Abstract
Neuroimaging, especially BOLD fMRI, has begun to identify how variability in brain function contributes to individual differences in complex behavioral traits. In parallel, pharmacological fMRI and multimodal PET/fMRI are identifying how variability in molecular signaling pathways influences individual differences in brain function. Against this background, functional genetic polymorphisms are being utilized to understand the origins of variability in signaling pathways as well as to model efficiently how such emergent variability impacts behaviorally relevant brain function. This article provides an overview of a research strategy seeking to integrate these complementary technologies and utilizes existing empirical data to illustrate its effectiveness in illuminating the neurobiology of individual differences in complex behavioral traits. The article also discusses how such efforts can contribute to the identification of predictive markers that interact with environmental factors to precipitate disease and to develop more effective and individually tailored treatment regimes.
Keywords: amygdala, ventral striatum, serotonin, dopamine, temperament, genes
INTRODUCTION
Individual differences in trait affect, personality, and temperament are critical in shaping complex human behaviors, successfully navigating social interactions, and overcoming challenges from our ever-changing environments. Such individual differences may also serve as important predictors of vulnerability to neuropsychiatric disorders, including depression, anxiety, and addiction, especially upon exposure to environmental adversity. Accordingly, identifying the biological mechanisms that give rise to individual trait differences affords a unique opportunity to develop a deeper understanding of complex human behaviors, disease liability, and treatment. Having established multiple modal neural processes supporting specific aspects of complex behavioral processes, human neuroimaging studies, especially those employing BOLD fMRI, have now begun to reveal the neural substrates of interindividual variability in these and related constructs. Moreover, recent studies have established that BOLD fMRI measures represent temporally stable and reliable indices of brain function. Thus, much like their behavioral counterparts, patterns of brain activation represent enduring, trait-like phenomena, which in and of themselves may serve as important markers of individual differences as well as disease liability and pathophysiology.
As neuroimaging studies continue to illustrate the predictive links between regional brain activation and trait-like behaviors (e.g., increased amygdala reactivity predicts trait anxiety), an important next step is to identify systematically the underlying mechanisms driving variability in brain circuit function. In this regard, recent neuroimaging studies employing pharmacological challenge paradigms, principally targeting monoamine neurotransmission, have revealed that even subtle alterations in dopaminergic, noradrenergic, and serotonergic signaling can have profound impacts on the functional response of brain circuitries supporting affect, personality, and temperament. Similarly, multimodal neuroimaging approaches have provided evidence for directionally specific links between key components of monoaminergic signaling cascades, assessed with radiotracer PET, and brain function, assessed with BOLD fMRI. Collectively, pharmacological fMRI and multimodal PET/fMRI are revealing how variability in behaviorally relevant brain activation emerges as a function of underlying variability in key brain signaling pathways (e.g., increased serotonin signaling predicting increased amygdala reactivity). The next logical step is to identify the sources of interindividual variability in these key neurochemical signaling mechanisms.
In the modern era of human molecular genetics, this step is firmly planted in the direction of identifying common variation in the genes that influence the functioning or availability of components in these pathways. Because DNA sequence variation across individuals represents the ultimate wellspring of variability in emergent molecular, neurobiological, and related behavioral processes, understanding the links among genes, brain, and behavior is important for establishing a mechanistic foundation for individual differences in behavior and related psychiatric disease. Moreover, such genetic polymorphisms can be readily identified from DNA collected via cells from individual blood or even saliva samples using relatively well-tolerated, inexpensive, and standardized laboratory protocols. Once collected and isolated, an individual's DNA can be amplified repeatedly, providing an almost endless reservoir of material for genotyping of additional candidate polymorphisms as they are identified. When a precise cascade of related neurobiological and behavioral effects are clearly established, common polymorphisms can serve as incredibly powerful predictive markers of such emergent properties that are more readily accessible (e.g., samples can be collected in doctors' offices), applicable (e.g., even newborns can be genotyped), and economical (e.g., costing only tens of dollars per sample in comparison to the hundreds and even thousands of dollars required for fMRI and PET) than their technological counterparts in neuroimaging and neuropharmacology. Of course, arriving at this ultimate reduction requires intensive and expansive efforts wherein all these technologies as well as epidemiological and clinical studies are first brought to bear on explicating the detailed biological mechanisms mediating individual differences in trait behaviors and related risk for neuropsychiatric disease. More importantly, the limitations of such genetic reductionism, especially the probabilistic nature of the biological impact of candidate functional polymorphisms whose likely effects are dynamically moderated by other genetic variants as well as environmental and epigenetic factors, should always be considered in evaluating and characterizing the potential real-world value of genetic markers. Unlike their Mendelian counterparts, most common genetic polymorphisms in studies of individual differences in behaviorally relevant brain function will not have absolute effects on their related emergent biological pathways.
- BOLD fMRI
blood oxygen level-dependent functional magnetic resonance imaging
- PET
positron emission tomography
- Multimodal PET/fMRI
multimodal neuroimaging data are collected in the same subjects to map the links between the density of a specific component of a molecular signaling pathway, assayed with radiotracer PET, and task-related brain function, assayed with BOLD fMRI
Through the careful integration of these complementary approaches and technologies, investigators have already made significant progress in describing the contributions of multiple common genetic polymorphisms to individual differences in complex behavioral phenotypes and disease liability—in particular, by identifying effects of functional genetic variation on the neural processes that mediate behavioral responses to environmental challenge (Caspi & Moffitt 2006, Hariri & Holmes 2006). This article reviews how the integration of psychology, neuroimaging, neuropharmacology, and molecular genetics can work toward the ultimate goal of understanding the detailed mechanisms mediating individual differences in human behavior and, in turn, establishing predictive markers of disease vulnerability. The vast potential of such an integrated approach is highlighted by reviewing recent studies whose collective results demonstrate that common sequence variations in human genes that bias key components of molecular signaling cascades results in altered brain circuit function that mediates individual differences in complex behavioral traits such as temperamental anxiety and impulsivity (Figure 1). With their increased utilization and continued expansion, each level of analysis in this integrative strategy—brain circuit function, neural signaling cascades, and molecular genetics—also has the potential to uniquely illuminate clinically relevant information that can be used to devise individually tailored treatment regimes and establish predictive disease markers. In lieu of further describing a general framework, three specific examples, detailed below and summarized in Table 1, are used to illustrate the effectiveness of this integrated strategy to parse biological mechanisms mediating individual differences in complex behaviors. In each, subjects were retrospectively genotyped for the candidate functional polymorphisms of interest from stored samples of DNA, and this information was used to group subjects on the basis of their individual genotypes. The behavioral assessments in all three examples were conducted as a component of a larger parent protocol that preceded measurement of task-related regional brain function with BOLD fMRI by an average interval of 29 weeks. The fact that robust brain-behavior correlations were observed despite the separation in time is consistent with the suggestion that both metrics (i.e., brain function and behavior) are remarkably stable, possibly indicative of trait-related variation. Such a link further underscores the likelihood that interindividual variability in brain-behavior associations is influenced by genetic polymorphisms affecting functioning of signaling pathways modulating underlying neural circuitries.
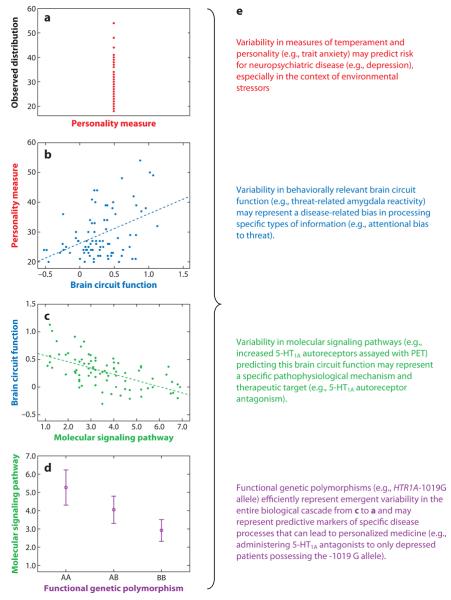
Figure 1.
Integration of complementary technologies can be used to reveal the neurobiology of individual differences in complex behavioral traits. (a) Individual differences in personality and temperament are critical to shaping complex human behaviors and may serve as important predictors of vulnerability to neuropsychiatric disorders. (b) Neuroimaging technologies, especially BOLD fMRI, can identify links between variability in brain circuit function and individual differences in personality and temperament. (c) Multimodal PET/fMRI (or pharmacological fMRI) can map individual differences in behaviorally relevant brain circuit function to variability in specific molecular signaling pathways. (d) Variability in specific molecular signaling pathways can be mapped to functional genetic polymorphisms, which inform their ultimate biological origins and can be used to model efficiently how such emergent variability impacts behaviorally relevant brain function. (e) Each level of analysis can potentially inform clinically relevant issues, provide guiding principles for the development of more effective and personalized treatment options, and represent predictive risk markers that interact with unique environmental factors to precipitate disease.
Table 1.
Summary of studies reviewed in this article that link individual differences in complex behavioral traits with underlying variability in brain circuit function, molecular signaling pathways and functional genetic polymorphisms1
| Behavioral trait | Brain circuitry | Signaling pathway |
Functional polymorphism |
|---|---|---|---|
| Trait anxiety | Amygdala | 5-HT | HTR1A −1019G allele associated with increased autoreceptor expression and reduced 5-HT release |
| Impulsivity | Ventral striatum | DA | DAT1 9-repeat allele associated with reduced DAT expression and increased synaptic DA |
| Trait anxiety and impulsivity | Amygdala and ventral striatum | eCB | FAAH 385A allele associated with reduced enzyme activity and increased eCB signaling |
Abbreviations: 5-HT, 5-hydroxytryptamine; DA, dopamine; eCB, endocannabinoids.
- Functional Polymorphism
a gene sequence variant present at >1% in a population that affects the regulation of the gene and/or the functioning of its protein product
Multiple mechanisms involving de novo biosynthesis, vesicular release, active reuptake, metabolic degradation, and a myriad of both pre- and postsynaptic receptors contribute to the regulation of neurotransmission and its subsequent modulation of brain function. In general, component processes that affect the magnitude of signaling (e.g., biosynthesis, reuptake, autoregulation, degradation) rather than localized effects on target neurons (e.g., postsynaptic receptors) represent key bottlenecks in neurotransmitter regulation of neural circuit function. To illustrate the powerful capacity of functional genetic polymorphisms to model emergent variability in signaling pathways, each of the three exemplars below focuses on a different critical node in regulating the magnitude of neurotransmission: autoregulatory negative feedback, active synaptic reuptake, and enzymatic degradation. In the first example, individual differences in trait anxiety are mapped onto threat-related amygdala reactivity. Variability in amygdala reactivity is, in turn, mapped to serotonin signaling. Finally, variability in serotonin signaling is mapped to a common functional polymorphism impacting the capacity for negative feedback inhibition of serotonergic neurons in the midbrain. In the second example, similar links are described among variability in impulsivity, reward-related ventral striatum reactivity, dopamine signaling, and a polymorphism impacting synaptic clearance of striatal dopamine. In the third and last example, a common polymorphism affecting the enzymatic degradation of endocannabinoids is linked to divergent effects on threat-related amygdala and reward-related ventral striatum reactivity.
TRAIT ANXIETY, THE AMYGDALA, AND SEROTONIN
The experience of anxiety is commonplace among both human and nonhuman primates as well as other highly social animals. In the context of social interactions, especially within delimited social hierarchies consisting of dominant and subordinate individuals, anxiety shapes appropriate and often opposing responses to precipitating events such as competition for limited resources (e.g., food, water, reproductive partners). Sensitivity to potentially threatening social cues (e.g., affective facial expressions) varies considerably among individuals and represents a core component of commonly employed constructs representing trait anxiety. Individuals with high trait anxiety exhibit a propensity to appraise situations as more threatening than do others and are generally more sensitive to social cues, including those representing both explicit and implicit threat (e.g., angry and fearful facial expressions). In turn, these individuals are at increased risk for developing neuropsychiatric disorders characterized by abnormal social and emotional behaviors such as depression and often precipitated by exposure to chronic or severe stressors. Examining the neural correlates of individual variability in dispositional temperament such as trait anxiety represents an important step in understanding key socioemotional behaviors as well as an effective means of elucidating pathophysiological processes contributing to related disordered states.
Converging evidence from animal and human studies clearly demonstrates that the amygdala is centrally involved in mediating both physiological (e.g., autonomic reactivity) and behavioral (e.g., reallocation of attentional resources) effects that allow an individual to respond adaptively to varied environmental and social challenges (LeDoux 2000). A large corpus of human neuroimaging research reveals that the amygdala is robustly engaged by varied biologically salient stimuli, most notably emotional facial expressions, especially those representing threat. However, individuals differ appreciably in the magnitude of amygdala activation on exposure to emotionally expressive facial expressions, and these individual differences appear to be stable over time (Johnstone et al. 2005, Manuck et al. 2007). Thus, they may contribute to the emergence of stable differences in temperament such as trait anxiety.
Recent neuroimaging studies have reported positive links between the magnitude of amygdala reactivity to affective, especially threatening, stimuli and interindividual variability in indices of trait (Dickie & Armony 2008, Etkin et al. 2004, Haas et al. 2007, Killgore & Yurgelun-Todd 2005, Most et al. 2006, Ray et al. 2005) and also state anxiety (Bishop et al. 2004, Somerville et al. 2004). In one study, Stein et al. (2007) report that high trait anxiety is associated with greater amygdala reactivity not only to angry and fearful but also to happy facial expressions. Consistent with this pattern of normal variability, various mood and anxiety disorders (e.g., unipolar and bipolar depression, generalized anxiety disorder, social phobia) have been linked with greater amygdala responses to facial expressions depicting fear and anger, as well as sadness and disgust, and, more variably, to emotionally neutral facial expressions (Cooney et al. 2006, Evans et al. 2008, Phan et al. 2006, Phillips et al. 2003, Stein et al. 2002, Whalen et al. 2002). Such findings demonstrate that anxiety-related psychopathology is associated with a heightened amygdala response to diverse affective stimuli. More importantly, in the absence of such disorders, variability in the magnitude of threat-related amygdala reactivity is an important predictor of individual differences in trait anxiety.
Having first established a predictive link between amygdala reactivity and trait anxiety, factors that drive such behaviorally relevant variability in brain function can be now be identified in the broader context of detailing the biological mechanisms mediating individual differences in temperamental anxiety. Converging preclinical and clinical evidence indicates that amygdala functioning is sensitive to the effects of central serotonin (Sadikot & Parent 1990), whose principal forebrain innervation is provided by the midbrain dorsal raphe nuclei (DRN). Available data from animal studies indicate that relative increases in local 5-HT result in potentiation of amygdala activation and associated behavioral phenomena, such as fear conditioning (Amat et al. 1998, 2004; Burghardt et al. 2004, 2009; Forster et al. 2006; Maier & Watkins 2005). As advanced in the introduction of this article, recent neuroimaging studies using multimodal PET/fMRI or pharmacological challenge BOLD fMRI have provided direct evidence for parallel effects of 5-HT in humans. Specifically, in vivo PET has revealed that decreased endogenous capacity for local 5-HT reuptake (Rhodes et al. 2007) is associated with relatively increased amygdala reactivity. Acute IV administration of a selective serotonin reuptake inhibitor, which reduces capacity for 5-HT reuptake, during BOLD fMRI is likewise associated with not only increased amygdala reactivity but also decreased habituation of amygdala reactivity over time (Bigos et al. 2008). These data clearly indicate that variability in the regulation of 5-HT signaling is an important source of individual differences in amygdala reactivity.
- 5-HT
5-hydroxytryptamine (serotonin)
Crucial among components regulating 5-HT neurotransmission and its subsequent modulation of brain function is activation of somatodendritic 5-HT1A autoreceptors, which mediate negative feedback on DRN neurons resulting in decreased 5-HT release at postsynaptic targets in the forebrain (Sharp et al. 2007). Using multimodal PET/fMRI, we previously reported that the density of 5-HT1A autoreceptors accounts for 30%–44% of variability in amygdala reactivity in healthy adults (Fisher et al. 2006), confirming the important role of 5-HT1A autoreceptors in modulating the activity of serotonergic target regions. Given the critical role of 5-HT1A autoreceptors in regulating 5-HT signaling and its resulting influence on the functioning of major brain targets, such as the amygdala, as well as complex behavioral processes (Cowen et al. 1994, Hansenne et al. 2002, Lesch & Gutknecht 2004), it is important to identify sources of emergent variability in 5-HT1A function.
Common sequence variation in the human 5-HT1A gene (HTR1A) represents one potential source of such interindividual variability. Recently, a relatively frequent single nucleotide polymorphism, C(−1019)G, in the promoter region of HTR1A affected transcriptional regulation of the gene through altered binding of the transcription factors. Specifically, the −1019G allele abolishes or impairs transcriptional repression of the promoter and, as a consequence, is associated with increased 5-HT1A expression (Lemonde et al. 2003), a phenomenon that appears to be specific to autoreceptors (Czesak et al. 2006). Consistent with this finding, in vivo human PET has revealed specifically increased 5-HT1A autoreceptor density in both healthy adults and depressed patients carrying the −1019G allele (Parsey et al. 2006). However, a similar effect was not observed in an earlier PET study (David et al. 2005). Regardless, the in vitro effects of the HTR1A −1019G allele and the more general link documented between increased 5-HT1A autoreceptor density and decreased amygdala reactivity (Fisher et al. 2006) suggest that this common functional genetic variation may contribute significantly to the emergence of interindividual variability in serotonin signaling, which, in turn, biases amygdala reactivity.
Consistent with the existing data (i.e., increased 5-HT1A autoreceptors leading to increased negative feedback inhibition of DRN and decreased 5-HT release), we recently demonstrated that the HTR1A −1019G allele is associated with significantly decreased threat-related amygdala reactivity (Fakra et al. 2009). In addition, we found that HTR1A genotype effects on trait anxiety were mediated through its impact on threat-related amygdala reactivity, which presumably reflects genotype-dependent modulation of postsynaptic 5-HT release. Specifically, although path models revealed no significant direct genotype effect on trait anxiety, they demonstrated that HTR1A C(−1019)G and amygdala reactivity indirectly predicted a significant proportion (9.2%) of individual differences in trait anxiety through their respective indirect and direct paths (Figure 2). The data from this study is remarkably consistent with that reported for other common functional polymorphisms also associated with relatively increased 5-HT signaling, most notably the 5-HTTLPR short allele (Hariri et al. 2002b, Munafo et al. 2008) and MAOA low-activity alleles (Meyer-Lindenberg et al. 2006). More importantly, these findings represent an important step in this avenue of research by providing empirical documentation for the basic premise that genetically driven variation in neural signaling cascades indirectly impacts emergent behavioral processes by biasing the response of underlying neural circuitries (Hariri et al. 2006b, Hariri & Weinberger 2003).
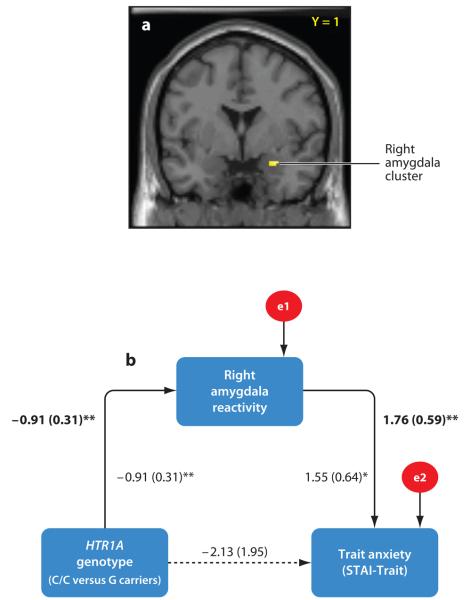
Figure 2.
The HTR1A genotype indirectly predicts trait anxiety through amygdala reactivity (adapted from Fakra et al. 2009). (a) Statistical parametric map illustrating the right amygdala cluster correlated with both HTR1A genotype and trait anxiety. Single-subject activation values from the maximal voxel in this cluster were entered into path analyses. (b) Path model testing indirect effects of the HTR1A genotype on trait anxiety. Lines are labeled with nonstandardized path coefficients and standard errors in parentheses. Coefficients in bold above the line represent values from the trimmed model, whereas coefficients in Roman represent values from the full model with all paths included. Indirect effects of the HTR1A genotype on trait anxiety were significant (αβ = −1.60, SE = 0.73, P < 0.05), whereas direct effects were nonsignificant and dropped from the model. e1 and e2 represent the residual variances not explained by model variables. *P < 0.05, **P < 0.01.
IMPULSIVITY, THE VENTRAL STRIATUM, AND DOPAMINE
Discounting future outcomes underlies much of human decision making and figures prominently in several overlapping psychological constructs, such as self-regulation, impulse-control, delay of gratification, and intertemporal choice (Manuck et al. 2003). Moreover, individuals who strongly prefer immediate over deferred rewards of larger nominal value are often generally impulsive or lacking in self-control and are at risk for addictive disorders such as pathological gambling, cigarette smoking, and drug and alcohol abuse (Alessi & Petry 2003, Bickel et al. 1999, Kirby et al. 1999, Madden et al. 1997). In experimental research on intertemporal choice, discounting of future rewards or delay discounting (DD) is a well-characterized behavioral measure of preference for immediate over delayed rewards and provides an index of impulsive tendencies in humans (Green & Myerson 2004). Behavioral tests used to derive estimates of DD commonly ask participants to choose between multiple immediate rewards that vary in value and a constant, larger reward available after varying intervals of delay. In such tasks, rates of discounting often differ appreciably and consistently among individuals (Simpson & Vuchinich 2000). Thus, DD represents a potentially important psychometric index of individual differences in present- versus future-oriented tendencies.
Similar to the research on trait anxiety, explication of the underlying neural processes that give rise to such interindividual variability may allow for a more comprehensive understanding of the mechanisms leading to not only normal variability in such behaviors but also the pathophysiology of addiction and related disorders. Through reciprocal cortical and subcortical connections, the nucleus accumbens (NAcc) and, more broadly, the ventral striatum (VS) contribute to the motivational salience of stimuli and abet appetitive or reward-dependent behaviors (Berridge & Robinson 2003). VS activity increases in response to both the anticipation and the receipt of rewarding stimuli including primary (e.g., food) and secondary (e.g., money) reinforcers (O'Doherty 2004). In addicts, craving and compulsive drug seeking as well as sensitivity to drug cues are associated with dysregulated increases in VS activity (Kalivas & Volkow 2005). Because the VS exhibits an immediate response to rewards, the magnitude of VS activity may contribute to individual differences in a relative preference for immediate, compared with delayed, rewards.
Using BOLD fMRI, we have demonstrated that the magnitude of VS reactivity predicts individual differences in a simple laboratory measure of DD (Hariri et al. 2006a). Specifically, analyses revealed that individual differences in DD correlate positively with the magnitude of VS activation, in response to both positive and negative feedback, as well as with differential reward-related VS activation in response to positive compared with negative feedback. Consistent with the strong general correlation between DD and traditional self-report measures of impulsivity (De Wit et al. 2008), we have also found that reward-related VS reactivity is positively correlated with scores from the Barratt Impulsiveness Scale (Forbes et al. 2009). Collectively, our results suggest that increased self-reported impulsivity as well as the preference for smaller immediate over larger delayed rewards reflect both a relatively indiscriminate and a hyper-reactive VS circuitry. Similar variability in VS function has also been associated with more complex measures of incentive-based decision making (Knutson et al. 2007). Moreover, dysregulation of the VS contributes to addiction, perhaps by affecting impulsive decision making (Kalivas & Volkow 2005). As such, interindividual variability in VS reactivity to reward-related stimuli likely contributes to the emergence of differences in the intermediate behavioral risk factors for, as well as the clinical expression of, addiction. Identifying variability in neural signaling pathways, which contributes to individual differences in VS function, offers additional traction in the search for underlying biological mechanisms.
Dopamine (DA) modulation of neuronal activity, especially in the VS (i.e., mesolimbic system), serves as a nexus for the expression of DA signaling at the level of reward-related behaviors (Cardinal et al. 2004, Kelley 2004). Functioning of the DA system has been linked to normal individual differences in reward-related traits (Depue et al. 1994), and disorders involving enhanced reward seeking, such as addiction, may reflect maladaptive alterations of this mesolimbic reward system (Hyman et al. 2006, Volkow et al. 1999). Multimodal and pharmacological neuroimaging studies of DA effects on brain function again offer a unique opportunity to more directly evaluate underlying molecular mechanisms regulating this circuitry. A recent in vivo human study reported a direct link between striatal DA synthesis, assessed with PET, and brain activity, assessed with BOLD fMRI (Siessmeier et al. 2006). Acute increase of DA release via oral amphetamine has also been linked with a relatively increased extent of activation in the VS (Menon et al. 2007). More generally, an acute pharmacologic increase of DA in both healthy volunteers (Hariri et al. 2002a) and patients with Parkinson disease (Tessitore et al. 2002) results in relatively increased BOLD fMRI–assessed activity in closely related limbic brain regions, namely the amygdala. Given the importance of DA in modulating this behaviorally relevant neural circuitry, identifying factors that determine interindividual variability in DA signaling and its related impact on the reactivity of the VS will facilitate our understanding of the neurobiological mechanisms governing reward-related behaviors and augment efforts to improve the treatment and even prevention of pathological behaviors such as drug abuse and addiction.
We have explored the role of altered DA signaling, resulting from a common functional polymorphism impacting active synaptic reuptake in the striatum, in determining interindividual variability in reward-related VS reactivity and correlated variability in behavioral impulsivity. Consistent with the research on serotonin signaling, amygdala reactivity, and trait anxiety, our selection of the candidate polymorphism was driven by available in vitro and/or in vivo assays demonstrating significant impact of the variant on aspects of biological function related to DA neurotransmission and not on available data from association studies with behavioral (e.g., impulsivity) or clinical (e.g., alcoholism) phenotypes. Although association studies are necessary for understanding the ultimate contribution of genetic polymorphisms to variability in behavioral and clinical phenomena, they do not readily allow us to make inferences regarding polymorphic effects on gene or protein function. Such inferences are instrumental to developing biologically plausible and tractable hypotheses regarding the impact of genetic variation on interindividual variability in brain function and associated behaviors such as those pursued in our current work (Hariri et al. 2006b, Hariri & Weinberger 2003).
The DA transporter (DAT) is responsible for the active clearance of synaptic DA and, thus, plays a critical role in regulating the duration of postsynaptic DA signaling, especially in the striatum (Sesack et al. 1998). Accumulating evidence indicates that a 40-base-pair variable number of tandem repeats polymorphism (DAT1) in the 3′ untranslated region of the DAT gene (DAT1) influences the expression and availability of DAT (Bannon et al. 2001). Although investigators have not consistently observed a genotype effect across all studies (Martinez et al. 2001, Michelhaugh et al. 2001, Mill et al. 2005, van Dyck et al. 2005), several suggest that in comparison to the 9-repeat allele, the 10-repeat is associated with relatively increased levels of DAT both in vivo (Cheon et al. 2005, Heinz et al. 2000) and in vitro (Mill et al. 2002, VanNess et al. 2005). We hypothesized that there would be relatively greater VS reactivity associated with the 9-repeat allele, which is linked with reduced DAT expression and presumably greater striatal synaptic DA, in comparison with the 10-repeat allele. Consistent with our hypothesis, the DAT1 9-repeat allele was associated with relatively greater VS reactivity and accounted for nearly 12% of the interindividual variability. In contrast, genetic variation directly affecting DA signaling only in the prefrontal cortex (i.e., COMT Val158Met) was not associated with variability in VS reactivity. These results highlight an important role for a genetic polymorphism affecting striatal DA neurotransmission in mediating interindividual differences in reward-related VS reactivity. They further suggest that altered VS reactivity may represent a key neurobiological pathway through which these polymorphisms contribute to variability in behavioral impulsivity and related risk for substance use disorders.
- DA
dopamine
ENDOCANNABINOIDS, THREAT-, AND REWARD-RELATED BRAIN FUNCTION
Modern neuroscience methodologies have greatly advanced our understanding of the intrinsic mechanisms mediating and regulating endogenous cannabinoid or endocannabinoid signaling in the CNS (Piomelli 2003). Such eCB signaling has emerged as a potent modulator of neural circuitries mediating both basic physiological (Calignano et al. 1998, Meng et al. 1998) and advanced behavioral responses (Maldonado et al. 2006, Scherma et al. 2008, Viveros et al. 2005). Experimental manipulation of these mechanisms has revealed significant behavioral effects, especially in threat- and reward-related domains, which are generally consistent with the effects of Cannabis intoxication, driven largely by the constituent chemical Δ9-tetrahydrocannabinol (Robson 2005). The elucidation of molecular mechanisms regulating eCB signaling, akin to that for serotonin and DA, has motivated attempts to understand its possible contribution to the emergence of variability in brain circuit function and related individual differences in behavioral attributes (e.g., anxious or impulsive temperament) associated with increased risk for psychiatric disorders.
After their biosynthesis from arachidonic acid, eCBs such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG) typically modulate synaptic neurotransmission through stimulation of CB1, the principal CNS cannabinoid receptor widely expressed on multiple neuronal subtypes and their distributed circuitries. In turn, the duration and intensity of eCB signaling, especially for AEA, are regulated by two complementary mechanisms: enzymatic degradation via fatty acid amide hydrolase (Cravatt et al. 1996) and active synaptic clearance via the AEA transporter (Piomelli et al. 1999). The psychotropic and THC-like effects of AEA, however, appear to be coupled with fatty acid amide hydrolase (FAAH) but not AEA transporter function (Solinas et al. 2007). Thus, FAAH, an integral membrane enzyme, may uniquely regulate behaviorally relevant eCB signaling by mediating the hydrolytic breakdown of AEA into arachidonic acid and ethanolamine.
Again, common genetic variation (i.e., polymorphisms) affecting the functioning of components involved in eCB neurotransmission (e.g., AEA, CB1, FAAH) may represent a significant potential source of interindividual variability in eCB signaling that mediates emergent differences in emotion- and reward-related behaviors (Onaivi et al. 2002). Because of its critical role in regulating the signaling duration and intensity of AEA (Cravatt et al. 1996) and its selective contribution to the psychotropic effects of AEA (Solinas et al. 2007), we have recently examined the neurobiological and behavioral effects of a common functional nonsynonymous SNP resulting in the conversion of a conserved proline residue to threonine (P129T) in the amino acid sequence of FAAH (Hariri et al. 2008). In vitro, FAAH 385A is associated with normal catalytic properties but reduced cellular expression of FAAH, possibly through enhanced sensitivity to proteolytic degradation (Chiang et al. 2004, Sipe et al. 2002). Moreover, the C385A is the only common mutation in FAAH (Flanagan et al. 2006) and the 385A, which putatively augments AEA signaling via decreased enzymatic degradation and has been associated with reward-related pathologies, including street drug use and problem drug/alcohol abuse, as well as being overweight and obese (Flanagan et al. 2006, Sipe et al. 2002).
- eCB
endocannabinnoids
- AEA
anandamide
- FAAH
fatty acid amide hydrolase
- SNP
single nucleotide polymorphism
In animal models, both pharmacologic and genetic disruption of FAAH function result in decreased anxiety-like behaviors, as well as increased consumption and preference for ethanol (Basavarajappa et al. 2006, Blednov et al. 2007, Kathuria et al. 2003, Moreira et al. 2008, Solinas et al. 2007). Moreover, a recent pharmacological fMRI study in human subjects has reported that acute oral administration of THC is associated with reduced amygdala reactivity to threat-related facial expressions (Phan et al. 2008). Consistent with these effects, we hypothesized that the FAAH 385A would be associated with relatively decreased threat-related amygdala but increased reward-related VS reactivity. Analyses revealed that carriers of the FAAH 385A, associated with reduced enzyme expression and, presumably, increased AEA signaling, have decreased threat-related amygdala reactivity. In contrast, carriers of the FAAH 385A exhibited increased reward-related VS reactivity in comparison with C385 homozygotes. Moreover, divergent effects of the FAAH C385A genotype on brain function were manifest in a consistent manner at the level of brain-behavior links (Figure 3). Relative to C385 homozygotes, FAAH 385A carriers showed a diminished correlation between amygdala reactivity and trait anxiety. In contrast, 385A carriers exhibited a markedly increased correlation between VS reactivity and delay discounting.
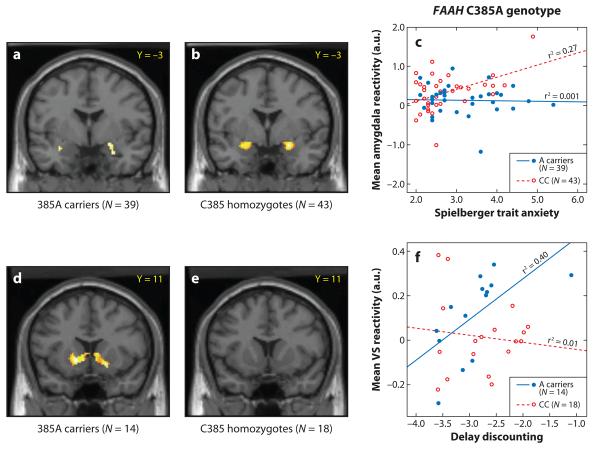
Figure 3.
Divergent effects of the FAAH genotype on threat- and reward-related brain function (adapted from Hariri et al. 2008). Statistical parametric maps illustrating the correlation between fear-related amygdala reactivity and trait anxiety in (a) FAAH 385A carriers and (b) C385 homozygotes. (c) Plots of the differential correlation between fear-related amygdala reactivity and trait anxiety as a function of the FAAH C385A genotype (β = 0.38, P = 0.03; 385A carriers: r = −0.03, P = 0.84; C385 homozygotes: r = 0.52, P < 0.001). Statistical parametric maps illustrating the correlation between reward-related VS reactivity and delay discounting in (d ) FAAH 385A carriers and (e) C385 homozygotes (no significant correlation). (f) Plots of the differential correlation between reward-related VS reactivity and delay discounting as a function of the FAAH C385A genotype (β = −0.54, P = 0.04; 385A carriers: r = 0.63, P = 0.02) and C385 homozygotes (r = 0.11, P = 0.65).
It is important to note that this study showed no direct associations between the FAAH genotype and behavioral phenotypes (i.e., anxiety or impulsivity), a common occurrence when working with relatively small samples, possibly reflecting the minimal effect the proximal biological impact associated with any genotype has on any distal behavioral phenotype (Hariri et al. 2006b, Hariri & Weinberger 2003) and the importance of environmental stressors in unmasking genetically driven effects on behavior (Caspi & Moffitt 2006). However, there were robust differences in the correlations between regional brain function and complex behaviors as a function of the FAAH C385A genotype. These observed brain-behavior patterns may reflect the influence of FAAH C385A-associated differences in endogenous eCB tone on stimulus-driven neural circuit function mediating complex behavioral processes. Relatively higher levels of AEA in the amygdala of FAAH 385A carriers may reduce the responsivity of this structure to salient input (possibly through CB1-mediated potentiation of local GABAergic interneurons) and may, as a consequence, reduce anxiety-like behaviors predicted by amygdala function. In contrast, higher levels of AEA may increase the responsivity of the VS in FAAH 385A carriers (possibly through CB1-mediated increased DA release and potentiation of VS neuron activity), leading to increased reward sensitivity predicted by VS function. Support for this speculation exists in studies reporting a failure of restraint stress to effect changes in amygdala activation in knockouts lacking FAAH or animals treated with FAAH inhibitors (Patel et al. 2005) and increased food intake as a result of local FAAH inhibition in the nucleus accumbens (Sorice-Gomez et al. 2007). Thus, the endogenous state of eCB signaling associated with either constitutive genetic variation such as the FAAH C385A or acute pharmacologic manipulation likely biases the responsivity of neural circuits to behaviorally relevant information and their subsequent regulation of complex behaviors.
Decreased threat-related amygdala reactivity and associated trait anxiety may contribute to the emergence of pathologies such as addiction and obesity, previously associated with the FAAH 385A (Flanagan et al. 2006, Sipe et al. 2002, Tyndale et al. 2007), by reducing the sensitivity of these individuals to potential environmental threat or harm. In fact, blunted amygdala reactivity has been reported in individuals at high familial risk for alcoholism, which may contribute to decreased threat sensitivity and subsequently increased risk-taking behaviors in these genetically predisposed individuals (Glahn et al. 2007). An increase in reward-related VS reactivity and associated impulsivity (e.g., steeper discounting of future, relative to immediate rewards) may likewise contribute to disinhibitory psychopathologies through heightened reward sensitivity and impulsive decision making. Studies in addicted patients have generally reported a sensitization of the neural circuitry for reward, including the VS (Kalivas & Volkow 2005). Increased behavioral impulsivity and reward sensitivity are significant risk factors for addiction (de Wit & Richards 2004). Thus, through divergent effects on both threat- and reward-related brain function, the influence of FAAH C385A on eCB signaling may have a compound and accelerated effect on risk for related pathologies.
- Pharmacological fMRI
a drug is administered to challenge a specific component of a molecular signaling pathway (e.g., serotonin transporter) implicated in modulating the functional circuitry being assayed with BOLD fMRI
SUMMARY AND FUTURE DIRECTIONS
As detailed above, neuroimaging technologies, especially BOLD fMRI, have begun to identify how variability in neural substrates associated with processing specific forms of information contributes to emergent individual differences in stable and enduring aspects of human behaviors such as personality and temperament. In parallel, the application of pharmacological fMRI and multimodal PET/fMRI allows investigators to understand how variability in specific molecular signaling pathways influences individual differences in the function of these behaviorally relevant brain circuitries. Moreover, information on DNA sequence variation in humans and related identification of functional genetic polymorphisms are now being utilized to understand the biological origins of variability in component processes of molecular signaling pathways as well as to model efficiently how such emergent variability impacts behaviorally relevant brain function. Such ongoing efforts to understand the detailed mechanisms that mediate individual differences in complex behavioral traits and related neuropsychiatric diseases at the level of brain circuit function, molecular signaling pathways, and functional genetic polymorphisms may inform clinically relevant issues and provide guiding principles to develop more effective and individually tailored treatment regimes. In addition, the elucidation of such mechanisms, especially those mapped to functional genetic polymorphisms, can help identify predictive risk markers that interact with unique environmental factors to precipitate disease.
Although the three examples highlighted in this article support the potential of an informed and integrated research strategy to identify the neurobiology of individual differences in complex behavioral traits and their related clinical end points, much work is left to be done. First, to allow for tractable experimental designs and testable hypotheses in existing samples, the studies highlighted above have focused on the effects of a single signaling pathway on behaviorally relevant brain circuitry. Of course, it is very clear that there are numerous complex interactions between signaling pathways and that more than one pathway contributes to the regulation of brain circuitry. For example, we know that DA plays an important role in modulating amygdala function and anxiety (Hariri et al. 2002a, Tessitore et al. 2002) and that 5-HT can influence reward-related brain circuitry and impulsivity (Manuck et al. 1998). However, existing studies lack the power and sophistication to model such complex interactions while effectively controlling for other important modulatory factors (e.g., age, sex). To do so, we must aggressively expand the scale and scope of our studies to include hundreds and, preferably, thousands of subjects. Achieving this will afford opportunities to examine the impact of interactions between signaling pathways (e.g., 5-HT and DA) on brain function and behavior through modeling of multiple functional polymorphisms (e.g., HTR1A −1019 and DAT1).
A second important consideration is that existing studies have been conducted largely in ethnically and racially homogenous populations. Thus, the observed effects may not generalize to other populations. This possibility is especially true of studies utilizing functional genetic polymorphisms because the potential effect of any single genetic variant on a complex biological and behavioral phenotype is likely small against the background of the ~20,000–25,000 human genes and the multitude of other neurobiologically relevant functional variants they likely harbor. In fact, we have already seen that the well-replicated effects of a common functional polymorphism affecting 5-HT signaling on amygdala reactivity in Caucasian subjects may be reversed in those of Asian ancestry (Lee & Ham 2008, Munafo et al. 2008). Our most recent studies have experimentally controlled for occult genetic stratification independent of self-reported race or ethnicity as well as the independence of the target genotype from other functional polymorphisms impacting the brain functions under study. Although such efforts allow investigators to attribute emergent variability in brain and behavior to the candidate variant of interest and not to other possible polymorphisms or more general differences between genotype groups in genetic background, it is important to test explicitly the independence of functional polymorphisms through rigorous statistical modeling in larger samples and also to test the validity of any associations derived in one sample population (e.g., Caucasian) in populations with different genetic backgrounds (e.g., Asian).
A third important consideration for the future of this research is the need to conduct large-scale prospective studies beginning in childhood to determine any developmental shifts in neurogenetic pathways mediating individual differences in behavior as well as their predictive utility in identifying neuropsychiatric disease risk as a function of environmental or other stressors. All the studies described above and most of the studies available in the literature as a whole have been conducted in adults carefully screened for the absence of psychopathology. Because of this limitation, these findings identify mechanisms contributing to variability in the normative range of behavior only. Whether they are neural, molecular, or genetic, the utility of these markers of individual differences in behavior for predicting vulnerability to neuropsychiatric disorder is unclear. Such predictive utility is ideally tested through prospective studies beginning with premorbid populations that account for the moderating effects of environmental stress in the emergence of clinical disorders over time (Caspi & Moffitt 2006, Viding et al. 2006).
A fourth issue is the need to further integrate pharmacological challenge protocols with multimodal PET/fMRI to determine if variability in molecular components of signaling pathways mediates effects of specific neurotransmitters or neuromodulators on individual differences in behaviorally relevant brain circuit function. For example, despite the remarkable convergence of findings implicating variability in eCB signaling in threat- and reward-related brain function, the exact nature of the downstream signaling pathways through which FAAH C385A may modulate neuronal and neural circuit function cannot be determined from the available results. FAAH catalyzes the hydrolysis of other biologically active endogenous fatty acid amides (e.g., oleamide and oleoylethanolamide), which impact threat- and reward-related behaviors independently of AEA (LoVerme et al. 2005, Wei et al. 2007). Although FAAH has high selectivity for AEA (Desarnaud et al. 1995), the effects of FAAH C385A cannot be specifically linked to AEA neurotransmission without additional data. If the neural and behavioral effects of FAAH C385A are mediated by genotype-driven differential availability of AEA, then these effects should be sensitive to manipulation of CB1 receptors. An interesting test of this putative mechanism would be to examine the impact of CB1 antagonists, such as rimonabant, on neural phenotypes associated with FAAH C385A genotype using pharmacological fMRI. The availability of a PET radiotracer for CB1 (Burns et al. 2007) also allows investigators to observe any FAAH C385A effects on endogenous receptor concentrations. If this polymorphism biases brain function through AEA stimulation of CB1, then antagonism of the receptor should eliminate its divergent effects on amygdala and VS reactivity. Any genotype-related alterations in AEA concentrations may also be reflected in relative up- or downregulation of CB1 receptors assayed via PET. If CB1 antagonism fails to abolish the differential effects of FAAH C385A on brain function or if there are no differences in CB1 concentrations on the basis of the genotype, then the existing effects are likely mediated by non-eCB fatty acid amides. In addition to testing this mechanistic hypothesis with pharmacological fMRI and multimodal PET/fMRI, future studies with substantially increased sample sizes can model allele load effects of FAAH 385A, as well as potential FAAH interactions with functional genetic polymorphisms affecting other components of eCB neurotransmission (Chakrabarti et al. 2006).
Finally, there is tremendous potential in developing large databases (again preferably thousands of subjects) with detailed measures of behavioral traits, neuroimaging-based measures of multiple brain circuitries, and extensive genotyping. One of the most exciting applications of molecular genetics is in identifying novel biological pathways contributing to the emergence of complex traits (Gibson & Goldstein 2007, McCarthy et al. 2008). The continued refinement of a detailed map of sequence variation across the entire human genome (i.e., SNPs that tag every gene) and production of technologies supporting efficient high-throughput identification of such variation in individuals have dramatically accelerated the discovery of genes involved in the emergence of complex disease processes (Fellay et al. 2007, Link et al. 2008) as well as normal variability in continuous traits (Lettre et al. 2008). More recently, copy number variations (CNVs) including insertions, deletions, or duplications of relatively large expanses of DNA (e.g., >1Kb) have been found to exist in nearly all regions of the human genome (Redon et al. 2006). Although currently more difficult to assay than SNPs, CNVs, especially those occuring as biallelic polymorphisms (e.g., deleted versus not-deleted), have already been linked to individual differences in complex traits and related disease processes including several neuropsychiatric disorders (Kehrer-Sawatzki 2007). Many of the genes identified in studies using either tag SNPs or CNVs have illuminated novel pathways not previously implicated in the target behavioral or clinical phenomenon of interest, spurring intensive efforts to understand the potential biological effects of the proteins produced by these genes. As such, these genome-wide screens represent an opportunity to leap forward beyond the available pool of candidate molecules and pathways in parsing the mechanisms of complex biological processes. Because neuroimaging-based measures of brain function reveal key mechanisms involved in the emergence of individual differences in behavioral traits and are closer to the biological effects of functional genetic polymorphisms, they are ideal substrates for genome-wide screens. For example, BOLD fMRI estimates of amygdala reactivity predicting variability in temperamental anxiety can be used as the continuous trait in a genome-wide screen. Any significant associations that emerge between genetic variation and amygdala reactivity may confirm existing links (e.g., the importance of genes biasing 5-HT signaling) or, more importantly, may reveal unexpected candidate molecules or pathways (e.g., a gene producing a molecule that is expressed in the brain and may function in second-messenger signaling cascades). Once identified and, ideally, replicated in large-scale databases that effectively address confounds common to genome-wide screens (e.g., controlling for multiple comparisons resulting from testing the association of a phenotype with hundreds of thousands of SNPs), the impact of variation in novel genes associated with amygdala reactivity can be explored at each level of the biological cascade leading to trait anxiety (i.e., can be fed back into the discovery framework outlined in Figure 1). In addition to improving exponentially our understanding of neurobiological pathways leading to individual differences in complex behavioral traits, these efforts may lead to the discovery of novel therapeutic strategies targeting related disease processes.
SUMMARY POINTS.
Neuroimaging technologies, especially BOLD fMRI, have begun to identify how variability in neural substrates associated with processing specific forms of information contributes to emergent individual differences in stable and enduring aspects of human behaviors such as personality and temperament.
The application of pharmacological fMRI and multimodal PET/fMRI helps investigators understand how variability in specific molecular signaling pathways influences individual differences in behaviorally relevant brain circuit function.
Detailed information on DNA sequence variation in humans and related identification of functional genetic polymorphisms are now being utilized to understand the biological origins of variability in component processes of molecular signaling pathways as well as to model efficiently how such emergent variability impacts behaviorally relevant brain function.
Existing efforts to understand the detailed mechanisms that mediate individual differences in complex behavioral traits and related neuropsychiatric diseases at the level of brain circuit function, molecular signaling pathways, and functional genetic polymorphisms have the potential to inform clinically relevant issues and provide guiding principles for the development of more effective and individually tailored treatment regimes. In addition, the elucidation of such mechanisms, especially those mapped to functional genetic polymorphisms, can lead to identification of predictive risk markers that interact with unique environmental factors to precipitate disease.
FUTURE DIRECTIONS.
Aggressively expanding the scale and scope of studies will allow investigators to examine the effects of interactions between signaling pathways (e.g., 5-HT and DA) on behaviorally relevant brain function by modeling multiple functional polymorphisms (e.g., HTR1A −1019 and DAT1).
Extending ongoing studies to ethnically and racially diverse populations will allow investigators to determine if observed mechanisms, especially those rooted in functional genetic polymorphisms, are generally involved in shaping behavior or operate only against a specific genetic background.
Conducting large-scale prospective studies beginning in childhood will allow investigators to determine any developmental shifts in neurogenetic pathways mediating individual differences in behavior as well as their predictive utility in identifying neuropsychiatric disease risk as a function of environmental or other stressors.
Integrating pharmacological challenge protocols with multimodal PET/fMRI will allow investigators to determine if variability in molecular components of signaling pathways mediate effects of specific neurotransmitters or neuromodulators on individual differences in behaviorally relevant brain circuit function.
Developing large databases of neuroimaging-based measures of behaviorally relevant brain function and using genome-wide screens will allow investigators to map neural phenotypes to genes and genetic variation that will illuminate new biological pathways important in shaping brain function and behavior.
ACKNOWLEDGMENTS
The author thanks Patrick Fisher, Karen Muñoz, Adam Gorka, Luke Hyde, and Maggie Sweitzer for their invaluable contributions to the preparation of this review.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav. Process. 2003;64:345–54. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–20. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, et al. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–19. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Michelhaugh SK, Wang J, Sacchetti P. The human dopamine transporter gene: gene organization, transcriptional regulation, and potential involvement in neuropsychiatric disorders. Eur. Neuropsychopharmacol. 2001;11:449–55. doi: 10.1016/s0924-977x(01)00122-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–44. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl.) 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein H, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–25. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. 2004;24:10364–68. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–82. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Bush DEA, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT2C receptor antagonist. Biol. Psychiatry. 2007;62:1111–18. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reup-take inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol. Psychiatry. 2004;55:1171–78. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc. Natl. Acad. Sci. USA. 2007;104:9800–5. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–81. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann. N. Y. Acad. Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 2006;7:583–90. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Kent L, Suckling J, Bullmore E, Baron-Cohen S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur. J. Neurosci. 2006;23:1944–48. doi: 10.1111/j.1460-9568.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim JW, Cho DY. The homozygosity for 10-repeat allele at dopamine transporter gene and dopamine transporter density in Korean children with attention deficit hyperactivity disorder: relating to treatment response to methylphenidate. Eur. Neuropsychopharmacol. 2005;15:95–101. doi: 10.1016/j.euroneuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum. Mol. Genet. 2004;13:2113–19. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Res. 2006;148:55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Power AC, Ware CJ, Anderson IM. 5-HT1A receptor sensitivity in major depression. A neuroendocrine study with buspirone. Br. J. Psychiatry. 1994;164:372–79. doi: 10.1192/bjp.164.3.372. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J. Neurosci. 2006;26:1864–71. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafo MR, Johnstone EC, et al. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J. Neurosci. 2005;25:2586–90. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Flory JD, Acheson A, McLoskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personal. Individ. Differ. 2007;42:111–21. [Google Scholar]
- de Wit H, Richards JB. Dual determinants of drug use in humans: reward and impulsivity. Nebr. Symp. Motiv. 2004;50:19–55. [PubMed] [Google Scholar]
- Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: relation of agonist-induced dopamine activity to positive emotionality. J. Pers. Soc. Psychol. 1994;67:485–98. doi: 10.1037//0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- Desarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. J. Biol. Chem. 1995;270:6030–35. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: interaction between sex and trait anxiety. Psychiatry Res. 2008;162:51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress. Anxiety. 2008;25:496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch. Gen. Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–47. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Hariri AR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat. Neurosci. 2006;9:1362–63. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Flanagan JM, Gerber AL, Cadet JL, Beutler E, Sipe JC. The fatty acid amide hydrolase 385 A/A (P129T) variant: haplotype analysis of an ancient missense mutation and validation of risk for drug addiction. Hum. Genet. 2006;120:581–88. doi: 10.1007/s00439-006-0250-x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, et al. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–55. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Gibson G, Goldstein DB. Human genetics: the hidden text of genome-wide associations. Curr. Biol. 2007;17:R929–32. doi: 10.1016/j.cub.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol. Psychiatry. 2007;61:1306–9. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol. Bull. 2004;130:769–92. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav. Neurosci. 2007;121:249–56. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Hansenne M, Pitchot W, Pinto E, Reggers J, Scantamburlo G, et al. 5-HT1A dysfunction in borderline personality disorder. Psychol. Med. 2002;32:935–41. doi: 10.1017/s0033291702005445. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J. Neurosci. 2006a;26:13213–17. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol. Psychiatry. 2006b;59:888–97. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat-and reward-related brain function. Biol. Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.10.047. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn. Sci. 2006;10:182–91. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002a;27:1036–40. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002b;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. Br. Med. Bull. 2003;65:259–70. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–39. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, et al. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25:1112–23. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kehrer-Sawatzki H. What a difference copy number variation makes. Bioessays. 2007;29(4):311–13. doi: 10.1002/bies.20554. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–79. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–75. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–56. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee BT, Ham BJ. Serotonergic genes and amygdala activity in response to negative affective facial stimuli in Korean women. Genes Brain Behav. 2008;7:899–908. doi: 10.1111/j.1601-183X.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J. Neurosci. 2003;23:8788–99. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Gutknecht L. Focus on the 5-HT1A receptor: emerging role of a gene regulatory variant in psychopathology and pharmacogenetics. Int. J. Neuropsychopharmacol. 2004;7:381–85. doi: 10.1017/S1461145704004845. [DOI] [PubMed] [Google Scholar]
- Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, et al. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat. Genet. 2008;40:584–91. doi: 10.1038/ng.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E, Parish S, Armitage J, Bowman L, Heath S, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N. Engl. J. Med. 2008;359:789–99. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. Regulation of food intake by oleoylethanolamide. Cell Mol. Life Sci. 2005;62:708–16. doi: 10.1007/s00018-004-4494-0. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp. Clin. Psychopharmacol. 1997;5:256–62. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–32. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am. J. Psychiatry. 2007;164:1613–14. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19:287–99. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Muldoon MF, Ferrell RE. A neurobiology of intertemporal choice. In: Loewenstein G, Read D, Baumeister RF, editors. Time and Decision: Economic and Psychological Perspectives on Intertemporal Choice. Sage; New York: 2003. pp. 139–72. [Google Scholar]
- Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, et al. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology. 2001;24:553–60. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet. 2008;9:356–69. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–83. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, et al. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biol. Psychiatry. 2007;62:765–72. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. USA. 2006;103:6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelhaugh SK, Fiskerstrand C, Lovejoy E, Bannon MJ, Quinn JP. The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J. Neurochem. 2001;79:1033–38. doi: 10.1046/j.1471-4159.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am. J. Med. Genet. 2002;114:975–79. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Craig I, D'Souza UM. Transient expression analysis of allelic variants of a VNTR in the dopamine transporter gene (DAT1) BMC Genet. 2005;6:3. doi: 10.1186/1471-2156-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–50. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Johnson MR, Kiehl KA. Attentional modulation of the amygdala varies with personality. Neuroimage. 2006;31:934–44. doi: 10.1016/j.neuroimage.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol. Psychiatry. 2008;63:852–57. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Leonard CM, Ishiguro H, Zhang PW, Lin Z, et al. Endocannabinoids and cannabinoid receptor genetics. Prog. Neurobiol. 2002;66:307–44. doi: 10.1016/s0301-0082(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol. Psychiatry. 2006;59:106–13. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J. Neurosci. 2008;28:2313–19. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol. Psychiatry. 2006;59:424–29. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, et al. Structural determinants for recognition and translocation by the anandamide transporter. Proc. Natl. Acad. Sci. USA. 1999;96:5802–7. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn. Affect. Behav. Neurosci. 2005;5:156–68. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Rhodes RA, Murthy NV, Dresner MA, Selvaraj S, Stavrakakis N, et al. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J. Neurosci. 2007;27:9233–37. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P. Human studies of cannabinoids and medicinal cannabis. Handb. Exp. Pharmacol. 2005;168:719–56. doi: 10.1007/3-540-26573-2_25. [DOI] [PubMed] [Google Scholar]
- Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–54. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot AF, Parent A. The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience. 1990;36:431–47. doi: 10.1016/0306-4522(90)90439-b. [DOI] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, et al. The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology. 2008;54:129–40. doi: 10.1016/j.neuropharm.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Guido MA, Levey AI. Cellular and subcellular localization of the dopamine transporter in rat cortex. Adv. Pharmacol. 1998;42:171–74. doi: 10.1016/s1054-3589(08)60720-6. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007;28:629–36. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Siessmeier T, Kienast T, Wrase J, Larsen JL, Braus DF, et al. Net influx of plasma 6-[18F]fluoro-l-DOPA (FDOPA) to the ventral striatum correlates with prefrontal processing of affective stimuli. Eur. J. Neurosci. 2006;24:305–13. doi: 10.1111/j.1460-9568.2006.04903.x. [DOI] [PubMed] [Google Scholar]
- Simpson CA, Vuchinich RE. Reliability of a measure of temporal discounting. Psychol. Rec. 2000;50:3–16. [Google Scholar]
- Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc. Natl. Acad. Sci. USA. 2002;99:8394–99. doi: 10.1073/pnas.082235799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J. Pharmacol. Exp. Ther. 2007;321:370–80. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol. Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sorice-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br. J. Pharmacol. 2007;151:1109–16. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch. Gen. Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am. J. Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, et al. Dopamine modulates the response of the human amygdala: a study in Parkinson's disease. J. Neurosci. 2002;22:9099–103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, Payne JI, Gerber AL, Sipe JC. The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: studies of drug use and dependence in Caucasians. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144:660–66. doi: 10.1002/ajmg.b.30491. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J. Nucl. Med. 2005;46:745–51. [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Williamson DE, Hariri AR. Developmental imaging genetics: challenges and promises for translational research. Dev. Psychopathol. 2006;18:877–92. doi: 10.1017/s0954579406060433. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005;81:331–42. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J. Psychopharmacol. 1999;13:337–45. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- Wei XY, Yang JY, Dong YX, Wu CF. Anxiolytic-like effects of oleamide in group-housed and socially isolated mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:1189–95. doi: 10.1016/j.pnpbp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Semin. Clin. Neuropsychiatry. 2002;7:234–42. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]