) diestrus, consisting predominantly of leucocytes. Nucleated epithelial cells (N), leucocytes (L), cornified cell (C).
2. Estrus
This stage is distinctively characterized by cornified squamous epithelial cells, which occur in clusters (Fig 1b). There is no visible nucleus; the cytoplasma is granular; and the shape is irregular. E2 remains elevated throughout the morning and falls back to basal levels in the afternoon (Walmer et al, 1992).
3. Metestrus
In this stage, there is a mix of cell types with a predominance of leucocytes and a few nucleated epithelial and/or cornified squamous epithelial cells (Fig 1c). Plasma E2 concentration is low (Walmer et al, 1992).
4. Diestrus
This stage consists predominantly of leukocytes (Fig 1d). During this stage, E2 levels start to increase (Walmer et al, 1992).
During estrus, metestrus and diestrus, the plasma circulation of LH and FSH are low (Parkening et al., 1982).
Note: Because animals may exhibit initial stress to handling, it may be difficult to distinguish the stages of estrous cycle in the first three days of analysis. After this period the animals become accustomed to manipulations, and consequently the collection of material tends to improve.
Basic Protocol 1: Assessment of Vaginal Opening in Mice
Vaginal opening is an apoptosis-mediated event (Rodriguez et al., 1997) used as an external index of puberty onset. It occurs as a result of increasing estradiol secretion and can be stimulated by injection of estradiol into immature mice or rats (Ojeda and Urbancki, 1994). Whereas vaginal opening in the rat occurs simultaneously with the first ovulation, vaginal opening in the mouse may occur up to 10 days before the first vaginal cornification and the onset of estrus cycle (Nelson et al., 1982).
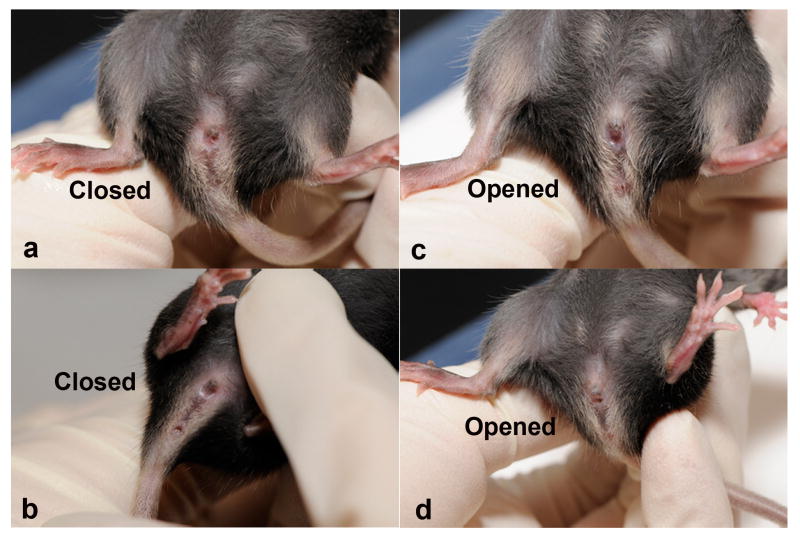
The age of vaginal opening in mice is documented by monitoring mice every morning from 24 days to 30 days of age. Usually the opening is detected through a simple visual examination of the vulva. In mice, vaginal opening occurs around 26 days old.
Materials
Female mice from 23 days old
Cotton balls
Saline 0.9%
Choose a method to identify each animal. Ear punch and toe clip are routinely used. Proper handling of mice is essential to minimize the stress of the mice while protecting the handler from injury such as bites.
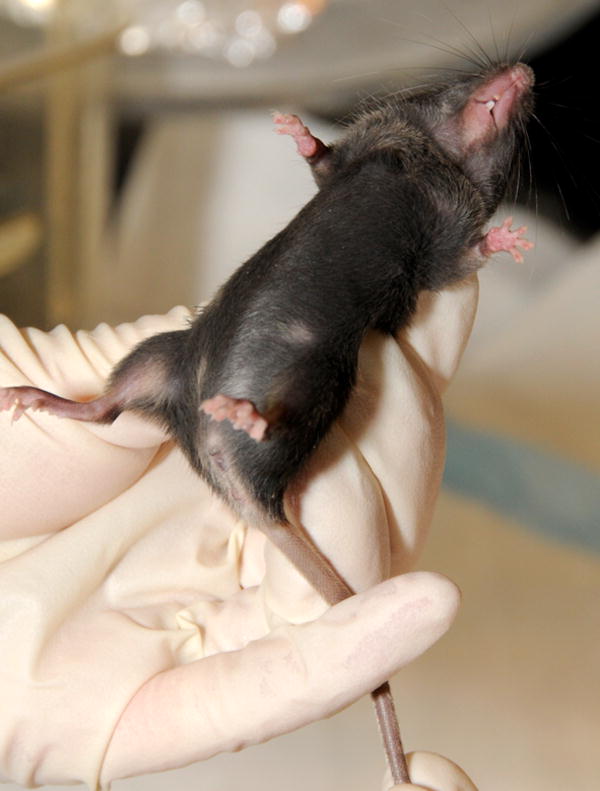
Lift the mouse by the bas e of the tail with the dominant hand; then, use the opposite hand to grasp the loose skin at the nape of the neck (between one's thumb and forefinger). Lift the animal and restrain with one hand. [This is made easier by lifting the mouse, allowing the mouse to grasp a wire cage top or other surface with the forelimbs, then the handler can grasp the skin of the nape (scruff) area.] Invert the mouse so that the weight of the mouse rests in the palm of the hand. Restrain the lower half of the mouse by placing the tail between the handler's fourth and fifth fingers (Fig 2).
Once the mouse is restrained with one hand, use the other hand to examine the vulva and see if the vagina is completely open (Fig 3). It is important to make sure that there is no secretion occluding the entrance to the vagina. For this reason it is important to clean the vulva with a piece of cotton damp with saline.
Figure 2.
Proper means of holding a mouse for vaginal opening analysis and for the collection of vaginal secretion. Using the non-dominant hand, grasp the loose skin at the nape of the neck between one's thumb and forefinger and restrain the animal with one hand. Then invert the animal in the palm of the hand and place the tail between the handler's fourth and fifth fingers.
Figure 3.
Vaginal opening in mice at approximately 26 days old, showing closed vaginal cavity (A, B) and opened vaginal cavity (C, D).
Basic Protocol 2: Collection and Analyses of Vaginal Secretion (“Wet Smears”)
This protocol describes a rapid method to obtain material for analyzing the stage of estrous cycle in rodents. Because some stages of estrous cycle are very short (e.g., metestrus), it is important to collect the material at the same time each day. If the objective of the study is to use cycling mice in different phases or to test the effects of drugs on cycling, at least two consecutive baseline cycles should be recorded prior to manipulation.
Materials
Female mice post-puberal (approximately 40 days old)
Fine tip plastic pipettes, tip inner diameter 1mm
Phosphate buffer saline (PBS) or saline 0.9% (0.15M)
Light microscope with 10× objective
Cleaned slides or printed slides
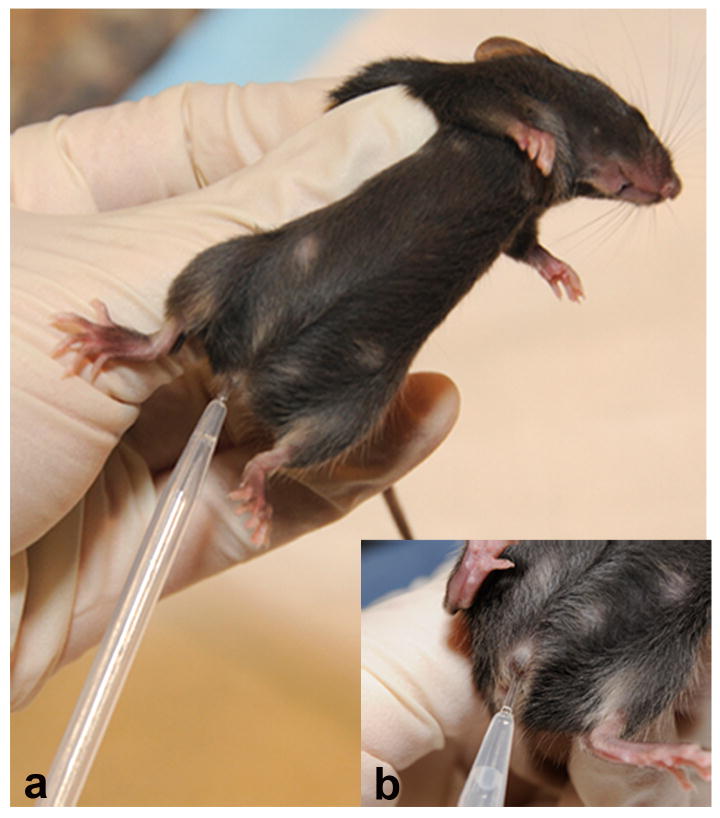
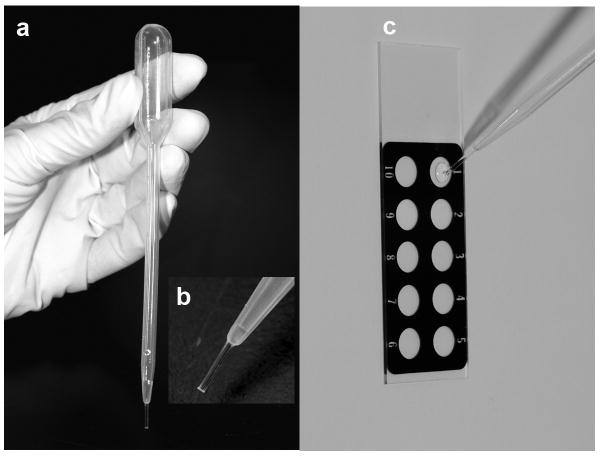
Identify and restrain mouse (see BASIC PROTOCOL 1, Fig 2). Place the tip of plastic pipette, filled with PBS or saline (∼10μL), into the vagina. Flush the vagina gently three to five times with same PBS/saline solution (fig 4). Collect final flush in pipette tip. A volume of 10 μL of saline solution allows collecting sufficient material for observation of vaginal cytology
Place final flush containing vaginal fluid on a glass slide. Printed slides are very useful, because they help to avoid mixing the samples of different animals (Fig 5). However, a regular cytological slide can be used with one slide for each animal.
Observe unstained material under light microscope with a 10× objective. It is not necessary to use a cover slip. If the drop dries, it is possible to add more saline (<10 μl) with another clean pipet tip to dampen it. To visualize the cells, use low illumination in the microscope, without the use of the condenser lens to assure good contrast.
Figure 4.
Collection of vaginal secretion in mice. After the female mouse has been restrained with the non-dominant hand of the handler, using the dominant hand, the tip of plastic pipette (∼5mm) filled with saline 0.9% or PBS is introduced in the vagina of the mouse (a, detail in b) and the liquid is flushed to collect the sample.
Figure 5.
Plastic pipette filled with saline 0.9% (a) with a detail of the tip (b). Vaginal fluid is placed on a printed slide (c) for further analysis on microscope.
Using the 40 × objective lens, the characterization of the cell types is easier than using the 10 × objective. However, the determination of the estrous cycle phase is based on the proportion among the three cell types, which is easier to distinguish if the 10 × objective is used.
Figure 1 shows representative photomicrographs of the cells present in the vaginal secretion in the four stages of estrous cycle in mice. Although, the pictures are not in color, studies made in rats assessing the reliability of the “wet smears” by comparing it with classical staining techniques (Papanicoloau and methylene blue) show that the same result can be obtained with direct examination (“wet smear”) technique. So, there is no need to use time-consuming, less practical and more expensive techniques such as Papanicolaou or methylene blue (Yener et al., 2007).
Commentary
Critical Parameters and Troubleshooting
In the beginning, the animals may be aggressive so it is crucial that handling be firm and secure, otherwise the animal can bite the holder.
Urination and defecation are common stress responses to handling. It is important to clean the area around the vagina and avoid contaminating the vaginal secretion collected in the pipette. Such contamination will make subsequent analysis more difficult.
Anticipated Results
Table 1 shows the estrous cycle of 4 mice during 19 consecutives days. The sequence of the stages are: proestrus, estrus, metestrus and diestrus. In mice it is normal to observe extra diestrus and estrus stages. In order to make sure they are cycling, it is important to check the proestrus (pre-ovulatory day) followed by the estrus, For example, the animal number 55 shows three cycles (there are three proestrus followed by estrus and then, metestrus and diestrus). If the question is: is it cycling? The answer is yes, and the researcher can compare it with a knockout animal, for example. Also, if the question is to analyze the effects of hormones variation in determinated parameter, [for example: “Oxytocin secretion induced by osmotic stimulation in rats during the estrous cycle and after ovariectomy and hormone replacement therapy” (Caligioni and Franci, 2002)] the mouse could be killed at proestrus, estrus, metestrus or diestrus, in the third cycle. There is no data in the literature (for mice), showing differences in hormones levels (estrogen, progesterone, LH, FSH) during two or more consecutives estrus (or metestrus/diestrus). So, if the researcher is choosing mice in different stages of the estrous cycle, it is better to kill in the first estrus (or metestrus/diestrus). In the table 1, the mice numbers 38 and 39 are cycling as well, with estrus, metestrus and diestrus varying in the cycles. The mouse number 33 shows only 2 cycles (the others show 3 and 4 cycles). In this case, the researcher should do the vaginal smears for one more week (approximately) to check if she will show the third cycle (it means another proestrus followed by estrus). In this animal, the first cycle is longer; this is why more time is required for analyzing the smears.
Table 1.
Sample of 4 mice identified by 55, 38, 39 and 33, showing their estrous cycle for 19 days.
| Day 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | #P | #E | #M | #D | # Cycle | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | ||||||||||||||||||||||||
| 55 | E | D | D | P | E | M | D | D | P | E | E | D | D | P | E | E | M | M | M | 3 | 6 | 4 | 6 | 3 |
| 38 | E | M | M | P | E | D | P | E | E | D | D | P | E | E | D | D | D | D | D | 3 | 6 | 2 | 8 | 3 |
| 39 | M | D | P | E | E | M | P | E | E | D | D | P | E | E | E | E | D | P | E | 4 | 9 | 2 | 4 | 4 |
| 33 | D | P | E | M | E | E | E | M | E | E | E | E | D | P | E | D | D | E | E | 2 | 11 | 2 | 4 | 2 |
P, proestrus; E, estrus; M, metestrus and D, diestrus.
Literature Cited
- Caligioni CS, Franci CR. Oxytocin secretion induced by osmotic stimulation in rats during the estrous cycle and after ovariectomy and hormone replacement therapy. Life Sci. 2002;71:2821–2831. doi: 10.1016/s0024-3205(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Freeman ME. The neuroendocrine control of the ovarian cycle in the rat. In: Knobil E, Neill JD, editors. Physiology of Reproduction. Raven; New York: 1994. pp. 613–658. [Google Scholar]
- Long JA, Evans HM. The estrous cycle in the rat and its associated phenomena. Memories of University of California. 1922;6:1–148. [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbancki HF. Puberty in the rat. In: Knobil E, Neill JD, editors. Physiology of Reproduction. Raven Press; New York: 1994. pp. 363–409. [Google Scholar]
- Parkening TA, Collins TJ, Smith ER. Plasma and pituitary concentrations of LH, FSH, and prolactin in aging C57BL/6 mice at various times of the estrous cycle. Neurobiol Aging. 1982;3:31–5. doi: 10.1016/0197-4580(82)90058-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Araki K, Khatib K, Martinou JC, Vassalli P. Mouse vaginal opening in an apoptosis-dependent process which can be prevented by the overexpression of Bcl2. Dev Biol. 1997;1997;184:115–121. doi: 10.1006/dbio.1997.8522. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature. 1976;264:461–463. doi: 10.1038/264461a0. [DOI] [PubMed] [Google Scholar]
- Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinol. 1992;131:1458–1466. doi: 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- Yener T, Turkkani Tunc A, Aslan H, Aytan H, Cantug Caliskan A. Determination of oestrous cycle of the rats by direct examination: how reliable? Anat Histol Embryol. 2007;36:75–77. doi: 10.1111/j.1439-0264.2006.00743.x. [DOI] [PubMed] [Google Scholar]