Abstract
Mercury is a major toxic metal ranking top in the Toxic Substances List. Cinnabar (contains mercury sulfide) has been used in traditional medicines for thousands years as an ingredient in various remedies, and 40 cinnabar-containing traditional medicines are still used today. Little is known about toxicology profiles or toxicokinetics of cinnabar and cinnabar-containing traditional medicines, and the high mercury content in these Chinese medicines raises justifiably escalations of public concern. This minireview searched the available database of cinnabar, compared cinnabar with common mercurials, such as mercury vapor, inorganic mercury, and organic mercury, and discusses differences in their bioavailability, disposition, and toxicity. The analysis showed that cinnabar is insoluble and poorly absorbed from the gastrointestinal tract. Absorbed mercury from cinnabar is mainly accumulated in kidney, resembling the disposition pattern of inorganic mercury. Heating cinnabar results in release of mercury vapor, which in turn can produce toxicity similar to inhalation of these vapors. The doses of cinnabar required to produce neurotoxicity are thousands 1000 times higher than methyl mercury. Following long-term use of cinnabar, renal dysfunction may occur. Dimercaprol and succimer are effective chelation therapies for general mercury intoxication including cinnabar. Pharmacology studies of cinnabar suggest sedative and hypnotic effects, but the therapeutic basis of cinnabar is still not clear. In summary, cinnabar is chemically inert with a relatively low toxic potential when taken orally. In risk assessment, cinnabar is less toxic than many other forms of mercury, but the rationale for its inclusion in traditional Chinese medicines remains to be fully justified.
Keywords: Cinnabar, Traditional medicines, Elementary mercury, Mercuric chloride, Methylmercury, Bioavailability, Disposition, Toxicology
Introduction
Cinnabar (contains mercury sulfide) has been used for 2000 years in traditional Chinese medicines and in Indian Ayurvedic medicines (1–3). Mercury is a well-known toxic heavy metal, ranking high on the CDC Toxic Substances List (http://www.atsdr.cdc.gov). Mercury content found in traditional medicines justifiably alarms the public (4–7), and many mercury-containing traditional medicines have been banned. However, some are still in use today (1–2).
Mercury in traditional Chinese medicines mainly comes from cinnabar, deliberately included for therapeutic purposes based on Pharmacopeia of China (1). Chinese Ministry of Health has paid close attention to the mercury contents in traditional Chinese remedies, and the allowable amounts of cinnabar in these preparations have been dramatically decreased by as much as 65%, from a daily allowable dose of 0.3 – 1.5 g in the 1977 Pharmacopeia to 0.1 – 0.5 g in the 2005 Pharmacopeia of China (1, 8), but the mercury in these traditional medicines can still be thousands folds higher than what is considered safe in Western countries including the USA. The question becomes, is cinnabar toxicologically similar to common mercurials?
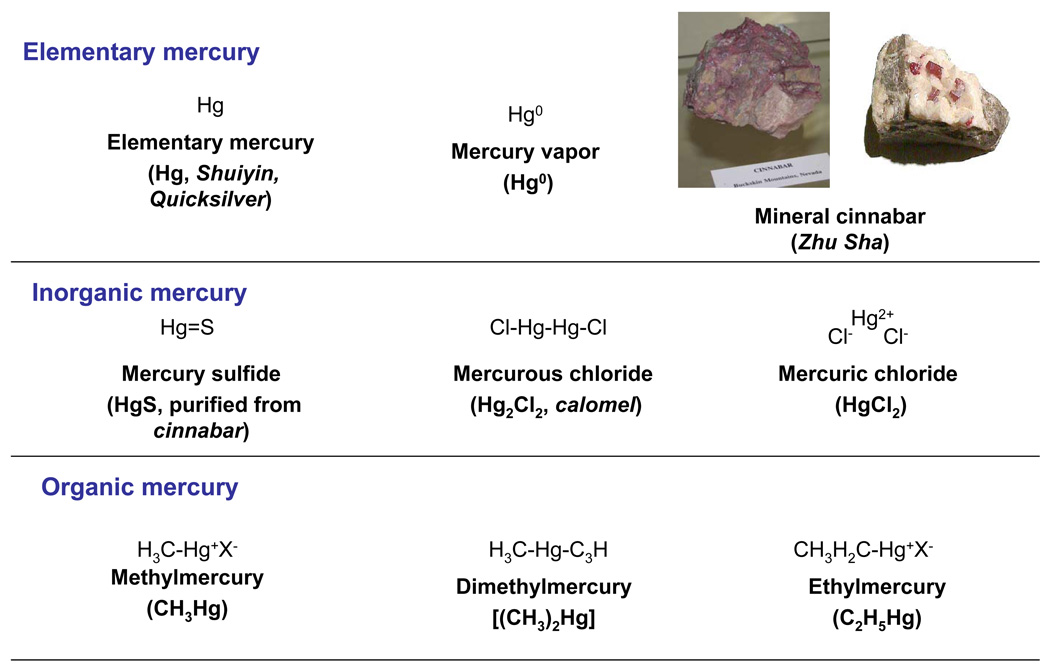
Mercurials are commonly grouped as elementary, inorganic, and organic mercurials (Figure 1). Mercury disposition and toxicity are highly dependent on the chemical forms and physical status, and the three major mercurial forms must be distinguished when discussing their toxicity (9, 10). For examples, mercury vapor (Hg0) is much more hazardous than the liquid form of elementary mercury (Hg, also called Shui Yin and Quicksilver). Mercury ores are often found as cinnabar which contains mercuric sulfide, HgS) (11). Mercury binds to other elements, such as chlorine, sulfur, or oxygen, to form inorganic mercurous (Hg1+) or mercuric (Hg2+) salts, such as mercury sulfide (HgS, purified from cinnabar), mercurous chloride (Hg2Cl2, also called calomel) and mercuric chloride (HgCl2). Mercury can form a number of stable organic metallic compounds by attaching to one or two carbon atoms. Methyl mercury (CH3Hg+) is the toxicologically most important organic form (11), but dimethyl mercury [(CH3)2Hg] is the most toxic mercurial (12). Ethyl mercury (C2H5Hg+) is the major component of thimerosal, used as preservative in many vaccine preparations routinely administered to infants (13). However, little is known about the disposition and toxicity of cinnabar used in traditional medicines. Based on available data on cinnabar from the literature, this minireview compared the human exposure, disposition, and toxicology of cinnabar with mercury vapor, mercuric chloride, and methyl mercury. All mercurial compounds have characteristic toxicokinetics and quite different health effects depending on oxidation state, physical status, and associated organic species (9–13). Thus, total mercury content alone seems to be insufficient for safety evaluation of cinnabar and cinnabar-containing traditional medicines, and their chemical characteristics should be taken into consideration.
Fig. 1.
Chemical structure of common mercurials
Human exposure of mercurial compounds
Elementary mercury is the pure form of mercury, also called metallic mercury. Metallic mercury is a shinny, silver-white metal that is in liquid state at room temperature, so called Quicksilver or Shui Yin. The first emperor of China, Qin-Shi-Huang, was driven insane and killed by mercury pills intended to give him eternal life and buried in a tomb fill with mercury (http://wikipedia.org). Mercury amalgam has been used for centuries in tooth filling and it is now gradually being replaced by other materials. In the processing of gold, especially in developing countries, large quantities of metallic mercury are used to form a gold amalgam. The amalgam is then heated to drive off the mercury, resulting in a substantial atmospheric mercury release (11). Metallic mercury is also used in some religious practices, and is sold under the name “azogue” in botanicas stores. Botanicas are common in Hispanic and Haitian communities where azogue may be used as an herbal remedy or for spiritual practices (11). However, metallic mercury is not used in traditional Chinese medicines.
Mercury vapor is colorless and odorless. The higher the temperature the more vapor will be released from liquid metallic mercury. Inhalation of large amount of mercury vapor can be fatal (14). Elemental mercury spills can occur in many ways, such as from broken elemental mercury containers, medicinal devices, barometers, and from melting tooth amalgam fillings to recover silver (14), or from smelting gold-mercury amalgam (11). When cinnabar is inappropriately overheated, mercury vapor can also be released, and in Chinese Pharmacopeia, heating cinnabar is never a part of preparation technique (1, 8)
Cinnabar
Cinnabar is the naturally occurring mineral with mercury in combination with sulfur, and is red in color so called red mercury sulfide, Zhu Sha or China Red. Cinnabar ores are the major source for metallic mercury production. Cinnabar is insoluble and stable, and cinnabar powder has been used as an important ingredient in traditional Chinese medicines (1) and in Indian Ayurvedic medicines (3, 15). Cinnabar-gold was used as an alchemical drug of longevity, called Makaradhwaja in India (16). Cinnabar is used to color paints and as one of red coloring agents used in tattoo dyes. Approximately 40 traditional Chinese medicines contain some cinnabar according to Pharmacopeia of China (1), and it is the major source of mercury found in traditional medicines.
Mercurous mercury also called calomel, was used as diuretics, antiseptics, skin ointments, vitiligo, and laxatives for centuries. Calomel was also used in traditional medicines, but now theses uses have largely been replaced by safer therapies. Other preparations containing mercury are still used as antibacterials (11). Very few traditional remedies contain calomel, and no calomel-containing oral Chinese remedy is listed in Pharmacopeia of China (1).
Mercuric mercury was once used as a disinfectant and antiseptic agent (9, 11). Occupational exposure may occur from chloralkali industry where mercury is used as a cathode in the electrolysis of brine or from manufacturing scientific instruments and electrical control devices. Some degree of exposure of mercuric mercury may also occur from diet, such as from consumption of mercury-contaminated fish (11). Mercuric chloride is not used in traditional medicines.
Methyl mercury is produced primarily by microorganisms (bacteria and fungi) in the environment, rather by human activity. Fish consumption is the major route of exposure to methyl mercury. Until the 1970s, methyl mercury and ethyl mercury compounds were used to protect grain seeds from fungal infection. This use was banned after consumption of organomercury-treated grains in Iraq and China in 1970s leading to mass poisonings with hundreds of deaths (12, 13). No methyl mercury is used in any of traditional medicines.
Dimethyl mercury is the most toxic form among mercurial compounds. Contact with even a small amount of dimethyl mercury can penetrate laboratory gloves and resulted in rapid transdermal absorption, causing delayed cerebella damage and death (13). The use of dimethyl mercury as a laboratory standard is now strictly regulated.
Ethyl mercury was used as preservatives. Thimerosal contains the ethyl mercury radical attached to the sulfur group of thiosalicylate (49.6% mercury by weight as ethyl mercury), and has been used as a preservative in many children vaccines since 1930s. Thimerosal has been removed from US vaccines by switching to single-dose vials that do not require any preservatives, but it is approved by WHO to use as preservatives in multidose vials in developing countries (17). No ethyl mercury is used in any of traditional medicines.
Thus, cinnabar is the only form of mercury used in traditional Chinese medicines today. How cinnabar differs from other forms of mercury will be discussed below.
Disposition of cinnabar as compared to mercury vapor, mercuric chloride and methyl mercury
The solubility and bioavailability of cinnabar are quite low. The water solubility of mercuric chloride is 30–70 g/L, but cinnabar is less than 0.001 g/L at 20°C (11, 18). In the stomach, the lower pH the more cinnabar dissolution as Hg2SOH+ formation occurs. In the intestine, sulfur (Na2S and S0) increase cinnabar dissolution as mercury-sulfur complexes such as Hg (SH)+, HgS (OH) −, HgS2 (OH) −, and HgS3 (OH) − are formed (19). Sonochemical dissolution of cinnabar was investigated by measuring sulfur oxidation products and dissolved Hg2+ released into the aqueous solution (20). Dissolved S2− was not detected and SO4 2− was the main S species, but Hg2+ release was much lower than S species. Ultrasound can reduce cinnabar particle size and increase cinnabar surface area and isoelectric point. Humic acid acts synergistically to enhance cinnabar dissolution (20). The insoluble property of cinnabar or mercury sulfide made its accumulation in the body quite different from common mercurials (Table 2).
Table 2.
Disposition of cinnabar, mercury vapor, mercuric chloride, and methyl mercury
| Name | Absorption | Distribution | Biotransformation | Excretion | References |
|---|---|---|---|---|---|
| Cinnabar | GI, <0.2% | Kidney, spleen, liver | HgS to Hg2+ | Feces and urine | 21–29 |
| Mercury vapor | Lung 80%, GI<0.01% | Lung, brain, kidney | Hg0 to Hg2+ | Urine and feces | 9–10, 14 |
| Mercuric chloride | GI, 7–15% | Kidney, liver, spleen | Hg2+ to Hg0 | Urine and feces | 9–11 |
| Methyl mercury | GI, > 95% | Brain, kidney, liver | CH3Hg to Hg2+ | Feces and urine | 9–13,17 |
Absorption
Absorption of cinnabar (0.2%) from the gastrointestinal tract is much less than mercuric chloride (7–15%), and methyl mercury ( >95%). Oral administration of powered cinnabar or mercury sulfide to mice resulted in 10- to 100-fold less tissue mercury accumulation as compared to the similar dose given as mercuric chloride either acutely (21), or chronically (18). When powdered cinnabar or mercury sulfide-containing diet was given to mice for 5 days, less than 0.02% of the dose was found in the kidney and liver (22). In general, bioavailability of cinnabar is 30- to 60-fold less than mercuric chloride (23). In comparison to methyl mercury, oral cinnabar or mercury sulfide administration results in at least 1000-fold less tissue mercury accumulation in mice (24–27), and in rats (28). Synthetic mercury sulfide was reported to have better bioavailability than cinnabar in mice (18, 21), but in other studies, synthetic mercury sulfide was reported to have less oral bioavailability than cinnabar in mice (27) and in guinea pigs (29). This discrepancy could be due to differences in cinnabar processing methods, as well as to animal species or strain variation. Nonetheless, both crude cinnabar and synthetic mercury sulfide have very low oral bioavailability and are poorly absorbed from the gastrointestinal tract as compared to mercuric chloride and methyl mercury, but are better than liquid elementary mercury (less than 0.01%). Mercury vapor is readily absorbed (80%) through diffusion in the lungs. When cinnabar is heated, mercury vapor is released, and is easily absorbed to produce local and systemic toxicity. This is why in Pharmacopeia of China (1), heating cinnabar is restricted. Cinnabar is not used in injectable preparations. Little is known about cinnabar absorption via the skin, or from parenteral administration.
Distribution and biotransformation
The distribution of mercury from absorbed cinnabar basically follows the distribution pattern for inorganic mercurials. The highest concentration of mercury is found in kidney, a major target of inorganic mercury exposure (18, 21–27). Renal uptake of mercury salts is through two routes: from luminal membranes in renal proximal tubule in the form of the cysteine S-conjugates (Cys-S-Hg-S-Cys) or from the basolateral membrane through organic anion transporters (30). Inorganic mercury salts do not readily pass blood-brain barrier or placenta. However, a small portion of absorbed inorganic mercury can be reduced in tissues and exhaled as mercury vapor. A significant portion of mercury vapor crosses the blood-brain barrier and placenta before it is re-oxidized to divalent inorganic mercury by tissue and erythrocyte catalase (9–12, 17). Oral cinnabar or synthetic mercury sulfide administration results in brain distribution (about 10% of renal accumulation), mainly to the cerebral cortex and cerebellum (26–29). Accumulation of mercury from cinnabar in liver ranged from 5% to 50% of that in kidneys depending on experimental conditions (18, 21; 26–29). In comparison, methyl mercury is more uniformly distributed to various tissues upon absorption (9, 13). Methyl mercury is bound to thiol-containing molecules such as cysteine (CH3Hg-S-Cys), which mimic methionine to cross the blood-brain barrier and placenta through the neutral amino acid carrier (30). Methyl mercury is slowly metabolized to inorganic mercury by microflora in intestine (about 1% of the body burden per day), resulting in increased kidney accumulation (13).
In the Chinese literature, it is assumed that cinnabar could be converted to methyl mercury in the intestine under anaerobic conditions at pH 7 (8). However, no evidence is available to support this assumption. Unlike arsenic methylation reactions, mercury methylation reaction does not occur in humans, and little is known about biotransformation of inorganic mercurial salts to methyl mercury in the body; instead, intestinal bacteria can convert methyl mercury to inorganic mercury (9–13). From over 1000- to 5000-fold differences in bioavailability between cinnabar and methyl mercury (28), such assumed reaction, if it exits, is very minor accounting for less than 0.02% of dosed cinnabar.
Inorganic mercury salts non-uniformly distributed to kidney and are excreted in urine and feces, with a half-life of about 2 months. Methyl mercury undergoes extensive enterohepatic recycling which can be interrupted to enhance fecal excretion. 90% of the methyl mercury is eliminated from the body in the feces, and less than 10% is in the urine, with a half life of 45–70 days (9–13).
It is quite clear that solubility and bioavailability of cinnabar is quite different from mercury vapor, mercuric chloride, and methyl mercury. The bioavailability is a critical determinant of toxicity of mercurial compounds. Thus, it is not surprising that cinnabar has quite different toxicology potentials from common mercurials. To better understand toxicokinetics of cinnabar is very important for appropriate safety assessment of mineral cinnabar used in traditional medicines.
Toxicological profiles of cinnabar, mercury vapor, mercuric chloride and methylmercury
The toxicity potentials for mercurial compounds, including cinnabar and cinnabar-containing Chinese medicines, vary greatly dependent on the chemical forms of these mercurials (9–13).
Cinnabar-containing traditional medicines are generally relatively non-toxic at therapeutic doses. The correct preparation methods, appropriate doses, disease status, age and drug combinations are important factors impacting cinnabar toxicity (1, 8, 31). In general, the adverse effects at therapeutic doses of cinnabar-containing traditional medicines are rare and are largely tolerable and reversible. The cinnabar poisoning cases are associated with overdose, long-term uses, and improper processing such as heating, decocting, fumigating, or in combination with other drugs (31). For example, heating cinnabar resulted in mercury vapor release, and acute inhalation of mercury fumes can be fatal (32). Grinding cinnabar using aluminum utensils or in combination of iodide- and bromide-containing drugs could increase mercury toxicity (31), but the mechanisms of such interactions are not completely known. The long-term use of cinnabar-containing traditional medicines could result in renal dysfunction due to accumulation of mercury in the kidney. Blurred vision due to accumulation of mercury in brain is possible, gastrointestinal symptoms also often occur following long-term administration (9–11, 31). Skin allergic reaction may occur when cinnabar is used in tattoo dyes (33).
Oral administration of cinnabar at a high dose (1.0 g/kg/d for 7d) produced reversible hearing dysfunction, learning memory deficit, and other behavioral abnormalities in mice (24), rats (25, 28), and guinea pigs (29). In comparison, the ototoxicity produced by methyl mercury was so dramatic and irreversible, even at doses 1/1000 to 1/5000 of cinnabar (24–29). It should also be pointed out that the dose of cinnabar or mercury sulfide (1.0 g/kg) used in these studies is at least 100–500 times higher than human daily dose (i.e., 50 g/50 kg person, while allowable daily human oral dose is 0.1 – 0.5 g) (1). At lower cinnabar doses (10 mg/kg/d) for a longer time (up to 11 weeks), cinnabar did not produce neurotoxic effects in mice until 7 weeks of continuous administration (27). The cerebellum appeared to be the most vulnerable brain region (27). Long-term (4 weeks) oral administration of mercury sulfide in mice increased renal mercury burden, and decreased circulating thyroxin (T4) levels (34). However, no data on nephrotoxicity was reported from this study.
Inhalation of mercury vapor produces acute corrosive bronchitis and interstitial pneumonitis and, if not fatal, may be associated with central nervous system effects such as tremor or increased excitability (9–11). With chronic exposure to mercury vapor, the major effects are on the central nervous system. The triad of tremors, gingivitis and erethism (memory loss, increased excitability, insomnia, depression and shyness) has been recognized historically as the major manifestation of mercury poisoning from inhalation of mercury vapor. Sporadic instances of proteinuria and even nephrotic syndrome may occur in persons with exposure to mercury vapor, particularly with chronic occupational exposure (9–11). A case report of chronic mercury poisoning from burning a traditional medicine mixture composed of cinnabar and calomel in the treatment of vitiligo, blood mercury levels were elevated to 1100 µg/L (normal < 20 µg/L), and central nervous system toxicity and renal toxicity typical to chronic mercury poisoning occurred. After chelation treatment with dimercaprol for 4 weeks, her blood mercury levels decreased with improved mercury intoxication symptoms (32).
Kidney is the major target organ for inorganic mercury in humans and in experimental animals (9–11). Although a high dose of mercuric chloride is directly toxic to renal tubular cells, chronic low-dose exposure to mercuric salts may induce an immunologic glomerular disease (36). Exposed persons may develop proteinuria that is reversible after workers are removed from exposure. Experimental studies have shown that the pathogenesis has two phases: an early phase characterized by an anti–basement membrane glomerulonephritis, followed by a superimposed immune-complex glomerulonephritis with transiently raised concentrations of circulating immune complexes (37). The pathogenesis of the nephropathy in humans appears similar, although antigens have not been characterized. In humans, the early glomerular nephritis may progress to interstitial immune-complex nephritis (36). Acrodynia has occurred in children chronically exposed to inorganic mercury compounds in teething powder and diaper disinfectants, as well as to organomercurials. Acordynia is characterized by pink hands and feet (also called Pink Disease). These subjects are photophobic and suffer from joint pains (11–13). The long-term use of cinnabar-containing traditional medicines could result in accumulation of mercury in the kidney and renal dysfunction similar to mercuric mercury exposure may occur (8, 31, 35).
The major human health effect from exposure to methyl mercury is neurotoxicity. Clinical manifestations of neurotoxicity include paresthesia (a numbness and tingling sensation around the mouth, lips) and ataxia, manifested as a clumsy, stumbling gait, difficulty in swallowing and articulating words. Other signs include neurasthenia (a generalized sensation of weakness), vision and hearing loss, and spasticity and tremor. There may finally progress to coma and death (9–13). Neuropathological observations have shown that the cerebral cortex and cerebellum are selectively involved with focal necrosis of neurons, lysis and phagocytosis, and replacement by supporting glial cells. These changes are most prominent in the deeper fissures (sulci), as in the visual cortex and insula. The overall acute effect is cerebral edema, but with prolonged destruction of gray matter and subsequent gliosis, cerebral atrophy results (9–13, 17). There is as yet no available report on cinnabar-induced neurotoxicity in humans.
Children are sensitive to mercury toxicity
Early life stages are particularly vulnerable to mercury intoxication (38). In Minamata, Japan, pregnant women who consumed fish contaminated with methyl mercury manifested mild or minimal symptoms, but gave birth to infants with severe developmental disabilities, raising initial concerns for mercury as a developmental toxicant. Methyl mercury crosses the placenta and reaches the fetus, and is concentrated in fetal brain at least 5 to 7 times that of maternal blood (13). Prenatal methyl mercury exposure at high levels can induce widespread damage to the fetal brain. However, the effects from low-level exposures are inconsistent (38, 39). In the Seychelles Children Development Study, a group with significant methyl mercury exposure from a diet predominantly of fish was studied for developmental adverse effects. These children were examined 6 times over 11 years using extensive batteries of age-appropriate developmental endpoints, but no convincing associations were found except for delayed walking (38). The National Research Council reviewed the epidemiologic studies relating in utero methyl mercury exposure and fetal neurological development. It concluded that the current EPA reference dose for methyl mercury of 0.1 µg/kg per day or 5.8 µg/L cord blood is scientifically justifiable for protection of human health (40). The RfD is equivalent to 12 ppm methyl mercury in maternal hair (10, 40).
More than 12 cinnabar-containing Chinese medicines are used in pediatrics, mainly for their sedative and hypnotic effects. Toxicity has been reported from inappropriate use of cinnabar and cinnabar-containing medicines in infants and preschool children (7, 31). Thus, caution should be taken when cinnabar-containing Chinese medicines are used for children, as children are susceptible to mercury toxicity.
Treatment
Therapy for mercury poisoning should be directed toward lowering the concentration of mercury at the critical organ or site of injury. For the most severe cases, particularly with acute renal failure, hemodialysis may be the first measure, along with administration of chelating agents for mercury, such as dimercaprol (BAL), 2, 3-dimercaptosuccinic acid (DMSA, succimer), EDTA (calcium disodium, edentate calcium disodium), or D-penicillamine (NAP). Chelation therapy is not very helpful for methyl mercury exposure (9, 13, 17). Biliary excretion and reabsorption by the intestine can be interrupted by oral administration of a nonabsorbable thiol resin, which can bind mercury and enhance fecal excretion (17). Succimer (DMSA) is a FDA-approved pediatric use in the treating mercury poisoning (41).
Pharmacology studies of cinnabar
The effects of cinnabar on anxiety-like behaviors in mice were studied using the elevated plus maze test. Cinnabar at the oral dose of 50 and 100 mg/kg/d for 10 days significantly improved the performance in the elevated maze test, but at the 1000 mg/kg, a dose 100-fold higher than the human daily dose, it was ineffective (42). This pharmacological effect is associated with the decreased in serotonin levels in mouse brain, but the dose-dependent relationship is not clear (42). In mice given low dose of cinnabar (10 mg/kg/d) for 11 weeks of continuous administration, the locomotor activity was reduced and pentobarbital sleeping time was increased, suggesting sedative or hypnotic effects (27). Induction of renal metallothionein in rats by cinnabar is dose- and time-dependent, but induction of hepatic metallothionein was lower and independent of dose and time (43). This fortifies the notion that cinnabar is poorly absorbed and the kidney is the major organ of mercury accumulation, despite the fact that the doses used in this study were 1000 times higher than human daily dose (2.5– 5.0 g/kg, po, for 2–4 weeks). In general, little is known about the therapeutic effects of cinnabar, and available pharmacology literature is limited.
Cinnabar is not used alone in traditional medicines, and it is usually used as an ingredient in traditional Chinese medicine recipes (1). Some pharmacological studies on cinnabar-containing traditional medicines are available in the Chinese literature, but not in PubMed. The available studies on the pharmacological effects of cinnabar-containing traditional medicines are inconsistent. For example, the inclusion of cinnabar in An-Gong-Niu-Huang Wan, a famous cinnabar-containing Chinese medicine, has been reported to be essential (44), to have some beneficial effects (45), or not to be important at all (46). Considering the very poor bioavailability of mercury in cinnabar, whether the mercury in these preparations is of any therapeutic value is highly questionable (47). To extensively comment on these studies is beyond the scope of this minireview, and much more studies are needed to fully justify the therapeutic basis for inclusion mercury in any form of traditional medicines.
Summary
This minireview comments on natural mineral cinnabar used in traditional medicines. Cinnabar is insoluble, has very low bioavailability and thus is poorly absorbed from the gastrointestinal tract. Once absorbed into the blood, the mercury disposition from cinnabar follows the pattern for inorganic mercury salts and preferentially distributed to kidney, with a small portion to the brain. The heating, overdose and the long-term use of cinnabar are major causes of mercury intoxication, but at the therapeutic doses, the adverse effects cinnabar-containing traditional medicines seem to be tolerable and reversible. In safety evaluation of cinnabar-containing traditional medicines, total mercury content alone is insufficient, and chemical forms of mercurial compounds should be taken into consideration. Toxicologically, cinnabar or synthetic mercury sulfide should be distinguished from mercury vapor, mercuric chloride, and methyl mercury.
Table 1.
Human Exposure of Mercurial Compounds
| Name | Symbol, popular names | Major source of exposure | Traditional Uses | References |
|---|---|---|---|---|
| Elementary mercury | Hg, Quicksilver, Shui Yin | Barometer, Dental amalgams | Religious | 11 |
| Mercury vapor | Hg0 | Occupational, accidental | 14 | |
| Cinnabar | Shu Sha, China red Contains mercury sulfide, HgS | Traditional medicines | Sedative, disinfection | 1–3, 15–16 |
| Mercurous chloride | Hg2Cl2, Calomel | Medicinal uses External use | Antiseptics, diuretics Anti-parasites, detoxication | 9,11 |
| Mercuric chloride | HgCl2 | Chloralkali industry | Industry | 9–11 |
| Methyl mercury | CH3Hg | Fish consumption | Preservative in vaccine | 9–13, 17 |
| Ethyl mercury | C2H5Hg | Preservatives, | Vaccines, agriculture | 9–13 |
Table 3.
Toxicological profiles of cinnabar, mercury vapor, mercuric chloride, and methyl mercury
| Name | Acute toxicity | Chronic toxicity | Treatment | References |
|---|---|---|---|---|
| Cinnabar | Heating cinnabar, death | Neurotoxicity, Renal and GI symptoms | BAL | 8, 31–33 |
| Mercury vapor | Death, lung and brain | Pneumonitis, bronchitis Neurotoxicity, nephrotoxicity | DMSA, BAL | 9–11, 14,41 |
| Mercuric chloride | Renal failure | Kidney injury and immunopathy Skin irritation, Acordynia | BAL, EDTA | 9–11 |
| Methyl mercury | Death, brain | Neuropathy, developmental toxicity | No Chelators | 9–13, 17,41 |
BAL= dimercaprol; DMSA= 2,3-dimercaptosuccinic acid, succimer; EDTA = Ethylenediaminetetraacetic acid, its sodium salt edentate disodium, and closely related edentate calcium disodium.
Acknowledgements
The authors thank Drs. Yang Sun, Wei Qu and Larry Keefer for their critical review of this minireview. This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and National Institute of Environmental Health Sciences. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Reference
- 1.Pharmacopedia of China. Beijing: People’s Press; 2005. pp. 1–586. [Google Scholar]
- 2.Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353–361. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Nair AG, Reddy AV, Garg AN. Bhasmas: unique ayurvedic metallic-herbal preparations, chemical characterization. Biol Trace Elem Res. 2006;109:231–254. doi: 10.1385/bter:109:3:231. [DOI] [PubMed] [Google Scholar]
- 4.Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci. 2002;23:136–139. doi: 10.1016/S0165-6147(00)01972-6. [DOI] [PubMed] [Google Scholar]
- 5.Lynch E, Braithwaite R. A review of the clinical and toxicological aspects of 'traditional' (herbal) medicines adulterated with heavy metals. Expert Opin Drug Saf. 2005;4:769–778. doi: 10.1517/14740338.4.4.769. [DOI] [PubMed] [Google Scholar]
- 6.Cooper K, Noller B, Connell D, Yu J, Sadler R, Olszowy H, Golding G, Tinggi U, Moore MR, Myers S. Public health risks from heavy metals and metalloids. J Toxicol Environ Health A. 2007;70:1694–1699. doi: 10.1080/15287390701434885. [DOI] [PubMed] [Google Scholar]
- 7.Kang-Yum E, Oransky SH. Chinese patent medicine as a potential source of mercury poisoning. Vet Hum Toxicol. 1992;34:235–238. [PubMed] [Google Scholar]
- 8.Liang AH, Shang MF. General situation of the study on the toxicity of cinnabaris. Zhongguo Zhong Yao Za Zhi. 2005;30:249–252. [PubMed] [Google Scholar]
- 9.Klaassen CD. Heavy metals and heavy-metal antagonists. In: Hardman JG, Limbird LE, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 1851–1876. [Google Scholar]
- 10.Liu J, Goyer R, Waalkes MP. Toxic effects of metals. In: Klaassen CD, editor. Casarett and Doull’s Toxicology-The Basic Science of Poisons. 7th Edition. McGraw Hill: 2007. pp. 900–950. [Google Scholar]
- 11.ATSDR. Toxicological Profile for Mercury (update) Atlanta: Agency for Toxic Substances and Disease Registry; 1999. pp. 1–485. [Google Scholar]
- 12.Risher JF, Murray HE, Prince GR. Organic mercury compounds: human exposure and its relevance to public health. Toxicol Ind Health. 2002;18:109–160. doi: 10.1191/0748233702th138oa. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baughman TA. Elemental mercury spills. Environ Health Perspect. 2006;114:147–152. doi: 10.1289/ehp.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, Davis RB, Phillips RS. Heavy metal content of ayurvedic herbal medicine products. JAMA. 2004;292:2868–2873. doi: 10.1001/jama.292.23.2868. [DOI] [PubMed] [Google Scholar]
- 16.Mahdihassan S. Cinnabar-gold as the best alchemical drug of longevity, called Makaradhwaja in India. Am J Chin Med. 1985;13:93–108. doi: 10.1142/S0192415X85000149. [DOI] [PubMed] [Google Scholar]
- 17.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 18.Sin YM, Lim YF, Wong MK. Uptake and distribution of mercury in mice from ingesting soluble and insoluble mercury compounds. Bull Environ Contam Toxicol. 1983;31:605–612. doi: 10.1007/BF01605483. [DOI] [PubMed] [Google Scholar]
- 19.Zeng KW, Wang Q, Yang XD, Wang K. Investigation on dissolution of cinnabar in vitro] Zhongguo Zhong Yao Za Zhi. 2007;32:231–234. [PubMed] [Google Scholar]
- 20.He Z, Traina SJ, Weavers LK. Sonochemical dissolution of cinnabar (alpha-HgS) Environ Sci Technol. 2007;41:773–778. doi: 10.1021/es0613299. [DOI] [PubMed] [Google Scholar]
- 21.Sin YM, Teh WF, Wong MK. Absorption of mercuric chloride and mercuric sulphide and their possible effects on tissue glutathione in mice. Bull Environ Contam Toxicol. 1989;42:307–314. doi: 10.1007/BF01699416. [DOI] [PubMed] [Google Scholar]
- 22.Yeoh TS, Lee AS, Lee HS. Absorption of mercuric sulphide following oral administration in mice. Toxicology. 1986;41:107–111. doi: 10.1016/0300-483x(86)90108-3. [DOI] [PubMed] [Google Scholar]
- 23.Schoof RA, Nielsen JB. Evaluation of methods for assessing the oral bioavailability of inorganic mercury in soil. Risk Anal. 1997;17:545–555. doi: 10.1111/j.1539-6924.1997.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 24.Chuu JJ, Hsu CJ, Lin-Shiau SY. Abnormal auditory brainstem responses for mice treated with mercurial compounds: involvement of excessive nitric oxide. Toxicology. 2001;162:11–22. doi: 10.1016/s0300-483x(01)00348-1. [DOI] [PubMed] [Google Scholar]
- 25.Chuu JJ, Liu SH, Lin-Shiau SY. Effects of methyl mercury, mercuric sulfide and cinnabar on active avoidance responses, Na+/K+-ATPase activities and tissue mercury contents in rats. Proc Natl Sci Counc Repub China B. 2001;25:128–136. [PubMed] [Google Scholar]
- 26.Yen CC, Liu SH, Chen WK, Lin RH, Lin-Shiau SY. Tissue distribution of different mercurial compounds analyzed by the improved FI-CVAAS. J Anal Toxicol. 2002;26:286–295. doi: 10.1093/jat/26.5.286. [DOI] [PubMed] [Google Scholar]
- 27.Huang CF, Liu SH, Lin-Shiau SY. Neurotoxicological effects of cinnabar (a Chinese mineral medicine, HgS) in mice. Toxicol Appl Pharmacol. 2007;224:192–201. doi: 10.1016/j.taap.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Chuu JJ, Hsu CJ, Lin-Shiau SY. Differential neurotoxic effects of methylmercury and mercuric sulfide in rats. Toxicol Lett. 2007;169:109–120. doi: 10.1016/j.toxlet.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Young YH, Chuu JJ, Liu SH, Lin-Shiau SY. Neurotoxic mechanism of cinnabar and mercuric sulfide on the vestibulo-ocular reflex system of guinea pigs. Toxicol Sci. 2002;67:256–263. doi: 10.1093/toxsci/67.2.256. [DOI] [PubMed] [Google Scholar]
- 30.Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmaco. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang AH, Xu YJ, Shang MF. Analysis of adverse effects of cinnabar. Zhongguo Zhong Yao Za Zhi. 2005;30:1809–1811. [PubMed] [Google Scholar]
- 32.Ho BS, Lin JL, Huang CC, Tsai YH, Lin MC. Mercury vapor inhalation from Chinese red (Cinnabar) J Toxicol Clin Toxicol. 2003;41:75–78. doi: 10.1081/clt-120018275. [DOI] [PubMed] [Google Scholar]
- 33.Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. Granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar) Arch Dermatol. 1987;123(1557):1560–1561. doi: 10.1001/archderm.123.11.1557b. [DOI] [PubMed] [Google Scholar]
- 34.Sin YM, Teh WF, Wong WF. Effect of long-term uptake of mercuric sulphide on thyroid hormones and glutathione in mice. Bull Environ Contam Toxicol. 1992;49:847–854. doi: 10.1007/BF00203157. [DOI] [PubMed] [Google Scholar]
- 35.Hardy AD, Sutherland HH, Vaishnav R, Worthing MA. A report on the composition of mercurials used in traditional medicines in Oman. J Ethnopharmacol. 1995;49:17–22. doi: 10.1016/0378-8741(95)01296-6. [DOI] [PubMed] [Google Scholar]
- 36.Bigazzi PE. Metals and kidney autoimmunity. Environ Health Perspect. 1999;107(Suppl 5):753–765. doi: 10.1289/ehp.99107s5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry GA, Jarnot BM, Steinhoff MM, Bigazzi PE. Mercury-induced renal autoimmunity in the MAXX rat. Clin Immunol Immunopathol. 1988;49:187–203. doi: 10.1016/0090-1229(88)90109-2. [DOI] [PubMed] [Google Scholar]
- 38.Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol Appl Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 39.Davidson PW, Myers GJ, Weiss B, Shamlave CF, Cox C. Prenatal methyl mercury exposure from fish consumption and child development: A review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicology. 2006;27:951–969. doi: 10.1016/j.neuro.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 40.NRC. Toxicological effects of methylmercury / Committee on the Toxicological Effects of Methylmercury, Board on Environmental Studies and Toxicology, Commission on Life Sciences. Washington DC: National Research Council; 2000. pp. 1–344. National Academy. [Google Scholar]
- 41.Risher JF, Amler SN. Mercury exposure: evaluation and intervention the inappropriate use of chelating agents in the diagnosis and treatment of putative mercury poisoning. Neurotoxicology. 2005;26:691–699. doi: 10.1016/j.neuro.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q, Yang X, Zhang B, Yang X, Wang K. The anxiolytic effect of cinnabar involves changes of serotonin levels. Eur J Pharmacol. 2007;565:132–137. doi: 10.1016/j.ejphar.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 43.Huang ZY, Shen JC, Zhuang ZX, Wang XR, Lee FS. Investigation of metal-binding metallothioneins in the tissues of rats after oral intake of cinnabar. Anal Bioanal Chem. 379:427–432. doi: 10.1007/s00216-004-2624-z. [DOI] [PubMed] [Google Scholar]
- 44.Zhu KJ, Sun JN, Ma CH, Geng Y. Effect of angong niuhuang pill and heavy metal constituents on EcoG of brain damage caused by LPS in rats. Zhongguo Zhong Yao. 2007;32:949–953. [PubMed] [Google Scholar]
- 45.Tang YS, Lin PY, Ou WP. Effects of cinnabar and realgar in angong niuhuang powder on lactate dehydrogenase and its isoenzymes in rats with infectious cerebral edema. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:436–440. [PubMed] [Google Scholar]
- 46.Zhao Y, Cao CY, Wang XZ, Cui HF, Wang YS, Wang ZM, Ye ZG, Du GY. Effects of realgar and cinnabar in Angong Niuhumang Pill on ischemia brain injury in rats. Zhongguo Zhong Xi Yu Jiehe Za zhi. 2002;22:684–688. [Google Scholar]
- 47.Wang JH, Ye ZG. Current research on angong niuhuang pills. Zhongguo Zhong Yao Za Zhi. 2004;29:119–122. [PubMed] [Google Scholar]