Abstract
OBJECTIVE
To evaluate whether 6 months of raloxifene was effective in treatment of chronic pelvic pain in women with endometriosis.
METHODS
Women with chronic pelvic pain and no endometriosis treatment for 6 months underwent laparoscopy for excision of all lesions. Those with biopsy-proven endometriosis were randomly allocated to raloxifene (180 mg) or placebo daily. A second laparoscopy was performed at 2 years, or earlier, if pain returned. Return of pain was defined as 2 months of pain equal to or more severe than that at study entry. Menstrual cycles and adverse events were recorded. The log rank test was used to compare the time to return of pain by drug group. Analyses were done as intent-to-treat.
RESULTS
A total of 127 of 158 women underwent surgery. Of these, 93 had biopsy-confirmed endometriosis and were randomly assigned to study treatment. Menstrual cycle length, pelvic pain severity, quality of life, bone mineral density, and adverse events did not differ between treatment groups. The Data Safety Monitoring Committee terminated the study early when the raloxifene group experienced pain (P=.03) and had second surgery (P=.016) significantly sooner than the placebo group. Interestingly, biopsy-proven endometriosis was not associated with return of pain (P=.6).
CONCLUSION
Raloxifene significantly shortened the time to return of chronic pelvic pain. Because recurrence of endometriosis lesions did not correlate with return of pain, other factors are implicated in pelvic pain.
CLINICAL TRIAL REGISTRATION
ClinicalTrials.gov, www.cliicaltrials.gov, NCT00001848 (Obstet Gynecol 2008;111:88–96)
LEVEL OF EVIDENCE
I
Many women with chronic pelvic pain have endometriosis, a condition in which endometrial glands and stroma implant and grow outside the uterus.1,2 Chronic pelvic pain is estimated to cost 25 billion dollars to the U.S. health system each year,3,4 and 14 billion dollars in lost productivity.5 Medical therapy with gonadotropin-releasing hormone agonists decreases circulating estrogen concentrations and is associated with pain relief, but adverse effects and bone loss limit long-term use without the addition of a hormonal add-back regimen.1,2,6–8 The ideal medical therapy for endometriosis would reduce growth of lesions, preserve bone mass and fertility, and reduce pelvic pain. While gonadotropin-releasing hormone agonists with estrogen and/or progestin have this potential, their expense often precludes long-term use.
Selective estrogen receptor modulators (SERMs) are associated with beneficial estrogen agonist effects in some tissues without undesirable effects in others.9,10 In postmenopausal women, the SERM raloxifene shows the beneficial effects of estrogens on bone density, but stimulates neither the endometrium nor breasts.11–14 In menstruating women, at a daily dose of 200 mg, it did not alter cycle length or stimulate endometrial growth, despite elevated plasma estrogen levels.15 The beneficial effect on bone density without endometrial stimulation suggested that raloxifene might be an effective medical therapy for women with endometriosis. In this randomized, placebo-controlled, double-blind prospective study we sought to evaluate whether surgical excision of endometriosis followed by 6 months of treatment with raloxifene would eliminate the return of pelvic pain during the subsequent 18 months.
MATERIALS AND METHODS
Women with chronic pelvic pain with or without a history of endometriosis referred themselves to the study. The study and most laparoscopies were conducted at the Clinical Center of the National Institutes of Health from January 1999 to December 2004 after approval by the Institutional Review Board of the National Institute of Child Health and Human Development (ClinicalTrials.gov Identifier: NCT00001848). Some laparoscopies were performed at Georgetown University Medical Center after institutional review board approval by that institution.
The study entry criteria included women aged 18 years to 45 years who had a 3-month history of pelvic pain, biopsy-proven endometriosis at study laparoscopy, and a significant postoperative pelvic pain reduction. They had to be in excellent health with a body mass index less than 40 kg/m2, except for use of antidepressants, medications for migraines and headaches, and allergy medications. All participants agreed to postpone pregnancy during the study and use abstinence, barrier contraception, or sterilization as a method of contraception.
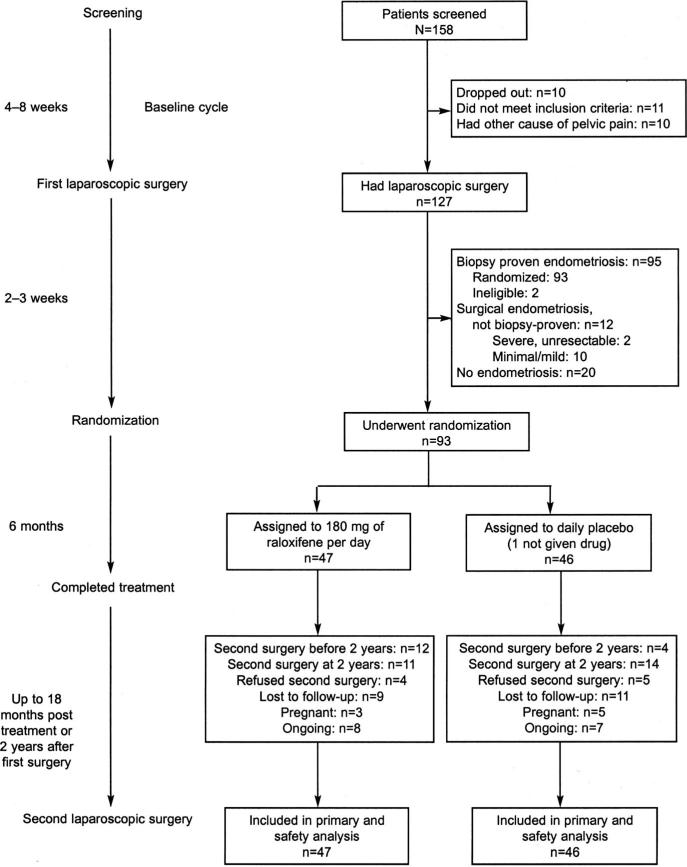
After giving informed consent, women underwent a standardized diagnostic evaluation for causes of pelvic pain; those without treatment for endometriosis within 6 months who met inclusion and exclusion criteria were enrolled (Fig. 1). Participants charted pain and symptoms for at least 1 month before surgery as described below.
Fig. 1. Flowchart of patients in the study.
Stratton. Chronic Pelvic Pain and Raloxifene Treatment. Obstet Gynecol 2008.
Patients had a history of regular cyclic menses. In November 2002, after 70 women did not have a significant change in menstrual cycle length on treatment, women with irregular cycles were allowed if their fasting insulin, prolactin, and testosterone levels were normal. Participants who had a hysterectomy or bilateral salpingo-oophorectomy or who were pregnant or lactating were excluded.
Women were excluded if they had chronic pelvic pain resulting only from infectious, gastrointestinal, musculoskeletal, neurologic, or psychiatric causes. In January 2000, screening for myofascial diseases using serologic tests of antinuclear antibody, rheumatoid factor, creatinine kinase, erythrocyte sedimentation rate, thyroid stimulating hormone, and free thyroxine was begun, and those with abnormal results were referred for evaluation. They were also excluded if they had significant abnormalities in the physical or laboratory examination, including renal and liver function more than twice the normal range.
Patients were not allowed to use hormonal contraception, selective estrogen receptor modulators, progestins, estrogens, steroids, or ovulation induction in the past 3 months or have had other medical or surgical treatment for endometriosis in the past 6 months. Those with untreated abnormal cervical cytology or other gynecologic condition were excluded. Women with a history of venous thrombosis events, stroke, transient ischemic attack, manic depressive illness, or untreated major depression were excluded.
Using a standardized surgical approach, one investigator (P.S.) operated at 125 of 127 laparoscopic surgeries to guarantee consistency. To ensure accurate diagnosis of endometriosis, all apparent endometriosis lesions, including endometriomas and nonclassic lesions (eg, clear or red), were excised using a neodymium:yttrium argon garnet contact laser (Surgical Laser Technologies, The Oaks, PA). Pelvic adhesions were lysed. After removal, sites of large endometriomas were wrapped with the adhesion barrier Interceed (Johnson & Johnson, Cincinnati, OH). Findings were recorded using the American Society for Reproductive Medicine diagrams, scoring, and staging system for lesions and adhesions.18
Participants with biopsy-proven endometriosis, defined as endometrial glands and stroma in at least one specimen, and some postoperative pain reduction were randomly assigned to study treatment by three weeks after surgery (Fig. 1). Participants were instructed to take two capsules at the same time daily until the first menses after the 6th-month visit.
Raloxifene tablets purchased commercially were ground and blended with lactose. The mixture was put into pink opaque capsules, so each contained 90 mg of raloxifene. Identically appearing capsules for placebo contained lactose and inert materials. The Pharmaceutical Development Service created the allocation sequence, using a table of random numbers and alternating blocks of 8 and 10, which was accessible only to the pharmacy. Treatment assignment was concealed from study staff and participants until the study ended.
Participants had study visits and completed standardized quality-of-life16 and pelvic pain17 questionnaires preoperatively and then every 3 months until pelvic pain had returned or for 18 months after finishing study medication. After November 2002, a telephone interview substituted for the 3-, 9-, 15-, and 21-month visits for asymptomatic participants. Women were offered a second laparoscopy, performed just as the initial procedure, at 2 years or when pain returned earlier (as defined below).
Participants indicated whether pain returned for 2 consecutive months either at study visits or by contacting study staff to arrange an interval evaluation. Pain severity was determined by visual analogue scale with a score of 0 indicating no pain and 10 the worst pain imaginable for dysmenorrhea, dyspareunia, and nonmenstrual pelvic pain. Subjective verbal ratings for nonmenstrual pelvic pain, dysmenorrhea, and dyspareunia were defined as follows: Nonmenstrual pain was mild if it was occasional, moderate if it occurred most of the time, and severe if it was daily. Dysmenorrhea was considered minimal if participants were able to work, but reported reduced efficiency; moderate if participants were in bed or could not work part of 1 day; or severe if participants were in bed more than 1 day or incapacitated. Dyspareunia was defined as mild if discomfort could be tolerated during intercourse, moderate if intercourse was interrupted by pain, or severe if intercourse was avoided because of pain.
Participants kept a daily diary of menses and untoward experiences. At study visits, they were asked about contraceptive use and adverse effects. When indicated, pregnancy testing was performed; study medication was stopped if pregnancy was confirmed. Women with possible adverse effects were sometimes advised to reduce or stop study medication.
The study endpoint, return of pain, was defined as 2 consecutive months of pelvic pain equal to or more severe than that at study entry. Secondary outcomes included assessment of adverse events, the presence and severity of pelvic pain,17 quality of life,16 menstrual cycle length, and bone mineral density.
The rate of return of pain at 2 years was estimated to be 40% after surgical excision of endometriosis (personal communication regarding a retrospective study, C. Winkel, July 1998). The study required a sample size of 63 participants per group for 80% power to detect a 50% reduction in the rate of return of pain at 2 years (40% compared with 20%, excision compared with excision and raloxifene), with a two-tailed alpha level of 0.05, using a log rank analysis that estimated time to return of pain.19
Annual interim efficacy analyses by the Data Safety Monitoring Committee started when one quarter of patients (n=30) completed study treatment, using a two-sided O'Brien-Fleming group sequential boundary, based on a Lan-DeMets alpha-spending function of 0.005.20 In December 2004, the Data Safety Monitoring Committee noted that the raloxifene group had significantly earlier return of pain and the study was stopped.
Data were analyzed according to the intention-to-treat principle. Noncompleting participants were categorized based on their pain status at last contact. The time to recurrence of pain was analyzed using the log rank test.19 For other analyses, χ2 or Fisher exact test was used for categorical variables, nonparametric tests for ordinal variables, two-sample or paired t tests, as appropriate, and analysis of variance or analysis of covariance for a combination of variables. Paired analyses were used whenever possible. P<.05 was considered statistically significant, and descriptive statistics are expressed as mean±standard error.
In considering whether treatment was associated with a difference in the return of pain, the Cochran-Mantel-Haenszel test was used to adjust for whether drug dosage was reduced or stopped. Nominal logistic regression models were used to control for endometriosis at second surgery.
RESULTS
A total of 158 women were enrolled (Fig. 1). Thirty-one dropped out, failed to meet inclusion criteria, or had other causes of pelvic pain and therefore did not undergo surgery. At staging laparoscopy, 93 of 127 (73%) women had biopsy-proven endometriosis and were randomly assigned. Treatment groups were similar with respect to age, race, body mass index, pregnancy history, depression history, duration of pelvic pain, prior diagnosis of endometriosis, number of prior surgical procedures, and extent of endometriosis (Table 1). Participants taking placebo were more likely than those on raloxifene to have a history of migraine headaches (P=.05).
Table 1.
Baseline Characteristics and Extent of Endometriosis at First Study Surgery
| Raloxifene (n=47) | Placebo (n=46) | P | |
|---|---|---|---|
| Age (y) | 31.1 ± 1.1 | 32.0 ± 1.1 | .57 |
| Race | .13 | ||
| Asian | 1 (2.1) | 0 (0) | |
| African-American | 11 (23.4) | 4 (8.7) | |
| Hispanic | 1 (2.1) | 1 (2.2) | |
| White | 34 (36.6) | 41 (44.1) | |
| Body mass index | 25.3 ± 0.7 | 25.2 ± 0.9 | .93 |
| Gravida | 1.0 ± 0.3 | 1.0 ± 0.2 | .86 |
| Parity | 0.5 ± 0.2 | 0.5 ± 0.1 | .88 |
| History of surgical diagnosis of endometriosis | 37 (78.7) | 35 (76.1) | .81 |
| Number of laparoscopies for endometriosis (range) | 1.2 ± 0.1 (0–4) | 1.2 ± 0.2 (0–3) | .98 |
| 1 | 22 (46.8) | 22 (47.8) | |
| 2 or more | 15 (31.9) | 14 (30.4) | |
| History of laparotomy | 7 (14.9) | 4 (8.7) | .80 |
| Duration of pelvic pain (range) (y) | 10.6 ± 0.9 (0.75–32) | 10.8 ± 1.3 (1–28) | .90 |
| Menstrual cycle length (d) | 29.5 ± 1.0 | 27.8 ± 0.6 | .37 |
| Bone mineral density (g/cm2) | 1.030 ± 0.016 | 1.027 ± 0.018 | .89 |
| T score | 0.37 ± 0.15 | 0.26 ± 0.16 | .63 |
| ASRM stage | .87 | ||
| I | 13 (27.7) | 15 (32.6) | |
| II | 19 (40.4) | 17 (37.0) | |
| III | 9 (19.2) | 10 (21.7) | |
| IV | 6 (12.8) | 4 (8.7) | |
| History of | |||
| Headaches | 32 (68.1) | 39 (84.8) | .09 |
| Migraines | 25 (53.2) | 34 (73.9) | .05 |
| Headaches on hormones | 3 (6.4) | 9 (19.6) | .07 |
| Depression | 17 (36.2) | 21 (45.7) | .4 |
| Depression on hormones | 9 (19.2) | 6 (13.0) | .57 |
ASRM, American Society for Reproductive Medicine.
Data are mean ± standard error or n (%).
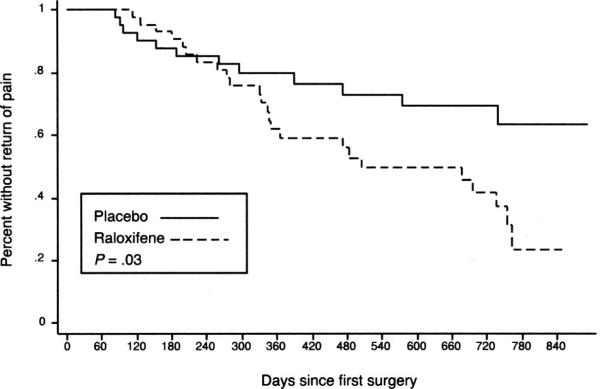
The raloxifene group had significantly earlier return of pain than the placebo group (P=.03, Fig. 2). This finding persisted after controlling for whether drug dosage was reduced or stopped (P=.01).
Fig. 2. Time to return of pain.
Stratton. Chronic Pelvic Pain and Raloxifene Treatment. Obstet Gynecol 2008.
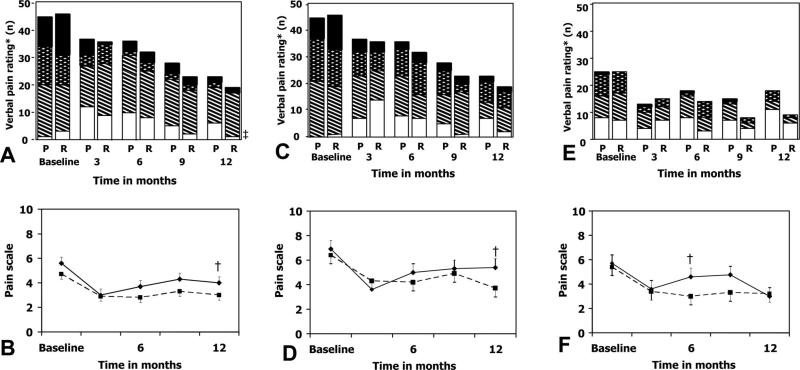
Nearly all participants had dysmenorrhea and nonmenstrual pain at baseline (Fig. 3). These significantly decreased in severity (by visual analogue scale and subjective ratings) in both groups after surgery, with gradual return by 6–12 months depending on the type of pain.
Fig. 3.
Nonmenstrual pelvic pain, dysmenorrhea, and dyspareunia. Nonmenstrual pain is depicted in panels A and B, dysmenorrhea in panels C and D, and dyspareunia in panels E and F. Subjective rating for pain from pain questionnaires is depicted in the top panels and verbal rating on a scale from 0 to 10 in the bottom panels. In panels B, D, and F, raloxifene participants are indicated by a solid circle and solid line; placebo participants are indicated by a solid square and dotted line. The number of women responding regarding nonmenstrual pain and dysmenorrhea at baseline, 3, 6, 9, and 12 months for questions was 46, 36, 32, 23, and 19 women taking raloxifene and 45, 36, 36, 28, and 23 women taking placebo, respectively. The number of women responding regarding dyspareunia at baseline, 3, 6, 9, and 12 months were 25, 15, 14, 4, and 9 women taking raloxifene and 25, 13, 18, 7, and 18 taking placebo, respectively. * Subjective rating for nonmenstrual pelvic pain, dysmenorrhea, and dyspareunia were defined as follows: Nonmenstrual pain was mild if it was occasional, moderate if it occurred most of the time, and severe if it was daily. Dysmenorrhea was considered minimal if participants were able to work, but reported reduced efficiency; moderate if participants were in bed or could not work part of 1 day; or severe if participants were in bed more than 1 day or incapacitated. Dyspareunia was defined as mild if discomfort could be tolerated during intercourse, moderate if intercourse was interrupted by pain, or severe if intercourse was avoided because of pain. † Both nonmenstrual pain and dysmenorrhea significantly improved after surgery, but returned to baseline levels by 9 and 12 months in the raloxifene and placebo groups, respectively. Dyspareunia also significantly decreased in severity, returning to baseline levels after 6 and 9 months in the raloxifene and placebo groups, respectively. Treatment groups did not differ at any time point in pain severity and debility by pain type. R, raloxifene group; P, placebo group.
Stratton. Chronic Pelvic Pain and Raloxifene Treatment. Obstet Gynecol 2008.
In general, quality-of-life measures were similar between groups at baseline and during the study. The groups differed only in mental health quality-of-life scores, which were similar at baseline; these improved significantly by 12 months in the placebo compared with raloxifene group (P<.05; change 5.8 placebo compared with –5.3 raloxifene).
Treatment did not affect the likelihood of second laparoscopy, at which most, 16 of 23 raloxifene and 13 of 17 placebo-treated women, had recurrence of lesions that were biopsy-proven endometriosis (Fig. 1; one woman had second surgery elsewhere). Biopsy-proven endometriosis was not associated with return of pain (P=.6). Women taking raloxifene, had second surgery earlier than those taking placebo (530±48 days compared with 682±46 days; P=.016). Logistic regression analysis of recurrence of pain, controlling for second surgery, revealed a strong treatment effect (P=.02; odds ratio [OR] 1.71, 95% confidence interval [CI] 1.10–2.71). Logistic regression analysis also showed that raloxifene was associated with return of pain (P=.006; OR 2.81, 95% CI 1.41–6.19), but treatment was not associated with endometriosis confirmed histologically at second surgery (P=.52; OR 1.29, 95% CI 0.59–2.95). The groups did not differ in the rate of major or minor surgical complications, which were rare.
Of women without adverse effects, eight delayed starting, stopped medication, or took fewer capsules than recommended; all others reported taking study drug as directed. Overall, 37 patients with adverse effects either reduced dose or stopped study medication early at a similar rate between groups (Table 2).
Table 2.
Adverse Events on Study Drug
| Events | Raloxifene (n=47) | Placebo*(n=45) | P |
|---|---|---|---|
| Pelvic pain | 14 (30.0) | 11 (24.4) | .64 |
| Ovarian cyst | 8 (17.0) | 5 (11.1) | .55 |
| Headaches | 10 (21.3) | 9 (20.0) | 1 |
| Migraines | 6 (12.8) | 8 (17.8) | .21 |
| Depression | 8 (17.0) | 4 (8.9) | .35 |
| Pregnant | 1 (2.1) | 3 (6.7) | .45 |
| Number who reduced dosage or stopped study drug | 15 (31.9) | 22 (48.9) | .09 |
| Reasons some reduced dosage or stopped study drug | |||
| Pelvic pain | 8 (17.0) | 7 (15.6) | 0.23 |
| Ovarian cyst | 4 (8.5) | 4 (8.9) | 0.53 |
| Headaches | 4 (8.5) | 6 (13.3) | 0.58 |
| Migraines | 3 (6.4) | 5 (11.1) | 0.60 |
| Depression | 3 (6.4) | 2 (4.4) | 0.43 |
Data are n (%).
Those taking placebo reported various symptoms, including body allergy (n=1), hand pain (n=1), increased breast size (n=1), more frequent headaches (n=1), red face (n=1), and weight gain (n=1).
Menstrual cycle length and the frequency of adverse effects were similar between groups throughout the study (Table 1 and 2). Cycle length was similar between groups and did not change significantly during (29.8±1.1 days raloxifene compared with 27.7±0.5 days placebo) or after treatment (30.4±1.2 days raloxifene compared with 29.4±1.1 days placebo). As a result of these similarities, participants and study staff remained blinded to treatment assignment. In the raloxifene group, a 44-year-old woman became amenorrheic with an elevated follicle-stimulating hormone but resumed menstruating after its discontinuation; two others reported vaginal dryness, and one had generalized rash and abdominal cramps.
Pregnancy was diagnosed at the time of missed menses in four participants, who stopped treatment immediately. Three were taking placebo. Four other became pregnant after completing study medication. Of these eight, only one (taking placebo) reported pain had returned at missed menses. All women had uncomplicated pregnancies and normal infants.
Women had bone densitometry studies before (n=87) and at 6 (n=63) and 12 months (n=42) after surgery. Bone mineral density and T-score were similar between groups at study entry (Table 1) and did not change between the 6-month intervals. Although women taking placebo had an increase in bone mineral density and T score at 12 months compared with baseline (0.013±0.004 g/cm2 and 0.116±0.044, respectively), those taking raloxifene had a decrease (–0.007±0.007 g/cm2 and –0.061±0.063, respectively), which was statistically significantly different (P=.01 for bone mineral density and P=.02 for T score). However, this difference was not clinically relevant.
DISCUSSION
In this prospective, randomized, double-blind, placebo-controlled study of women with biopsy-proven lesions, women treated with the SERM raloxifene had pelvic pain sooner than those taking placebo. This result was unexpected. The more rapid return of pain was associated with raloxifene treatment, but not with histologically confirmed lesions at second surgery, and the majority of women in either treatment group had recurrent lesions at second surgery. This earlier return of pain months after the 6-month treatment period had ended suggested that raloxifene affects processes associated with pain long after its discontinuation.
Higher circulating estradiol (E2) levels correlate with pelvic pain, suggesting a role in its development and maintenance.1,2 Ovulatory women or those receiving postmenopausal estrogen experience more pain compared with those with lower, nonfluctuating estrogen levels as a result of oral contraceptive therapy or menopause.21,22 Increased pain and nervous system activation may reflect estrogen interaction with its α or β receptors in nerves within lesions or within the spinal cord, sensory ganglia or pelvic autonomic ganglia.23 In experimental pain models, estrogen seemed to increase brain-derived neurotrophic factor–mediated neuropathic and nociceptive pain, suggesting a hypothetical downstream mechanism of action.24
It is unknown how raloxifene triggered return of pain. We used a higher dose (180 mg) of raloxifene because safety data were available, postmenopausal women were using either 60 or 120 mg of raloxifene when we designed the study, and younger women are often given even higher doses of hormones than postmenopausal ones.15,25 We were unable to test the possibility that raloxifene increased circulating E2 levels as Baker et al15 had reported, which complicates interpretation of the study results. Furthermore, the prolonged exposure at this dose of raloxifene may stimulate the endometrium or endometriosis lesions. Also, raloxifene effects on the central nervous system are not fully understood, because both agonist and antagonist actions in have been reported.26 Thus, raloxifene's deleterious effect on pain may represent either a synergy or antagonism of E2 action on implants, menstrual cycle events, or the nervous system.
Compared with placebo-treated women, bone density significantly decreased at 12 months in women who took raloxifene. Since all values remained in the normal range, this effect was not clinically significant. Others have reported a decrease in bone mass in premenopausal women taking either tamoxifen or raloxifene.27,28 Thus, raloxifene and other SERMs seem to have estrogen antagonist or weak agonist effects in bone in premenopausal women while acting as an agonist in postmenopausal women.
Two randomized, controlled studies have shown that laparoscopic excision or ablation of endometriosis improves pain symptoms in women with mild-to-moderate endometriosis compared with diagnostic laparoscopy alone.29,30 Lesion excision has the advantage of biopsy confirmation of endometriosis.
Endometriosis may have a nociceptive effect on pelvic neurons. Ectopic endometrial lesions are innervated, and nerves, which may play a pathogenetic role, seem to form when lesions are established in the rat model and women.31,32 The presence of pain in women with few visualized lesions, as in this study, suggests a neuropathic mechanism involving changes in the peripheral nervous system that sensitize the central response.23,33 Neuropathic pain also is suggested by less symptom relief with surgical treatment in those with minimal or mild endometriosis.29
A major strength of our study was the rigorous design. Randomized, masked placebo-controlled clinical trials and long-term follow-up of women with endometriosis are important, because both medical and surgical treatment are effective short-term, and a third of women receiving placebo initially report pain improvement.30 Few studies have been prospective or well-designed,29,34 and most have limited follow-up.35,36 Those that combined surgery and systemic medical treatment demonstrated a significant decrease in lesion size and disease recurrence, but not in pain, although most had insufficient statistical power.35 Unlike most other studies of endometriosis, we only included participants with biopsy-positive lesions, which avoided inaccurate diagnostic assignment, because only 30% to 70% of lesion biopsies contain endometriosis (Martin DC. Endometriosis: correlation between histologic and visual findings at laparoscopy [letter]. Am J Obstet Gynecol 2003;188:1663; author reply 1663–4).37
We also standardized the surgical approach and documentation, limiting variation inherent in most surgical studies. However, the consistency of surgical treatment might be viewed as a weakness, because our results might not be applicable to surgical teams who have different skills or ability to identify endometriosis lesions. Most women had endometriosis at second surgery regardless of treatment, and the extent of disease did not differ between the groups (data not presented).
The standardized assessment of the subjective symptom of pain was an important strength of the study. By excluding women with other causes of pain, we limited the study population to women whose chronic pelvic pain was believed to be caused by endometriosis. Postoperative improvement in all types of pain was more significant and persisted longer for the placebo group. Although the improvement of pain after surgery suggested that pain was related to endometriosis, many women reported other types of pain, with a high proportion reporting headaches at study entry and painful adverse effects during treatment. Thus, as in all studies of chronic pelvic pain and endometriosis, pain was associated with endometriosis, but may not be solely due to endometriosis, especially in those with minimal disease and other painful conditions.
Surprisingly, none of the quality-of-life measurements differed significantly between women on placebo and raloxifene. The Duke quality-of-life scale16 was chosen for its ease of use and various quality of life measures because, at the time of the study inception, no validated quality-of-life scale for women with pain and endometriosis was identified. This lack of sensitivity may be overcome by the recently developed endometriosis quality-of-life scale by Kennedy et al.38
We have conducted the first placebo-controlled study of a SERM to treat women with pain from endometriosis after complete excision of disease. Raloxifene was associated with return of pain earlier than placebo and that effect was independent of return of endometriosis. Thus, the estrogen hypothesis, which suggests that estrogen is critical for developing endometriosis, perhaps should be expanded to indicate that estrogen and SERMs may modulate chronic pelvic pain through a direct action on the nervous system.
Acknowledgments
The authors thank the Reproductive Endocrinology Staff and Fellows for their support in the operating room (Alicia Armstrong, MD, Mark Leondires MD, William Catherino, MD, PhD, Rose Christian, MD, John Fratterelli, MD, Rob Gustofson, MD, Rhonda Hearns-Stokes, MD, Andy Levi, MD, Cindy Murdock, MD, Adrienne Neithardt, MD, Jason Parker, MD, and Mark Payson, MD), the data management staff for their help in creating electronic data management systems for the protocol (Louis Battuello, Linda Hazlehurst, Asma Idress, Shelly Mashburn, Pat Moyer, PhD, and Tim Stitely), the Operating Room nurses for assistance in the operating room (Barb Gallagher, Jose Garcia, Maureen George, Jeanne O'Donnell, Marru Rodriguez, Tessa Rodriguez, and Juanita Washington), the Research staff for their help with specimens and data entry (Heidi Godoy, MD, Nadine Idress, MD, Shannon Liu, Nancy Kim, Vanessa Lopez, Sheila Mahoney, CNM, Kelly Morrissey, Katherine Plumb, Clariss Potlog-Nahari, MD, Stacey Spechler, and Victoria Shanmugan, MD), and the Clinical Center nurses for assistance with study visits (Janice Wilson, RN, and Donna Hardwick, RN).
Supported by the Intramural Program of the National Institute of Child Health and Human Development and the National Institutes of Health.
Footnotes
Presented at the 9th World Congress Society of Endometriosis, Maastricht, the Netherlands, September 14–17, 2005.
Financial Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med. 2001;345:266–75. doi: 10.1056/NEJM200107263450407. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 3.Association of Professors of Gynecology and Obstetrics . APGO educational series on women's health issues: chronic pelvic pain: an integrated approach. Association of Professors of Gynecology and Obstetrics; Washington (DC): 2000. [Google Scholar]
- 4.Wenof M. The International Pelvic Pain Society. [October 15, 2007];Chronic pelvic pain: a patient education booklet. 1999 Available at: http://www.pelvicpain.org/patients/patientbooklet.asp.
- 5.Winkel CA. Modeling of medical and surgical treatment costs of chronic pelvic pain: new paradigms for making clinical decisions. Am J Manag Care. 1999;5:S276–90. [PubMed] [Google Scholar]
- 6.Barbieri RL. Endometriosis and the estrogen threshold theory. Relation to surgical and medical treatment. J Reprod Med. 1998;43(suppl):287–92. [PubMed] [Google Scholar]
- 7.Sagsveen M, Farmer JE, Prentice A, Breeze A. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database Syst Rev. 2003;(4):CD001297. doi: 10.1002/14651858.CD001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstet Gynecol. 2002;99:709–19. doi: 10.1016/s0029-7844(02)01945-2. [DOI] [PubMed] [Google Scholar]
- 9.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [published erratum appears in N Engl J Med 2003;348:1192].
- 10.Fuchs-Young R, Glasebrook AL, Short LL, Draper MW, Rippy MK, Cole HW, et al. Raloxifene is a tissue-selective agonist/antagonist that functions through the estrogen receptor. Ann N Y Acad Sci. 1995;761:355–60. doi: 10.1111/j.1749-6632.1995.tb31392.x. [DOI] [PubMed] [Google Scholar]
- 11.Eng-Wong J, Zujewski JA. Raloxifene and its role in breast cancer prevention. Expert Rev Anticancer Ther. 2004;4:523–32. doi: 10.1586/14737140.4.4.523. [DOI] [PubMed] [Google Scholar]
- 12.Francucci CM, Romagni P, Boscaro M. Raloxifene: bone and cardiovascular effects. J Endocrinol Invest. 2005;28(suppl):85–9. [PubMed] [Google Scholar]
- 13.Silfen SL, Ciaccia AV, Bryant HU. Selective estrogen receptor modulators: tissue selectivity and differential uterine effects. Climacteric. 1999;2:268–83. doi: 10.3109/13697139909038087. [DOI] [PubMed] [Google Scholar]
- 14.Thiebaud D, Secrest RJ. Selective estrogen receptor modulators: mechanism of action and clinical experience. Focus on raloxifene. Reprod Fertil Dev. 2001;13:331–6. doi: 10.1071/rd00109. [DOI] [PubMed] [Google Scholar]
- 15.Baker VL, Draper M, Paul S, Allerheiligen S, Glant M, Shifren J, et al. Reproductive endocrine and endometrial effects of raloxifene hydrochloride, a selective estrogen receptor modulator, in women with regular menstrual cycles. J Clin Endocrinol Metab. 1998;83:6–13. doi: 10.1210/jcem.83.1.4448. [DOI] [PubMed] [Google Scholar]
- 16.Parkerson GR, Jr, Broadhead WE, Tse CK. The Duke Health Profile. A 17-item measure of health and dysfunction. Med Care. 1990;28:1056–72. doi: 10.1097/00005650-199011000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Brosens I, Donnez J, Benagiano G. Improving the classification of endometriosis. Hum Reprod. 1993;8:1792–5. doi: 10.1093/oxfordjournals.humrep.a137936. [DOI] [PubMed] [Google Scholar]
- 18.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–21. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 19.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121–9. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 20.Lan KK, Rosenberger WF, Lachin JM. Use of spending functions for occasional or continuous monitoring of data in clinical trials. Stat Med. 1993;12:2219–31. doi: 10.1002/sim.4780122307. [DOI] [PubMed] [Google Scholar]
- 21.Hapidou EG, Rollman GB. Menstrual cycle modulation of tender points. Pain. 1998;77:151–61. doi: 10.1016/S0304-3959(98)00087-6. [DOI] [PubMed] [Google Scholar]
- 22.Fillingim RB, Edwards RR. The association of hormone replacement therapy with experimental pain responses in postmenopausal women. Pain. 2001;92:229–34. doi: 10.1016/s0304-3959(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 23.Papka RE, Mowa CN. Estrogen receptors in the spinal cord, sensory ganglia, and pelvic autonomic ganglia. Int Rev Cytol. 2003;231:91–127. doi: 10.1016/s0074-7696(03)31003-4. [DOI] [PubMed] [Google Scholar]
- 24.Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81:193–9. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- 25.Walsh BW, Kuller LH, Wild RA, Paul S, Farmer M, Lawrence JB, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–51. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
- 26.Bernardi F, Pluchino N, Stomati M, Pieri M, Genazzani AR. CNS: sex steroids and SERMs. Ann N Y Acad Sci. 2003;997:378–88. doi: 10.1196/annals.1290.041. [DOI] [PubMed] [Google Scholar]
- 27.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and post-menopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 28.Eng-Wong J, Reynolds JC, Venzon D, Liewehr D, Gantz S, Danforth D, et al. Effect of raloxifene on bone mineral density in premenopausal women at increased risk of breast cancer. J Clin Endocrinol Metab. 2006;91:3941–6. doi: 10.1210/jc.2005-2827. [DOI] [PubMed] [Google Scholar]
- 29.Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- 30.Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil Steril. 2004;82:878–84. doi: 10.1016/j.fertnstert.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101:11094–8. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–9. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain. 2003;4:372–80. doi: 10.1016/s1526-5900(03)00720-x. [DOI] [PubMed] [Google Scholar]
- 34.Abbott JA, Hawe J, Clayton RD, Garry R. The effects and effectiveness of laparoscopic excision of endometriosis: a prospective study with 2–5 year follow-up. Hum Reprod. 2003;18:1922–7. doi: 10.1093/humrep/deg275. [DOI] [PubMed] [Google Scholar]
- 35.Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev. 2004;(3):CD003678. doi: 10.1002/14651858.CD003678.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore J, Kennedy S, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. 2000;(2):CD001019. doi: 10.1002/14651858.CD001019. [update in Cochrane Database Syst Rev 2007;(3):CD001019].
- 37.Stratton P, Winkel CA, Sinaii N, Merino MJ, Zimmer C, Nieman LK. Location, color, size, depth, and volume may predict endometriosis in lesions resected at surgery. Fertil Steril. 2002;78:743–9. doi: 10.1016/s0015-0282(02)03337-x. [DOI] [PubMed] [Google Scholar]
- 38.Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the Endometriosis Health Profile Questionnaire: the EHP-30. Qual Life Res. 2004;13:705–13. doi: 10.1023/B:QURE.0000021316.79349.af. [DOI] [PubMed] [Google Scholar]